Effects of Altitude on Tea Composition: Dual Regulation by Soil Physicochemical Properties and Microbial Communities
Abstract
1. Introduction
2. Results
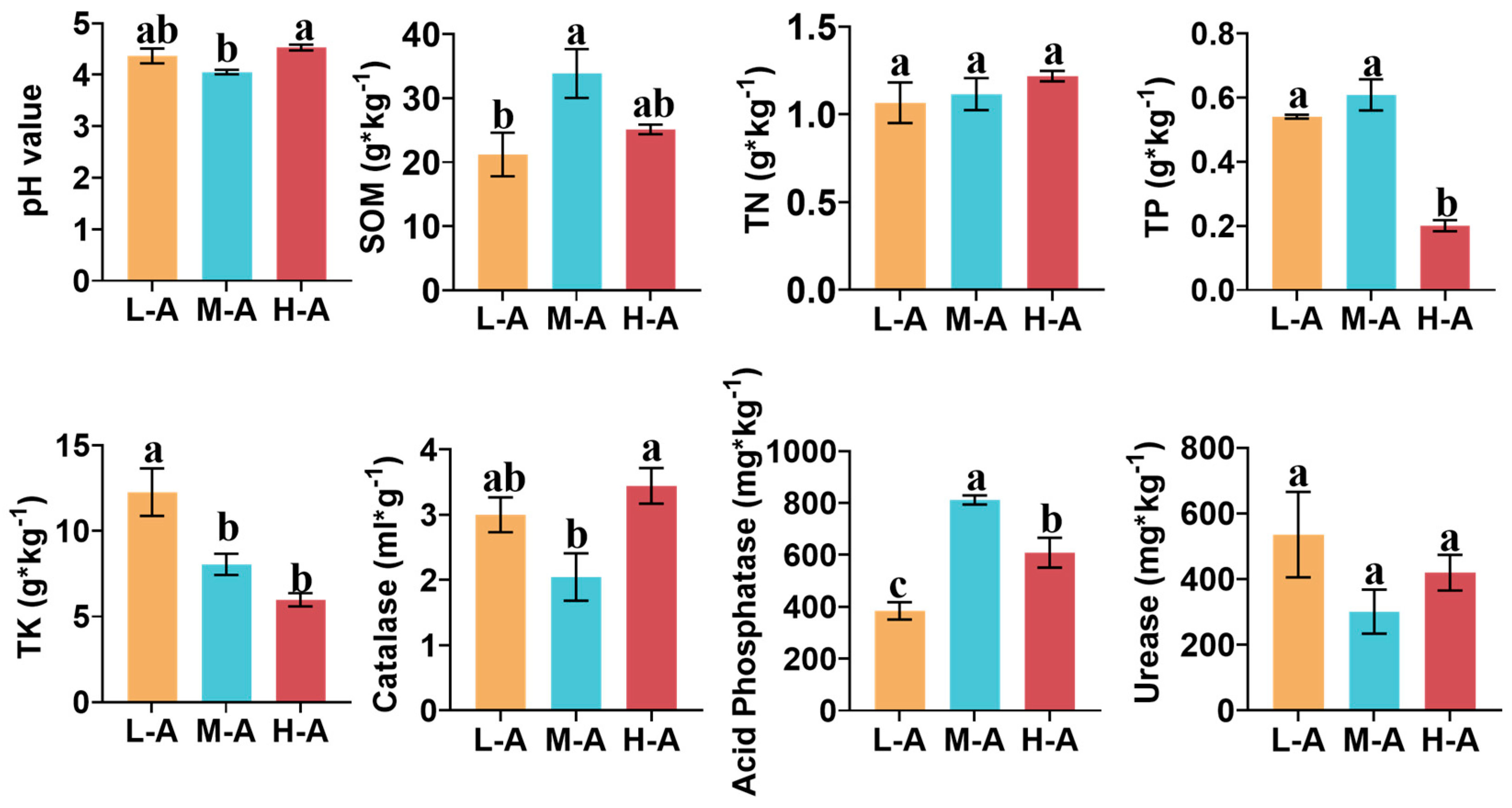
2.1. Rhizosphere Soil Properties and Root Traits Varied with Altitudes
2.2. Microbial Community Compositions and Diversity
2.3. Relationship Between Soil Chemical Properties and Microbial Relative Abundance
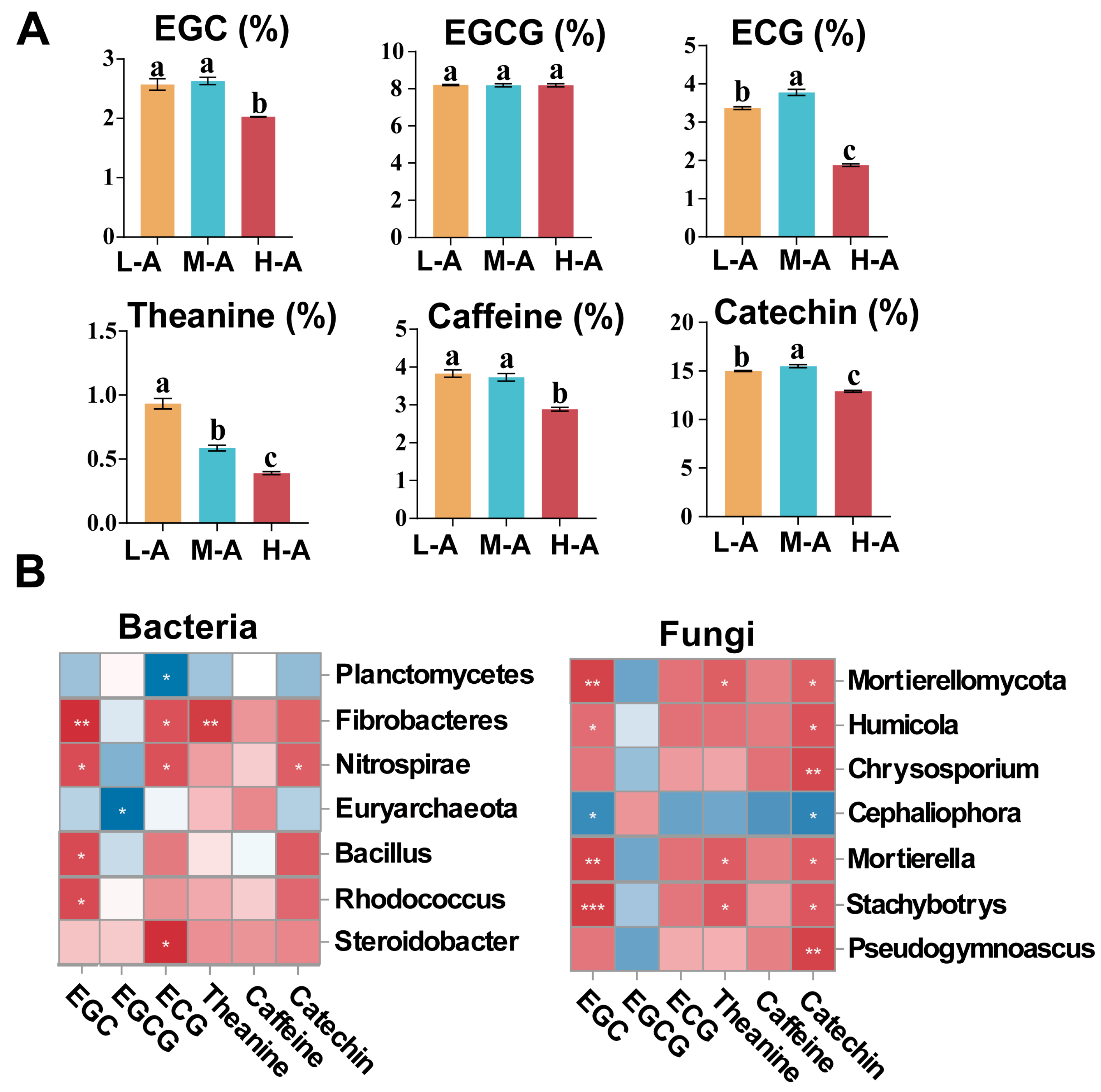
2.4. Relationship of Soil Chemical Properties and Microbial Communities with Tea Quality
3. Discussion
3.1. Soil Chemical Properties at Different Altitudes
3.2. Soil Microbial Composition at Different Altitudes
3.3. Key Drivers of Change in Soil Fungal and Bacterial Communities
3.4. Relationship Between Soil Chemical Properties and Tea Quality
3.5. Relationship Between Soil Microbial Community and Tea Quality
4. Materials and Methods
4.1. Study Sites and Sample Collection
4.2. Determination of Soil Physical and Chemical Properties
4.3. Leaf Composition
4.4. DNA Extraction and Illumina Sequencing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, R.; Redfern, S.P.; Kirkup, D.; Porter, E.A.; Kite, G.C.; Terry, L.A.; Berry, M.J.; Simmonds, M.S. Variation of theanine, phenolic, and methylxanthine compounds in 21 cultivars of Camellia sinensis harvested in different seasons. Food Chem. 2017, 220, 517–526. [Google Scholar] [CrossRef]
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stöger, V.; Reiner, A.; Hochkogler, C.M.; Köck, E.; Marchiori, A.; Hans, J.; et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Tai, Y.; Wang, X.; Ho, C.-T.; Wan, X. Gene Discovery of Characteristic Metabolic Pathways in the Tea Plant (Camellia sinensis) Using ‘Omics’-Based Network Approaches: A Future Perspective. Front. Plant Sci. 2018, 9, 480. [Google Scholar] [CrossRef]
- Bassiony, A.; Peng, Q.; Baldermann, S.; Feng, S.; Yang, K.; Zhang, Y.; Fu, J.; Lv, H.; Lin, Z.; Shi, J. Differential accumulation patterns of flavor compounds in Longjing 43 and Qunti fresh leaves and during processing responding to altitude changes. Food Res. Int. 2024, 187, 114392. [Google Scholar] [CrossRef]
- Liu, D.; Liu, G.; Chen, L.; Wang, J.; Zhang, L. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Sci. China Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef]
- Yin, X.; Song, Y.; Shen, J.; Sun, L.; Fan, K.; Chen, H.; Sun, K.; Ding, Z.; Wang, Y. The role of rhizosphere microbial community structure in the growth and development of different tea cultivars. Appl. Soil Ecol. 2025, 206, 105817. [Google Scholar] [CrossRef]
- Tang, W.; Xin, W.; Xu, T.; Yang, Z. Tea plant microorganisms in the improvement of tea quality. Trends Microbiol. (Regul. Ed.) 2025, 33, 11–14. [Google Scholar] [CrossRef]
- Huang, X.; Wang, B.; Li, P.; Chen, A.; Cui, J.; Chen, Y.; Gao, W. Organic management promotes nitrogen transformation in tea plantations soil: A case study from southwestern China. Appl. Soil Ecol. 2025, 206, 105878. [Google Scholar] [CrossRef]
- Shao, S.; Li, Z.; Ma, X.; Cui, J.; Zhu, Y.; Li, Y.; Wu, L.; Rensing, C.; Cai, P.; Zhang, J.; et al. Enhancing tea plant growth and soil microbial ecology through intercropping tea plants with Ophiopogon japonicus. Plant Soil 2025. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, Y.; Zheng, Z.; Xue, X.; Wang, J.; Lin, H.; Zhang, Q. Comparison of diversities, network patterns and potential functions of microbial communities in different soil type oolong tea growing areas. Environ. Technol. Innov. 2025, 37, 104039. [Google Scholar] [CrossRef]
- Bai, Y.; Li, B.-X.; Xu, C.-Y.; Raza, M.; Wang, Q.-Z.; Fu, Y.-N.; Hu, J.-Y.; Imoulan, A.; Hussain, M.; Xu, Y.-J. Intercropping Walnut and Tea: Effects on Soil Nutrients, Enzyme Activity, and Microbial Communities. Front. Microbiol. 2022, 13, 852342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Dong, C.; Bian, W.; Zhang, W.; Wang, Y. Effects of different fertilization practices on maize yield, soil nutrients, soil moisture, and water use efficiency in northern China based on a meta-analysis. Sci. Rep. 2024, 14, 6480. [Google Scholar] [CrossRef]
- Zhou, B.; Chen, Y.; Zeng, L.; Cui, Y.; Li, J.; Tang, H.; Liu, J.; Tang, J. Soil nutrient deficiency decreases the postharvest quality-related metabolite contents of tea (Camellia sinensis (L.) Kuntze) leaves. Food Chem. 2022, 377, 132003. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, K.; Rahman, N.S.N.A. The Microbial Connection to Sustainable Agriculture. Plants 2023, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Banik, A. Exploring tea (Camellia sinensis) microbiome: Insights into the functional characteristics and their impact on tea growth promotion. Microbiol. Res. 2022, 254, 126890. [Google Scholar] [CrossRef]
- Lv, J.; Yang, S.; Zhou, W.; Liu, Z.; Tan, J.; Wei, M. Microbial regulation of plant secondary metabolites: Impact, mechanisms and prospects. Microbiol. Res. 2024, 283, 127688. [Google Scholar] [CrossRef]
- Ren, C.; Zhou, Z.; Guo, Y.; Yang, G.; Zhao, F.; Wei, G.; Han, X.; Feng, L.; Feng, Y.; Ren, G. Contrasting patterns of microbial community and enzyme activity between rhizosphere and bulk soil along an elevation gradient. CATENA 2021, 196, 104921. [Google Scholar] [CrossRef]
- Xie, L.; Li, W.; Pang, X.; Liu, Q.; Yin, C. Soil properties and root traits are important factors driving rhizosphere soil bacterial and fungal community variations in alpine Rhododendron nitidulum shrub ecosystems along an altitudinal gradient. Sci. Total Environ. 2023, 864, 161048. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Niu, Y.; Jiang, W.; Wang, Y.; Liu, S.; Wei, W. 44-Years of Fertilization Altered Soil Microbial Community Structure by Changing Soil Physical, Chemical Properties and Enzyme Activity. J. Soil Sci. Plant Nutr. 2024, 24, 3150–3161. [Google Scholar] [CrossRef]
- Sadeghi, S.; Petermann, B.J.; Steffan, J.J.; Brevik, E.C.; Gedeon, C. Predicting microbial responses to changes in soil physical and chemical properties under different land management. Appl. Soil Ecol. 2023, 188, 104878. [Google Scholar] [CrossRef]
- Krahe, J.C.; Krahe, M.A.; Roach, P.D. Development of an objective measure of quality and commercial value of Japanese-styled green tea (Camellia L. sinensis): The Quality Index Tool. J. Food Sci. Technol. 2018, 55, 2926–2934. [Google Scholar] [CrossRef]
- Ivashchenko, K.; Sushko, S.; Selezneva, A.; Ananyeva, N.; Zhuravleva, A.; Kudeyarov, V.; Makarov, M.; Blagodatsky, S. Soil microbial activity along an altitudinal gradient: Vegetation as a main driver beyond topographic and edaphic factors. Appl. Soil Ecol. 2021, 168, 104197. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, D.-Q.; Yang, C.; Wu, W.; Liu, H.-B. The spatial variability of temporal changes in soil pH affected by topography and fertilization. CATENA 2022, 218, 106586. [Google Scholar] [CrossRef]
- Badía, D.; Ruiz, A.; Girona, A.; Martí, C.; Casanova, J.; Ibarra, P.; Zufiaurre, R. The influence of elevation on soil properties and forest litter in the Siliceous Moncayo Massif, SW Europe. J. Mt. Sci. 2016, 13, 2155–2169. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Li, L.; Hou, J.; Zhang, X.; Li, H.; Yang, C.; Yang, L. Absorptive root-multidimension strategy links air temperature and species distribution in a montane forest. For. Ecosyst. 2023, 10, 100113. [Google Scholar] [CrossRef]
- Yong, L.; Zhu, G.; Wan, Q.; Xu, Y.; Zhang, Z.; Sun, Z.; Ma, H.; Sang, L.; Liu, Y.; Guo, H.; et al. The Soil Water Evaporation Process from Mountains Based on the Stable Isotope Composition in a Headwater Basin and Northwest China. Water 2020, 12, 2711. [Google Scholar] [CrossRef]
- Tian, Q.; Jiang, Y.; Tang, Y.; Wu, Y.; Tang, Z.; Liu, F. Soil pH and Organic Carbon Properties Drive Soil Bacterial Communities in Surface and Deep Layers Along an Elevational Gradient. Front. Microbiol. 2021, 12, 646124. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Y.; Deng, L.-J.; Li, Q.-Q.; Wang, C.-Q.; Li, B.; Deng, O.-P.; Zeng, M. Spatial variability of soil total nitrogen, phosphorus and potassium in Renshou County of Sichuan Basin, China. J. Integr. Agric. 2019, 18, 279–289. [Google Scholar] [CrossRef]
- Wu, Y.; Tian, X.; Zhang, M.; Wang, R.; Wang, S. A Case Study of Initial Vegetation Restoration Affecting the Occurrence Characteristics of Phosphorus in Karst Geomorphology in Southwest China. Sustainability 2022, 14, 12277. [Google Scholar] [CrossRef]
- Tripathi, S.; Chakraborty, A.; Chakrabarti, K.; Bandyopadhyay, B.K. Enzyme activities and microbial biomass in coastal soils of India. Soil Biol. Biochem. 2007, 39, 2840–2848. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, K.; Xie, Y.; Li, X.; Zhang, S.; Liu, W.; Huang, Y.; Cui, L.; Wang, S.; Bao, P. Geographical, climatic, and soil factors control the altitudinal pattern of rhizosphere microbial diversity and its driving effect on root zone soil multifunctionality in mountain ecosystems. Sci. Total Environ. 2023, 904, 166932. [Google Scholar] [CrossRef]
- Yu, Z.; Lee, C.; Kerfahi, D.; Li, N.; Yamamoto, N.; Yang, T.; Lee, H.; Zhen, G.; Song, Y.; Shi, L.; et al. Elevational dynamics in soil microbial co-occurrence: Disentangling biotic and abiotic influences on bacterial and fungal networks on Mt. Seorak. Soil Ecol. Lett. 2024, 6, 240246. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Wang, D.; Zhang, Z. Soil Microbial Community, Soil Quality, and Productivity along a Chronosequence of Larix principis-rupprechtii Forests. Plants 2023, 12, 2913. [Google Scholar] [CrossRef]
- Whalen, E.D.; Grandy, A.S.; Geyer, K.M.; Morrison, E.W.; Frey, S.D. Microbial trait multifunctionality drives soil organic matter formation potential. Nat. Commun. 2024, 15, 10209. [Google Scholar] [CrossRef]
- Liu, S.; García-Palacios, P.; Tedersoo, L.; Guirado, E.; van der Heijden, M.G.A.; Wagg, C.; Chen, D.; Wang, Q.; Wang, J.; Singh, B.K.; et al. Phylotype diversity within soil fungal functional groups drives ecosystem stability. Nat. Ecol. Evol. 2022, 6, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Wu, J.H.; Sun, Y.X.; Zhang, Y.Y.; Zhao, Y.F.; Huang, Z.; Duan, W.H. Climate and geochemistry at different altitudes influence soil fungal community aggregation patterns in alpine grasslands. Sci. Total Environ. 2023, 881, 163375. [Google Scholar] [CrossRef]
- Hu, Z.; Delgado-Baquerizo, M.; Fanin, N.; Chen, X.; Zhou, Y.; Du, G.; Hu, F.; Jiang, L.; Hu, S.; Liu, M. Nutrient-induced acidification modulates soil biodiversity-function relationships. Nat. Commun. 2024, 15, 2858. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Caputo, F.; De Sabata, D.; Fornasier, F. The enzyme patterns of Ascomycota and Basidiomycota fungi reveal their different functions in soil. Appl. Soil Ecol. 2024, 196, 105323. [Google Scholar] [CrossRef]
- Zhou, Y.; Jia, X.; Han, L.; Tian, G.; Kang, S.; Zhao, Y. Spatial characteristics of the dominant fungi and their driving factors in forest soils in the Qinling Mountains, China. CATENA 2021, 206, 105504. [Google Scholar] [CrossRef]
- Corbett, D.; Wall, D.P.; Lynch, M.B.; Tuohy, P. The influence of phosphorus application and varying soil pH on soil and herbage properties across a range of grassland soils with impeded drainage. J. Agric. Sci. 2022, 160, 516–527. [Google Scholar] [CrossRef]
- Zhou, X.; Rahman, M.K.U.; Liu, J.; Wu, F. Soil acidification mediates changes in soil bacterial community assembly processes in response to agricultural intensification. Environ. Microbiol. 2021, 23, 4741–4755. [Google Scholar] [CrossRef]
- Datta, R. Enzymatic degradation of cellulose in soil: A review. Heliyon 2024, 10, e24022. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Huo, Y.; Tian, Y.; Yan, W.; George, T.S.; Huang, C.; Feng, G.; Zhang, L. The interplay of direct and mycorrhizal pathways for plants to efficiently acquire phosphorus from soil. Front. Agric. Sci. Eng. 2025, 12, 47–56. [Google Scholar]
- Riseh, R.S.; Fathi, F.; Vatankhah, M.; Kennedy, J.F. Catalase-associated immune responses in plant-microbe interactions: A review. Int. J. Biol. Macromol. 2024, 280 Pt 2, 135859. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Aye, N.S.; Butterly, C.R.; Sale, P.W.; Tang, C. Interactive effects of initial pH and nitrogen status on soil organic carbon priming by glucose and lignocellulose. Soil Biol. Biochem. 2018, 123, 33–44. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, H.; Shutes, B.; Rousseau, A.N.; Feng, W.-D.; Hou, S.-N.; Ou, Y.; Yan, B.-X. Soil aggregate-driven changes in nutrient redistribution and microbial communities after 10-year organic fertilization. J. Environ. Manag. 2023, 348, 119306. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.-N.; Chen, J.-X.; Wang, F.; Du, Q.-Z.; Yin, J.-F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, F.; Wu, W.; Yu, W.; Zhang, G.; Huang, X.; Hao, Y.; Luo, L. Identification and quality evaluation of Lushan Yunwu tea from different geographical origins based on metabolomics. Food Res. Int. 2024, 186, 114379. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, J.; Zhou, X.; Xiao, Y.; Liao, Y.; Tang, J.; Dong, F.; Zeng, L. Effects of temperature and light on quality-related metabolites in tea [Camellia sinensis (L.) Kuntze] leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef] [PubMed]
- Riga, P.; Benedicto, L.; Gil-Izquierdo, Á.; Collado-González, J.; Ferreres, F.; Medina, S. Diffuse light affects the contents of vitamin C, phenolic compounds and free amino acids in lettuce plants. Food Chem. 2019, 272, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Liu, M.; Shi, Y.; Zhang, H.; Ruan, J.; Zhang, Q.; Cao, M. Metabolomics Reveal That the High Application of Phosphorus and Potassium in Tea Plantation Inhibited Amino-Acid Accumulation but Promoted Metabolism of Flavonoid. Agronomy 2022, 12, 1086. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y. Potassium management in tea plantations: Its uptake by field plants, status in soils, and efficacy on yields and quality of teas in China. J. Plant Nutr. Soil Sci. 2013, 176, 450–459. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kapoor, B.; Kapoor, D.; Thakur, U.; Dolma, Y.; Raza, A. Manifold roles of potassium in mediating drought tolerance in plants and its underlying mechanisms. Plant Sci. 2025, 351, 112337. [Google Scholar] [CrossRef]
- Lin, Z.; Qi, Y.-P.; Chen, R.-B.; Zhang, F.-Z.; Chen, L.-S. Effects of phosphorus supply on the quality of green tea. Food Chem. 2012, 130, 908–914. [Google Scholar] [CrossRef]
- Gou, X.; Ren, Y.; Qin, X.; Wei, X.; Wang, J. Global patterns of soil phosphatase responses to nitrogen and phosphorus fertilization. Pedosphere 2024, 34, 200–210. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, W.; Xiao, H.; Zhai, Y.; Luo, Y.; Wang, Y.; Liu, Z.; Li, Q.; Huang, J. A Review on Rhizosphere Microbiota of Tea Plant (Camellia sinensis L): Recent Insights and Future Perspectives. J. Agric. Food Chem. 2023, 71, 19165–19188. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, J.; Yu, Y.; Tian, Y.; Li, H.; Chen, X.; Li, W.; Liu, Y.; Lu, T.; He, B.; et al. Root microbiota of tea plants regulate nitrogen homeostasis and theanine synthesis to influence tea quality. Curr. Biol. 2024, 34, 868–880.e6. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, J.; Chung, J.-O.; Cho, D.; Roh, J.-H.; Hong, Y.-D.; Kim, W.-G.; Kang, H. Variations in the composition of tea leaves and soil microbial community. Biol. Fertil. Soils 2022, 58, 167–179. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and Plant Phenylalanine Ammonia-lyase. Mycobiology 2018, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Devi, C.J.; Thakur, D. Unlocking Rhizosphere Dynamics: Exploring Mechanisms of Plant–Microbe Interactions for Enhanced Tea (Camellia sinensis (L.) O. Kuntze) Productivity. Curr. Microbiol. 2025, 82, 257. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Chen, Y.; Wang, S.; Wang, M.; Xie, T.; Wang, G. Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci. Rep. 2016, 6, 31616. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, E. Effects of agricultural land use on soil nutrients and its variation along altitude gradients in the downstream of the Yarlung Zangbo River Basin, Tibetan Plateau. Sci. Total Environ. 2023, 905, 167583. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.; Ou, J.; Liu, F.; Cai, G.; Tan, K.; Wang, X. Nitrogen substitution practice improves soil quality of red soil (Ultisols) in South China by affecting soil properties and microbial community composition. Soil Tillage Res. 2024, 240, 106089. [Google Scholar] [CrossRef]
- Qiu, Z.; Liao, J.; Chen, J.; Li, A.; Lin, M.; Liu, H.; Huang, W.; Sun, B.; Liu, J.; Liu, S.; et al. Comprehensive analysis of fresh tea (Camellia sinensis cv. Lingtou Dancong) leaf quality under different nitrogen fertilization regimes. Food Chem. 2024, 439, 138127. [Google Scholar] [CrossRef]
- Mei, S.; Yu, Z.; Chen, J.; Zheng, P.; Sun, B.; Guo, J.; Liu, S. The Physiology of Postharvest Tea (Camellia sinensis) Leaves, According to Metabolic Phenotypes and Gene Expression Analysis. Molecules 2022, 27, 1708. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Wei, Y.; Meng, H.; Cao, Y.; Lead, J.; Hong, J. Effects of fertilization and reclamation time on soil bacterial communities in coal mining subsidence areas. Sci. Total Environ. 2020, 739, 139882. [Google Scholar] [CrossRef] [PubMed]


| Phylum/Genus | pH | SOM | TN | TP | TK | Catalase | Acid Phosphatase | Urease |
|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||
| Patescibacteria | 0.38 | −0.43 | −0.52 | −0.24 | −0.10 | 0.17 | 0.02 | 0.76 * |
| Cyanobacteria | −0.29 | 0.55 | −0.02 | 0.12 | 0.02 | −0.36 | 0.81 * | −0.14 |
| Fibrobacteres | −0.71 * | 0.24 | 0.05 | 0.90 ** | 0.86 ** | −0.55 | −0.26 | −0.24 |
| Nitrospirae | −0.74 * | 0.71 * | 0.33 | 0.64 | 0.29 | −0.50 | 0.52 | −0.79 * |
| Rhodococcus | −0.60 | 0.07 | −0.17 | 0.83 * | 0.81 * | −0.50 | −0.21 | −0.02 |
| Fungi | ||||||||
| Mortierellomycota | −0.73 * | 0.47 | 0.00 | 0.85 ** | 0.55 | −0.58 | 0.17 | −0.57 |
| Humicola | −0.68 * | 0.48 | 0.28 | 0.54 | 0.30 | −0.68 * | 0.23 | −0.22 |
| Cephaliophora | 0.73 * | −0.55 | −0.18 | −0.36 | −0.27 | 0.53 | −0.18 | 0.37 |
| Mortierella | −0.73 * | 0.47 | 0.00 | 0.85** | 0.55 | −0.58 | 0.17 | −0.57 |
| Stachybotrys | −0.80 ** | 0.57 | 0.23 | 0.89 ** | 0.48 | −0.68* | 0.27 | −0.50 |
| Pseudogymnoascus | −0.70 * | 0.35 | 0.12 | 0.41 | 0.33 | −0.57 | −0.02 | −0.27 |
| Microbial Diversity Index | Theanine | Caffeine | Catechin | Theanine/Caffeine | Theanine/Catechin |
|---|---|---|---|---|---|
| Bacterial richness | 0.33 | 0.15 | 0.02 | 0.37 | 0.33 |
| Fungal Shannon diversity | −0.27 | −0.05 | 0.20 | −0.33 | −0.27 |
| Fungal richness | 0.80 ** | 0.40 | 0.13 | 0.85 ** | 0.80 ** |
| Fungal Shannon diversity | −0.35 | −0.58 | −0.23 | −0.27 | −0.36 |
| Low Altitude | Mid-Altitude | High Altitude | |
|---|---|---|---|
| Latitude | 116°44′48″ N | 116°41′54″ N | 116°41′7″ N |
| Longitude | 23°55′7″ E | 23°55′44″ E | 23°56′35″ E |
| Altitude (m) | 396.59 | 517.97 | 623.44 m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Lin, M.; Liu, J.; Khan, W.; Zhao, H.; Sun, B.; Liu, S.; Zheng, P. Effects of Altitude on Tea Composition: Dual Regulation by Soil Physicochemical Properties and Microbial Communities. Plants 2025, 14, 1642. https://doi.org/10.3390/plants14111642
Ren X, Lin M, Liu J, Khan W, Zhao H, Sun B, Liu S, Zheng P. Effects of Altitude on Tea Composition: Dual Regulation by Soil Physicochemical Properties and Microbial Communities. Plants. 2025; 14(11):1642. https://doi.org/10.3390/plants14111642
Chicago/Turabian StyleRen, Xirong, Minyao Lin, Jiani Liu, Waqar Khan, Hongbo Zhao, Binmei Sun, Shaoqun Liu, and Peng Zheng. 2025. "Effects of Altitude on Tea Composition: Dual Regulation by Soil Physicochemical Properties and Microbial Communities" Plants 14, no. 11: 1642. https://doi.org/10.3390/plants14111642
APA StyleRen, X., Lin, M., Liu, J., Khan, W., Zhao, H., Sun, B., Liu, S., & Zheng, P. (2025). Effects of Altitude on Tea Composition: Dual Regulation by Soil Physicochemical Properties and Microbial Communities. Plants, 14(11), 1642. https://doi.org/10.3390/plants14111642







