Abstract
The imbalance of phosphodiesterase 5 (PDE5) enzyme in the male body, or excessive PDE5 enzyme levels, can occur due to factors such as aging, diseases (e.g., cardiovascular disease, diabetes, depressive disorder), and physical behaviors (e.g., alcoholism, smoking, stress). PDE5 is directly associated with erectile dysfunction disease. Currently, many studies aim to find natural PDE5 inhibitors as an alternative to commercial drugs. This study is the first to demonstrate that the ethanolic leaf extract of D. cochinchinensis exhibits potent PDE5-inhibitory activity. The PDE5-inhibitory activity of five plant parts was evaluated: leaf (IC50 = 1.53 ± 0.12 µg/mL), twig (3.37 ± 0.54), fruit (14.92 ± 2.85), heartwood (19.05 ± 5.60), and bark (16.03 ± 2.92). However, there is still uncertainty about which compounds in leaf extract are responsible for the PDE5 inhibition. Therefore, the purpose of this study is to identify the chemical constituents in the leaf of D. cochinchinensis, including determining which of these compounds may act as PDE5 inhibitors. This study was achieved using at-line LC-QTOF-MS2.
1. Introduction
Erectile dysfunction is a prevalent male sexual disorder characterized by the inability to achieve or maintain an erection sufficient for satisfactory sexual performance [1,2]. The risk factors contributing to ED include aging, lifestyle choices, medications, chronic diseases, pelvic injuries, and abnormalities in endocrine and cardiovascular systems [3]. ED involves cyclic contraction and the relaxation of corpus cavernosum smooth muscle, which is regulated by phosphodiesterase 5 (PDE5)—mediated cGMP hydrolysis [1,4,5,6]. PDE5 is a cyclic guanosine monophosphate (cGMP)-specific enzyme predominantly found in the lungs, smooth muscles, platelets, and corpus cavernosum [4,5]. It hydrolyzes cGMP to its inactive form, guanosine monophosphate (GMP), leading to decreased cGMP levels and vascular smooth muscle contraction. Conversely, inhibiting PDE5 promotes smooth muscle relaxation, which is essential for penile erection [7]. Although FDA-approved PDE5 inhibitors (e.g., sildenafil, tadalafil) effectively restore erection, adverse effects (e.g., headache, flushing) and contraindications (e.g., nitrate co-administration) limit their use [8,9,10].
Research interest in natural products has been steadily growing as scientists aim to discover safer and more affordable PDE5 inhibitors [11]. Flavonoids in medicinal plants have been reported as potential sources of PDE5 inhibitors [12,13,14]. In addition, curcuminoids [15], xanthones [16], phenanthrenes [17], and alkaloids [18] have been reported to exhibit PDE5 inhibitory activity. Several species of the Dalbergia genus are traditionally used in various medicinal systems and have been reported to exhibit a wide range of bioactivities, such as anti-inflammatory, osteogenic, antioxidant, anti-androgenic, antibacterial, anti-allergic, antidiarrheal, antifungal, anti-tumor, and blood stasis [19,20]. Phytoconstituents isolated from heartwood, including stem and seeds of D. cochinchinensis, have been reported to include flavonoids (flavones, isoflavones, flavans, isoflavans, flavanones, chalcones, neoflavonoids, and pterocarpans) and simple phenols as major compounds, along with terpenoids (sesquiterpenoids and triterpenoids), quinones, benzofurans, benzophenols, phytosterols, stilbenes, xanthones, and lignans [21,22,23,24]. The D. cochinchinensis seed is rich in glycosidase enzymes, with both β-glucosidase and β-fucosidase activities [25]. From reviews, chemical compounds in the leaf have been reported in only four species of Dalbergia, namely D. sissoo, where flavonoids (including flavones and isoflavones), triterpenoids, and phytosterols have been identified [26,27,28,29], D. stipulacea, which has been found to contain luteolin, D. spruceana, which has been reported to contain luteolin 4′-rutinoside, and D. spinosa, which has been noted to contain prunasin [30,31]. D. cochinchinensis has demonstrated its various bioactivities, including antioxidant [19], antimicrobial effects against Staphylococcus aureus and Aspergillus niger, cytotoxicity against lung and pericardial cancers [32], 5α-reductase inhibition [33], and anti-inflammation effects [34].
Using a chemotaxonomic approach to guide the selection of plants for PDE5 inhibition screening, D. cochinchinensis was chosen based on reports of its chemical composition, particularly its flavonoid content—compounds previously reported to exhibit PDE5 inhibitory activity. Additionally, ethnopharmacological records indicate the traditional use of D. cochinchinensis, especially its heartwood, for treating tumors and blood stasis [20]. Furthermore, based on our literature review, no previous studies have reported the PDE5 inhibitory activity of this plant. These findings collectively supported the selection of this species for further investigation. To the best of our knowledge, no previous studies have reported the PDE5 inhibitory activity of D. cochinchinensis. This study is the first to demonstrate such activity, providing new insights into the pharmacological potential of this species. In preliminary screening, we found that the hydro-ethanolic leaf extract inhibited PDE5 most potently, motivating detailed LC-QTOF-MS2 profiling.
2. Results
2.1. PDE5 Inhibitory Activity of Various Parts of D. cochinchinensis
In our preliminary study, five parts of D. cochinchinensis (leaf, twig, fruit, heartwood, and bark) were extracted with 95% ethanol and screened for PDE5 inhibitory activity. Table 1 shows that all extracts exhibited over 70% inhibition at a concentration of 25 µg/mL. Extracts exceeding 70% inhibition were considered effective and selected for IC50 determination. Among them, the leaf extract demonstrated the highest potency and was chosen for further identification of PDE5 inhibitors.

Table 1.
PDE5 inhibition and IC50 value of five parts of D. cochinchinensis extract. The values represent the means ± standard deviations from triplicate experiments.
2.2. At-Line Screening and Identification of PDE5 Inhibitors in D. cochinchinensis Leaf Ethanolic Extract
The leaf ethanolic extract underwent rapid screening to identify the chemical compounds in the leaf of D. cochinchinensis using at-line LC-QTOF-MS2. Figure 1 displays the extracted ion chromatogram of the leaf extract in panel A, and the corresponding bioactivity chromatogram representing the PDE5 inhibitory activity of collected micro-fractions is displayed in panel B. The zoom-in of this chromatogram highlights the bioactive region, between 36 and 64 min of retention time. In this area, the micro-fractions demonstrated PDE5 inhibition of 80% or greater, as shown in Figure 2.
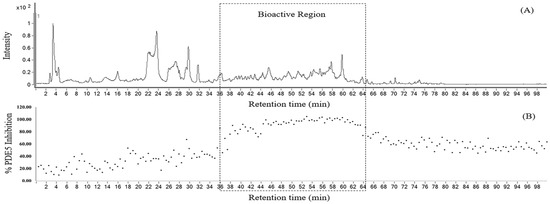
Figure 1.
Extracted ion chromatogram from at-line LC-QTOF-MS of D. cochinchinensis leaf extract (A), and the corresponding bioactivity chromatogram representing the PDE5 inhibitory activity of collected micro-fractions (B).
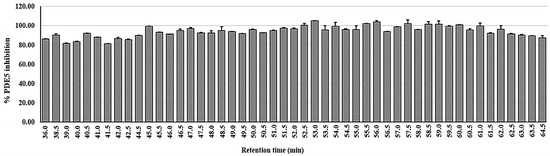
Figure 2.
Graphical presentation of PDE5 inhibitory activities for at-line micro-fractions of D. cochinchinensis leaf ethanolic extract, with data expressed as mean ± standard deviation (n = 3).
2.3. Identification of Chemical Compounds in the Leaf of D. cochinchinensis Using LC-QTOF-MS2
For the tentative identification of constituents of the leaf extract, the micro-fractions were analyzed using m/z fragmentation and MassHunter Data Acquisition Software Version B.05.01 and MassHunter Qualatative Analysis Software B 06.0 respectively (Agilent Technologies, Santa Clara, CA, USA). The chemical compounds in the leaf extract were identified based on m/z fragmentation and are listed in Table 2. From previous studies, a summary of compounds isolated from the leaves of various Dalbergia species is shown in Table 3.

Table 2.
Tentative identification by MS/MS fragmentation (negative mode) of D. cochinchinensis Leaf ethanolic extract.

Table 3.
Reviews of phytochemicals in the leaves of various species of genus Dalbergia [19,24].
2.4. Liquid–Liquid Partition of D. cochinchinensis Leaf Extract
The study initially conducted at-line screening to assess both the biological activity and chemical composition in the leaf extract of D. cochinchinensis. The results revealed the chemical composition of the leaf extract and identified a group of substances expected to be the PDE5 inhibitor. Liquid–liquid partitioning was used to separate groups of compounds in the leaf extract based on the polarity of the solvents used. D. cochinchinensis leaf extract was fractionated using the partition method to segregate it into groups based on the polarity of the solvent. For sequential partitioning, 25 g of the extract were utilized. Liquid–liquid partition produced an extract of hexane (HE), ethyl acetate (EE), and water (WE) at 2.7 g, 12.8 g, and 9.4 g, respectively. The process of partition and the dry weight of samples obtained are stated in Scheme 1. Subsequently, these extracts were analyzed using HPLC, with the system conditions set to match those used for the at-line technique. The HPLC chromatograms of each extract are shown in Figure 3.
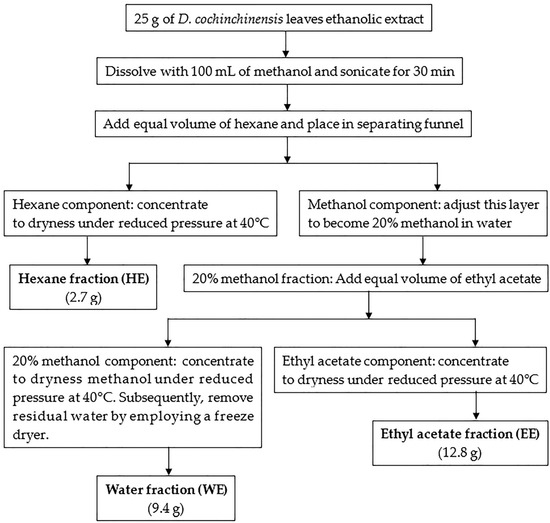
Scheme 1.
The flow chart indicates the sequential partitioning of D. cochinchinensis leaf ethanolic extracts.
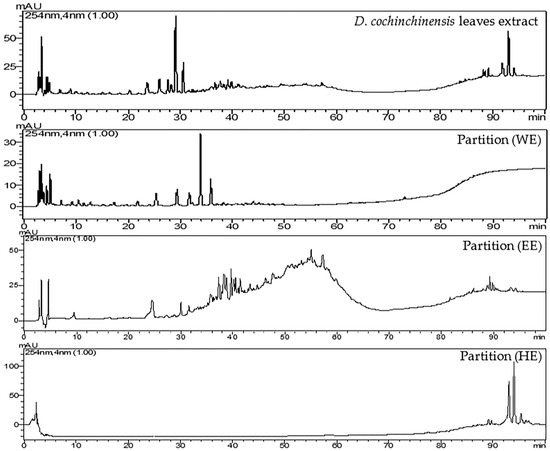
Figure 3.
HPLC chromatograms of D. cochinchinensis leaf ethanolic extract and three fractions from partition with solvents: water (WE), ethyl acetate (EE), and hexane (HE) at 254 nm.
HPLC chromatograms of the partitioned extracts were compared with the crude extract. The chromatograms show that the extracts obtained from partitioning with different solvents clearly separate into distinct groups, including highly polar compounds (represented by the water extract, WE), moderately polar compounds (represented by the ethyl acetate extract, EE), and non-polar compounds (represented by the hexane extract, HE). All three partition extracts were tested for PDE5 inhibitory activity in triplicate at the final concentration of 5 μg/mL. The results revealed that the EE extract (95.69 ± 2.97%) demonstrated the highest efficacy in inhibiting PDE5, followed by the HE (65.57 ± 3.13%) and WE extracts (39.61 ± 2.75%), respectively. Upon examining the HPLC chromatogram of the EE extract, it was observed that it corresponds to the bioactive region identified in the at-line analysis, which contains compounds with more than 80% efficacy in inhibiting PDE5 (Figure 4). The study results confirm that the PDE5 inhibitors are indeed located in the bioactive region, and this also supports the validity of the at-line analysis technique.
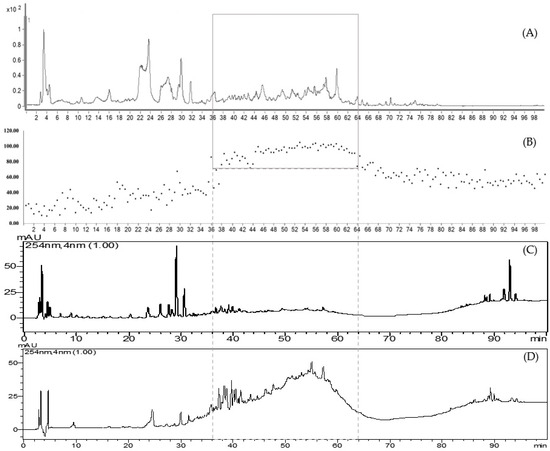
Figure 4.
Comparison of the bioactive regions in the at-line technique (A,B), the HPLC chromatogram showing the chemical profile of the leaf extract (C), and the compounds in the EE partition extract (D) from D. cochinchinensis.
2.5. Identification of Chemical Compounds of Ethyl Acetate Partition (EE) Using LC-QTOF-MS2
The results indicate that the EE extract contains compounds with potential PDE5 inhibitory activity. Therefore, the EE extract was analyzed using LC-MS/MS to identify the tentative compounds based on m/z fragmentation patterns. The extracted ion chromatogram from at-line LC-QTOF-MS of EE partition (negative mode) is shown in Figure 5. The tentative identifications based on m/z fragmentation of the EE partition from D. cochinchinensis are listed in Table 4.
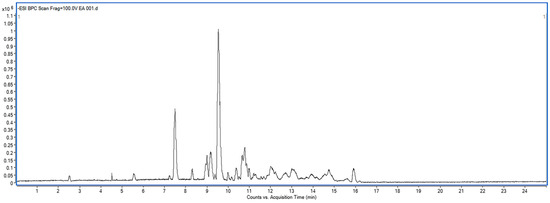
Figure 5.
Extracted ion chromatogram from at-line LC-QTOF-MS of EE partition (negative mode).

Table 4.
Tentative identification by MS/MS fragmentation (negative mode) of EE partition from D. cochinchinensis.
3. Discussion
Dalbergia genus includes 274 accepted species worldwide, with 26 native species found in Thailand [32]. D. cochinchinensis, traditionally known as Thai Rosewood or Siamese Rosewood, was described in 1898. It belongs to the family of Fabaceae and is widely distributed across Southeast Asia, including Vietnam, Thailand, Laos, and Cambodia. D. cochinchinensis is a large tree known for its durable heartwood, which is resistant to termites. This high economic value makes it sought after for various commercial purposes, including seeds, living trees, heartwood, handicrafts, and furniture [36].
The screening of PDE5 inhibitory activity in D. cochinchinensis revealed that the leaf extract exhibited the lowest IC50 values, indicating the highest efficacy, followed by the twig, fruit, bark, and heartwood, respectively. This study demonstrates, for the first time, that the leaf of D. cochinchinensis possesses the most potent PDE5 inhibitory activity. Therefore, it was of interest to further investigate the active compounds in the leaf, as no reports have yet identified chemical compounds from D. cochinchinensis acting as PDE5 inhibitors.
The at-line LC-QTOF-MS2 technique was used for rapid screening of bioactivity coupled with mass fragmentation. The use of at-line analysis allowed for the identification of potential PDE5 inhibitory zones in the chromatogram of the leaf extract. However, it was not possible to isolate pure compounds from the collected micro-fractions, with the amount of the fractions remaining after evaporation being insufficient for further study. Thus, the use of at-line techniques serves as a rapid screening method, providing both biological activity assessment and predictive analysis of potential PDE5 inhibitors based on m/z data. This study presents a faster approach for lead identification and the dereplication of known compounds compared to conventional fractionation techniques. However, despite the efficiency of at-line analysis, there is a concern that compounds present in low natural abundance may be overlooked.
This study found that the leaf predominantly contains flavonoids, a class of compounds known for their significant biological properties. Additionally, other substances have also been found, including sugar group, phenyl propanoic acid, quinic acid, benzodioxoles, sesquiterpene, xanthone, hydroxycinnamic acid, proanthocyanidin, quinoline alkaloid, stilbene, chalcone, and lipid. The compounds found in the bioactive region include flavonoids such as kaempferol and quercetin glycosides, as well as other substances like guibourtinidol-(4α→6)-catechin, niaziminin, and liquiritigenin. Previous reports have isolated flavonoid glucosides, including kaempferol-3-O-β-D-glucopyranoside, kaempferol-3-O-rutinoside, quercetin-3-β-D-glucopyranoside, and quercetin-3-O-rutinoside from the leaf of D. sissoo, as well as liquiritigenin from the heartwood of D. cochinchinensis [21,37]. Literature reviews indicate that the compounds isolated from the leaf are predominantly flavonoids (including flavanones, flavones, and isoflavones), along with other compounds such as triterpenoids, phytosterols, and lignans [19,24]. The results from the at-line technique have revealed the presence of several of these compounds in the leaf of D. cochinchinensis. However, these data are preliminary, based on mass spectrometry, and provide an initial analysis of the compound groups. The results of our experiment can be used to hypothesize potential PDE5 inhibitory compounds (in the bioactive region) and serve as a basis for further isolation of pure substances from the leaf extract.
The ethanolic leaf extract was further subjected to sequential partitioning to isolate the bioactive fraction. The partitioning process revealed that the bioactive fraction was obtained using the ethyl acetate (EE) partition. The compounds predominantly found in the EE partition include flavonol glycosides and procyanidins. In addition, other substances were also found, such as polyphenol, O-glycosyl, phenolic acid, monocarboxylic acid, phenylpropanoid, phenolic aldehyde, procyanidin, cyanogenic glycoside, and alkaloid. The partitioning process enabled the separation of compound groups, allowing us to identify those with PDE5 inhibitory activity. This helped specify the active groups more precisely and guided the selection of fractions for further isolation in a more targeted manner. This study has led to the discovery of candidate compounds for PDE5 inhibitors in D. cochinchinensis, which is being reported for the first time. Especially, the flavonol glycosides include kaempferol and quercetin glycosides, along with procyanidins, which consist of catechin-afzelechin, afzelechin dimers and trimers, and afzelechin-guibourtinidol. These compounds may have promise in becoming a PDE5 inhibitor. This research provides information about compounds in the leaves of D. cochinchinensis that have the potential to act as PDE5 inhibitors. Nevertheless, further studies are needed to identify the active ingredients and to complete the research in the future.
4. Materials and Methods
4.1. Chemicals and Materials
4.1.1. Plant Material
D. cochinchinensis was sourced from the Phra Mae Ya Sukhothai Botanical Garden in Sukhothai Province, Thailand. The voucher specimens (No. 004549) were authenticated by Assistant Professor Dr. Pranee Nangngam, Department of Biology, Faculty of Sciences, Naresuan University, Phitsanulok Province, Thailand.
4.1.2. Solvents
The following chemicals were purchased from RCI Labscan (Bangkok, Thailand): 95% ethanol, ethyl acetate, hexane, acetonitrile, water, dimethyl sulfoxide (DMSO), 98% formic acid (analytical grade), acetonitrile (HPLC grade), and methanol (LC-MS grade).
4.1.3. Reagents
Bovine serum albumin (BSA), crude snake venom (Crotalus atrox), dulbecco’s modified eagle medium (DMEM), dithiothreitol (DTT), ethylenediamine tetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), fetal bovine serum (FBS), imidazole, magnesium chloride (MgCl2), phenylmethylsulfonyl fluoride (PMSF), QAE Sephadex™ A-25, and tris (hydroxymethyl) aminomethane (Tris) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Geneticin (G418) was purchased from Gibco by Life Technologies (Paisley, Scotland). [3H]-cGMP and Ultima Gold cocktail were obtained from PerkinElmer (Waltham, MA, USA).
4.1.4. Tools Used for Analysis
ESI mass spectra were determined using an Agilent-6540 UHD accurate mass LC-QTOF-MS equipped with an electrospray ionization (ESI) interface (Agilent Technologies, Singapore). HPLC analysis was performed using the Prominence HPLC chromatograph system (Shimadzu, Kyoto, Japan). The chromatograph system was comprised of a binary pump system (LC-20AT) coupled with a UV/VIS detector (SPD-20A), communicator (CBM-20A), and degasser (DGU-20A3). HPLC analysis was conducted on a Phenomenex Luna® C18 column (150 mm × 4.6 mm, 5 μm) (Phenomenex, Torrance, CA, USA).
4.2. Plant Extraction
4.2.1. Crude Hydro-Ethanolic Extract
The fresh leaves of D. cochinchinensis were thoroughly cleaned and rinsed with water, then cut into small pieces and dried in a hot-air oven (Memmert UF 75, Schwabach, Germany) at 60 °C for 3 days. Subsequently, the dried leaves were ground into a rough powder using a grinder. The particle size of the powder was controlled by passing it through 100 mesh sieves (Retsch GmbH, Haan, Germany). The powdered leaves of D. cochinchinensis were macerated with 95% ethanol (EtOH) under shaking for 3 days at room temperature. Afterwards, the plant residue was separated by filtration through a filter paper (Whatman International Ltd., Chalfont St. Giles, UK). The filtrate was then concentrated by evaporating the ethanol using a BÜCHI Rotavapor R-124 (BÜCHI Labortechnik AG, Flawil, Switzerland). The crude extract was stored at −20 °C to maintain its integrity and stability until further analysis.
4.2.2. Liquid–Liquid Partition
The partitioning process was utilized to separate a group of compounds based on solvent polarity. The D. cochinchinensis leaf extract (25 g) was dissolved in 100 mL of methanol (MeOH) and sonicated for 30 min using an ultrasonic bath (Elmasonic S60H, Singen, Germany). It was then partitioned with hexane. The remaining MeOH fraction was adjusted with water to achieve a 20% MeOH solution, which was subsequently partitioned with ethyl acetate [8]. The resulting fractions were processed further: the hexane and EA fractions were concentrated to dryness at 40 °C using a rotary evaporator, while the 20% MeOH fraction was further dried using a freeze dryer (Thermo Electron Co., Ltd., Waltham, MA, USA) to remove the water. All extracts were stored in the dark at −20 °C until further analysis. Three fractions were obtained from the solvent partition: hexane (HE), 20% MeOH (WE), and ethyl acetate (EE).
4.3. Sample Analysis
4.3.1. High-Performance Liquid Chromatography (HPLC)
The chemical profile of D. cochinchinensis leaf extract was investigated by preparing a solution at a concentration of 10 mg/mL, dissolved in 50% methanol. This solution, as well as extracts obtained from liquid–liquid partitioning (also prepared at 10 mg/mL and dissolved in methanol), was filtered through nylon syringe filters with a pore size of 0.45 µm before analysis. For HPLC analysis, the system conditions were set to match those used for the LC-MS/MS analysis.
4.3.2. Liquid Chromatography Mass Spectrometry (LC-MS/MS)
For the at-line technique, the D. cochinchinensis leaf extract was prepared at a concentration of 20 mg/mL by dissolving it in 50% acetonitrile. The solution was then sonicated for 15 min and filtered through nylon syringe filters with a pore size of 0.45 µm.
For LC-MS/MS analysis of the ethyl acetate extract obtained from partitioning, the extract was prepared at a concentration of 10 mg/mL in 50% methanol. Chemical compounds were identified based on m/z data. Separations were performed using a Luna C18(2) column (4.6 mm × 150 mm, 5 μm particle size) from Phenomenex (Torrance, CA, USA), with the temperature maintained at 35 °C. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), with a gradient system increasing the proportion of solvent B from 5% to 95% over 25 min.
4.4. At-Line Technique
The at-line assay employing LC-MS/MS analysis utilized an Agilent 1260 Infinity Series HPLC system coupled to an Agilent 6540 UHD accurate mass QTOF-LC/MS, which was equipped with an electrospray interface (ESI). The leaf extract of D. cochinchinensis (20 μL of 20 mg/mL to 400 μg) was analyzed using the at-line technique with modifications based on the protocols outlined by Bhandari et al. [38]. The D. cochinchinensis leaf extract was injected into the LC-MS/MS system, and micro-fractions were collected in a 96-well plate. This procedure was repeated three times. The separations were conducted using a Luna C18(2) column (4.6 mm × 150 mm, 5 μm particle size) from Phenomenex (Torrance, CA, USA), with the temperature held constant at 35 °C. The mobile phase consisted of a mixture of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient program began with 10% solvent B, which was increased to 15% over 20 min, followed by a gradual increase to 30% over 25 min, 50% over 15 min, and 70% over 20 min. Finally, solvent B was ramped up to 100% over a total duration of 100 min. A post-time of 5 min was allowed for system re-equilibration before the next injection. The injection volume was set at 20 μL, and the flow rate was maintained at 0.5 mL/min. The eluent was split into two flows in 1:9 ratios. The majority of the eluent was collected in a 96-well plate micro-fractionation system with 30 s per well, while the minor portion was directed to an ESI-QTOF-MS system. The micro-fractions were dried using a sample concentrator (Techne, Cambridge, UK) and kept at −20 °C until test day. The ion source was operated in negative mode with the following conditions: drying gas flow of 10 L/min, gas temperature set to 350 °C, nebulizer pressure at 30 psig, capillary voltage of 3500 V, skimmer voltage set to 65 V, octapole RFV at 750 V, and fragmentor voltage at 250 V. All acquisition data were analyzed using MassHunter software (Agilent Technologies, Santa Clara, CA, USA). The MS and MS/MS fragmentation patterns of PDE5 inhibitor active micro-fractions were compared using a library search, including MassHunter Metlin metabolite PCD/PCDL (Agilent Technologies), the Human Metabolomics Database (https://www.hmdb.ca), ChemSpider (https://www.chemspider.com), and MassBank (https://www.massbank.eu). Molecular formulas were generated using Agilent MassHunter Qualitative Analysis Software B 06.0.
4.5. PDE5 Inhibitory Activity
4.5.1. Sample Preparation for PDE5 Inhibitory Activity
The micro-fractions in the 96-well plate were dried using a sample concentrator. Afterward, the micro-fractions were dissolved in 25 μL of 5% DMSO for the assay, resulting in a final concentration of 1% DMSO.
4.5.2. Enzyme Preparation
The human PDE5 plasmid, received from Professor Dr. Joseph A. Beavo of the University of Washington, Seattle, WA, USA, was sub-cloned into a pcDNA3 vector with an ampicillin-resistant gene. These plasmids were then scaled up and purified using the Invitrogen™ PureLink™ HiPure Plasmid Maxiprep Kit (Thermo Fisher Scientific, Waltham, MA, USA)). HEK293 cells were transfected with human PDE5 plasmid using Lipofectamine® 2000 reagent as per the protocol. After 2 days of transfection, the cells underwent treatment with a selective antibiotic (G418, 1 mg/mL) for 7 days. Surviving cells were sub-cultured in DMEM supplemented with 10% FBS in 175 mm flasks until they reached 90–100% confluence. Cell harvesting was performed using a scraper and lysed with a sonicate probe in 1 mL of Tris buffer [50 mM of Tris pH 7.5, 2 mM of EDTA, 1 mM of DTT, and 1:100 of 100 mM of PMSF]. The homogenate was centrifuged at 14,000 rpm, 4 °C for 20 min, and the supernatant was utilized as a source of PDE5 enzyme.
4.5.3. Experimental Protocols
The enzymatic assay consisted of a two-step process [13]. In the first step, the PDE5 enzyme was mixed with negatively charged [3H]-cGMP, either in the presence or absence of an inhibitor. The reaction mixture consisted of 25 μL of 10 mM EGTA, 25 μL of buffer C (containing 100 mM of Tris–HCl (pH 7.5), 100 mM of imidazole, 15 mM of MgCl2, and 1.0 mg/mL of BSA), and 25 μL of either, the extract or the solvent (5% DMSO), as a control. The substrate, 25 μL of [3H]-cGMP, was added to the reaction mixture and incubated at 30 °C for 10 min. After incubation, the reaction was stopped by placing the tube in boiling water for 1 min, followed by cooling for 5 min. In the second enzymatic reaction, 25 μL of snake venom containing 5′-nucleotidase were added to the mixture and incubated at 30 °C for 5 min to break the 5′ nucleotide bond of the product (negatively charged [3H] -5′GMP), releasing negatively charged phosphate and uncharged guanosine. Afterward, 250 μL of 20 mM of Tris–HCl (pH 6.8) were added to the reaction mixture, which was then applied to a QAE anion exchange resin column. The uncharged guanosine was eluted through the column and collected. These eluents were then mixed with Ultima Gold (scintillation cocktail), and the radioactivity was measured using a β counter (Tri-Crab® 2910 TR) (Perkin Elmer, Inc., Waltham, MA, USA). Higher amounts of uncharged guanosine led to lower inhibition, and counter. The hydrolysis activity of the PDE5 enzymes was standardized to represent 20–30% of the total substrate counts. The percentage of hydrolysis and PDE5 inhibition were calculated using the following Equations (1) and (2).
CPMsample is the radioactive count rate of the assay with an enzyme. CPMbackground is the radioactive count rate of the assay, but without enzymes. CPMcontrol is the radioactive count rate of the assay with enzymes but without any sample. The CPMtotal count is a count rate of 25 μL of substrate, plus 2 mL of low-salt buffer.
The percentage of hydrolysissample and percentage of hydrolysiscontrol are the enzyme activities of the sample and solvent in the assay, respectively.
5. Conclusions
This is the first report demonstrating PDE5 inhibitory activity in D. cochinchinensis leaf extract, a high-value plant native to Asia. This finding introduces a novel biological activity for this species. Additionally, the study identifies the chemical constituents in the leaf that have the potential to act as PDE5 inhibitors. The at-line technique was employed to rapidly determine the chemical composition and assess the biological activity. Our study was able to isolate specific compounds in the bioactive region from partitioning, identifying them as potential candidates for PDE5 inhibitors in this plant. It would be highly beneficial to conduct further studies in the future on the aspects of compound purification, standardization of extract, stability study, and cytotoxicity testing to enhance the completeness of the study. Based on the overall conclusions, this study identified a novel natural PDE5 inhibitor in the leaf extract of D. cochinchinensis. Future research will focus on isolating the active compounds based on the data obtained in this study, determining their IC50 values, elucidating their chemical structures, and standardizing the extract. Additionally, comprehensive efficacy and safety evaluations will be conducted to support the development of this extract in addressing male erectile dysfunction.
Author Contributions
Conceptualization, R.C., C.G., F.S., and P.T.; methodology, R.C. and P.T.; software, R.C.; validation, R.C. and P.T.; formal analysis, R.C., P.T., and N.N.; investigation, R.C.; resources, P.T. and K.I.; data curation, R.C. and P.T.; writing—original draft preparation, R.C. and P.T.; writing—review and editing, R.C., C.G., F.S., N.N., K.I., and P.T.; visualization, R.C., C.G., F.S., and P.T.; supervision, P.T.; project administration, P.T.; funding acquisition, P.T. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for financial support from Research and Researchers for Industries, which is under the National Research Council of Thailand (NRCT) [grant number N41A650401]; the JKKN supply and services Co., Ltd., the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [grant number B16F640099] and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Global and Frontier Research University Fund, Naresuan University; [grant number R2566C053].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the Pra Mae Ya Sukhothai Botanical Garden in Sukhothai Province, Thailand, for providing the plant materials. We are grateful to Pranee Nangngam from the Faculty of Science, Naresuan University, Phitsanulok, Thailand, for conducting plant identification. Special thanks to Corine Girard and François Senejoux from the Université de Franche-Comté for editing the grammar, syntax, and overall English expression in this document. The authors thank Nitra Nuengchamnong for technical support from the Science Laboratory Centre, Faculty of Science, Naresuan University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PDE5 | Phosphodiesterase 5 |
| HE | Hexane-partition extract |
| EE | Ethyl acetate-partition extract |
| WE | Water-partition extract |
References
- Maggi, M.; Filippi, S.; Ledda, F.; Magini, A.; Forti, G. Erectile dysfunction: From biochemical pharmacology to advances in medical therapy. Eur. J. Endocrinol. 2000, 143, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Rösing, D.; Klebingat, K.-J.; Berberich, H.J.; Bosinski, H.A.G.; Loewit, K.; Beier, K.M. Male sexual dysfunction: Diagnosis and treatment from a sexological and interdisciplinary perspective. Dtsch. Arztebl. Int. 2009, 106, 821–828. [Google Scholar] [CrossRef]
- Feldman, H.A.; Johannes, C.B.; Derby, C.A.; Kleinman, K.P.; Mohr, B.A.; Araujo, A.B.; McKinlay, J.B. Erectile Dysfunction and Coronary Risk Factors: Prospective Results from the Massachusetts Male Aging Study. Prev. Med. 2000, 30, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int. J. Impot. Res. 2004, 16 (Suppl. 1), S11–S14. [Google Scholar] [CrossRef]
- Feneck, R. Phosphodiesterase inhibitors and the cardiovascular system. Cont. Educ. Anaesth. Crit. Care Pain 2007, 7, 203–207. [Google Scholar] [CrossRef]
- Lue Tom, F. Erectile Dysfunction. N. Engl. J. Med. 2000, 342, 1802–1813. [Google Scholar] [CrossRef]
- Leite, R.; Giachini, F.R.; Carneiro, F.S.; Nunes, K.P.; Tostes, R.C.; Webb, R.C. Targets for the treatment of erectile dysfunction: Is NO/cGMP still the answer? Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 119–132. [Google Scholar] [CrossRef]
- Eardley, I.; Donatucci, C.; Corbin, J.; El-Meliegy, A.; Hatzimouratidis, K.; McVary, K.; Munarriz, R.; Lee, S.W. Pharmacotherapy for erectile dysfunction. J. Sex. Med. 2010, 7, 524–540. [Google Scholar] [CrossRef]
- Huang, S.A.; Lie, J.D. Phosphodiesterase-5 (PDE5) Inhibitors In the Management of Erectile Dysfunction. Pharm. Ther. 2013, 38, 407–419. [Google Scholar] [PubMed]
- Moreira, S.G., Jr.; Brannigan, R.E.; Spitz, A.; Orejuela, F.J.; Lipshultz, L.I.; Kim, E.D. Side-effect profile of sildenafil citrate (Viagra) in clinical practice. Urology 2000, 56, 474–476. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Kim, H.J.; Kwak, J.H.; Chun, H.O.; Kim, J.H.; Park, H.; Kim, D.H.; Lee, Y.S. A prenylated flavonol, sophoflavescenol: A potent and selective inhibitor of cGMP phosphodiesterase 5. Bioorganic Med. Chem. Lett. 2002, 12, 2313–2316. [Google Scholar] [CrossRef] [PubMed]
- Temkitthawon, P.; Hinds, T.R.; Beavo, J.A.; Viyoch, J.; Suwanborirux, K.; Pongamornkul, W.; Sawasdee, P.; Ingkaninan, K. Kaempferia parviflora, a plant used in traditional medicine to enhance sexual performance contains large amounts of low affinity PDE5 inhibitors. J. Ethnopharmacol. 2011, 137, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Chaichamnong, N.; Temkitthawon, P.; Khorana, N.; Pitpakdeeanan, P.; Taepavarapruk, P.; Nuengchamnong, N.; Siriwattanasathien, Y.; Suksamrarn, A.; Ingkaninan, K. Phosphodiesterase 5 Inhibitors from Derris scandens. Planta Med. 2018, 84, 1134–1140. [Google Scholar] [CrossRef]
- Kruangtip, O.; Chootip, K.; Temkitthawon, P.; Changwichit, K.; Chuprajob, T.; Changtam, C.; Suksamrarn, A.; Khorana, N.; Scholfield, C.N.; Ingkaninan, K. Curcumin analogues inhibit phosphodiesterase-5 and dilate rat pulmonary arteries. J. Pharm. Pharmacol. 2015, 67, 87–95. [Google Scholar] [CrossRef]
- Sabphon, C.; Temkitthawon, P.; Ingkaninan, K.; Sawasdee, P. Phosphodiesterase inhibitory activity of the flavonoids and xanthones from Anaxagorea luzonensis. Nat. Prod. Commun. 2015, 10, 301–303. [Google Scholar] [CrossRef]
- Temkitthawon, P.; Changwichit, K.; Khorana, N.; Viyoch, J.; Suwanborirux, K.; Ingkaninan, K. Phenanthrenes from Eulophia macrobulbon as Novel Phosphodiesterase-5 Inhibitors. Nat. Prod. Commun. 2017, 12, 79–82. [Google Scholar] [CrossRef]
- Nam, K.W.; Je, K.H.; Shin, Y.J.; Kang, S.S.; Mar, W. Inhibitory effects of furoquinoline alkaloids from Melicope confusa and Dictamnus albus against human phosphodiesterase 5 (hPDE5A) in vitro. Arch. Pharm. Res. 2005, 28, 675–679. [Google Scholar] [CrossRef]
- Saha, S.; Shilpi, J.; Mondal, H.; Hossain, F.; Anisuzzman, M.; Hasan, M.; Cordell, G. Ethnomedicinal, phytochemical, and pharmacological profile of the genus Dalbergia L. (Fabaceae). Phytopharmacology 2013, 4, 291–346. [Google Scholar]
- Palasuwan, A.; Soogarun, S.; Lertlum, T.; Pradniwat, P.; Wiwanitkit, V. Inhibition of Heinz body induction in an in vitro model and total antioxidant activity of medicinal Thai plants. Asian Pac. J. Cancer Prev. 2005, 6, 458–463. [Google Scholar]
- Liu, R.-H.; Li, Y.-Y.; Shao, F.; Chen, L.-Y.; Wen, X.-C.; Zhang, P.-Z.; Huang, H.-L. A New Chalcone from the Heartwood of Dalbergia cochinchinensis. Chem. Nat. Compd. 2016, 52, 405–408. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, J.; Mo, X.; Xu, Z.; Qiu, S.; Liu, X. A New Isoflavan From the Heartwood of Dalbergia cochinchinensis. Nat. Prod. Commun. 2021, 16, 1–4. [Google Scholar] [CrossRef]
- Shirota, O.; Pathak, V.; Sekita, S.; Satake, M.; Nagashima, Y.; Hirayama, Y.; Hakamata, Y.; Hayashi, T. Phenolic constituents from Dalbergia cochinchinensis. J. Nat. Prod. 2003, 66, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Son, N.T.; Manh Ha, N. Siamese, Indian, and Brazilian Rosewoods: A Review on Phytochemistry, Applications, and Pharmacology. Nat. Prod. Commun. 2022, 17, 1934578X221096962. [Google Scholar] [CrossRef]
- Srisomsap, C.; Svasti, J.; Surarit, R.; Champattanachai, V.; Sawangareetrakul, P.; Boonpuan, K.; Subhasitanont, P.; Chokchaichamnankit, D. Isolation and characterization of an enzyme with beta-glucosidase and beta-fucosidase activities from Dalbergia cochinchinensis Pierre. J. Biochem. 1996, 119, 585–590. [Google Scholar] [CrossRef]
- Kothari, P.; Tripathi, A.K.; Girme, A.; Rai, D.; Singh, R.; Sinha, S.; Choudhary, D.; Nagar, G.K.; Maurya, R.; Hingorani, L.; et al. Caviunin glycoside (CAFG) from Dalbergia sissoo attenuates osteoarthritis by modulating chondrogenic and matrix regulating proteins. J. Ethnopharmacol. 2022, 282, 114315. [Google Scholar] [CrossRef]
- Dixit, P.; Chillara, R.; Khedgikar, V.; Gautam, J.; Kushwaha, P.; Kumar, A.; Singh, D.; Trivedi, R.; Maurya, R. Constituents of Dalbergia sissoo Roxb. leaves with osteogenic activity. Bioorg. Med. Chem. Lett. 2012, 22, 890–897. [Google Scholar] [CrossRef]
- Farag, S.F.; Ahmed, A.S.; Terashima, K.; Takaya, Y.; Niwa, M. Isoflavonoid glycosides from Dalbergia sissoo. Phytochemistry 2001, 57, 1263–1268. [Google Scholar] [CrossRef]
- Sarg, T.; Ateya, A.-M.; Abdel-Ghani, A.; Badr, W.; Shams, G. Phytochemical and Pharmacological Studies of Dalbergia sissoo Growing in Egypt. Pharm. Biol. 1999, 37, 54–62. [Google Scholar] [CrossRef]
- Borai, P.; Dayal, R. A flavone glycoside from Dalbergia stipulacea leaves. Phytochemistry 1993, 33, 731–732. [Google Scholar] [CrossRef]
- Narayanan, V.; Nacarajan, N.S. Two isoflavone galactosides from Dalbergia spinosa. Phytochemistry 1988, 27, 2364–2365. [Google Scholar] [CrossRef]
- Pham, T.L.; Tran, H.T.; Tran, T.B. Some biological, ecological characteristics and bioactivity of some species of Dalbergia genus in Vietnam (in Vietnamese). In Proceedings of the 4th National Scientific Conference on Ecology and Biological Resources, Ha Noi, Vietnam, 21 October 2011. [Google Scholar]
- Pathak, V.; Shirota, O.; Sekita, S.; Hirayama, Y.; Hakamata, Y.; Hayashi, T.; Yanagawa, T.; Satake, M. Antiandrogenic phenolic constituents from Dalbergia cochinchinensis. Phytochemistry 1997, 46, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, R.; Chen, L.; Quyang, C.; Wei, X.; Liu, Y.; Ren, J. Anti-inflammatory Benzofurans from the Heartwood of Dalbergia cochinchinensis Pierre ex Laness. Rec. Nat. Prod. 2023, 17, 549–554. [Google Scholar] [CrossRef]
- Kaufmann, A.; Walker, S. Evaluation of the interrelationship between mass resolving power and mass error tolerances for targeted bioanalysis using liquid chromatography coupled to high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 347–356. [Google Scholar] [CrossRef]
- Nhung, N.P.; Chi, N.M.; Thu, P.Q.; Thuong, B.H.; Ban, D.V.; Dell, B. Market and policy setting for the trade in Dalbergia tonkinensis, a rare and valuable rosewood, in Vietnam. Trees For. People 2020, 1, 100002. [Google Scholar] [CrossRef]
- Liu, R.-H.; Wen, X.-C.; Shao, F.; Zhang, P.-Z.; Huang, H.-L.; Zhang, S. Flavonoids from Heartwood of Dalbergia cochinchinensis. Chin. Herb. Med. 2016, 8, 89–93. [Google Scholar] [CrossRef]
- Bhandari, S.; Nuengchamnong, N.; Chaichamnong, N.; Seasong, T.; Ingkaninan, K.; Temkitthawon, P. At-line LC-QTOF-MS micro-fractionation of Derris scandens (Roxb.) Benth, coupled to radioassay for the early identification of PDE5A1 inhibitors. Phytochem. Anal. 2020, 31, 297–305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).