Effects of Climate Change Scenarios on Growth, Flowering Characteristics, and Honey Production Potential of Pseudolysimachion rotundum var. subintegrum
Abstract
1. Introduction
2. Results
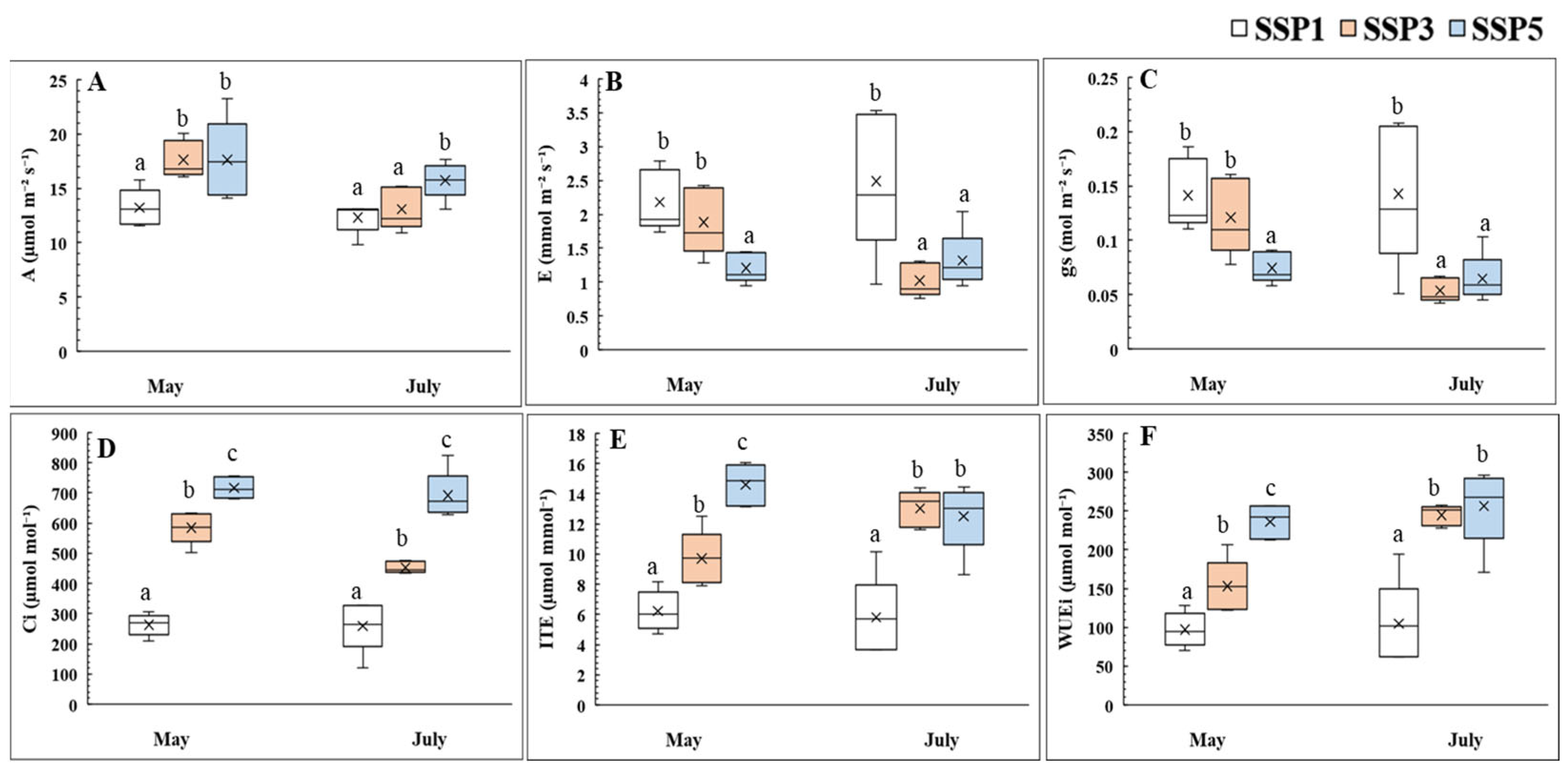
2.1. Photosynthesis and Stomatal Responses
2.2. Analysis of the Chlorophyll Content
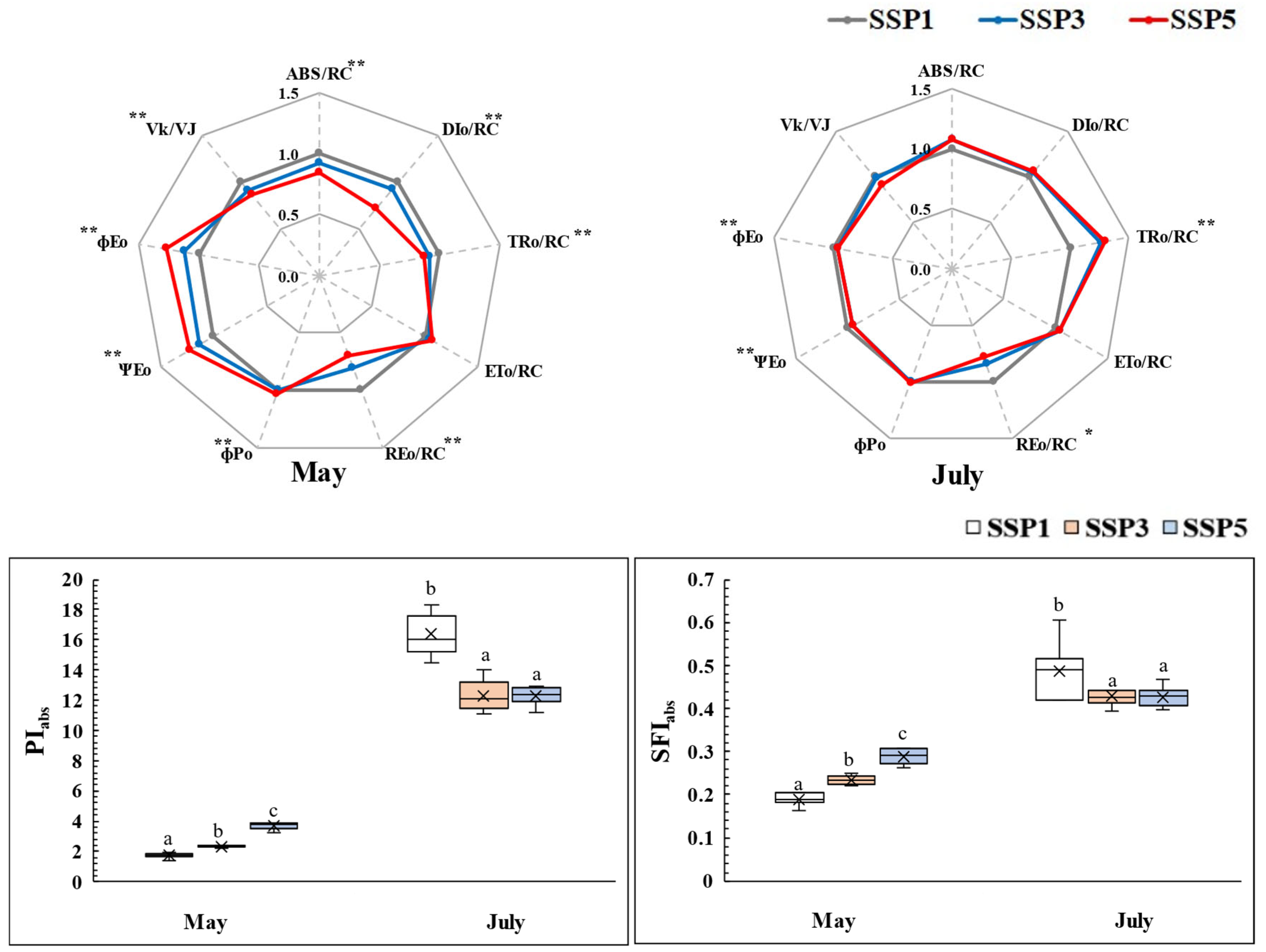
2.3. Chlorophyll Fluorescence Responses
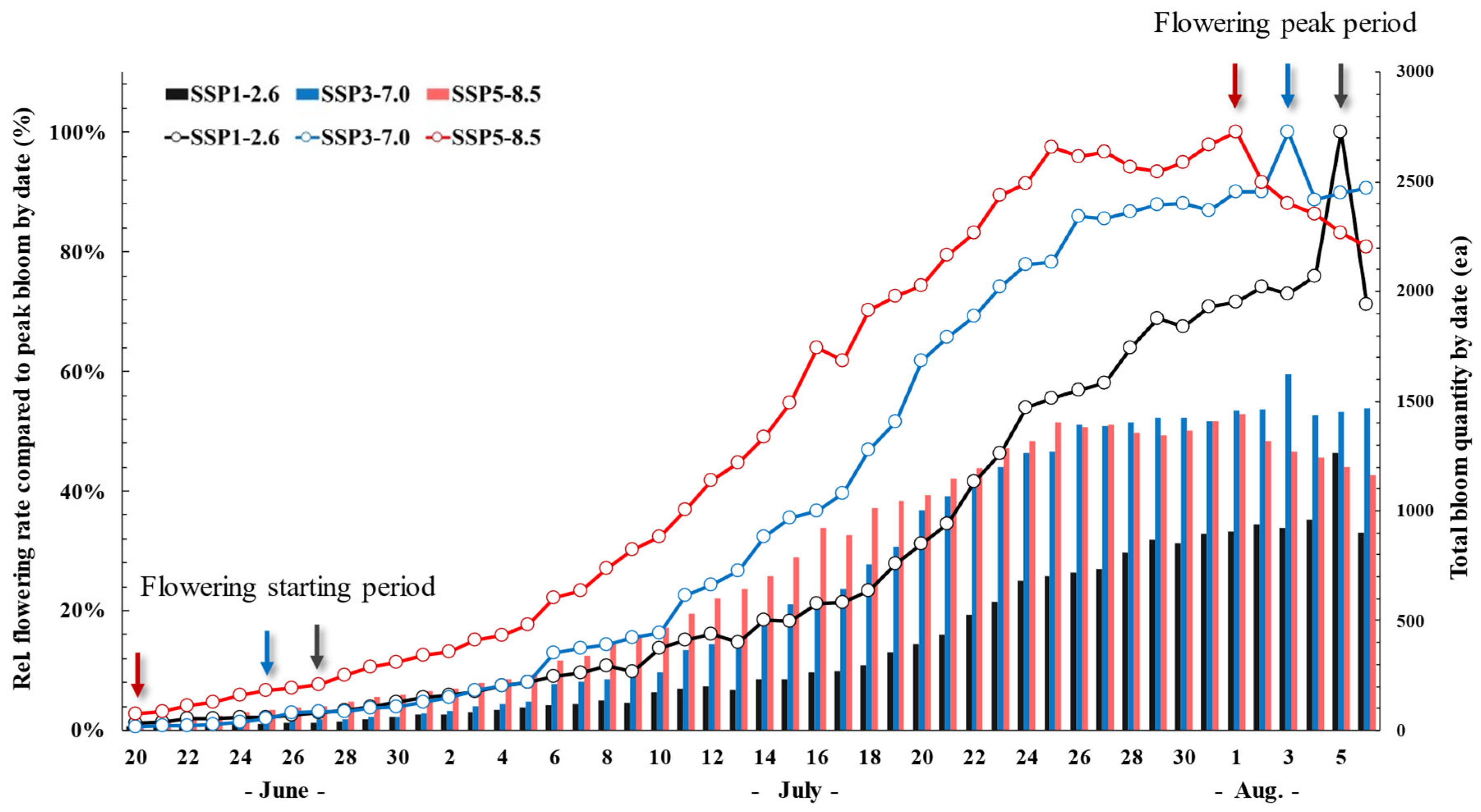
2.4. Growth and Flowering Characteristics
2.5. The Abundance of Nectar
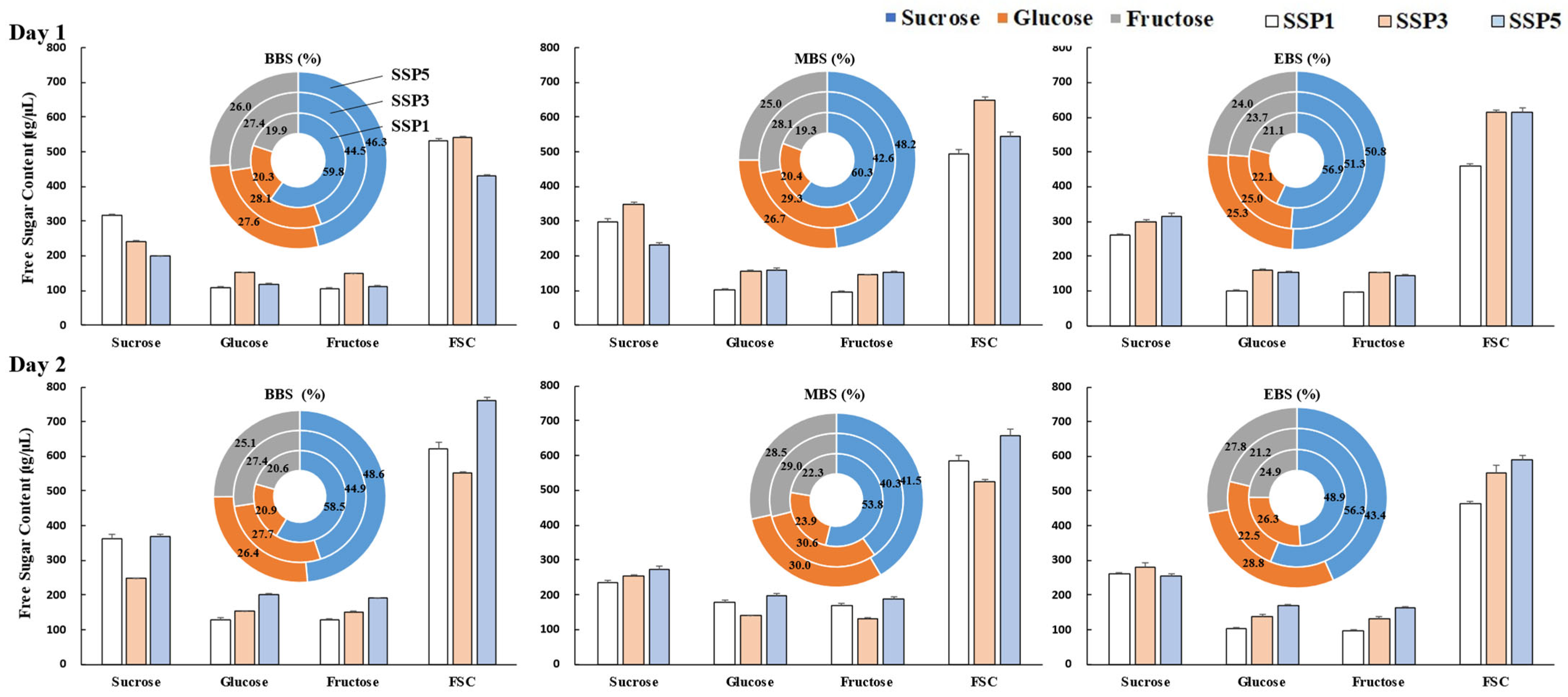
2.6. Floral Nectar Content and Composition
2.7. The Amino Acid Content and Composition
2.8. Estimation of Honey Production
3. Discussion
3.1. Photosynthesis and Stomatal Response
3.2. Analysis of the Chlorophyll Content
3.3. Analysis of Chlorophyll Fluorescence
3.4. Growth and Flowering Characteristics
3.5. Nectar Characteristics
3.6. Changes in Nectar’s Sugar Composition
3.7. Changes in Amino Acid Composition
3.8. Estimation of Honey Production
4. Materials and Methods
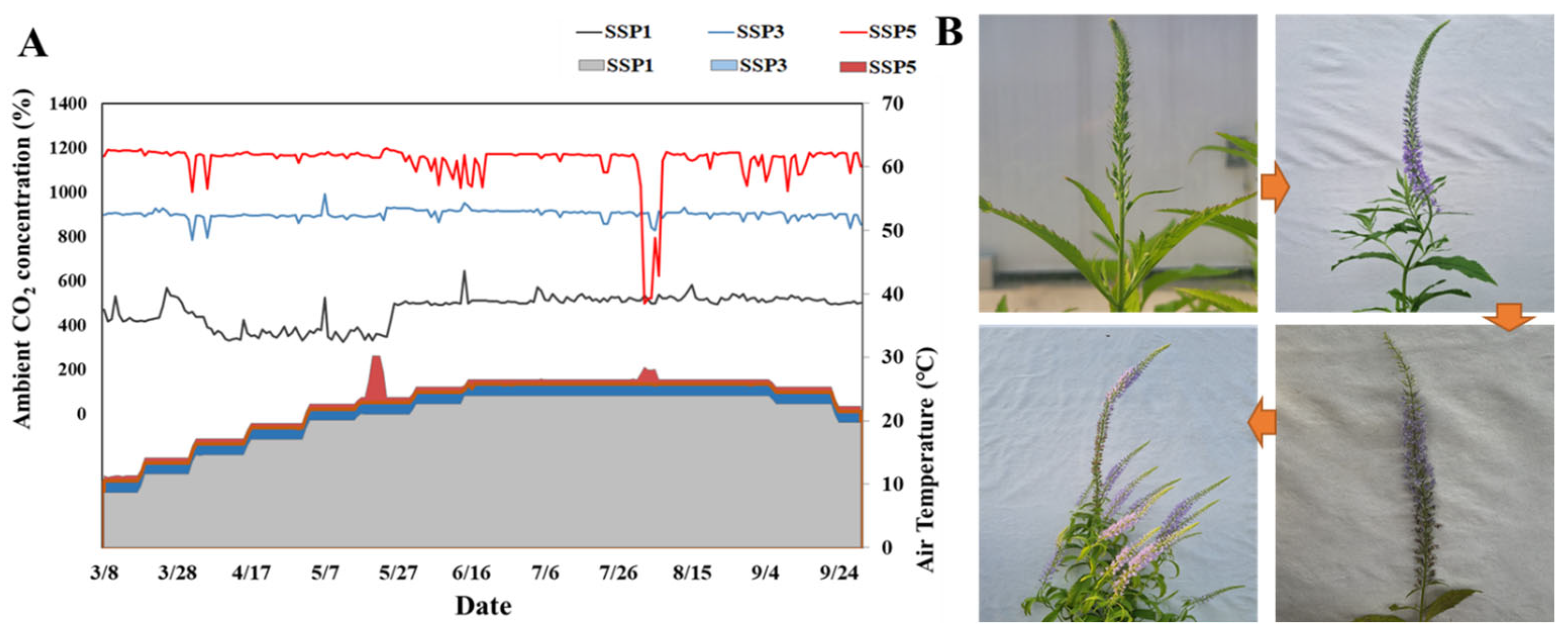
4.1. TheClimate Change Scenario Setup
4.2. Photosynthetic and Stomatal Responses
4.3. Analysis of the Chlorophyll Pigment Content
- Chlorophyll a = 12.7 A663 − 2.69 A645;
- Chlorophyll b = 22.9 A645 − 4.68 A663;
- Total chlorophyll = 20.29 A645 + 8.02 A663;
- Total carotenoids = (1000 A470 − 1.82 Chl a − 85.02 Chl b)/198.
4.4. Analysis of the Chlorophyll Fluorescence
4.5. Growth and Flowering Characteristics
4.6. Nectar Secretion Characteristics
4.7. Free Sugar Analysis
4.8. Free Amino Acid Analysis
4.9. Estimation of Honey Production
population (ea/ha) × 0.001 (for unit conversion: g to kg) × Honey potential b
4.10. The Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borghi, M.; Perez de Souza, L.; Yoshida, T.; Fernie, A.R. Flowers and climate change: A metabolic perspective. New Phytol. 2019, 224, 1425–1441. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK, 2023. [Google Scholar]
- KMA. 2020: Korean Climate Change Assessment Report; Korea Meteorological Administration: Seoul, Republic of Korea, 2020; p. 40.
- Meinshausen, M.; Nicholls, Z.R.; Lewis, J.; Gidden, M.J.; Vogel, E.; Freund, M.; Wang, R.H. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 2022, 13, 3571–3605. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuern, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Chang. 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Takkis, K.; Tscheulin, T.; Tsalkatis, P.; Petanidou, T. Climate change reduces nectar secretion in two common Mediterranean plants. AoB Plants 2015, 7, plv111. [Google Scholar] [CrossRef]
- Dorji, T.; Hopping, K.A.; Meng, F.; Wang, S.; Jiang, L.; Klein, J.A. Impacts of climate change on flowering phenology and production in alpine plants: The importance of end of flowering. Agric. Ecosyst. Environ. 2020, 291, 106795. [Google Scholar] [CrossRef]
- Lee, K.C.; An, J.; Hwang, J.E.; Kim, P.B.; Park, H.B.; Kim, S.; Park, H.J.; Lee, C.W.; Lee, B.D.; Kim, N.Y. Effects of Light Condition on Growth and Physiological Characteristics of the Endangered Species Sedirea japonica under RCP 6.0 Climate Change Scenarios. Plants 2021, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.G.; Hwang, J.E.; An, J.; Kim, P.B.; Park, H.B.; Park, H.J.; Lee, K.C. The growth and physiological characteristics of the endangered CAM Plant, Nadopungnan (Sedirea japonica), under drought and climate change scenarios. Forests 2022, 13, 1823. [Google Scholar] [CrossRef]
- Yun, Y.B.; Koo, H.J.; Kwon, H.Y.; Song, Y.; Lee, K.C.; Um, Y. Effects of CO2 Concentration and Temperature on Growth Characteristics, Physiological Activities, and Antioxidant Activities of Angelica gigas Nakai. J. Plant Biol. 2024, 65, 357–376. [Google Scholar] [CrossRef]
- Maggi, T.; Pardo, L.; Chreil, R. Pollinator Diversity: A Key to Ecosystem Resilience and Food Security. Pollinators 2023, 6, 33–48. [Google Scholar]
- Na, S.J.; Kim, Y.K.; Park, J.M. Nectar characteristics and honey production potential of five rapeseed cultivars and two wildflower species in South Korea. Plants 2024, 13, 419. [Google Scholar] [CrossRef]
- Kim, Y.K.; Na, S.J.; Kwon, H.Y.; Park, W.G. Evaluation of Honey Production of Ligustrum japonicum and Viburnum odoratissimum var. awabuki in the Southern Part of Korea. J. Apic. 2022, 37, 35–44. [Google Scholar]
- Lee, S.I.; Yeon, S.H.; Cho, J.S.; Jeong, M.J.; Lee, C.H. Optimization of Cultivation Conditions on Effective Seedlings of Veronica rotunda var. subintegra (Nakai) T. Yamaz. J. Bio-Environ. Control 2020, 29, 181–188. [Google Scholar] [CrossRef]
- Springer, C.J.; Ward, J.K. Flowering time and elevated atmospheric CO2. New Phytol. 2007, 176, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, M.T.; Tamburrino, Í.; Pliscoff, P.; Robles, V.; Colldecarrera, M.; Guerrero, P.C. Flowering phenology adjustment and flower longevity in a South American alpine species. Plants 2021, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Pacini, E.; Nepi, M.; Vesprini, J.L. Nectar biodiversity: A short review. Plant Syst. Evol. 2003, 238, 7–21. [Google Scholar] [CrossRef]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Yoo, H.W.; Kwon, H.Y.; Na, S.J. Estimation of Nectar Secretion, Sugar Content and Honey Production of Prunus mume (Siebold) Siebold & Zucc. J. Apic. Res. 2021, 36, 141–147. [Google Scholar]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of agricultural crops to free-air CO2 enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE): A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; John Wiley and Sons: New York, NY, USA, 2008; p. 522. [Google Scholar]
- Song, Y.; Kim, C.W.; Kweon, H.; Yoon, K.K.; Shim, Y.J.; Lee, U.; Lee, K.C. Effects of Drought Stress on the Growth and Physiological Characteristics of Grafted Ziziphus jujuba var. inermis under RCP 6.0 Climate Change Scenarios. Hortic. Sci. Technol. 2023, 41, 11–26. [Google Scholar]
- Curtis, P.; Wang, X. A meta-analysis of elevated CO2 effects on woodyplant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Ann. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 378. [Google Scholar] [CrossRef]
- Yun, Y.B.; Um, Y.; Huh, J.H.; Son, H.J.; Song, Y.G.; Lee, K.C. Changes in growth characteristics and physiological activity of Cnidium officinale Makino according to the climate change scenario (RCP). Korean J. Med. Crop Sci. 2022, 30, 450–461. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, S.; Lee, J.C.; Kim, K.W.; Kim, P.G. Effects of Elevated CO2 Concentration and Temperature on Physiological Characters of Liriodendron tulipifera. Korean J. Agric. For. Meteorol. 2013, 15, 145–152. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.; Jordan, G.J.; Field, T.S. Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytol. 2009, 183, 839–847. [Google Scholar] [CrossRef]
- Hopping, K.A.; Knapp, A.K.; Dorji, T.; Klein, J.A. Warming and land use change concurrently erode ecosystem services in Tibet. Glob. Chang. Biol. 2018, 24, 5534–5548. [Google Scholar] [CrossRef]
- Van den Ende, W.; Peshev, D. Sugars as antioxidants in plants. In Crop Improvement Under Adverse Conditions; Tuteja, N., Gill, S.S., Eds.; Springer: New York, NY, USA, 2013; pp. 285–307. [Google Scholar]
- Terashima, I.; Evans, J.R. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 1988, 29, 143–155. [Google Scholar]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Lee, K.C.; Kweon, H.; Sung, J.W.; Kim, Y.S.; Song, Y.G.; Cha, S.; Koo, N. Physiological response analysis for the diagnosis of drought and waterlogging damage in Prunus yedoensis. For. Sci. Technol. 2022, 18, 14–25. [Google Scholar] [CrossRef]
- Guha, A.; Sengupta, D.; Reddy, A.R. Polyphasic chlorophyll a fluorescence kinetics and leaf protein analyses to track dynamics of photosynthetic performance in mulberry during progressive drought. J. Photochem. Photobiol. B Biol. 2013, 119, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Boureima, S.; Oukarroum, A.; Diouf, M.; Cisse, N.; Van Damme, P. Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ. Exp. Bot. 2013, 81, 37–43. [Google Scholar] [CrossRef]
- Lee, K.M.; Kim, H.R.; Lim, H.; You, Y.H. Effect of elevated CO2 concentration and temperature on the growth and ecophysiological responses of ginseng (Panax ginseng CA Meyer). Korean J. Crop Sci. 2012, 57, 106–112. [Google Scholar] [CrossRef]
- Xu, Z.; Shimizu, H.; Yagasaki, Y.; Ito, S.; Zheng, Y.; Zhou, G. Interactive effects of elevated CO2, drought, and warming on plants. J. Plant Growth Regul. 2013, 32, 692–707. [Google Scholar] [CrossRef]
- Tun, W.; Yoon, J.; Jeon, J.S.; An, G. Influence of climate change on flowering time. J. Plant Biol. 2021, 64, 193–203. [Google Scholar] [CrossRef]
- Lim, S.H.; Im, N.H.; An, S.K.; Lee, H.B.; Kim, K.S. Korean native Veronica rotunda and Veronica longifolia are day-neutral plants with no vernalization requirements. Hortic. Environ. Biotechnol. 2021, 62, 859–869. [Google Scholar] [CrossRef]
- Lee, K.B. Plant Morphology, 3rd ed.; Lifescience: Seoul, Republic of Korea, 2016; p. 301. [Google Scholar]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Springer, C.J.; Orozco, R.A.; Kelly, J.K.; Ward, J.K. Elevated CO2 influences the expression of floral-initiation genes in Arabidopsis thaliana. New Phytol. 2008, 1781, 63–67. [Google Scholar] [CrossRef]
- He, J.S.; Wolfe-Bellin, K.S.; Bazzaz, F.A. Leaf-level physiology, biomass, and reproduction of Phytolacca americana under conditions of elevated CO2 and altered temperature regimes. Int. J. Plant Sci. 2005, 166, 615–622. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef]
- Adgaba, N.; Al-Ghamdi, A.; Tadesse, Y.; Getachew, A.; Awad, A.M.; Ansari, M.J.; Owayss, A.A.; Mohammed, S.E.A.; Alqarni, A.S. Nectar secretion daynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Galetto, L.; Bernardello, G. Floral Nectaries, Nectar Production Dynamics and Chemical Composition in Six Ipomoea Species (Convolvulaceae) in Relation to Pollinators. Ann. Bot. 2004, 94, 269–280. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoo, H.W.; Kwon, H.Y.; Na, S.J. Honeybee Foraging Activity and Evaluation of Honey Value from Chaenomeles speciosa (sweet) Nakai. J. Apic. Res. 2021, 36, 161–168. [Google Scholar]
- Petanidou, T.; Van Laere, A.J.; Smets, E. Change in floral nectar components from fresh to senescent flowers of Capparis spinosa (Capparidaceae), a nocturnally flowering Mediterranean shrub. Plant Syst. Evol. 1996, 199, 79–92. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.H.; Song, J.H.; Nam, J.I.; Song, J.M.; Kim, M.S. Analysis of secreted nectar volume, sugar and amino acid content in Prunus yedoensis Matsum. and Prunus sargentii Rehder. J. Apic. 2019, 34, 225–232. [Google Scholar]
- Han, J.; Kim, S.H. Flowering and nectar secretion characteristics of honey plant, Hovenia dulcis var. koreana Nakai. J. Apic. 2008, 23, 199–205. [Google Scholar]
- Han, J.; Kim, S.H.; Kang, M.S.; Kim, C.S.; Baik, E.S. Flowering and nectar secretion characteristics of Tilia amurensis Rupr. and Tilia manshurica Rupr. Et Max. J. Apic. 2010, 25, 217–221. [Google Scholar]
- Kim, S.H.; Lee, A.; Kwon, H.Y.; Lee, W.; Kim, M.S. Analysis of flowering and nectar characteristics of major four chestnut cultivars (Castanea spp.). J. Apic. 2017, 32, 237–246. [Google Scholar]
- Cawoy, V.; Kinet, J.M.; Jacquemart, A.L. Morphology of nectaries and biology of nectar production in the distylous species Fagopyrum esculentum. Ann. Bot. 2008, 102, 675–684. [Google Scholar] [CrossRef]
- Burquez, A.; Corbet, S.A. Dynamics of production and exploitation of nectar: Lessons from Impatiens glandulifera Royle. In Nectary Biology; Bahadur, B., Ed.; Dattsons: Nagpur, India, 1998; pp. 130–152. [Google Scholar]
- Kim, M.S.; Kim, S.H.; Song, J.H.; Kim, H.; Oh, D.S. Honeybee visit and secreted nectar characteristics on flowers of Ligustrum japonicum Thunb. J. Apic. 2014, 29, 279–285. [Google Scholar] [CrossRef]
- Nepi, M.; Stpiczynska, M. Nectar resorption and translocation in Cucurbita pepo L. and Platanthera chlorantha Custer (Rchb.). Plant Biol. 2007, 9, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Wolff, D.; Witt, T.; Jurgens, A.; Gottsberger, G. Nectar dynamics and reproductive success in Saponaria officinalis(Caryophyllaceae) in southern Germany. Flora 2006, 201, 353–364. [Google Scholar] [CrossRef]
- Fahn, A. Secretory tissue in vascular plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef]
- Horner, H.T.; Healy, R.A.; Ren, G.; Fritz, D.; Klyne, A.; Seames, C.; Thornburg, R.W. Amyloplast to chromoplast conversion in developing ornamental tobacco floral nectaries provides sugar for nectar and antioxidants for protection. Am. J. Bot. 2007, 94, 12–24. [Google Scholar] [CrossRef]
- Ren, G.; Healy, R.A.; Klyne, A.M.; Horner, H.T.; James, M.G.; Thornburg, R.W. Transient starch metabolism in ornamental tobacco floral nectaries regulates nectar composition and release. Plant Sci. J. 2007, 173, 277–290. [Google Scholar] [CrossRef]
- Lin, I.W.; Sosso, D.; Chen, L.Q.; Gase, K.; Kim, S.G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.H.; Qu, X.Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.E.; Worthington, R.D.; Jackson, M.S. Floral nectar sugar compositions of some South and Southeast Asian species. Biotropica 1991, 23, 568–574. [Google Scholar] [CrossRef]
- Stiles, F.G.; Freeman, C.E. Patterns in floral nectar characteristics of some bird-visited plant species from Costa Rica. Biotropica 1993, 25, 191–205. [Google Scholar] [CrossRef]
- Baker, H.G. Chemical constituents of nectar in relation to pollination mechanisms and phylogeny. In Biochemical Aspects of Evolutionary Biology, Proceedings of the 4th Annual Spring Systematics Symposium, Chicago, IL, USA, 8–9 May 1981; University of Chicago Press: Chicago, IL, USA, 1982; pp. 131–171. [Google Scholar]
- Kim, Y.K.; Yoo, H.W.; Park, M.S.; Kwon, H.Y.; Kim, M.J.; Na, S.J. Evaluation of Honey Production of Camellia japonica L. J. Apic. Res. 2021, 36, 149–159. [Google Scholar]
- Lohaus, G.; Schwerdtfeger, M. Comparison of sugars, iridoid glycosides and amino acids in nectar and phloem sap of Maurandya barclayana, Lophospermum erubescens, and Brassica napus. PLoS ONE 2014, 9, e87689. [Google Scholar] [CrossRef]
- Pacini, E.; Nepi, M. Nectar production and presentation. In Nectaries and Nectar; Springer: Dordrecht, The Netherlands, 2007; pp. 167–214. [Google Scholar]
- Erhardt, A.; Rusterholz, H.P.; Stöcklin, J. Elevated carbon dioxide increases nectar production in Epilobium angustifolium L. Oecologia 2005, 146, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Cun, Z.; Zhang, J.Y.; Hong, J.; Yang, J.; Gao, L.L.; Hao, B.; Chen, J.W. Integrated metabolome and transcriptome analysis reveals the regulatory mechanism of low nitrogen-driven biosynthesis of saponins and flavonoids in Panax notoginseng. Gene 2024, 901, 148163. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J., Jr. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Change 2014, 4, 477–480. [Google Scholar] [CrossRef]
- de Groot, A.P. Protein and amino acid requirements of the honeybee Apis mellifera. Physiol. Comp. Oecol. 1953, 3, 195–285. [Google Scholar]
- Hendriksma, H.P.; Oxman, K.L.; Shafir, S. Amino acid and carbohydrate tradeoffs by honey bee nectar foragers and their implications for plant–pollinator interactions. J. Insect Physiol. 2014, 69, 56–64. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, H.; Kim, S.D.; Park, S.J.; Song, J.H.; Kim, S.H. Pollinator Visit, Characteristics of Secreted Nectar and Analysis of Nectar Sugar and Amino Acid Contents in Flower of Dendropanax morbifera Lev. Korean J. Apic. 2015, 30, 307–314. [Google Scholar] [CrossRef]
- Avitabile, C.; D’Andrea, L.D.; Romanelli, A. Circular dichroism studies on the interactions of antimicrobial peptides with bacterial cells. Sci. Rep. 2014, 4, 4293. [Google Scholar] [CrossRef]
- Micheu, S.; Crailsheim, K.; Leonhard, B. Importance of proline and other amino acids during honeybee flight (Apis mellifera carnica POLLMANN). Amino Acids 2000, 18, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Bertazzini, M.; Medrzycki, P.; Bortolotti, L.; Maistrello, L.; Forlani, G. Amino acid content and nectar choice by forager honeybees (Apis mellifera L.). Amino Acids 2010, 39, 315–318. [Google Scholar] [CrossRef]
- Bertazzini, M.; Forlani, G. Intraspecific variability of floral nectar volume and composition in rapeseed (Brassica napus L. var. oleifera). Front. Plant Sci. 2016, 7, 288. [Google Scholar] [CrossRef]
- Anraku, M.; Shintomo, R.; Taguchi, K.; Kragh-Hansen, U.; Kai, T.; Maruyama, T.; Otagiri, M. Amino acids of importance for the antioxidant activity of human serum albumin as revealed by recombinant mutants and genetic variants. Life Sci. 2015, 134, 36–41. [Google Scholar] [CrossRef]
- Duan, J.; Yin, J.; Ren, W.; Liu, T.; Cui, Z.; Huang, X.; Wu, L.; Kim, S.W.; Liu, G.; Wu, X.; et al. Dietary supplementation with L-glutamate and L-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 2016, 48, 53–64. [Google Scholar] [CrossRef]
- Petanidou, T.; Van Laere, A.J.; Ellis, W.N.; Smets, E. What shapes amino acid and sugar composition in Mediterranean floral nectars? Oikos 2006, 115, 155–169. [Google Scholar] [CrossRef]
- Gardener, M.C.; Gillman, M.P. The effects of soil fertilizer on amino acids in the floral nectar of corncockle, Agrostemma githago (Caryophyllaceae). Oikos 2001, 92, 101–106. [Google Scholar] [CrossRef]
- Gijbels, P.; Ceulemans, T.; Van den Ende, W.; Honnay, O. Experimental fertilization increases amino acid content in floral nectar, fruit set and degree of selfing in the orchid Gymnadenia conopsea. Oecologia 2015, 179, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Mevi-Schütz, J.; Erhardt, A. Amino acids in nectar enhance butterfly fecundity: A long-awaited link. Am. Nat. 2005, 165, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Hoover, S.E.; Ladley, J.J.; Shchepetkina, A.A.; Tisch, M.; Gieseg, S.P.; Tylianakis, J.M. Warming, CO2, and nitrogen deposition interactively affect a plant-pollinator mutualism. Ecol. Lett. 2012, 15, 227–234. [Google Scholar] [CrossRef]
- Filipiak, Z.M.; Filipiak, M. The scarcity of specific nutrients in wild bee larval food negatively influences certain life history traits. Biology 2020, 9, 462. [Google Scholar] [CrossRef]
- Ceulemans, T.; Hulsmans, E.; Vanden Ende, W.; Honnay, O. Nutrient enrichment is associated with altered nectar and pollen chemical composition in Succisa pratensis Moench and increased larval mortality of its pollinator Bombus terrestris L. PLoS ONE 2017, 12, e0175160. [Google Scholar] [CrossRef]
- Jakobsen, H.B.; Kristja’nsson, K. Influence of temperature and floret age on nectar secretion in Trifolium repens L. Ann. Bot. 1994, 74, 327–334. [Google Scholar] [CrossRef]
- Nocentini, D.; Pacini, E.; Guarnieri, M.; Martelli, D.; Nepi, M. Intrapopulation heterogeneity in floral nectar attributes and foraging insects of an ecotonal Mediterranean species. Plant Ecol. 2013, 214, 799–809. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Petanidou, T. Introducing plants for bee-keeping at any cost? Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions. Plant Syst. Evol. 2003, 238, 155–168. [Google Scholar] [CrossRef]

| Month | SSP Scenarios | Chlorophyll (mg·g−1) | Carotenoid (mg·g−1) | Chl a/b | Total Chl/Car | ||
|---|---|---|---|---|---|---|---|
| a | b | a + b | |||||
| May | SSP1 | 12.55 ± 0.75 b | 2.88 ± 0.19 ns | 15.43 ± 0.83 b | 3.74 ± 0.39 ns | 4.37 ± 0.20 ns | 4.12 ± 0.29 b |
| SSP3 | 10.38 ± 0.29 a | 2.54 ± 0.15 | 12.92 ± 0.43 a | 3.71 ± 0.13 | 4.09 ± 0.11 | 3.50 ± 0.07 a | |
| SSP5 | 11.90 ± 0.55 ab | 2.94 ± 0.13 | 14.84 ± 0.65 ab | 3.49 ± 0.25 | 4.07 ± 0.18 | 4.28 ± 0.28 b | |
| July | SSP1 | 17.88 ± 1.91 c | 4.40 ± 0.49 c | 22.28 ± 2.39 c | 4.51 ± 0.52 c | 4.07 ± 0.22 ns | 4.96 ± 0.22 b |
| SSP3 | 14.10 ± 1.40 b | 3.43 ± 0.27 b | 17.53 ± 1.66 b | 3.74 ± 0.29 b | 4.11 ± 0.23 | 4.69 ± 0.45 b | |
| SSP5 | 10.67 ± 0.24 a | 2.55 ± 0.26 a | 13.21 ± 0.49 a | 3.09 ± 0.29 a | 4.19 ± 0.30 | 4.28 ± 0.35 a | |
| Treatments | Dry Weight (g) | S/R Ratio (g g−1) | Inflorescence | Number of Inflorescences per Plant | Number of Flowers per Inflorescence | |||
|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Total | Length (cm) | Width (cm) | ||||
| SSP1 | 104.5 ± 3.3 a | 56.4 ± 5.1 ns | 160.9 ± 7.7 a | 1.9 ± 0.1 a | 20.2 ± 2.4 a | 1.6 ± 0.4 ns | 50.1 ± 30.3 a | 205.0 ± 63.2 a |
| SSP3 | 125.6 ± 9.7 ab | 45.2 ± 3.3 | 170.8 ± 6.7 ab | 2.8 ± 0.4 b | 30.4 ± 5.3 b | 1.8 ± 0.2 | 72.2 ± 31.3 b | 264.1 ± 50.6 a |
| SSP5 | 143.0 ± 10.4 b | 52.2 ± 5.0 | 195.2 ± 15.6 b | 2.7 ± 0.1 b | 25.8 ± 6.3 b | 1.5 ± 0.2 | 89.0 ± 47.4 b | 347.4 ± 86.8 b |
| Flowering Time | Treatments | Nectar Volume per Flower (μL/Flower) | ||
|---|---|---|---|---|
| BBS | MBS | EBS | ||
| 1 day | SSP1 | 0.15 ± 0.01 a | 0.13 ± 0.05 ns | 0.21 ± 0.04 b |
| SSP3 | 0.15 ± 0.04 a | 0.10 ± 0.03 | 0.14 ± 0.05 ab | |
| SSP5 | 0.26 ± 0.02 b | 0.15 ±0.07 | 0.09 ± 0.01 a | |
| 2 day | SSP1 | 0.37 ± 0.09 ns | 0.28 ± 0.08 ns | 0.41 ± 0.05 b |
| SSP3 | 0.38 ± 0.10 | 0.28 ± 0.02 | 0.27 ± 0.05 a | |
| SSP5 | 0.33 ± 0.22 | 0.31 ± 0.05 | 0.24 ± 0.03 a | |
| Source | Day 1 | Day 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Sucrose | Glucose | Fructose | FSC | Sucrose | Glucose | Fructose | FSC | |
| Flowering stage (FS) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Treatment (T) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| FS × T | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Flowering Time | Treatments | BBS | MBS | EBS | Flowering Stage (FS) | Treatment (T) | FS × T |
|---|---|---|---|---|---|---|---|
| 1 day (mg/L) | ssp1 | 2406.1 ± 23.2 | 4430.3 ± 187.1 | 2383.2 ± 179.2 | <0.001 | <0.001 | <0.001 |
| ssp3 | 4419.8 ± 83.1 | 3520.1 ± 128.9 | 2788.1 ± 129.0 | ||||
| ssp5 | 1585.9 ± 16.4 | 1476.6 ± 72.2 | 2513.4 ± 60.4 | ||||
| 2 day (mg/L) | ssp1 | 2373.6 ± 121.8 | 4335.0 ± 157.7 | 2693.2 ± 281.7 | <0.001 | <0.001 | <0.001 |
| ssp3 | 1836.8 ± 37.9 | 3385.8 ± 178.8 | 2872.3 ± 184.0 | ||||
| ssp5 | 2257.3 ± 59.3 | 1198.5 ± 89.5 | 2437.6 ± 52.3 |
| Treatments | Nectar Sugar Content (μg/Flower) | Estimated Honey Production (g/Plant) | Estimated Honey Yield (kg/ha) |
|---|---|---|---|
| SSP1 | 0.22 ± 0.03 ns | 2.6 ± 0.3 a | 285.8 ± 36.1 a |
| SSP3 | 0.17 ± 0.02 | 3.6 ± 0.5 a | 401.1 ± 56.3 a |
| SSP5 | 0.20 ± 0.05 | 7.1 ± 1.6 b | 772.8 ± 178.8 b |
| Trait Category | Indicator | SSP1 | SSP3 | SSP5 |
|---|---|---|---|---|
| Physiological responses | Net photosynthetic rate | Lowest | Moderate in May; decreased in July | Highest (↑1.3 × SSP1) |
| Intercellular CO2 concentration | Baseline | Increased | Highest | |
| Instantaneous transpiration efficiency | Lowest | ↑2.2–2.4 × SSP1 | ||
| PIABS/SFIABS | Increased from May to July | Moderate increase | Highest in May, lower in July | |
| Total chlorophyll contents | Highest | Decreased to 78.0–78.8% of that in SSP1 | Decreased to 57.8–59.6% of the value in SSP1 | |
| Growth characteristics | Aboveground biomass | Baseline | +20.2% | +36.9% |
| Number of inflorescences | 50.1 ± 30.3 | 72.2 ± 31.3 | 89.0 ± 47.4 | |
| Number of flowers per inflorescence | 205.0 ± 63.2 | 264.1 ± 50.6 | 347.4 ± 86.8 | |
| Flowering onset | 28 June | 26 June | 21 June | |
| Nectar quantity | Nectar volume per flower (EBS, day 2) | Highest | Moderate | 59.9% lower than that in SSP1 |
| Total estimated honey per plant | 2.6 g | 3.6 g | 7.1 g | |
| Estimated yield per ha | 317.0 kg | 401.1 kg | 772.8 kg | |
| Nectar quality | Sucrose content (EBS, day 2) | 279.9 µg/µL | 314.6 µg/µL | 8.6% lower than that in the early stage |
| Free sugar content | 431.1–555.5 µg/µL | Variable | Peaked in the BBS, day 2 | |
| Amino acids | Total amino acid content (MBS, day 2) | Highest (4335.0 mg/L) | High | Lowest (1198.5 mg/L) |
| Phenylalanine | 62.5–75.5% | |||
| Parameters | Description |
|---|---|
| VK/VJ | The ratio of variable fluorescence in a time of 0.3 ms to the variable fluorescence in a time of 2 ms as an indicator of the PSII’s donor-side limitation |
| ABS/RC | Absorption flux per reaction center |
| TR0/RC | Trapped energy flux per reaction center at t = 0 |
| ET0/RC | Electron transport flux from QA to QB per reaction center at t = 0 |
| DI0/RC | Energy dissipation flux per reaction center at t = 0 |
| RE0/RC | Electron transport flux until PSI acceptors per reaction center at t = 0 |
| ΦPO | Probability that an absorbed photon leads to a reduction further than QA- |
| ΦEO | Probability that an absorbed photon leads to electron transport further than QA- |
| ΨO | Probability that an absorbed photon leads to a reduction in QA- |
| PIABS | The performance index on an absorption basis |
| SFIABS | The structure function index on an absorption basis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-C.; Song, Y.-G.; Koo, H.-J.; Kim, K.-J.; Kim, H.-J.; Baek, H.-Y.; Na, S.-J. Effects of Climate Change Scenarios on Growth, Flowering Characteristics, and Honey Production Potential of Pseudolysimachion rotundum var. subintegrum. Plants 2025, 14, 1647. https://doi.org/10.3390/plants14111647
Lee K-C, Song Y-G, Koo H-J, Kim K-J, Kim H-J, Baek H-Y, Na S-J. Effects of Climate Change Scenarios on Growth, Flowering Characteristics, and Honey Production Potential of Pseudolysimachion rotundum var. subintegrum. Plants. 2025; 14(11):1647. https://doi.org/10.3390/plants14111647
Chicago/Turabian StyleLee, Kyeong-Cheol, Yeong-Geun Song, Hyun-Jung Koo, Kyung-Jun Kim, Hyung-Joo Kim, Ha-Young Baek, and Sung-Joon Na. 2025. "Effects of Climate Change Scenarios on Growth, Flowering Characteristics, and Honey Production Potential of Pseudolysimachion rotundum var. subintegrum" Plants 14, no. 11: 1647. https://doi.org/10.3390/plants14111647
APA StyleLee, K.-C., Song, Y.-G., Koo, H.-J., Kim, K.-J., Kim, H.-J., Baek, H.-Y., & Na, S.-J. (2025). Effects of Climate Change Scenarios on Growth, Flowering Characteristics, and Honey Production Potential of Pseudolysimachion rotundum var. subintegrum. Plants, 14(11), 1647. https://doi.org/10.3390/plants14111647






