Comparative Analysis of Moringa oleifera Lam. Leaves Ethanolic Extracts: Effects of Extraction Methods on Phytochemicals, Antioxidant, Antimicrobial, and In Ovo Profile
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification and Quantification of Phenolic Compounds in Moringa Ethanolic Extracts
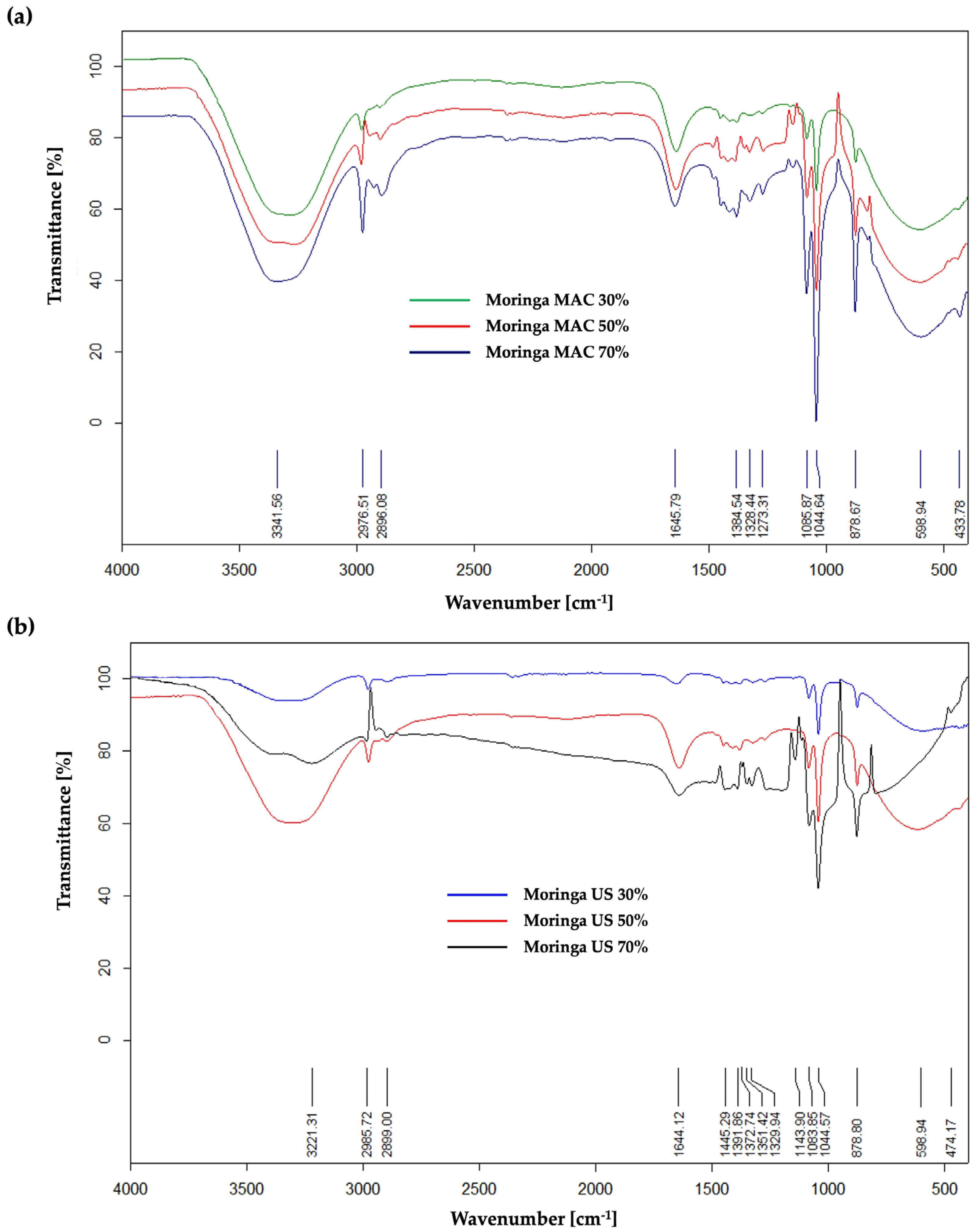
2.2. Qualitative Characterization Technique (ATR FT–IR) for MO Ethanolic Extracts
2.3. Antioxidant Activity of MO Ethanolic Extracts Using DPPH Radical Scavenging Assay
2.4. Antimicrobial Activity of Moringa MAC and Moringa US Extracts
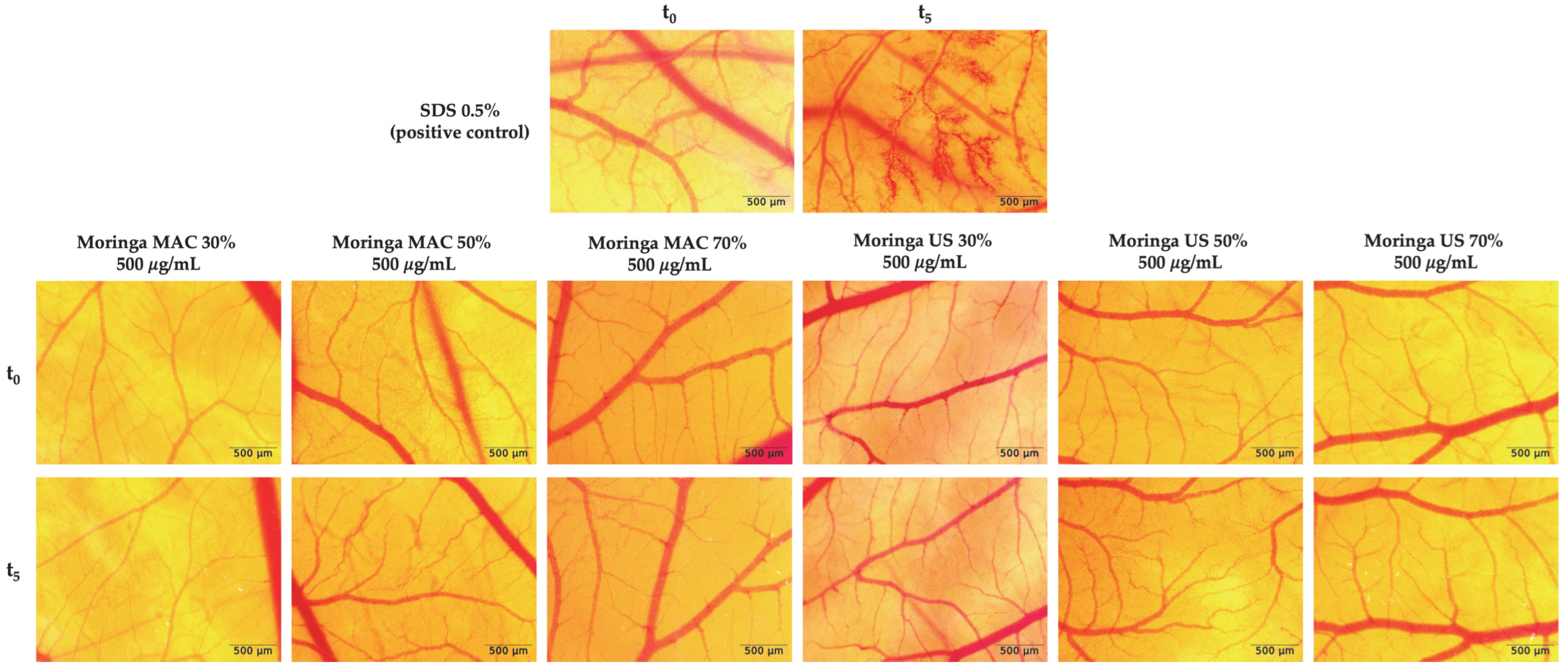
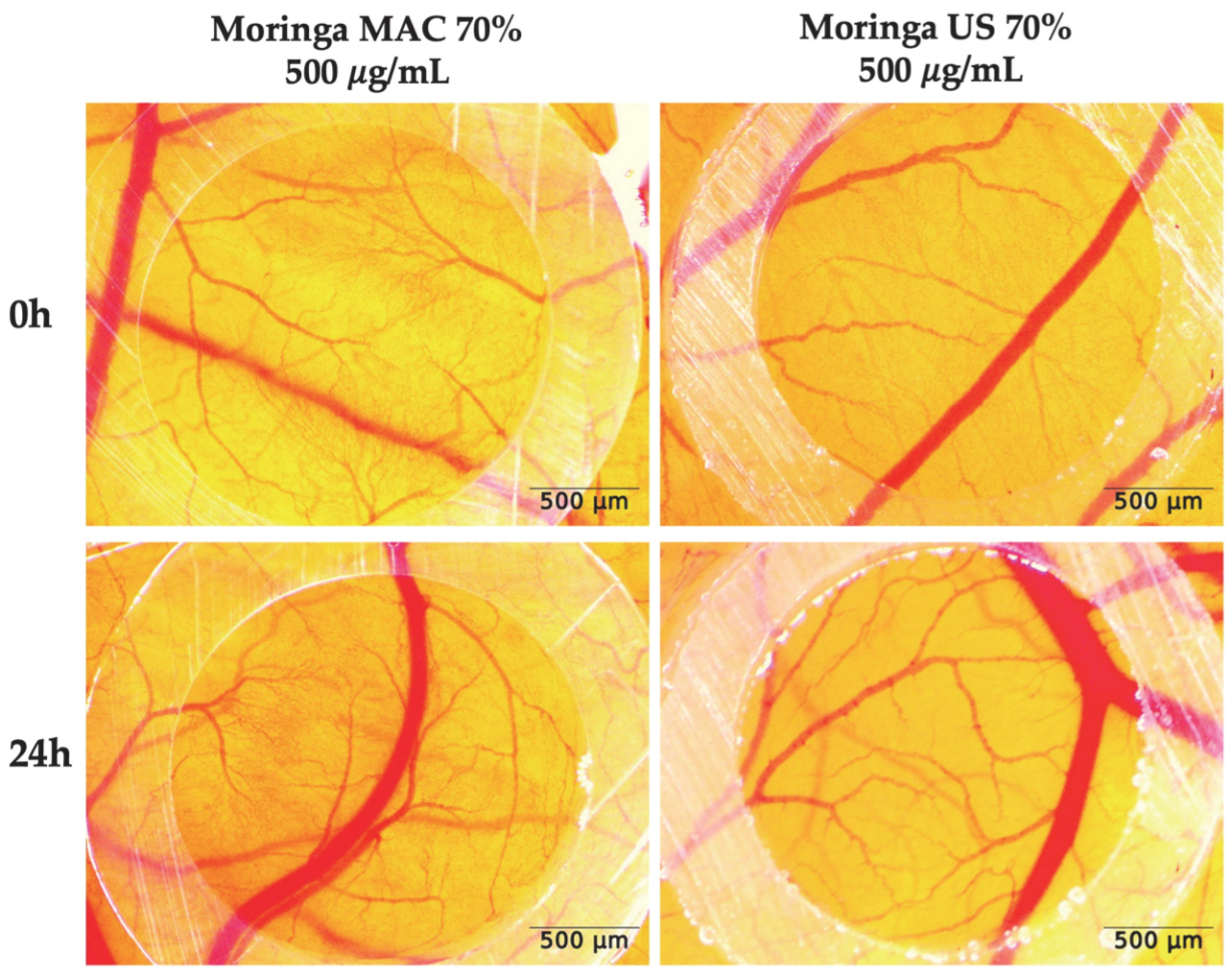
2.5. In Ovo Safety Profile of MO Ethanolic Extracts
3. Materials and Methods
3.1. Plant Materials and Extraction Protocol
3.2. Chemicals and Reagents
3.3. Phytochemical Profile of Moringa oleifera Lam. Ethanolic Extracts
3.3.1. HPLC/LC–MS Identification and Quantification of Polyphenolic Compounds
3.3.2. Fourier Transform–Infrared Spectroscopy (FT–IR) Characterization Technique
3.4. Antioxidant Activity—2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
3.5. Antimicrobial Potential
3.6. In Ovo CAM Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elkordy, A.A.; Haj-Ahmad, R.R.; Awaad, A.S.; Zaki, R.M. An Overview on Natural Product Drug Formulations from Conventional Medicines to Nanomedicines: Past, Present and Future. J. Drug Deliv. Sci. Technol. 2021, 63, 102459. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-Derived Natural Products for Drug Discovery: Current Approaches and Prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Kesarwani, K.; Gupta, R. Bioavailability Enhancers of Herbal Origin: An Overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253–266. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Choi, S.H. WHO Traditional Medicine Strategy and Activities “Standardization with Evidence-Based Approaches”. JAMS J. Acupunct. Meridian Stud. 2008, 1, 153–154. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa Oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Mallenakuppe, R.; Homabalegowda, H.; Gouri, M.D.; Basavaraju, P.S.; Chandrashekharaiah, U.B. History, Taxonomy and Propagation of Moringa oleifera—A Review. SSR Inst. Int. J. Life Sci. 2019, 5, 2322–2327. [Google Scholar] [CrossRef]
- Pareek, A.; Pant, M.; Gupta, M.M.; Kashania, P.; Ratan, Y.; Jain, V.; Pareek, A.; Chuturgoon, A.A. Moringa Oleifera: An Updated Comprehensive Review of Its Pharmacological Activities, Ethnomedicinal, Phytopharmaceutical Formulation, Clinical, Phytochemical, and Toxicological Aspects. Int. J. Mol. Sci. 2023, 24, 2098. [Google Scholar] [CrossRef]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Azlan, U.K.; Mediani, A.; Rohani, E.R.; Tong, X.; Han, R.; Misnan, N.M.; Jam, F.A.; Bunawan, H.; Sarian, M.N.; Hamezah, H.S. A Comprehensive Review with Updated Future Perspectives on the Ethnomedicinal and Pharmacological Aspects of Moringa oleifera. Molecules 2022, 27, 5765. [Google Scholar] [CrossRef] [PubMed]
- Gandji, K.; Chadare, F.J.; Idohou, R.; Salako, V.K.; Assogbadjo, A.E.; Kakaï, R.L.G. Status and Utilisation of Moringa oleifera Lam: A Review. Afr. Crop. Sci. J. 2018, 26, 137. [Google Scholar] [CrossRef]
- du Toit, E.S.; Sithole, J.; Vorster, J. Pruning Intensity Influences Growth, Flower and Fruit Development of Moringa oleifera Lam. under Sub-Optimal Growing Conditions in Gauteng, South Africa. South Afr. J. Bot. 2020, 129, 448–456. [Google Scholar] [CrossRef]
- Alavilli, H.; Poli, Y.; Verma, K.S.; Kumar, V.; Gupta, S.; Chaudhary, V.; Jyoti, A.; Sahi, S.V.; Kothari, S.L.; Jain, A. Miracle Tree Moringa Oleifera: Status of the Genetic Diversity, Breeding, In Vitro Propagation, and a Cogent Source of Commercial Functional Food and Non-Food Products. Plants 2022, 11, 3132. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An Updated Comprehensive Review of the Medicinal, Phytochemical and Pharmacological Properties of Moringa oleifera. South Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Sharma, P.; Wichaphon, J.; Klangpetch, W. Antimicrobial and Antioxidant Activities of Defatted Moringa oleifera Seed Meal Extract Obtained by Ultrasound-Assisted Extraction and Application as a Natural Antimicrobial Coating for Raw Chicken Sausages. Int. J. Food Microbiol. 2020, 332, 108770. [Google Scholar] [CrossRef]
- Yang, M.; Tao, L.; Kang, X.R.; Li, L.F.; Zhao, C.C.; Wang, Z.L.; Sheng, J.; Tian, Y. Recent Developments in Moringa oleifera Lam. Polysaccharides: A Review of the Relationship between Extraction Methods, Structural Characteristics and Functional Activities. Food Chem. X 2022, 14, 100322. [Google Scholar] [CrossRef] [PubMed]
- Gharsallah, K.; Rezig, L.; Rajoka, M.S.R.; Mehwish, H.M.; Ali, M.A.; Chew, S.C. Moringa oleifera: Processing, Phytochemical Composition, and Industrial Application. South Afr. J. Bot. 2023, 160, 180–193. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Gaweł-Bęben, K.; Rutka, A.; Blicharska, E.; Tatarczak-Michalewska, M.; Kulik-Siarek, K.; Kukula-Koch, W.; Malinowska, M.A.; Szopa, A. Moringa oleifera (Drumstick Tree)—Nutraceutical, Cosmetological and Medicinal Importance: A Review. Front. Pharmacol. 2024, 15, 1288382. [Google Scholar] [CrossRef]
- Fahey, J.W. Moringa Oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic, and Prophylactic Properties. Part 1. Available online: https://scispace.com/pdf/moringa-oleifera-a-review-of-the-medical-evidence-for-its-2tpm5yyogd.pdf (accessed on 23 April 2025).
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, Nutritional, and Therapeutic Significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Saleem, A.; Saleem, M.; Akhtar, M.F. Antioxidant, Anti-Inflammatory and Antiarthritic Potential of Moringa oleifera Lam: An Ethnomedicinal Plant of Moringaceae Family. South Afr. J. Bot. 2020, 128, 246–256. [Google Scholar] [CrossRef]
- Anzano, A.; Ammar, M.; Papaianni, M.; Grauso, L.; Sabbah, M.; Capparelli, R.; Lanzotti, V. Moringa oleifera Lam.: A Phytochemical and Pharmacological Overview. Horticulturae 2021, 7, 409. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, S.M.R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M.R.; Karim, R. Moringa Oleifera Is a Prominent Source of Nutrients with Potential Health Benefits. Int. J. Food Sci. 2021, 2021, 6627265. [Google Scholar] [CrossRef]
- Duru, K.C.; Kovaleva, E.G.; Danilova, I.G.; van der Bijl, P.; Belousova, A.V. The Potential Beneficial Role of Isoflavones in Type 2 Diabetes Mellitus. Nutr. Res. 2018, 59, 1–15. [Google Scholar] [CrossRef]
- Prajapati, C.; Ankola, M.; Upadhyay, T.K.; Sharangi, A.B.; Alabdallah, N.M.; Al-Saeed, F.A.; Muzammil, K.; Saeed, M. Moringa oleifera: Miracle Plant with a Plethora of Medicinal, Therapeutic, and Economic Importance. Horticulturae 2022, 8, 492. [Google Scholar] [CrossRef]
- Kashyap, P.; Kumar, S.; Riar, C.S.; Jindal, N.; Baniwal, P.; Guiné, R.P.F.; Correia, P.M.R.; Mehra, R.; Kumar, H. Recent Advances in Drumstick (Moringa oleifera) Leaves Bioactive Compounds: Composition, Health Benefits, Bioaccessibility, and Dietary Applications. Antioxidants 2022, 11, 402. [Google Scholar] [CrossRef]
- Díaz-Prieto, L.E.; Gómez-Martínez, S.; Vicente-Castro, I.; Heredia, C.; González-Romero, E.A.; Martín-Ridaura, M.D.C.; Ceinos, M.; Picón, M.J.; Marcos, A.; Nova, E. Effects of Moringa oleifera Lam. Supplementation on Inflammatory and Cardiometabolic Markers in Subjects with Prediabetes. Nutrients 2022, 14, 1937. [Google Scholar] [CrossRef]
- Patil, S.V.; Mohite, B.V.; Marathe, K.R.; Salunkhe, N.S.; Marathe, V.; Patil, V.S. Moringa Tree, Gift of Nature: A Review on Nutritional and Industrial Potential. Curr. Pharmacol. Rep. 2022, 8, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Dalhoumi, W.; Guesmi, F.; Bouzidi, A.; Akermi, S.; Hfaiedh, N.; Saidi, I. Therapeutic Strategies of Moringa oleifera Lam. (Moringaceae) for Stomach and Forestomach Ulceration Induced by HCl/EtOH in Rat Model. Saudi J. Biol. Sci. 2022, 29, 103284. [Google Scholar] [CrossRef] [PubMed]
- Louisa, M.; Patintingan, C.G.H.; Wardhani, B.W.K. Moringa oleifera Lam. in Cardiometabolic Disorders: A Systematic Review of Recent Studies and Possible Mechanism of Actions. Front. Pharmacol. 2022, 13, 792794. [Google Scholar] [CrossRef]
- Sultan, R.; Ahmed, A.; Wei, L.; Saeed, H.; Islam, M.; Ishaq, M. The Anticancer Potential of Chemical Constituents of Moringa oleifera Targeting CDK-2 Inhibition in Estrogen Receptor Positive Breast Cancer Using in-Silico and in Vitro Approches. BMC Complement. Med. Ther. 2023, 23, 396. [Google Scholar] [CrossRef]
- Giugliano, R.; Ferraro, V.; Chianese, A.; Della Marca, R.; Zannella, C.; Galdiero, F.; Fasciana, T.M.A.; Giammanco, A.; Salerno, A.; Cannillo, J.; et al. Antiviral Properties of Moringa Oleifera Leaf Extracts against Respiratory Viruses. Viruses 2024, 16, 1199. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioall. Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Shri Chengama Raju, N.; Wing Kei, W. Anti-Angiogenic Screening of Moringa oleifera Leaves Extract Using Chorioallantoic Membrane Assay. Iraq J. Pharm. Sci. 2022, 31, 225–232. [Google Scholar] [CrossRef]
- Ogundipe, A.; Adetuyi, B.; Iheagwam, F.; Adefoyeke, K.; Olugbuyiro, J.; Ogunlana, O.; Ogunlana, O. In Vitro Experimental Assessment of Ethanolic Extract of Moringa oleifera Leaves as an α-Amylase and α-Lipase Inhibitor. Biochem. Res. Int. 2022, 2022, 4613109. [Google Scholar] [CrossRef]
- Reda, R.M.; Helmy, R.M.A.; Osman, A.; Ahmed, F.A.G.; Kotb, G.A.M.; El-Fattah, A.H.A. The Potential Effect of Moringa oleifera Ethanolic Leaf Extract Against Oxidative Stress, Immune Response Disruption Induced by Abamectin Exposure in Oreochromis niloticus. Environ. Sci. Pollut. Res. 2023, 30, 58569–58587. [Google Scholar] [CrossRef]
- Rajabi, L.; Ebrahimdoost, M.; Mohammadi, S.A.; Samarkhazan, H.S.; Khamisipour, G.; Aghaei, M. Aqueous and Ethanolic Extracts of Moringa oleifera Leaves Induce Selective Cytotoxicity in Raji and Jurkat Cell Lines by Activating the P21 Pathway Independent of P53. Mol. Biol. Rep. 2025, 52, 102. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Khalil, S.R.; Awad, A.; Zeid, E.H.A.; El-Aziz, R.A.; El-Serehy, H.A. Ethanolic Extract of Moringa oleifera Leaves Influences NF-Κb Signaling Pathway to Restore Kidney Tissue from Cobalt-Mediated Oxidative Injury and Inflammation in Rats. Nutrients 2020, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- El-Shehawi, A.M.; Alkafafy, M.; El-Shazly, S.; Sayed, S.; Farouk, S.; Alotaibi, S.; Madkour, D.A.; Khalifa, H.K.; Ahmed, M.M. Moringa oleifera Leaves Ethanolic Extract Ameliorates High Fat Diet-Induced Obesity in Rats. J. King Saud. Univ. Sci. 2021, 33, 101552. [Google Scholar] [CrossRef]
- Khalid, S.; Arshad, M.; Mahmood, S.; Siddique, F.; Roobab, U.; Ranjha, M.M.A.N.; Lorenzo, J.M. Extraction and Quantification of Moringa oleifera Leaf Powder Extracts by HPLC and FTIR. Food Anal Methods 2023, 16, 787–797. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, J.; Li, H.; He, Y.; Lan, T.; Wu, D.; Gong, H.; Zhang, Y.; Chen, Z. Optimization of Phenolic Compound Extraction from Chinese Moringa Oleifera Leaves and Antioxidant Activities. J. Food Qual. 2019, 2019, 5346279. [Google Scholar] [CrossRef]
- Oluewu, M.M.; Walker, L.T.; Ogutu, S.; Koko, C.O. Determination of Antioxidant Activities of Moringa oleifera Leaves from Selected Countries. Int. J. Biochem. Res. Rev. 2024, 33, 164–175. [Google Scholar] [CrossRef]
- Bennour, N.; Mighri, H.; Eljani, H.; Zammouri, T.; Akrout, A. Effect of Solvent Evaporation Method on Phenolic Compounds and the Antioxidant Activity of Moringa oleifera Cultivated in Southern Tunisia. South Afr. J. Bot. 2020, 129, 181–190. [Google Scholar] [CrossRef]
- Lin, X.; Wu, L.; Wang, X.; Yao, L.; Wang, L. Ultrasonic-Assisted Extraction for Flavonoid Compounds Content and Antioxidant Activities of India Moringa oleifera L. Leaves: Simultaneous Optimization, HPLC Characterization and Comparison with Other Methods. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100284. [Google Scholar] [CrossRef]
- Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; Vázquez-Espinosa, M.; González-De-peredo, A.V.; García-Alvarado, M.Á.; Palma, M.; Rodríguez-Jimenes, G.D.C.; Barbero, G.F. Optimization of an Ultrasound-Assisted Extraction Method Applied to the Extraction of Flavonoids from Moringa Leaves (Moringa oleífera Lam.). Agronomy 2022, 12, 261. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical Ethanol Extraction of Flavonoids from Moringa Oleifera Leaf and Evaluation of Antioxidant Activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Herman-Lara, E.; Rodríguez-Miranda, J.; Ávila-Manrique, S.; Dorado-López, C.; Villalva, M.; Jaime, L.; Santoyo, S.; Martínez-Sánchez, C.E. In Vitro Antioxidant, Anti-Inflammatory Activity and Bioaccessibility of Ethanolic Extracts from Mexican Moringa oleifera Leaf. Foods 2024, 13, 2709. [Google Scholar] [CrossRef]
- Divya, S.; Pandey, V.K.; Dixit, R.; Rustagi, S.; Suthar, T.; Atuahene, D.; Nagy, V.; Ungai, D.; Ahmed, A.E.M.; Kovács, B.; et al. Exploring the Phytochemical, Pharmacological and Nutritional Properties of Moringa oleifera: A Comprehensive Review. Nutrients 2024, 16, 3423. [Google Scholar] [CrossRef] [PubMed]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa oleifera Leaf Extract by the Appropriate Extraction Method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of Extraction Method to Obtain a Phenolic Compounds-Rich Extract from Moringa oleifera Lam Leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Setyani, W.; Murwanti, R.; Sulaiman, T.N.S.; Hertiani, T. Application of Response Surface Methodology (RSM) for the Optimization of Ultrasound-Assisted Extraction (UAE) of Moringa Oleifera: Extraction Yield, Content of Bioactive Compounds, and Biological Effects In Vitro. Plants 2023, 12, 2455. [Google Scholar] [CrossRef]
- Mehganathan, P.; Rosli, N.A. A Review on Extraction of Bioactive Compounds from Moringa Oleifera A Review on Extraction of Bioactive Compounds from Moringa oleifera Leaves: Their Principle, Advantages, and Disadvantages. Austin Chem. Eng. 2022, 9, 1090. [Google Scholar] [CrossRef]
- Jahan, S.; Shahjahan, M.; Rasna, S.S.; Aktar, M.; Sultana, S.; Ahmed, S.M.; Sabrin, F.; Nahar, S. Antibacterial Effect of Moringa (Moringa oleifera) Leaf Ethanolic Extract Against Staphylococcus aureus and Escherichia coli. Mymensingh Med. J. 2022, 31, 976–982. [Google Scholar] [PubMed]
- Enerijiofi, K.E.; Akapo, F.H.; Erhabor, J.O. GC–MS Analysis and Antibacterial Activities of Moringa oleifera Leaf Extracts on Selected Clinical Bacterial Isolates. Bull. Natl. Res. Cent. 2021, 45, 179. [Google Scholar] [CrossRef]
- Yang, F.; Lee, G.; Fan, Y. Navigating Tumor Angiogenesis: Therapeutic Perspectives and Myeloid Cell Regulation Mechanism. Angiogenesis 2024, 27, 333–349. [Google Scholar] [CrossRef]
- Batmomolin, A.; Ahsan, A.; Wiyasa, I.W.A.; Santoso, S. Ethanolic Extract of Moringa oleifera Leaves Improve Inflammation, Angiogenesis, and Blood Pressure in Rat Model of Preeclampsia. J. Appl. Pharm. Sci. 2020, 10, 52–57. [Google Scholar]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Asad, M.; Joseph, B. Moringa oleifera Leaf Extract Promotes Healing of Infected Wounds in Diabetic Rats: Evidence of Antimicrobial, Antioxidant and Proliferative Properties. Pharmaceuticals 2022, 15, 528. [Google Scholar] [CrossRef]
- Achondo Jr., C.; Acctanasiri, K.; Agraan, S.; Alingayao, L.; Anzures, Y.; Apalisok, M.; Araya, E.; Arenque, M.; Arocena, M.; Gupta, A.; et al. Anti-Angiogenic Activity of Moringa oleifera Ethanolic Leaf Extract in Chorioallantoic Membrane (CAM) Assay. Philipp. Sci. J. 2021, 54, 10–15. [Google Scholar]
- Vlase, A.M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2023, 12, 91. [Google Scholar] [CrossRef]
- Gligor, O.; Clichici, S.; Moldovan, R.; Muntean, D.; Vlase, A.M.; Nadăș, G.C.; Matei, I.A.; Filip, G.A.; Vlase, L.; Crișan, G. The Effect of Extraction Methods on Phytochemicals and Biological Activities of Green Coffee Beans Extracts. Plants 2023, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Solcan, M.B.; Fizeșan, I.; Vlase, L.; Vlase, A.M.; Rusu, M.E.; Mateș, L.; Petru, A.E.; Creștin, I.V.; Tomuțǎ, I.; Popa, D.S. Phytochemical Profile and Biological Activities of Extracts Obtained from Young Shoots of Blackcurrant (Ribes nigrum L.), European Blueberry (Vaccinium myrtillus L.), and Mountain Cranberry (Vaccinium vitis-idaea L.). Horticulturae 2023, 9, 1163. [Google Scholar] [CrossRef]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST Technical Note on the EUCAST Definitive Document EDef 7.2: Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts EDef 7.2 (EUCAST-AFST)*. Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; M07Ed11; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Corina, D.; Bojin, F.; Ambrus, R.; Muntean, D.; Soica, C.; Paunescu, V.; Cristea, M.; Pinzaru, I.; Dehelean, C. Physico-Chemical and Biological Evaluation of Flavonols: Fisetin, Quercetin and Kaempferol Alone and Incorporated in Beta Cyclodextrins. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 615–626. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.M.; Berger, D.; Pavel, I.Z.; Muntean, D.; Minda, D.; Dehelean, C.A.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological Evaluation of Black Chokeberry Extract Free and Embedded in Two Mesoporous Silica-Type Matrices. Pharmaceutics 2020, 12, 1–22. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane in the Study of Tumor Angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135. [Google Scholar] [PubMed]
- Avram, Ș.; Bora, L.; Vlaia, L.L.; Muț, A.M.; Olteanu, G.E.; Olariu, I.; Magyari-Pavel, I.Z.; Minda, D.; Diaconeasa, Z.; Sfirloaga, P.; et al. Cutaneous Polymeric-Micelles-Based Hydrogel Containing Origanum vulgare L. Essential Oil: In Vitro Release and Permeation, Angiogenesis, and Safety Profile In Ovo. Pharmaceuticals 2023, 16, 940. [Google Scholar] [CrossRef] [PubMed]
- Pavel, I.Z.; Csuk, R.; Danciu, C.; Avram, S.; Baderca, F.; Cioca, A.; Moaca, E.A.; Mihali, C.V.; Pinzaru, I.; Muntean, D.M.; et al. Assessment of the Antiangiogenic and Anti-Inflammatory Properties of a Maslinic Acid Derivative and Its Potentiation Using Zinc Chloride. Int. J. Mol. Sci. 2019, 20, 2828. [Google Scholar] [CrossRef] [PubMed]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM). ICCVAM Test Method Evaluation Report: Current Validation Status of In Vitro Test Methods Proposed for Identifying Eye Injury Hazard Potential of Chemicals and Products Interagency Coordinating Committee on the Validation of Alternative Methods National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods. 2010. Available online: https://ntp.niehs.nih.gov/sites/default/files/iccvam/docs/ocutox_docs/invitro-2010/tmer-vol1.pdf (accessed on 6 July 2024).
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]



| No. | Compounds | Moringa MAC EtOH30% | Moringa MAC EtOH50% | Moringa MAC EtOH70% | Moringa US EtOH30% | Moringa US EtOH50% | Moringa US EtOH70% |
|---|---|---|---|---|---|---|---|
| Results (μg/mL) | |||||||
| 1 | Gentisic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | ND |
| 2 | Caffeic acid | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.676 ± 0.067 |
| 3 | Chlorogenic acid | <LOQ | 2.768 ± 0.083 | 2.994 ± 0.179 | <LOQ | 1.786 ± 0.053 | 1.484 ± 0.074 |
| 4 | 4-O-caffeoylquinic acid | 38.582 ± 5.787 | 35.865 ± 2.511 | 48.148 ± 4.814 | 31.043 ± 4.656 | 38.849 ± 3.107 | 53.735 ± 5.373 |
| 5 | p-coumaric acid | 0.612 ± 0.048 | <LOQ | <LOQ | 1.876 ± 0.056 | <LOQ | 0.521 ± 0.067 |
| 6 | Ferulic acid | <LOQ | ND | ND | <LOQ | ND | ND |
| 7 | Vitexin | 8.418 ± 0.336 | 6.449 ± 0.709 | 8.961 ± 0.806 | 6.992 ± 0.699 | 7.739 ± 1.006 | 9.504 ± 0.475 |
| 8 | Iso-quercitrin | 186.491 ± 7.459 | 161.603 ± 16.161 | 223.323 ± 11.166 | 126.235 ± 13.885 | 151.4312 ± 9.085 | 223.015 ± 33.452 |
| 9 | Rutin | 31.454 ± 1.258 | 28.634 ± 2.004 | 40.287 ± 4.028 | 28.337 ± 2.833 | 32.791 ± 1.967 | 45.631 ± 3.194 |
| 10 | Quercitrin | 62.159 ± 7.459 | 50.568 ± 2.528 | 68.703 ± 8.931 | 41.594 ± 3.327 | 42.716 ± 6.407 | 66.085 ± 0.661 |
| 11 | Quercetin | 7.299 ± 1.021 | 1.904 ± 0.266 | 2.482 ± 0.248 | 17.787 ± 2.312 | 1.738 ± 0.173 | 2.206 ± 0.242 |
| 12 | Luteolin | ND | ND | ND | ND | ND | <LOQ |
| 13 | Kaempferol | 3.691 ± 0.147 | 1.037 ± 0.021 | 1.037 ± 0.134 | 5.813 ± 0.523 | 0.672 ± 0.006 | <LOQ |
| 14 | Apigenin | <LOQ | ND | ND | <LOQ | ND | ND |
| 15 | Epicatechin | 0.156 ± 0.012 | 0.101 ± 0.014 | 0.098 ± 0.002 | 0.149 ± 0.007 | 0.152 ± 0.016 | 0.184 ± 0.005 |
| 16 | Catechin | ND | 0.007 ± 0.001 | 0.021 ± 0.001 | ND | 0.012 ± 0.001 | ND |
| 17 | Gallic acid | 0.298 ± 0.041 | 0.223 ± 0.008 | 0.348 ± 0.031 | 0.296 ± 0.005 | 0.293 ± 0.002 | 0.497 ± 0.069 |
| 18 | Protocatechuic acid | 0.828 ± 0.091 | 0.5 ± 0.075 | 0.628 ± 0.069 | 0.694 ± 0.062 | 0.532 ± 0.005 | 0.386 ± 0.007 |
| 19 | Epigallocatechin | 0.41 ± 0.024 | 0.199 ± 0.013 | 0.151 ± 0.015 | 0.236 ± 0.007 | 0.145 ± 0.021 | 0.147 ± 0.011 |
| Bacterial and Yeast Strains | Ethanolic Extracts | MIC Value (mg/mL) | MBC/MFC (mg/mL) |
|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Moringa MAC 30% | 25 | 25 |
| Moringa MAC 50% | 25 | 25 | |
| Moringa MAC 70% | 12.5 | 12.5 | |
| Moringa US 30% | 25 | 25 | |
| Moringa US 50% | 25 | 25 | |
| Moringa US 70% | 12.5 | 12.5 | |
| Streptococcus pyogenes ATCC 19615 | Moringa MAC 30% | 25 | 25 |
| Moringa MAC 50% | 25 | 25 | |
| Moringa MAC 70% | 12.5 | 12.5 | |
| Moringa US 30% | 12.5 | 12.5 | |
| Moringa US 50% | 12.5 | 12.5 | |
| Moringa US 70% | 12.5 | 12.5 | |
| Escherichia coli ATCC 25922 | Moringa MAC 30% | 25 | 25 |
| Moringa MAC 50% | 25 | 25 | |
| Moringa MAC 70% | 12.5 | 12.5 | |
| Moringa US 30% | 25 | 25 | |
| Moringa US 50% | 25 | 25 | |
| Moringa US 70% | 12.5 | 12.5 | |
| Pseudomonas aeruginosa ATCC 27853 | Moringa MAC 30% | * NA | * NA |
| Moringa MAC 50% | |||
| Moringa MAC 70% | |||
| Moringa US 30% | * NA | * NA | |
| Moringa US 50% | |||
| Moringa US 70% | |||
| Candida parapsilosis ATCC 22019 | Moringa MAC 30% | 50 | 50 |
| Moringa MAC 50% | 50 | 50 | |
| Moringa MAC 70% | 25 | 25 | |
| Moringa US 30% | 25 | 25 | |
| Moringa US 50% | 25 | 25 | |
| Moringa US 70% | 25 | 25 |
| Test Compound and Controls | Irritation Score (IS) | * Irritation Category |
|---|---|---|
| Positive control: SDS 0.5% | 17.29 ± 0.16 | Strong irritant |
| Moringa MAC 30%, 500 μg/mL | 0 ± 0.0 | Non-irritant |
| Moringa MAC 50%, 500 μg/mL | 0 ± 0.0 | Non-irritant |
| Moringa MAC 70%, 500 μg/mL | 0 ± 0.0 | Non-irritant |
| Moringa US 30%, 500 μg/mL | 0 ± 0.0 | Non-irritant |
| Moringa US 50%, 500 μg/mL | 0 ± 0.0 | Non-irritant |
| Moringa US 70%, 500 μg/mL | 0 ± 0.0 | Non-irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liga, S.; Magyari-Pavel, I.Z.; Avram, Ș.; Minda, D.I.; Vlase, A.-M.; Muntean, D.; Vlase, L.; Moacă, E.-A.; Danciu, C. Comparative Analysis of Moringa oleifera Lam. Leaves Ethanolic Extracts: Effects of Extraction Methods on Phytochemicals, Antioxidant, Antimicrobial, and In Ovo Profile. Plants 2025, 14, 1653. https://doi.org/10.3390/plants14111653
Liga S, Magyari-Pavel IZ, Avram Ș, Minda DI, Vlase A-M, Muntean D, Vlase L, Moacă E-A, Danciu C. Comparative Analysis of Moringa oleifera Lam. Leaves Ethanolic Extracts: Effects of Extraction Methods on Phytochemicals, Antioxidant, Antimicrobial, and In Ovo Profile. Plants. 2025; 14(11):1653. https://doi.org/10.3390/plants14111653
Chicago/Turabian StyleLiga, Sergio, Ioana Zinuca Magyari-Pavel, Ștefana Avram, Daliana Ionela Minda, Ana-Maria Vlase, Delia Muntean, Laurian Vlase, Elena-Alina Moacă, and Corina Danciu. 2025. "Comparative Analysis of Moringa oleifera Lam. Leaves Ethanolic Extracts: Effects of Extraction Methods on Phytochemicals, Antioxidant, Antimicrobial, and In Ovo Profile" Plants 14, no. 11: 1653. https://doi.org/10.3390/plants14111653
APA StyleLiga, S., Magyari-Pavel, I. Z., Avram, Ș., Minda, D. I., Vlase, A.-M., Muntean, D., Vlase, L., Moacă, E.-A., & Danciu, C. (2025). Comparative Analysis of Moringa oleifera Lam. Leaves Ethanolic Extracts: Effects of Extraction Methods on Phytochemicals, Antioxidant, Antimicrobial, and In Ovo Profile. Plants, 14(11), 1653. https://doi.org/10.3390/plants14111653














