Abstract
The use of Artemisia species’ plant extracts and essential oils, which are rich in bioactive compounds (allelochemicals), could support weed management. This study focused on the chemical analysis and evaluation of the allelopathic potential of plant extracts (PEs) and essential oils (EOs) of Artemisia absinthium and A. vulgaris on the germination and early seedling growth of weeds (Amaranthus retroflexus and Setaria viridis) in vitro. The plant extract from A. vulgaris showed higher antioxidant activity (IC50 = 0.171 ± 0.01 mg/mL) and phenolic content than that from A. absinthium (IC50 = 0.263 ± 0.01 mg/mL). Chlorogenic acid was the most abundant phenol in both extracts. However, A. absinthium contained a higher amount (1.694 ± 0.081 mg/g) and exhibited a stronger inhibitory effect on the germination of A. retroflexus (EC50 = 0.54 ± 0.02%) and S. viridis (EC50 = 1.51 ± 0.07%) compared to A. vulgaris. The dominant components of A. absinthium essential oil were β-thujone (18.9%), cis-ocimene epoxide (7.88%), and bicyclogermacrene (7.04%), while the main constituents of A. vulgaris essential oil included gurjunene (10.41%), cis-crysanthenyl acetate (7.17%), and γ-humulene (6.67%). The lowest EC50 values for A. absinthium essential oil regarding seed germination and seedling length were estimated for S. viridis (0.28 ± 0.48% and 0.03 ± 0.00%, respectively), whereas A. retroflexus was the most sensitive to A. vulgaris essential oil (0.11 ± 0.04% and 0.02 ± 0.00%, respectively). All tested extracts showed allelopathic potential; however, the results indicate that the essential oils had a stronger inhibitory effect than the plant extracts.
1. Introduction
Sustainable environmental and production management in modern agriculture means facing up to the challenges of climate change, environmental pollution, depletion of natural resources, and dependence on agricultural inputs. The harmful effects of herbicides on the environment and human health, the ever-increasing number of herbicide-resistant weed populations, the increasing number of invasive alien weed species, the slower development of novel herbicides, the ban on the use of many herbicides, and the intensified focus on organic farming are some of the main factors that have promoted eco-friendly approaches to weed control in recent decades [1,2,3]. In the last two decades, the concept of allelopathy has been employed to reduce our heavy reliance on synthetic herbicides and to find a promising solution to the problems of environmental pollution and herbicide resistance as well as ecological weed management [4,5]. The phytotoxic properties of allelochemicals exuded by allelopathic plants mean they can be a source for the identification and isolation of a wide selection of potential new environmentally acceptable bioherbicides. More than 2000 plant species (39 families) have been found to have strong allelopathic potential [6], but only 3% of the approximately 400,000 known compounds in plants have herbicidal impact [7]. These allelochemicals can influence the germination and growth of weeds through different modes of action [7]. The allelochemicals can affect vital biochemical and physiological processes in plants, e.g., respiration, photosynthesis, cell division and elongation, membrane permeability, water balance, protein biosynthesis, and the activity of many enzymes [8].
Allelochemicals belong to various chemical families, including phenols, flavonoids, terpenoids, glucosinolates, benzoquinones, and cyanogenic compounds [9]. Essential oils and plant extracts have long been utilized as sources of bioactive molecules, particularly phenolic compounds and terpenes—two groups of allelochemicals recognized for their allelopathic potential. Generally, essential oils exhibit higher toxicity than extracts; however, if the extracts originate from plants known to produce toxic metabolites, these extracts may be more toxic than essential oils [10]. Although there is a lack of comparative studies on both types of extracts, published results indicate that essential oils have a stronger growth inhibitory effect on weeds than plant extracts [11]. Furthermore, essential oils demonstrate high biodegradability in the environment and relative safety for humans and other non-target organisms compared to synthetic pesticides [12]. The use of essential oils as biopesticides presents numerous challenges due to their inherent properties (lipophilicity and high volatility), production costs, and manufacturing limitations [3].
The Asteraceae family is a natural source of allelopathic sesquiterpenes and sesquiterpene lactones. They can inhibit the enzyme asparagine synthase, thereby preventing growth, and impair the respiration of mitochondrial cell organelles and the release of proteins into the plasma membrane [13]. In particular, the genus Artemisia L. comprises over 200 species worldwide, nine of which occur in the flora of Serbia: A. vulgaris, A. absinthium, A. annua, A. pontica, A. petrosa, A. lobelii, A. maritima, A. campestris, and A. scoparia [14]. Plants of this genus exhibit a broad spectrum of biological activities (antifungal, insecticidal, and herbicidal effects), including medicinal properties (anthelmintic, antimalarial, antispasmodic, anti-inflammatory, antirheumatic, and anticancer), due to the presence of different phytochemicals [15,16,17,18]. To our knowledge, there is no data in the literature regarding a dual approach to the allelopathic potential of plant extracts and essential oils from A. absinthium and A. vulgaris on seed germination and early seedling growth of weed species. Keeping all this in mind, the present study focused on (I) extraction of plant material from the aboveground parts of A. absinthium and A. vulgaris originating from Serbia; (II) analysis of total phenolic content (TPC) and evaluation of antioxidant activity of A. absinthium and A. vulgaris plant extracts (PEs); (III) identification and quantification of major phenolic compounds in the plant extracts (PEs) and terpenes in the essential oils (EOs) of A. absinthium and A. vulgaris; and (IV) in vitro evaluation of the allelopathic potential of the PEs and EOs of A. absinthium and A. vulgaris on seed germination and early seedling growth of two economically noxious weed species (Amaranthus retroflexus and Setaria viridis) widely distributed in arable fields.
2. Results
2.1. Chemical Analysis of A. absinthium and A. vulgaris Plant Extracts
The results of the UHPLC-DAD MS/MS analysis of the individual phenolic compounds in both Artemisia extracts are shown in Table 1. Chromatograms can be found in the Supplementary Materials (Figure S1). In A. absinthium, two tested compounds, p-coumaric acid and luteolin, were below the detection limit. The most abundant phenolic compound was chlorogenic acid (1.694 ± 0.081 mg/g d.e.), while kaempferol-3-O-glucoside (0.197 ± 0.032 mg/g d.e.), rutin (0.135 ± 0.019 mg/g d.e.), isorhamnetin-3-O-rutinoside (0.090 ± 0.013 mg/g d.e.), and hyperoside (0.066 ± 0.011 mg/g d.e.) were among the other significant phenolic compounds likely responsible for the high antioxidant activity of the plant. In A. vulgaris, isorhamnetin was below the detection limit among the tested compounds. In this extract, chlorogenic acid (1.381 ± 0.075 mg/g d.e.) also contributed the most to the high phenolic content, followed by rutin (0.821 ± 0.046 mg/g d.e.), kaempferol-3-O-glucoside (0.579 ± 0.030 mg/g d.e.), and hyperoside (0.212 ± 0.015 mg/g d.e.).

Table 1.
Quantification of identified phenols in plant extracts (PEs) of A. absinthium and A. vulgaris.
The analysis of the total phenolic content shows that these compounds were present in significant amounts in both PEs, with the total phenolic content being higher in A. vulgaris (73.7 ± 2.5 mg GAE/g d.e.) than in A. absinthium (58.4 ± 2.4 mg GAE/g d.e.). The scavenging effect of the PEs of A. absinthium and A. vulgaris on DPPH radicals is shown in Figure 1. The PE of A. vulgaris with an IC50 value of 0.171 ± 0.01 mg/mL showed a greater reduction of DPPH and inhibited almost 90% of DPPH radicals at the highest concentration used (0.5 mg/mL), compared to A. absinthium, which had an IC50 value of 0.263 ± 0.01 mg/mL and inhibited approximately 75% of DPPH radicals at the same concentration. Both PEs showed moderate DPPH reduction compared to ascorbic acid, which had an IC50 value of 0.018 mg/mL. The results of the reducing antioxidant power of ferric (FRAP) analysis showed that A. vulgaris also had a higher capacity to reduce metal ions than A. absinthium. The FRAP value for the PE of A. vulgaris was 180.1 ± 21.4 µmol Fe2+/g d.e., while the PE of A. absinthium had a FRAP value of 138.0 ± 17.6 µmol Fe2+/g d.e. However, the reduction potential of both plant PEs was significantly lower than that of the standard used, as the FRAP value of ascorbic acid was 2576 ± 21 μmol Fe2+/g. Overall, the results showed that the PEs from A. vulgaris and A. absinthium demonstrate lower metal-reducing ability compared to their free radical scavenging ability.
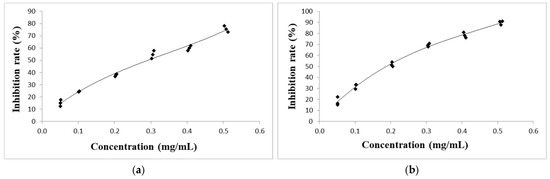
Figure 1.
The scavenging effect of PEs of A. absinthium (a) and A. vulgaris (b) on DPPH radical.
2.2. Chemical Analysis of A. absinthium and A. vulgaris Essential Oils
The EOs of A. absinthium and A. vulgaris were extracted with yields of 0.088% (v/w) and 0.012% (v/w), respectively. The main oil components (compounds with contents higher than 1%) are detailed in Table 2. Chromatograms can be found in the Supplementary Materials (Figure S2). The complete chemical analysis of the EOs from A. absinthium and A. vulgaris, which account for over 99% of the total oil mass, is presented in Supplementary Table S1. The EO of A. absinthium contained 19 compounds that exceeded 1% in quantity, representing 77.92% of the total oil mass. The most prominent chemical compound classes were monoterpenes (42.43%) and sesquiterpenes (28.58%), with mono- and sesquiterpene hydrocarbons contributing 7.90% and 18.96%, while oxygenated mono- and sesquiterpenes contributed 34.53% and 9.62%, respectively. The oil also contained geranyl-terpinene (1.51%) as a diterpene hydrocarbon, 13-epi-manool oxide (3.27%) as an oxygenated diterpene, and hexadecyl acetate (2.13%) as an ester compound. The most abundant components of the oil were trans-thujone (β-thujone) (18.9%), linalool (4.17%), and cis-ocimene epoxide (7.88%) as oxygenated monoterpenes; β-caryophyllene (6.0%), germacrene D (4.71%), and bicyclogermacrene (7.04%) as sesquiterpene hydrocarbons; and germacrene D-4-ol (5.35%) as an oxygenated sesquiterpene (alcohol).

Table 2.
The main components of A. absinthium and A. vulgaris essential oils (EOs).
In the EO of A. vulgaris, 21 components with contents of more than 1% were identified, accounting for 75.97% of the total oil mass. The most dominant components were gurjunene (10.41%), γ-humulene (6.67%), β-caryophyllene (5.81%), α-isocomene (5.15%), germacrene D (4.88%), and β-selinene (4.86%) as sesquiterpene hydrocarbons; cis-chrysanthenyl acetate (7.17%) as an oxygenated monoterpene; and davanone (5.62%) as an oxygenated sesquiterpene. Overall, the composition of the EO of A. vulgaris was dominated by sesquiterpene hydrocarbons (51.91%). The content of oxygenated sesquiterpenes and monoterpenes was 11.11% and 7.17%, respectively, with monoterpene hydrocarbons accounting for 3.93%, while geranyl benzoate, as a benzoate ester, accounted for 1.85% of the oil mass.
2.3. Assessment of the Allelopathic Potential of Plant Extracts of A. absinthium and A. vulgaris In Vitro
A. absinthium and A. vulgaris PEs at concentrations of 0.25%, 0.50%, 0.75%, 1.00%, 2.50%, and 5.00% significantly reduced the seed germination and early growth of seedlings of A. retroflexus and S. viridis (see Table S2). The inhibition of seed germination of A. retroflexus at the highest A. absinthium PE concentrations (0.75%, 1.00%, 2.50%, and 5.00%) ranged from 78% to 100%, while the inhibition at the lowest A. absinthium PE concentrations (0.25% and 0.5%) was 8% to 43% (Figure 2a). Similarly, inhibition of all seedling growth parameters decreased in proportion to decreasing PE concentrations. The radicle length parameter was more sensitive, and an inhibition from 6% to 100% was recorded, while the inhibition for shoot length was lower (16–100%), except at the 0.25% PE concentration, where a stimulation of 13% was noted (Figure 2b). In general, the seeds of S. viridis were less sensitive than those of A. retroflexus to the different concentrations of PE A. absinthium. The inhibition of seed germination at the two highest concentrations (2.50% and 5.00%) was 92% and 100%, respectively. On the other hand, the concentrations of 1.00%, 0.75%, 0.50%, and 0.25% caused a negligible inhibition from 1% to 13% (Figure 2a). The inhibition increased in proportion to the increasing PE concentrations of A. absinthium for the seedling growth parameters (radicle and shoot length) and was similar for both parameters and ranged from 14% to 100% (Figure 2b,c). Based on the estimated regression parameters (Table 3), it was found that the concentrations of 0.54 ± 0.02% (A. retroflexus) and 1.51 ± 0.07% (S. viridis) of A. absinthium PE were sufficient to cause 50% inhibition of seed germination of the test plants. The EC50 values for seedling length showed a similar sensitivity for both weeds (Figure 2d). An exception was the radicle sensitivity (Figure 2c), where lower values were determined for A. retroflexus (0.18 ± 0.02%) than for S. viridis (0.55 ± 0.01%).
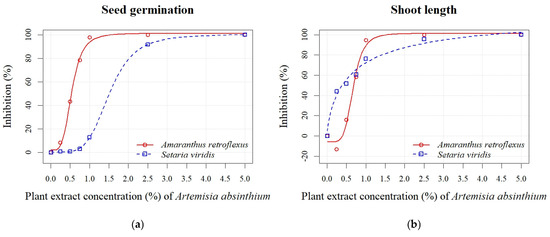
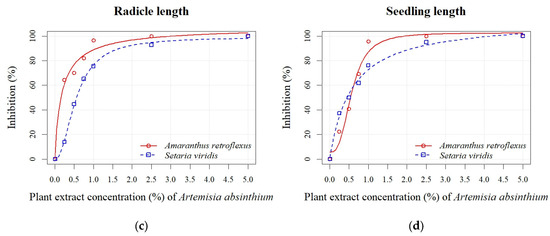
Figure 2.
Effects of different PE concentrations (0.25%, 0.50%, 0.75%, 1.00%, 2.50%, and 5.00%) of A. absinthium on seed germination (a), shoot length (b), radicle length (c), and seedling length (d) of A. retroflexus and S. viridis.

Table 3.
Regression parameters for seed germination, shoot length, radicle length, and seedling length for the test plants (A. retroflexus and S. viridis) after application of PEs of A. absinthium and A. vulgaris.
In general, the PE of A. vulgaris had a similar impact on seed germination and radicle length of A. retroflexus as A. absinthium. Conversely, the shoot length parameter was less sensitive, with stimulation of 19% and 35% recorded at concentrations of 0.50% and 0.25%, respectively (Figure 3b). Inhibition of seed germination of S. viridis was only noted at the highest concentrations (1.00%, 2.50%, and 5.00%) and ranged from 22% to 100% (Figure 3a). The parameters of seedling growth (radicle and shoot length) were more sensitive in both weed species in the treatment with PE of A. vulgaris than in the treatment with A. absinthium. The inhibition of radicle length ranged from 52% to 100% (Figure 3c), while shoot length ranged from 50% to 100% (Figure 3b). The calculated EC50 values of A. vulgaris PE for seed germination indicated slightly lower sensitivity for A. retroflexus (0.63 ± 0.01%) and S. viridis (1.74 ± 0.11%) in contrast to A. absinthium PE. The EC50 values for seed germination confirmed the higher sensitivity of A. retroflexus compared to S. viridis to both PEs. However, the EC50 values for seedling length revealed that both tested weeds were similarly sensitive to A. absinthium PE. On the other hand, S. viridis was more sensitive to PE of A. vulgaris than to A. absinthium (Figure 2d and Figure 3d).
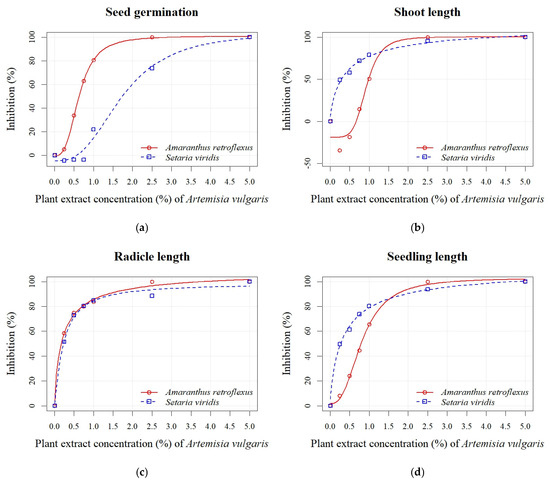
Figure 3.
Effects of different PE concentrations (0.25%, 0.50%, 0.75%, 1.00%, 2.50%, and 5.00%) of A. vulgaris on seed germination (a), shoot length (b), radicle length (c), and seedling length (d) of A. retroflexus and S. viridis.
2.4. Assessment of the Allelopathic Potential of Essential Oils of A. absinthium and A. vulgaris In Vitro
Increasing the EO concentrations of both Artemisia species enhanced the percentage of inhibition for all measured parameters in both weed species (Figure 4 and Figure 5). The absolute values of the measured parameters are shown in Supplementary Table S3. At the highest EO concentration of A. absinthium (0.50%), the seed germination of A. retroflexus was inhibited by 86%, while at lower concentrations (0.01–0.25%), the percentage of inhibition ranged from 1% to 48% (Figure 4a). All seedling growth parameters (shoot and radicle length) showed similar sensitivity, with inhibition of seedling length varying from 4% to 96% at all applied EO concentrations (Figure 4d). Inhibition of S. viridis germination at the highest concentrations of A. absinthium EO (0.10%, 0.25%, and 0.50%) ranged from 18% to 100%, with no significant differences observed between the lower concentrations (0.01%, 0.025%, and 0.05%) and the control. Although germination of S. viridis was inhibited by 18% at a concentration of 0.1%, seedling length was inhibited by 91% at the same EO concentration. The shoot length parameter also exhibited greater sensitivity, with inhibition ranging from 2% to 100% (Figure 4b). According to the estimated EC50 values for all measured parameters, S. viridis showed greater sensitivity than A. retroflexus (Table 4). Concentrations of 0.03 ± 0.00% and 0.28 ± 0.48% effectively achieved 50% inhibition of seedling length and seed germination of S. viridis, respectively.
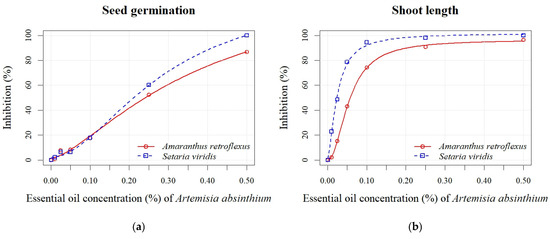
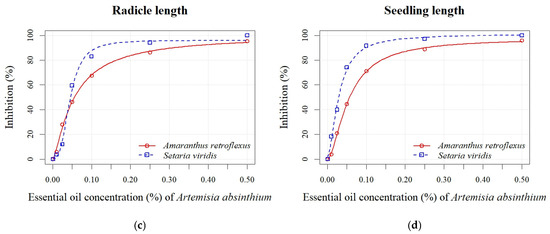
Figure 4.
Effects of different EO concentrations (0.01, 0.025, 0.05, 0.10, 0.25, and 0.50%) of A. absinthium on seed germination (a), shoot length (b), radicle length (c), and seedling length (d) of A. retroflexus and S. viridis.
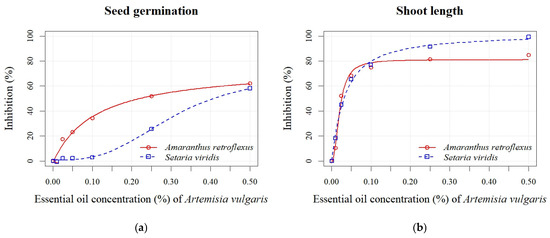
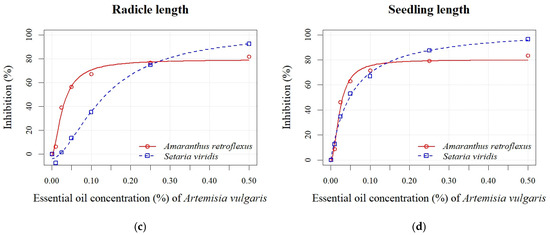
Figure 5.
Effects of different EO concentrations (0.01, 0.025, 0.05, 0.10, 0.25, and 0.50%) of A. vulgaris on seed germination (a), shoot length (b), radicle length (c), and seedling length (d) of A. retroflexus and S. viridis.

Table 4.
Regression parameters for seed germination, shoot length, radicle length, and seedling length for the test plants (A. retroflexus and S. viridis) after application of EOs of A. absinthium and A. vulgaris.
The inhibition of seed germination of A. retroflexus after applying various concentrations of A. vulgaris EO ranged from 0% to 62% (Figure 5a). The inhibitory effect on seed germination was observed even at the lowest concentration (0.01%). Inhibition increased proportionally with rising A. vulgaris EO concentration for all seedling growth parameters (radicle and shoot length). Inhibition of shoot length ranged from 10% to 85% (Figure 5b), while radicle length inhibition varied from 6% to 82% (Figure 5c). S. viridis showed lower sensitivity to the EO of A. vulgaris than to A. absinthium. Significant differences were found only between the control and the highest concentrations (0.25% and 0.50%), at which germination of S. viridis was inhibited by 26% and 58%, respectively (Figure 5a). Higher percentages of inhibition were recorded for seedling length, which ranged from 13% to 96% (Figure 5d). Similar to the application of A. absinthium EO, the radicle length parameter was less sensitive than the shoot length. A stimulating effect (8%) was also observed at the lowest concentration (0.01%) of A. vulgaris EO (Figure 5c). Based on the calculated EC50 values for both EOs across all measured parameters, seedling growth (shoot and radicle length) demonstrated greater sensitivity than seed germination (Table 4). In contrast to A. absinthium EO, lower EC50 values were found for seed germination and seedling length of A. retroflexus (0.11 ± 0.04% and 0.02 ± 0.00%, respectively) compared to S. viridis (0.31 ± 0.07% and 0.05 ± 0.00%, respectively) after the application of A. vulgaris EO.
3. Discussion
Due to their chemical diversity and biological activity, interest in the biological properties of Artemisia species has increased. The results demonstrate that the PEs and EOs of the two examined Artemisia species are rich in phenols and volatiles and possess allelopathic properties.
The findings revealed that the PE of A. vulgaris contained higher levels of phenols (73.7 ± 2.5 mg GAE/g d.e.) than A. absinthium (58.4 ± 2.4 mg GAE/g d.e.). Additionally, the extract of A. vulgaris was found to be effective in scavenging the DPPH radical and had a greater ability to reduce metal ions, suggesting superior antioxidant activity compared to A. absinthium. The high antioxidant potential of A. vulgaris extracts, with chlorogenic acid derivatives and flavonoids as main constituents, has also been reported [20].
Despite the higher content of phenols and antioxidant activity of A. vulgaris PE, the PE of A. absinthium showed a greater inhibitory effect on seed germination of both weed species. Furthermore, A. absinthium PE (EC50 = 0.57 ± 0.03%) proved to be more effective than A. vulgaris PE (EC50 = 0.82 ± 0.02%) in inhibiting seedling growth (radicle and shoot length) of A. retroflexus. An exception was S. viridis, where the EC50 values of A. vulgaris PE for shoot and radicle length (0.41 ± 0.04% and 0.23 ± 0.01%, respectively) were lower than those of A. absinthium (0.64 ± 0.15% and 0.55 ± 0.01%, respectively). A similar phenomenon was described by Marcinkeviciene et al. [21], where the aqueous extract of A. vulgaris leaves, which had a higher content of phenolics, showed weaker inhibition of seed germination, root, and shoot growth of winter wheat and oilseed rape than the aqueous extract of A. vulgaris roots with a lower content of phenolic compounds. Radicle length was the most sensitive parameter following the application of both PEs. Lower EC50 values for radicle compared to shoot length were also reported after applying the aqueous leaf extract of A. absinthium on Parthenium hysterophorus [18]. The shoot growth of A. retroflexus was stimulated after applying the lowest concentration (0.25%) of both PEs, and a relatively significant stimulation (19%) was also observed after the application of 0.50% of A. vulgaris PE. The stimulatory effects following the application of lower concentrations of various PEs are already well documented and have been observed in A. vulgaris [22,23]. In both PEs, chlorogenic acid was the main constituent, but a higher concentration of this phenolic acid was found in the PE of A. absinthium (1.694 ± 0.081 mg/g) than in the PE of A. vulgaris (1.381 ± 0.075 mg/g), which could explain the greater allelopathic effect of the PE of A. absinthium. The phytotoxic effect of chlorogenic acid and its impact on seed germination, root, and shoot length of various plants have already been reported [24]. In addition, the allelopathic potential of various solvent extracts of Artemisia species with chlorogenic acid as one of the dominant compounds has already been confirmed [25,26]. Xu et al. [27] discovered DHAR1 (dehydroascorbate reductase 1), an enzyme responsible for ascorbate regeneration in plants, as the target site of chlorogenic acid, whose inhibition of activity led to the accumulation of H2O2 and the reduction of proteins involved in water transport and photosynthesis. Chlorogenic acid was also identified as a dominant compound in other Artemisia species (A. alba, A. annua, A. campestris, A. ponica, and A. vulgaris) growing in Serbia [28].
The EO of A. absinthium consisted of 34.53% of components containing more than 1% belonging to oxygenated monoterpenes, while sesquiterpene hydrocarbon compounds, accounting for 51.91%, were the major constituents of the EO of A. vulgaris. Previous studies have demonstrated that monoterpenes, particularly oxygenated monoterpenes, can have toxic effects on the growth of numerous plants [29]. A comparison of the composition of A. vulgaris EO with that of the same species from Serbia shows relatively good agreement between the results [30,31,32], although certain differences in the composition and concentration of individual components can be explained by the various geo-eco-climatic conditions under which the plants were grown. Among other findings, these differences were corroborated by Ickovski et al. [31], who analyzed EOs extracted from A. vulgaris across 12 different localities. As in the case of the EO of A. vulgaris, certain differences in the composition and content of the individual components of the EO of A. absinthium can be explained by the different geo-eco-climatic conditions under which the plants were grown, which was confirmed by Ickovski et al. [31], who analyzed EOs extracted from 12 different locations, and Blagojević et al. [32], who analyzed 3 EOs isolated from plants grown in different localities. In general, oxygenated monoterpenes were the most dominant components in all analyzed EOs (from 72.3% to 89.1% by Blagojević et al. [32] and from 37.1% to 73.8% by Ickovski et al. [31]), with β-thujone being the most dominant component in most of them, which is consistent with our results. Additionally, the high content of β-thujone (25.75%) in the EOs isolated from the leaves of A. absinthium originating from Tunisia has already been reported [33]. Similarly, Fouad et al. [34] found that β-thujone (35.6%) was the main constituent of the EO of A. absinthium originating from Morocco.
Most studies investigating the allelopathic potential of Artemisia species have focused on examining the herbicidal properties of their EOs, with only a few exploring aqueous or alcoholic extracts [35]. The complex chemical composition of essential oils makes isolating their components and determining their potential mode of action difficult. The phytotoxic effects of essential oils have been associated with plant growth reduction, leaf chlorosis, changes in plant cells, mitosis, cellular respiration, chlorophyll content, membrane depolarization, ion leakage, cuticular waxes, oxidative stress, and microtubular polymerization [36]. This study confirms that the EOs of both Artemisia species exhibit a significantly higher inhibitory effect on the tested economically noxious weed species compared to the PEs, suggesting a greater allelopathic potential of the terpenes than the phenolic compounds. The calculated EC50 values for both EO and PE indicate that seedling growth is a more sensitive parameter than seed germination for both weed species. Based on the estimated regression parameters, the EC50 values for the investigated EOs ranged from 0.11 ± 0.04% to 0.37 ± 0.15% for seed germination and from 0.02 ± 0.00% to 0.06 ± 0.00% for seedling length. Other authors have also pointed out a greater sensitivity of seedling growth compared to seed germination [37,38]. The EC50 values determined for shoot and radicle length (0.02 ± 0.00% and 0.03 ± 0.00%) of A. retroflexus treated with A. vulgaris EO were slightly lower than the values reported by Han et al. [39] regarding the effects of this EO on the same test plant and parameters (EC50–0.356 mg/mL for shoot length and 0.308 mg/mL for root length). The lowest EC50 values for A. absinthium EO in terms of seed germination and seedling length were estimated for S. viridis (0.28 ± 0.48% and 0.03 ± 0.00%, respectively). The inhibitory effect of A. absinthium EO has already been confirmed in A. retroflexus, Poa annua [40], and Sinapis arvensis [34].
4. Materials and Methods
4.1. Collection and Extraction of Plant Material
Aboveground plant parts of A. absinthium and A. vulgaris were collected during the flowering period in July and August 2022 in Vratna (44°22′57″ N; 22°20′31″ E) and Rumenka (45°18′28″ N; 19°43′40″ E), respectively. The collected plant material was dried in the shade at a temperature of 22 ± 1 °C for three weeks and then stored in paper bags in a dry place until extraction. The seeds of A. retroflexus were collected in the field around Noćaj (44°55′33.5″ N 19°32′43.6″ E), and seeds of S. viridis were collected in the field around Majur (44°46′10.1″ N 19°40′30.0″ E) on non-arable habitat in October 2022.
The EOs were obtained from the dried aboveground plant material of A. absinthium (40 kg) and A. vulgaris (61 kg) by steam distillation in a small-scale distillation unit at the Institute of Field and Vegetable Crops in Novi Sad [41]. After drying over anhydrous sodium sulfate (Na2SO4), the EOs were stored in an amber bottle in a cold and dark place until use.
The PEs of A. absinthium and A. vulgaris were obtained by successive solvent extraction using seven solvents (hexane, ethyl acetate, acetone, acetonitrile, ethanol, methanol, and distilled water) to extract phenolic compounds with a broad polarity spectrum. The solvents were used successively in a serial manner in a 1:4 w/v ratio and sonicated for 15 min at 40 °C (frequency 70 kHz and power 240 W). After that, every aliquot of the extract was filtered separately through filter paper and evaporated to dryness at 40 °C using a vacuum rotary evaporator. After evaporation, the dry residue of each plant extract was merged and subjected to a lyophilization process. The PEs from both Artemisia species were stored dry until use.
4.2. Chemical Analysis of A. absinthium and A. vulgaris Plant Extracts
Quantitative and qualitative analyses of phenolic compounds in PEs were performed using a Dionex Ultimate 3000 UHPLC system equipped with a diode array detector (DAD) connected to a TSQ Quantum Access Max triple-quadrupole mass spectrometer (ThermoFisher Scientific, Basel, Switzerland), as previously described by Gašić et al. [42]. To prepare the extracts for analysis, 5 mg of plant extract was weighed and mixed with 96% methanol (w/v = 1:10) in an ultrasonic bath for 30 min. After centrifugation at 10,000× g for 10 min, the supernatants were filtered through 0.2 µm cellulose filters (Agilent Technologies, Santa Clara, CA, USA) and stored at 4 °C until analysis. The results were expressed as mg/g of dry extract.
The total phenolic content (TPC) of the PEs of A. absinthium and A. vulgaris was determined using the modified Folin–Ciocalteu method [43]. The TPC results were reported as mg gallic acid per g of dry extract (mg GAE/g d.e.). The antioxidant activity of the PEs was evaluated by determining the free radical scavenging activity using the modified DPPH method [44] and measuring the reducing potential using the modified FRAP assay [45]. The IC50 value (the concentration of the sample required to scavenge 50% of DPPH) was calculated, and the reducing potential results were expressed as mmol of ferric ions (Fe2+) per g of dry extract. Ascorbic acid served as a positive control for both methods.
4.3. Chemical Analysis of A. absinthium and A. vulgaris Essential Oils
Identification of the compounds in the EOs of A. vulgaris and A. absinthium was carried out using gas chromatography–mass spectrometry (GC-MS, model CP-3800/Saturn 2200), which was equipped with a split/splitless injector and a DB-5MS column (30 m × 0.25 mm and 0.25 µm film thickness). The Wiley 7.0 mass spectral library was utilized, and the obtained experimental retention indices (RIs) were compared with literature data [19]. Quantitative analysis of the EOs was performed on a gas chromatograph (GC, Agilent 7890A) with a split/splitless injector, a DB-5MS column (30 m × 0.25 mm, 0.25 µm film thickness), and a flame ionization detector (FID). In both cases, 1 µL of the hexane solution of the EO sample (1% solution) was injected in split mode (1:20). For the GC-MS analysis, the injector temperature was set to 250 °C, while the ion trap and transfer line temperatures were set to 250 °C and 280 °C, respectively. Helium was used as the carrier gas with a flow rate of 1 mL/min. The column temperature was programmed to increase linearly from 50 °C to 250 °C at a rate of 4 °C/min, with an isothermal hold at 250 °C for 10 min. The mass detector was operating in electron impact (EI) mode at 70 eV, with the mass range of 40–600 m/z. Total ion current (TIC) was used to record the chromatograms obtained. In the GC-FID analysis, the injector and detector temperatures were set to 250 °C and 300 °C, respectively. Hydrogen was used as the carrier gas at a flow rate of 1 mL/min, and the temperature program of the column was identical to that used in the GC-MS analysis.
4.4. Assessment of the Allelopathic Potential of Plant Extracts and Essential Oils of A. absinthium and A. vulgaris In Vitro
Three factors were considered in the in vitro evaluation of allelopathic potential: (I) weed seeds (A. retroflexus and S. viridis); (II) PEs of A. absinthium and A. vulgaris in the concentrations 0.25%, 0.50%, 0.75%, 1.00%, 2.50%, and 5.00% w/v (prepared in distilled water); and (III) EOs of A. absinthium and A. vulgaris in the concentrations 0.01, 0.025, 0.05, 0.10, 0.25, and 0.50% v/v (prepared in distilled water with the surfactant Tween 20, conc. 0.05% v/v). Distilled water and distilled water with added surfactant were used as controls. The test seeds were soaked in a 2% thiourea solution [SC(NH2)2] for 24 h to overcome their dormancy. The seeds were then surface sterilized in 3% hydrogen peroxide (H2O2) for 5 min and then rinsed with distilled water. Twenty-five soaked seeds were placed in a Petri dish (90 mm diameter) with filter paper, and 5 mL of the PE or EO solution was added. All Petri dishes were sealed with Parafilm to avoid evaporation and placed in an incubator (Memmert, Schwabach, Germany) at 27 ± 1 °C in the dark conditions. All treatments were carried out in four repetitions, and the experiment was repeated twice. The percentage of germinated seeds was calculated, and early seedling growth (radicle length, shoot length, and their sum, expressed as seedling length) was measured after seven days.
4.5. Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA) using the software package STATISTICA 8.0. When F values were statistically significant (p < 0.05), treatments were compared using Fisher’s least significant difference (LSD) test. Data on inhibition of germination and early seedling growth (seedling length, shoot, and radicle length) were analyzed with the program R using the statistical add-on package “drc” with a four-parameter log-logistic nonlinear regression model [46]:
where Y is the response (e.g., percent of inhibition), C is the lower limit, D is the upper limit, E is the dose resulting in a 50% response between the upper and lower limits (also known as inflection point I50 or EC50), the parameter B denotes the relative slope around E, and X is the concentration of PEs and EOs of A. absinthium and A. vulgaris.
Y = C + (D − C)/1 + exp (B (logX − logE))
5. Conclusions
The analysis of phenolic compounds from both Artemisia species growing in Serbia in this study demonstrates that A. absinthium and A. vulgaris PEs are rich sources of phenolic and flavonoid compounds with pronounced antioxidant activity. Additionally, this study reveals that A. absinthium and A. vulgaris EOs are rich sources of terpenes with high allelopathic potential. The EOs of both Artemisia species have a greater inhibitory effect on the germination and early seedling growth of A. retroflexus and S. viridis compared to PEs. The relevant data lead to the conclusion that the EOs of A. absinthium and A. vulgaris could serve as potential sources of natural-based weed control molecules. The prospects for practical application will be investigated through further studies, which will include the identification and isolation of the most effective allelochemicals from this source and validation of the present results in vivo and under field conditions, as well as regarding different weeds and crops.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14111663/s1, Table S1: The complete chemical composition of A. absinthium and A. vulgaris essential oil; Table S2: Effects of different concentrations of A. absinthium and A. vulgaris plant extract on seed germination and seedling growth of A. retroflexus and S. viridis; Table S3: Effects of different concentrations of A. absinthium and A. vulgaris essential oil on seed germination and seedling growth of A. retroflexus and S. viridis; Figure S1: Chromatogram of A. absinthium (a) and A. vulgaris (b) plant extract; Figure S2: Chromatogram of A. absinthium (a) and A. vulgaris (b) essential oil.
Author Contributions
Conceptualization, T.T., S.V. and M.S.-K.; methodology, all authors; software, T.T. and M.S.-K.; validation, S.V. and M.S.-K.; formal analysis, T.T. and M.S.-K.; investigation, S.V. and T.T.; data curation, all authors; writing—original draft preparation, T.T.; writing—review and editing, S.V., M.S.-K., T.Đ., R.Đ.-P., M.A., D.B. and L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education, Science, and Technological Development of the Republic of Serbia, grant numbers [451–03–137/2025–03/200116] to T.T., D.B. and S.V., [451-03-136/2025-03/200214] to T.Đ., R.Đ-P., Lj.R. and M.S.-K., and [451-03-136/2025-03/200032] to M.A.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PE | plant extract |
| EO | essential oil |
| TPC | total phenolic content |
| GAE | gallic acid |
References
- Hossard, L.; Guichard, L.; Pelosi, C.; Makowski, D. Lack of evidence for a decrease in synthetic pesticide use on the main arable crops in France. Sci. Total Environ. 2017, 575, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Anh, L.H.; Quan, N.V.; Nghia, L.T.; Xuan, T.D. Phenolic allelochemicals: Achievements, limitations, and prospective approaches in weed management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef]
- Li, Z.R.; Amist, N.; Bai, L.Y. Allelopathy in sustainable weeds management. Allelopathy J. 2019, 48, 109–138. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.B.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Assessment of allelopathic compounds to develop new natural herbicides: A review. Allelopathy J. 2021, 52, 21–39. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C.A. Ecotoxicity of plant extracts and essential oils: A review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef]
- Elghobashy, R.M.; El-Darier, S.M.; Atia, A.M.; Zakaria, M. Allelopathic Potential of Aqueous Extracts and Essential Oils of Rosmarinus officinalis L. and Thymus vulgaris L. J. Soil Sci. Plant Nutr. 2024, 24, 700–715. [Google Scholar] [CrossRef]
- Giunti, G.; Benelli, G.; Palmeri, V.; Laudani, F.; Ricupero, M.; Ricciardi, R.; Maggi, F.; Lucchi, A.; Guedes, R.N.C.; Desneux, N.; et al. Non-target effects of essential oil-based biopesticides for crop protection: Impact on natural enemies, pollinators, and soil invertebrates. Biol. Control 2022, 176, 105071. [Google Scholar] [CrossRef]
- Kaura, S.; Singhb, P.H.; Mittalb, S.; Batisha, R.D.; Kohlia, K.R. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crop. Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Josifović, M. (Ed.) Flora SR Srbije; VII Tom; SANU: Beograd, Srbija, 1975. (In Serbian) [Google Scholar]
- Julio, L.F.; Burillo, J.; Giménez, C.; Cabrera, R.; Díaz, C.E.; Sanz, J.; González-Coloma, A. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Ind. Crop. Prod. 2015, 76, 787–792. [Google Scholar] [CrossRef]
- Seixas, P.T.L.; Demuner, A.J.; Alvarenga, E.S.; Barbosa, L.C.A.; Marques, A.; de Sá Farias, E.; Picanço, M.C. Bioactivity of essential oils from Artemisia against Diaphania hyalinata and its selectivity to beneficial insects. Sci. Agric. 2018, 75, 519–525. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive Compounds and Health Benefits of Artemisia Species. Nat. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar] [CrossRef]
- Kapoor, D.; Rinzim; Tiwari, A.; Sehgal, A.; Landi, M.; Brestic, M.; Sharma, A. Exploiting the allelopathic potential of aqueous leaf extracts of Artemisia absinthium and Psidium guajava against Parthenium hysterophorus, a widespread weed in India. Plants 2019, 8, 552. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Pine, E.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Marcinkeviciene, A.; Kriauciunuene, Z.; Velicka, R.; Kosteckas, R.; Fujii, Y. Allelopathic effect of Artemisia vulgaris on winter wheat and winter oilseed rape. Fresenius Environ. Bull. 2018, 27, 727–732. [Google Scholar]
- Findura, P.; Kocira, S.; Hara, P.; Pawłowska, A.; Szparaga, A.; Kangalov, P. Extracts from Artemisia vulgaris L. in Potato Cultivation—Preliminary Research on Biostimulating Effect. Agriculture 2020, 10, 356. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Bashar, H.K.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Uddin, M.K.; Asib, N.; Anwar, M.P.; Karim, S.R.; Rahaman, F.; Haque, M.A.; Hossain, A. Documentation of Phytotoxic Compounds Existing in Parthenium hysterophorus L. Leaf and Their Phytotoxicity on Eleusine indica (L.) Gaertn. and Digitaria sanguinalis (L.) Scop. Toxins 2022, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Araniti, F.; Gulli, T.; Marrelli, M.; Statti, G.; Gelsomino, A.; Abenavoli, M.R. Artemisia arborescens L. leaf litter: Phytotoxic activity and phytochemical characterization. Acta Physiol. Plant. 2016, 38, 128. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Chen, Q.; Miao, Y.; Peng, Z.; Huang, B.; Guo, L.; Liu, D.; Du, H. Allelopathic effect of Artemisia argyi on the germination and growth of various weeds. Sci. Rep. 2021, 11, 4303. Available online: https://www.nature.com/articles/s41598-021-83752-6 (accessed on 22 February 2021). [CrossRef]
- Xu, J.; Chen, L.; Wang, S.; Zhang, W.; Liang, J.; Ran, L.; Deng, Z.; Zhou, Y. Chemoproteomic Profiling Reveals Chlorogenic Acid as a Covalent Inhibitor of Arabidopsis Dehydroascorbate Reductase 1. J. Agric. Food. Chem. 2025, 73, 908–918. [Google Scholar] [CrossRef]
- Radulović, M.; Unković, N.; Dimkić, I.; Janakiev, T.; Janaćković, P.; Gašić, U.; Knežević, B.; Radacsi, P.; Gavrilović, M. Phenolic profile and antimicrobial activity of leaf extracts from five Artemisia species (Asteraceae). Bot. Serb. 2024, 48, 7–16. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory effects of monoterpenes on seed germination and seedling growth. Z. Naturforsch. C. J. Biosci. 2007, 62, 207–214. [Google Scholar] [CrossRef]
- Radulović, M.; Rajčević, N.; Gavrilović, M.; Novaković, J.; Stešević, D.; Marin, P.; Janaćković, P. Five wild-growing Artemisia (Asteraceae) species from Serbia and Montenegro: Essential oil composition and its chemophenetic significance. J. Serb. Chem. Soc. 2021, 86, 1281–1290. [Google Scholar] [CrossRef]
- Ickovski, J.; Jovanović, O.; Zlatković, B.; Đorđević, M.; Stepić, K.; Ljupković, R.; Stojanović, G. Variations in the composition of essential oils of selected Artemisia species as a function of soil type. J. Serb. Chem. Soc. 2021, 86, 1259–1269. [Google Scholar] [CrossRef]
- Blagojević, P.; Radulović, N.; Palić, R.; Stojanović, G. Chemical composition of the essential oils of serbian wild-growing Artemisia absinthium and Artemisia vulgaris. J. Agric. Food Chem. 2006, 54, 4780–4789. [Google Scholar] [CrossRef]
- Riahi, L.; Chograni, H.; Elferchichi, M.; Zaouali, Y.; Zoghlami, N.; Mliki, A. Variations in Tunisian wormwood essential oil profiles and phenolic contents between leaves and flowers and their effects on antioxidant activities. Ind. Crop. Prod. 2013, 46, 290–296. [Google Scholar] [CrossRef]
- Fouad, R.; Bousta, D.; Lalami, A.E.O.; Chahdi, F.O.; Amri, I.; Jamoussi, B.; Greche, H. Chemical Composition and Herbicidal Effects of Essential Oils of Cymbopogon citratus (DC) Stapf, Eucalyptus cladocalyx, Origanum vulgare L and Artemisia absinthium L. cultivated in Morocco. J. Essent. Oil-Bear. Plants 2015, 18, 112–123. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roşu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides and Innovative Nano-Based Application Strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Sahraoui, A.L. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Shao, H.; Hu, Y.; Han, C.; Wei, C.; Zhou, S.; Zhang, C.; Zhang, C. Chemical composition and phytotoxic activity of Seriphidium terrae-albae (Krasch.) Poljakov (Compositae) essential oil. Chem. Biodivers. 2018, 15, e1800348. [Google Scholar] [CrossRef]
- Sarić-Krsmanović, M.; Tojić, T.; Gajić Umiljendić, J.; Đorđević, T.; Đurđević-Pejčev, R.; Radivojević, L.; Božić, D.; Vrbničanin, S. Phytochemical Investigation of Cuscuta campestris Yunck. Stem Extract and Evaluation of its Bioherbicidal Effect on Amaranthus retroflexus L. and Portulaca oleracea L. Chem. Biodivers. 2023, 20, e202300270. [Google Scholar] [CrossRef]
- Han, C.; Zhang, G.; Mei, Y.; Shan, Z.; Shi, K.; Zhou, S.; Shao, H. Chemical profile of Artemisia vulgaris L. essential oil and its phytotoxic, insecticidal, and antimicrobial activities. S. Afr. J. Bot. 2023, 162, 20–28. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, S.; Liu, L.; Toshmatov, Z.; Huang, L.; Shi, K.; Zhang, C.; Shao, H. Evaluation of the phytotoxic effect of the essential oil from Artemisia absinthium. Ecotoxicol. Environ. Saf. 2021, 226, 112856. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Cvetković, M.T.; Stanković Jeremić, J.M.; Pezo, L.L.; Varga, A.O.; Čabarkapa, I.S.; Kiprovski, B. Biological activity and profiling of Salvia sclarea essential oil obtained by steam and hydrodistillation extraction methods via chemometrics tools. Flavour Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Gašić, U.M.; Natić, M.M.; Mišić, D.M.; Lušić, D.V.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Lušić, D. Chemical markers for the authentication of unifloral Salvia officinalis L. honey. J. Food Compos. Anal. 2015, 44, 128–138. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Streibig, J.C. Herbicide bioassay. Weed Res. 1988, 28, 479–484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).