Quantification of Phenolic Compounds by HPLC/DAD and Evaluation of the Antioxidant, Antileishmanial, and Cytotoxic Activities of Ethanolic Extracts from the Leaves and Bark of Sarcomphalus joazeiro (Mart.)
Abstract
1. Introduction
2. Results
2.1. Quantification of Phenolic Acids by HPLC/DAD
2.2. Antioxidant Assay
2.2.1. Fe2+ Chelating Activity and Fe3+ Reducing Power
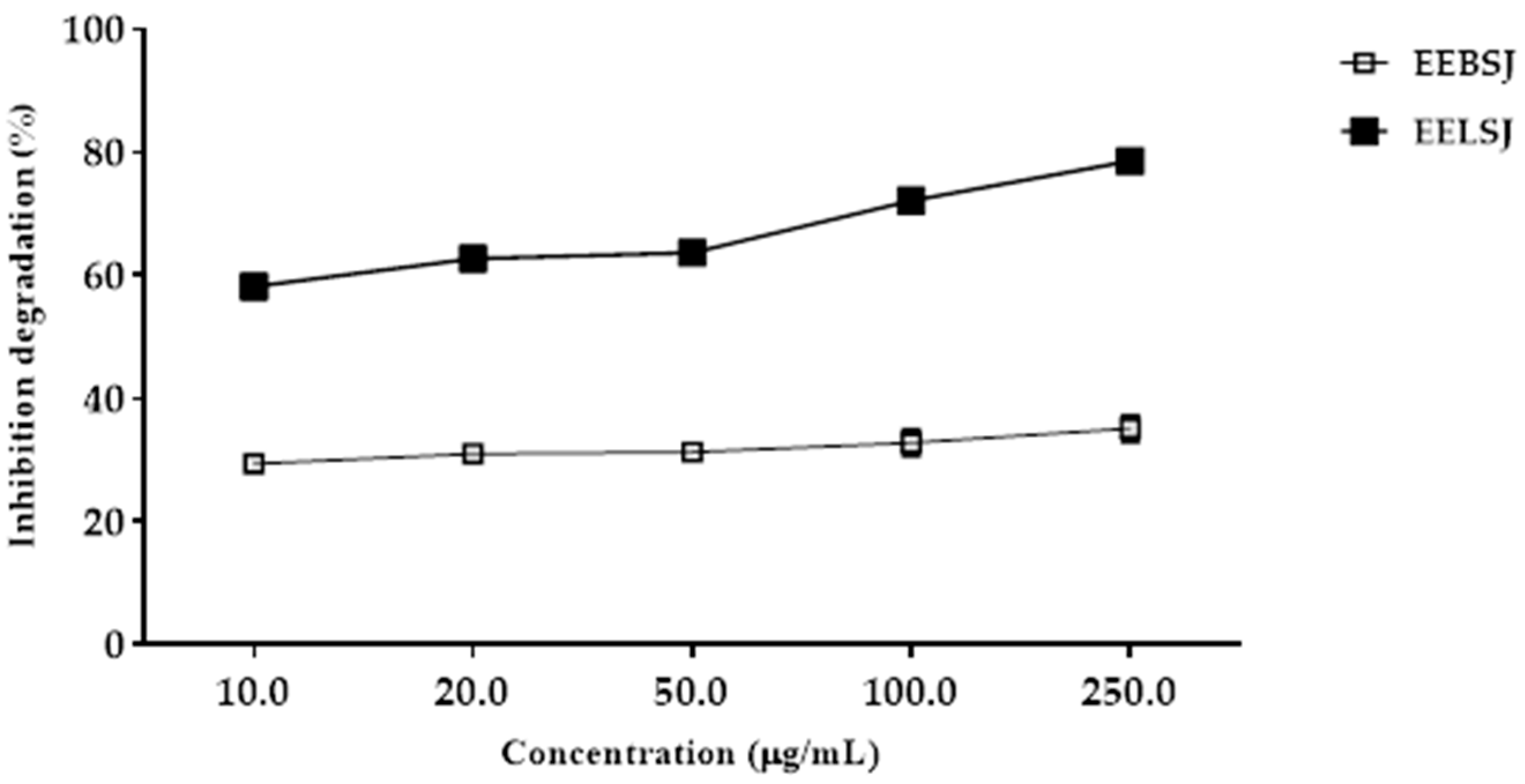
2.2.2. Deoxyribose Oxidative Degradation Assay
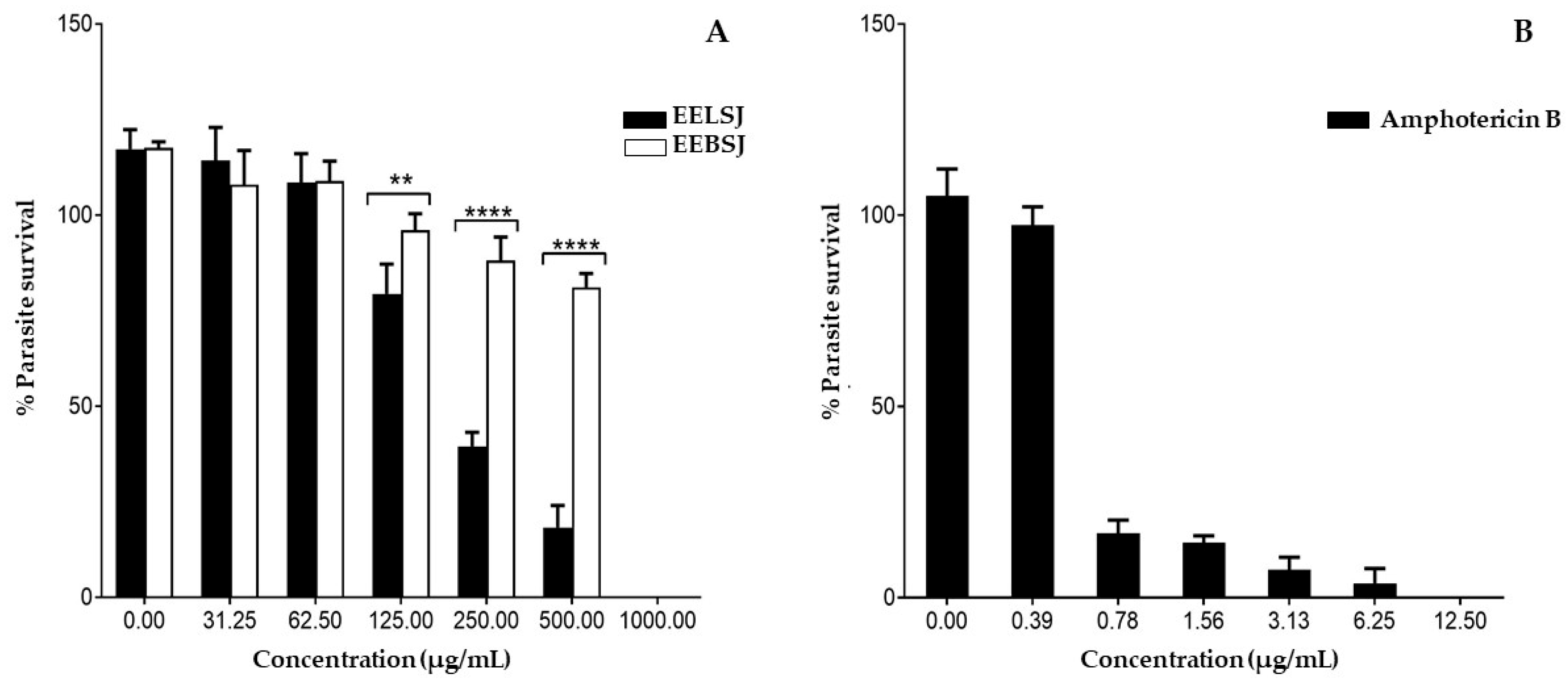
2.3. Antileishmanial Activity
2.4. Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Quantification of Phenolic Acids and Flavonoids by HPLC/DAD
4.2.1. Preparation of Standards
4.2.2. HPLC/DAD Analysis
4.3. Antioxidant Activity
4.3.1. Fe2+ Chelating Activity and Fe3+ Reducing Power
4.3.2. Deoxyribose Oxidative Degradation Assay
4.4. Antileishmanial and Cytotoxic Activity
4.4.1. Parasites
4.4.2. Cell Cultures
4.4.3. Activity Against Promastigote Forms
4.5. Cytotoxicity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| HPLC/DAD | high-performance liquid chromatography with a diode-array detector |
| IC50 | mean inhibitory concentration |
| 2-DR | Deoxyribose |
| SI | selectivity index |
| CC50 | cytotoxic concentration |
References
- Garcia, M.S.A.; Oliveira Filho, V.A.; Brioschi, M.B.C.; Minori, K.; Miguel, D.C. Leishmania (Leishmania) Amazonensis. Trends Parasitol. 2025, 41, 66–67. [Google Scholar] [CrossRef]
- OPAS. Leishmanioses: Informe Epidemiológico Das Américas. No° 11. Epidemiol. Rep. Am. 2022, 1–12. Available online: https://iris.paho.org/handle/10665.2/56832 (accessed on 27 April 2025).
- Ozbak, H.A.; Hemeg, H.A.; Afrin, F.; Chouhan, G.; Islamuddin, M.; Want, M.Y. Cinnamomum Cassia Exhibits Antileishmanial Activity against Leishmania Donovani Infection in Vitro and in Vivo. PLoS Negl. Trop. Dis. 2019, 13, e0007227. [Google Scholar] [CrossRef]
- Alshabanah, L.A.; Omran, N.; Elwakil, B.H.; Hamed, M.T.; Abdallah, S.M.; Al-Mutabagani, L.A.; Wang, D.; Liu, Q.; Shehata, N.; Hassanin, A.H.; et al. Elastic Nanofibrous Membranes for Medical and Personal Protection Applications: Manufacturing, Anti-COVID-19, and Anti-Colistin Resistant Bacteria Evaluation. Polymers 2021, 13, 3987. [Google Scholar] [CrossRef]
- Salazar, G.J.T.; Dias, F.J.; Ribeiro, P.R.V.; de Brito, E.S.; Canuto, K.M.; Coutinho, H.D.M.; Ribeiro-Filho, J.; Gallo, M.; Montesano, D.; Naviglio, D.; et al. Antioxidant Activity of Stryphnodendron Rotundifolium Mart. Stem Bark Fraction in an Iron Overload Model. Foods 2021, 10, 2683. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Mfopa, A.N.; Kemzeu, R.; Fokom, R.; Yamthe, L.R.T.; Dize, D.; Boyom, F.F. Phenolic Compounds, Antioxidant and Antileishmanial Activities of Kombucha as Affected by Fermentation Time. Heliyon 2024, 10, e40463. [Google Scholar] [CrossRef]
- do Nascimento, A.M.; da Fonseca, T.S.; Campos, M.F.; Moreira, L.O.; Marques, C.A.; Tavares, E.S.; Mendonça, S.C.; Leitão, G.G.; Simas, R.C.; Leitão, S.G. Ziziphus Joazeiro, a Saponin-Rich Brazilian Medicinal Plant: Pharmacognostic Characterization of Bark and Leaves. Rev. Bras. Farmacogn. 2020, 30, 756–764. [Google Scholar] [CrossRef]
- Tabarelli, M.; Leal, I.R.; Scarano, F.R.; Silva, J.M.C. da Caatinga: Legado, Trajetória e Desafios Rumo à Sustentabilidade. Cienc. Cult. 2018, 70, 25–29. [Google Scholar] [CrossRef]
- WFO. Ziziphus Joazeiro Mart. Available online: http://www.worldfloraonline.org/taxon/wfo-0000430296 (accessed on 8 April 2025).
- Andrade, J.C.; Silva, A.R.P.; Santos, A.T.L.; Freitas, M.A.; Carneiro, J.N.P.; Gonçalo, M.I.P.; de Souza, A.; Freitas, T.S.; Ribeiro, P.R.V.; Brito, E.S.; et al. UPLC-MS-ESI-QTOF Characterization and Evaluation of the Antibacterial and Modulatory Antibiotic Activity of Ziziphus Joazeiro Mart. Aqueous Extracts. S. Afr. J. Bot. 2019, 123, 105–112. [Google Scholar] [CrossRef]
- Carvalho, G.D.; Pereira, M.; Leite, D.; Javier, G.; Salazar, T.; Wellisson, J.; Marques, K.; Riceli, P.; Ribeiro, V.; Maria, A.; et al. UPLC-PDA-ESI-QDA characterization and evaluation of the antioxidant and anxiolytic activities of the ethanolic extract of Sarcomphalus joazeiro (Mart.) Hauenschild leaves. Pharmacol. Res.-Nat. Prod. 2025, 7, 100223. [Google Scholar] [CrossRef]
- Leite, D.O.D.; Camilo, C.J.; Nonato, C.d.F.A.; de Carvalho, N.K.G.; Salazar, G.J.T.; de Morais, S.M.; da Costa, J.G.M. Chemical Profile and Evaluation of the Antioxidant and Anti-Acetylcholinesterase Activities of Annona Squamosa l. (Annonaceae) Extracts. Foods 2021, 10, 2343. [Google Scholar] [CrossRef]
- Brito, S.M.O.; Coutinho, H.D.M.; Talvani, A.; Coronel, C.; Barbosa, A.G.R.; Vega, C.; Figueredo, F.G.; Tintino, S.R.; Lima, L.F.; Boligon, A.A.; et al. Analysis of Bioactivities and Chemical Composition of Ziziphus Joazeiro Mart. Using HPLC-DAD. Food Chem. 2015, 186, 185–191. [Google Scholar] [CrossRef]
- Macedo, J.M.; Souza, L.G.P.; Troncozo Valenzuela, V.d.C.; Oliveira, A.B.; Oliveira Castilho, R.; Ribeiro Jácome, R.L. Variação Sazonal Nos Teores de Flavonoides, Taninos e Atividade Antioxidante de Davilla Rugosa Poir. Rev. Cienc. Farm. Basica e Apl. 2013, 34, 585–590. [Google Scholar]
- da Silva, A.R.P.; Costa, M.d.S.; Araújo, N.J.S.; Alencar, G.G.; Ribeiro, P.R.V.; Tavares, J.F.; Pinheiro, J.C.A.; Coutinho, H.D.M. UPLC-QTOF-MS/MS Characterization of a Fraction Enriched in Saponins from Sarcomphalus Joazeiro Species and Evaluation of the Antibacterial Activity against Multidrug-Resistant Bacteria. S. Afr. J. Bot. 2024, 165, 324–330. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Hammi, K.M.; Essid, R.; Khadraoui, N.; Ksouri, R.; Majdoub, H.; Tabbene, O. Antimicrobial, Antioxidant and Antileishmanial Activities of Ziziphus Lotus Leaves. Arch. Microbiol. 2022, 204, 119. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Phenolic Composition, Ascorbic Acid Content, and Antioxidant Capacity of Spanish Jujube (Ziziphus jujube Mill.) Fruits. Food Chem. 2016, 201, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.L.; Cardoso; Costa, R.S.; Santana, S.; Gabriela, M.; Koblitz, B. Compostos Fenólicos, Carotenóides e Atividade Antioxidante Em Produtos Vegetais Phenolic Compounds, Carotenoids and Antioxidant Activity in Plant Products. Semin. Ciências Agrárias 2010, 31, 669–682. [Google Scholar] [CrossRef]
- Santi, A.M.M.; Murta, S.M.F. Antioxidant Defence System as a Rational Target for Chagas Disease and Leishmaniasis Chemotherapy. Mem. Inst. Oswaldo Cruz 2022, 117, e210401. [Google Scholar] [CrossRef]
- Belkhelfa-Slimani, R.; Djerdjouri, B. Caffeic Acid and Quercetin Exert Caspases-Independent Apoptotic Effects on Leishmania Major Promastigotes, and Reactivate the Death of Infected Phagocytes Derived from BALB/c Mice. Asian Pac. J. Trop. Biomed. 2017, 7, 321–331. [Google Scholar] [CrossRef]
- de Araújo, S.A.; Silva, C.M.P.; Costa, C.S.; Ferreira, C.S.C.; Ribeiro, H.S.; da Silva Lima, A.; Quintino da Rocha, C.; Calabrese, K.d.S.; Abreu-Silva, A.L.; Almeida-Souza, F. Leishmanicidal and Immunomodulatory Activity of Terminalia Catappa in Leishmania Amazonensis in Vitro Infection. Heliyon 2024, 10, e24622. [Google Scholar] [CrossRef]
- Albalawi, A.E. Antileishmanial Activity of Ziziphus Spina-Christi Leaves Extract and Its Possible Cellular Mechanisms. Microorganisms 2021, 9, 2113. [Google Scholar] [CrossRef]
- Faria, L.V.; Brígido, H.P.C.; Bentaberry-Rosa, A.A.; Correa-Barbosa, J.; Silva-Silva, J.V.; Bastos, M.L.C.; Costa, E.V.S.; Coelho-Ferreira, M.; Silveira, F.T.; Dolabela, M.F. Anti-leishmania activity of extract and fractions from the stem and leaf of Montrichardia linifera (Arruda) schott (Araceae) against Leishmania amazonensis. Res. Soc. Dev. 2021, 10, e9310212312. [Google Scholar] [CrossRef]


| Compounds | LD (mg/mL) | LQ (mg/mL) | EELSJ | EEBSJ | ||
|---|---|---|---|---|---|---|
| mg/g | % | mg/g | % | |||
| Caffeic acid | 0.0001 | 0.0003 | 0.0456 ± 0.000057 | 0.00456 | 0.0470 ± 0.020785 | 0.0047 |
| p-coumaric acid | 0.0007 | 0.0025 | 0.0369 ± 0.017609 | 0.00369 | - | - |
| Ferulic acid | 0.0048 | 0.0162 | 0.2313 ± 0.002367 | 0.02313 | 0.150 ± 0.008660 | 0.0150 |
| Cinnamic acid | 0.0009 | 0.0032 | - | - | - | - |
| Naringenin | 0.0011 | 0.0036 | - | - | - | - |
| Pinocembrin | 0.0030 | 0.0102 | 0.3928 ± 0.007621 | 0.03928 | - | - |
| Apigenin | 0.0027 | 0.0092 | - | - | - | - |
| Compounds | IC50 (µg/mL) | CC50 (µg/mL) | SI |
|---|---|---|---|
| Promastigote | J774.G8 | ||
| EELSJ | 246.2 ± 0.089 | 343.3 ± 0.98 | 1.3943 |
| EEBSJ | 880.7 ± 0.062 | 5.866 ± 1.027 | 0.0066 |
| Amphotericin B | 0.54 ± 0.122 | 4.9 ± 1.216 | 90.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, N.K.G.d.; Duarte Leite, D.O.; Colares, A.V.; Souza, F.A.; Calabrese, K.d.S.; Torres Salazar, G.J.; Nascimento, J.B.d.; Pereira da Silva, M.; Rodrigues, F.F.G.; Costa, J.G.M.d. Quantification of Phenolic Compounds by HPLC/DAD and Evaluation of the Antioxidant, Antileishmanial, and Cytotoxic Activities of Ethanolic Extracts from the Leaves and Bark of Sarcomphalus joazeiro (Mart.). Plants 2025, 14, 1733. https://doi.org/10.3390/plants14111733
Carvalho NKGd, Duarte Leite DO, Colares AV, Souza FA, Calabrese KdS, Torres Salazar GJ, Nascimento JBd, Pereira da Silva M, Rodrigues FFG, Costa JGMd. Quantification of Phenolic Compounds by HPLC/DAD and Evaluation of the Antioxidant, Antileishmanial, and Cytotoxic Activities of Ethanolic Extracts from the Leaves and Bark of Sarcomphalus joazeiro (Mart.). Plants. 2025; 14(11):1733. https://doi.org/10.3390/plants14111733
Chicago/Turabian StyleCarvalho, Natália Kelly Gomes de, Débora Odília Duarte Leite, Aracélio Viana Colares, Fernando Almeida Souza, Kátia da Silva Calabrese, Gerson Javier Torres Salazar, Joice Barbosa do Nascimento, Mariana Pereira da Silva, Fabiola Fernandes Galvão Rodrigues, and José Galberto Martins da Costa. 2025. "Quantification of Phenolic Compounds by HPLC/DAD and Evaluation of the Antioxidant, Antileishmanial, and Cytotoxic Activities of Ethanolic Extracts from the Leaves and Bark of Sarcomphalus joazeiro (Mart.)" Plants 14, no. 11: 1733. https://doi.org/10.3390/plants14111733
APA StyleCarvalho, N. K. G. d., Duarte Leite, D. O., Colares, A. V., Souza, F. A., Calabrese, K. d. S., Torres Salazar, G. J., Nascimento, J. B. d., Pereira da Silva, M., Rodrigues, F. F. G., & Costa, J. G. M. d. (2025). Quantification of Phenolic Compounds by HPLC/DAD and Evaluation of the Antioxidant, Antileishmanial, and Cytotoxic Activities of Ethanolic Extracts from the Leaves and Bark of Sarcomphalus joazeiro (Mart.). Plants, 14(11), 1733. https://doi.org/10.3390/plants14111733








