Do Microplastics in Soil Influence the Bioavailability of Sulfamethoxazole to Plants?
Abstract
1. Introduction
2. Results
2.1. Determination of the Effects of MPs and SMX Interactions on Germination and Early Plant Development
2.2. Analysis of the Impact of Microplastics on Changes in SMX Toxicity
2.3. Change in Accumulation of SMX Due to Presence of MPs in Soil
3. Discussion
4. Materials and Methods
4.1. Microplastics
4.2. Soil Characteristics
4.3. Assessing the Effects of MPs and SMX Interactions on Germination and Early Plant Development
4.4. Analysis of the Effect of MPs on Changes in SMX Accumulation
4.5. HPLC-MS Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| SMX | Sulfamethoxazole |
| PE | Polyethylene |
| PS | Polystyrene |
| ABS | Acrylonitrile–Butadiene–Styrene Copolymer |
| PLA | Polylactic Acid |
| ROS | Reactive Oxygen Species |
| CAT | Catalase |
| APX | Ascorbate Peroxidase |
| EC25 | Effective Concentration Causing 25% Inhibition |
| EC50 | Effective Concentration Causing 50% Inhibition |
| d.s.w. | Dry Soil Weight |
References
- Boxall, A.B.A.; Sinclair, C.J.; Fenner, K.; Kolpin, D.W.; Maund, S. When synthetic chemicals degrade in the environment. Environ. Sci. Technol. 2012, 38, 368A–375A. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.K.; Collins, T.J. Human Pharmaceuticals in the Aquatic Environment: A Challenge to Green Chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef]
- Koba, O.; Golovko, O.; Kodesova, R.; Fer, M.; Grabic, R. Antibiotics degradation in soil: A case of clindamycin, trimethoprim, sulfamethoxazole and their transformation products. Environ. Pollut. 2017, 220, 1251–1263. [Google Scholar] [CrossRef]
- Liu, F.; Ying, G.-G.; Yang, J.-F.; Li-Jun Zhou, L.-J.; Tao, R.; Wang, L.; Zhang, L.i.-Z.; Peng, P.-A. Dissipation of sulfamethoxazole, trimethoprim and tylosin in a soil under aerobic and anoxic conditions. Environ. Chem. 2010, 7, 370–376. [Google Scholar] [CrossRef]
- Rauseo, J.; Caracciolo, A.B.; Ademollo, N.; Cardoni, M.; Lenola, M.D.; Gaze, W.; Stanton, I.; Grenni, P.; Pescatorea, T.; Spataroa, F.; et al. Dissipation of the antibiotic sulfamethoxazole in a soil amended with anaerobically digested cattle manure. J. Hazard. Mat. 2019, 378, 120769. [Google Scholar] [CrossRef]
- Voogt, P.D.; Janex-Habibi, M.L.; Sacher, F.; Puijker, L.; Mons, M. Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci. Technol. 2009, 59, 39–46. [Google Scholar] [CrossRef]
- Oliveira, C.; Lima, D.L.D.; Silva, C.P.; Calisti, V.; Otero, M.; Esteves, V.I. Photodegradation of sulfamethoxazole in environmental samples: The role of pH, organic matter and salinity. Sci. Total Environ. 2019, 648, 1403–1410. [Google Scholar] [CrossRef]
- Wu, D.; Huang, Z.; Yang, K.; Graham, D.; Xie, B. Relationships between Antibiotics and Antibiotic Resistance Gene Levels in Municipal Solid Waste Leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128. [Google Scholar] [CrossRef]
- Göbel, A.; Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption 550 Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Goulas, A.; Sertillanges, N.; Brimo, K.; Garnier, P.; Bergheaud, V.; Dumény, V.; Benoit, P.; Haudin, C.S. Environmental availability of sulfamethoxazole and its acetylated metabolite added to soils via sludge compost or bovine manure. Sci. Total Environ. 2019, 651, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Morel, M.C.; Spadini, L.; Brimo, K.; Martins, J.M.F. Speciation study in the sulfamethoxazole–copper–pH–soil system: Implications for retention prediction. Sci. Total Environ. 2014, 481, 266–273. [Google Scholar] [CrossRef]
- Brain, R.A.; Ramirez, A.J.; Fulton, B.A.; Chambliss, C.K.; Brooks, B.W. Herbicidal Effects of Sulfamethoxazole in Lemna gibba: Using p-Aminobenzoic Acid as a Biomarker of Effect. Environ. Sci. Technol. 2008, 42, 8965–8970. [Google Scholar] [CrossRef]
- Xu, D.; Xie, Y.; Li, J. Toxic effects and molecular mechanisms of sulfamethoxazole on Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2022, 232, 113258. [Google Scholar] [CrossRef]
- Shatri, A.M.N.; Mumbengegwi, D.R. In vitro Cytotoxicity of Selected Medicinal Plant Extracts used for the Management of Gastroenteritis in Northern Namibia, and their Antibacterial Activity against Multidrug-resistant Pathogens. J. Pure Appl. Microbiol. 2024, 18, 2674–2687. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Caron, A.; Thomas, C.R.; Berry, K.; Motti, C.A.; Ariel, E.; Brodie, J.E. Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a sequential extraction protocol. Mar. Pollut. Bull. 2018, 127, 743–751. [Google Scholar] [CrossRef]
- Atugoda, T.; Wijesekara, H.; Werellagama, D.R.I.B.; Jinadasa, K.B.S.N.; Bolan, N.S.; Vithanage, M. Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: Implications for vector transport in water. Environ. Technol. Innov. 2020, 19, 100971. [Google Scholar] [CrossRef]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, T.; Gustave, W.; Li, B.; Cai, Y.; Ouyang, D.; Guo, T.; Zhang, H. Do microplastics affect sulfamethoxazole sorption in soil? Experiments on polymers, ionic strength and fulvic acid. Sci. Total Environ. 2023, 860, 160221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Chen, X.; Li, J.; Chen, J.; Li, J.; Chen, J.; Wang, H. The fate and risk of microplastic and antibiotic sulfamethoxazole coexisting in the environment. Environ. Geochem. Health 2023, 45, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, J.; Zhu, L.; Wang, Q.; Zhang, H. A contrasting alteration of sulfamethoxazole bioaccessibility in two different soils amended with polyethylene microplastic: In-situ measurement using diffusive gradients in thin films. Sci. Total Environ. 2022, 808, 152187. [Google Scholar] [CrossRef]
- Ren, S.; Xia, Y.; Jin, X.; Sun, D.; Luo, D.; Wei, W.; Yang, Q.; Ding, J.; Lv, M.; Chen, L. Influence of microplastics on the availability of antibiotics in soils. Sci. Total Environ. 2024, 924, 171514. [Google Scholar] [CrossRef]
- Syguda, A.; Gielnik, A.; Borkowski, A.; Woźniak-Karczewska, M.; Parus, A.; Piechalak, A.; Olejnik, A.; Marecik, R.; Ławniczak, Ł.; Chrzanowski, Ł. Esterquat Herbicidal Ionic Liquids (HILs) with Two Different Herbicides: Evaluation of Activity and Phytotoxicity. New J. Chem. 2018, 42, 9819–9827. [Google Scholar] [CrossRef]
- Duan, L.-Y.; Zhang, Y.; Li, Y.-Y.; Li, X.-Q.; Liu, Y.-Q.; Li, B.L.; Ding, C.-Y.; Ren, X.-M.; Duan, P.-F.; Han, H.; et al. Effects of combined microplastic and cadmium pollution on sorghum growth, Cd accumulation, and rhizosphere microbial functions. Ecotoxicol. Environ. Saf. 2024, 277, 116380. [Google Scholar] [CrossRef]
- Szczepaniak, Z.; Czarny, J.; Staninska-Pięta, J.; Lisiecki, P.; Zgoła-Grześkowiak, A.; Cyplik, P.; Chrzanowski, Ł.; Wolko, Ł.; Marecik, R.; Juzwa, W.; et al. Influence of Soil Contamination with PAH on Microbial Community Dynamics and Expression Level of Genes Responsible for Biodegradation of PAH and Production of Rhamnolipids. Environ. Sci. Pollut. Res. 2016, 23, 23043–23056. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Chen, Y.; Ren, X.-M.; Li, Y.-Y.; Chen, Z.-J. Plant growth-promoting bacteria modulate gene expression and induce antioxidant tolerance to alleviate synergistic toxicity from combined microplastic and Cd pollution in sorghum. Ecotoxicol. Environ. Saf. 2023, 264, 115439. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Application of a small scale-terrestrial model ecosystem (STME) for assessment of ecotoxicity of bio-based plastics. Sci. Total Environ. 2022, 828, 154353. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huang, Z.Y.; Li, Y.F.; Lu, X.L.; Li, G.R.; Qi, S.S.; Khan, I.U.; Li, G.L.; Dai, Z.C.; Du, D.L. The Degradability of Microplastics May Not Necessarily Equate to Environmental Friendliness: A Case Study of Cucumber Seedlings with Disturbed Photosynthesis. Agriculture 2024, 14, 53. [Google Scholar] [CrossRef]
- Roy, A.; Gerson, J. Effects of polyethylene microplastics on the growth of Arabidopsis thaliana & Phaseolus vulgaris and their soil. J. Emerg. Investig. 2022, 5, 1–7. [Google Scholar] [CrossRef]
- Parus, A.; Idziak, M.; Jacewicz, P.; Panasiewicz, K.; Zembrzuska, J. Assessment of environmental risk caused by the presence of antibiotics. En. Nan. Mon Mant. 2021, 16, 100533. [Google Scholar] [CrossRef]
- Marecik, R.; Wojtera-Kwiczor, J.; Ławniczak, Ł.; Cyplik, P.; Szulc, A.; Piotrowska-Cyplik, A.; Chrzanowski, Ł. Rhamnolipids Increase the Phytotoxicity of Diesel Oil Towards Four Common Plant Species in a Terrestrial Environment. Water Air Soil Pollut. 2012, 223, 4275–4282. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, D.K.; Syguda, A.; Borkowski, A.; Gajewska, M.; Gendaszewska-Darmach, E.; Pernak, J. Transformation of Indole-3-Butyric Acid into Ionic Liquids as a Sustainable Strategy Leading to Highly Efficient Plant Growth Stimulators. ACS Sustain. Chem. Eng. 2020, 8, 1591–1598. [Google Scholar] [CrossRef]
- Ai, T.; Yao, S.; Yu, Y.; Peng, K.; Jin, L.; Zhu, X.; Zhou, H.; Huang, J.; Sun, J.; Zhu, L. Transformation process and phytotoxicity of sulfamethoxazole and N4-acetyl-sulfamethoxazole in rice. Sci. Total Environ. 2024, 918, 170857. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Liu, Y.; Zhu, Y.; Jiang, T.; Liang, S.; Bian, S.; Cao, Y.; Wang, G.; Yang, J. Molecular mechanisms and physiological responses of rice leaves co-exposed to submicron-plastics and cadmium: Implication for food quality and security. J. Hazard. Mater. 2024, 463, 132957. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Wei, X.; Beiyuan, J.; Wang, L.; Liu, J.; Sun, H.; Zhang, G.; Xiao, T. Uptake, organ distribution and health risk assessment of potentially toxic elements in crops in abandoned indigenous smelting region. Chemosphere 2022, 292, 133321. [Google Scholar] [CrossRef]
- Liné, C.; Manent, F.; Wolinski, A.; Flahaut, E.; Larue, C. Comparative study of response of four crop species exposed to carbon nanotube contamination in soil. Chemosphere 2021, 274, 129854. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Zhou, S.; Li, K.; Niu, L.; Zhao, L.; Xu, D. Analysis of the effects of sulfamethoxazole on the secondary metabolites and antioxidants in oilseed rape (Brassica napus L.) and the underlying mechanisms. Sci. Total Environ. 2023, 902, 165768. [Google Scholar] [CrossRef]
- Li, K.; Zhao, M.; Zhou, S.; Niu, L.; Zhao, L.; Xu, D. Effects of antibiotics on secondary metabolism and oxidative stress in oilseed rape seeds. Environ. Sci. Pollut. Res. 2024, 31, 27689–27698. [Google Scholar] [CrossRef]
- Li, M.; Liu, G.; Cai, Y.; Guo, T.; Xu, Y.; Zhao, X.; Ji, H.; Ouyang, D.; Zhang, H. Decreased Sulfamethoxazole Uptake in Lettuce (Lactuca sativa L.) due to Transpiration Inhibition by Polypropylene Microplastics. Ecotoxicol. Environ. Saf. 2024, 286, 117201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, W.; Wang, X.; Zeb, A.; Wang, Q.; Mo, F.; Shi, R.; Liu, J.; Yu, M.; Li, J.; et al. Assessing stress responses in potherb mustard (Brassica juncea var. multiceps) exposed to a synergy of microplastics and cadmium: Insights from physiology, oxidative damage, and metabolomics. Sci. Total Environ. 2024, 907, 167920. [Google Scholar] [CrossRef]
- Ali Raza Khan, A.Z.; Ulhassan, Z.; Li, G.; Lou, J.; Iqbal, B.; Salam, A.; Azhar, W.; Batool, S.; Zhao, T.; Li, K.; et al. Micro/nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies. Sci. Total Environ. 2024, 912, 169420. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Werner, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Bakhshaee, A.; Babakhani, P.; Ashiq, M.M.; Bell, K.; Salehi, M.; Jazaei, F. Potential impacts of microplastic pollution on soil–water–plant dynamics. Sci. Rep. 2025, 15, 9784. [Google Scholar] [CrossRef]
- Aralappanavar, V.K.; Mukhopadhyay, R.; Yu, Y.; Liu, J.; Bhatnagar, A.; Praveena, S.M.; Li, Y.; Paller, M.; Adyel, T.M.; Rinklebe, J.; et al. Effects of microplastics on soil microorganisms and microbial functions in nutrients and carbon cycling—A review. Sci. Total Environ. 2024, 924, 171435. [Google Scholar] [CrossRef]
- Awet, T.T.; Kohl, Y.; Meier, F.; Straskraba, S.; Grün, A.-L.; Ruf, T.; Jost, C.; Drexel, R.; Tunc, E.; Emmerling, C. Effects of polystyrene nanoparticles on the microbiota and functional diversity of enzymes in soil. Environ. Sci. Eur. 2018, 30, 11. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, W.; Xu, E.G.; Li, L.; Zhang, H.; Yang, Y. Uptake, translocation, and biological impacts of micro(nano)plastics in terrestrial plants: Progress and prospects. Environ. Res. 2022, 203, 111867. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Wilms, W.; Parus, A.; Homa, J.; Batycka, M.; Niemczak, M.; Woźzniak-Karczewska, M.; Trzebny, A.; Zembrzuska, J.; Dabert, M.; Tancsics, A.; et al. Glyphosate versus glyphosate based ionic liquids: Effect of cation on glyphosate biodegradation, soxA and phnJ genes abundance and microbial populations changes during soil bioaugmentation. Chemosphere 2023, 316, 137717. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wan, S.; Liu, Z.; Xie, X.; Xiang, X.; Liao, L.; Zheng, W.; Fu, Z.; Liao, P.; Chen, R. Adsorption of antibiotics on microplastics (MPs) in aqueous environments: The impacts of aging and biofilms. J. Environ. Chem. Eng. 2024, 12, 111992. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Q.; Sun, Y.; Zhang, S.; Wang, F. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 2022, 424, 127364. [Google Scholar] [CrossRef] [PubMed]
- Archundia, D.; Duwig, C.; Spadini, L.; Morel, M.C.; Prado, B.; Perez, M.P.; Orsag, V.; Mart, J.M.F. Assessment of the Sulfamethoxazole mobility in natural soils and of the risk of contamination of water resources at the catchment scale. Environ. Int. 2019, 130, 104995. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, D. Identification and determination of metabolites and degradation products of sulfonamide antibiotics. TrAC Trends Anal. Chem. 2008, 27, 1008–1022. [Google Scholar] [CrossRef]
- Sun, X.; Tian, S.; You, L.; Huang, X.; Su, J.Q. UV-aging reduces the effects of biodegradable microplastics on soil sulfamethoxazole degradation and sul genes development. J. Environ. Sci. 2025, 138, 587–598. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, L.; Zhao, Y.; Xie, H.; Song, M.; Wu, H.; Hu, Z.; Liang, S.; Zhang, J. The critical role of microplastics in the fate and transformation of sulfamethoxazole and antibiotic resistance genes within vertical subsurface-flow constructed wetlands. J. Hazard. Mater. 2024, 465, 133222. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Z.; Zhang, Y.; Zhu, P.; Jiang, R.; Wang, M.; Wang, Y.; Lu, G. Co-exposure of microplastics and sulfamethoxazole propagated antibiotic resistance genes in sediments by regulating the microbial carbon metabolism. J. Hazard. Mater. 2024, 463, 132951. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhu, D.; Chen, Q.L.; O’Connor, P.; Yang, X.R.; Qiao, M.; Zhu, Y.G. Adsorbed sulfamethoxazole exacerbates the effects of polystyrene (~2 μm) on gut microbiota and the antibiotic resistome of a soil collembolan. Environ. Sci. Technol. 2019, 53, 12823–12834. [Google Scholar] [CrossRef]
- Lisiecka, N.; Ciesielski, T.; Sopata, O.; Parus, A.; Woźniak-Karczewska, M.; Simpson, M.; Frankowski, R.; Zgoła-Grześkowiak, A.; Kloziński, A.; Siwińska-Ciesielczyk, K.; et al. Sorption of ionic liquids in soil enriched with polystyrene microplastic reveals independent behavior of cations and anions. Chemosphere 2023, 341, 139927. [Google Scholar] [CrossRef] [PubMed]
- Lisiecka, N.; Woźniak-Karczewska, M.; Parus, A.; Simpson, M.; Frankowski, R.; Zgoła-Grześkowiak, A.; Siwińska-Ciesielczyk, K.; Niemczak, M.; Eberlein, C.; Heipieper, H.J.; et al. Effect of microplastic on sorption, toxicity, and mineralization of 2,4-dichlorophenoxyacetic acid ionic liquids. Appl. Microbiol. Biotechnol. 2024, 108, 523. [Google Scholar] [CrossRef] [PubMed]
- Lisiecka, N.; Parus, A.; Zembrzuska, J.; Simpson, M.; Frankowski, R.; Kloziński, A.; Zgoła-Grześkowiak, A.; Woźniak-Karczewska, M.; Siwińska-Ciesielczyk, K.; Niemczak, M.; et al. Unraveling the effects of acrylonitrile butadiene styrene (ABS) microplastic ageing on the sorption and toxicity of ionic liquids with 2,4-D and glyphosate herbicides. Chemosphere 2024, 341, 143271. [Google Scholar] [CrossRef]
- OECD. Guidelines for the testing of chemicals. In Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test, Test 208; OECD: Paris, France, 2006. [Google Scholar]
- Sun, H.; Shi, Y.; Zhao, P.; Long, G.; Li, C.; Wang, J.; Qiu, D.; Lu, C.; Ding, Y.; Liu, L.; et al. Effects of polyethylene and biodegradable microplastics on photosynthesis, antioxidant defense systems, and arsenic accumulation in maize (Zea mays L.) seedlings grown in arsenic-contaminated soils. Sci. Total Environ. 2023, 868, 161557. [Google Scholar] [CrossRef]
- Li, B.; Huang, S.; Wang, H.; Sun, K.; Yu, W.; Dai, J.; Wang, S.; Zhang, W.; Zhu, Z.; Wang, X. Effects of plastic particles on germination and growth of soybean (Glycine max): A pot experiment under field condition. Environ. Pollut. 2021, 272, 116418. [Google Scholar] [CrossRef]
- Parus, A.; Grzegorz, F. Impact of O-alkyl-pyridineamidoximes on the soil environment. Sci. Total Environ. 2018, 643, 1278–1284. [Google Scholar] [CrossRef]
- OECD. Organization of Economic Cooperation and development. In Guideline for the Testing Chemicals: Adsorption-Desorption Using a Catch Equilibrium Method, Guide 106; OECD: Belgirate, Italy, 2000. [Google Scholar]
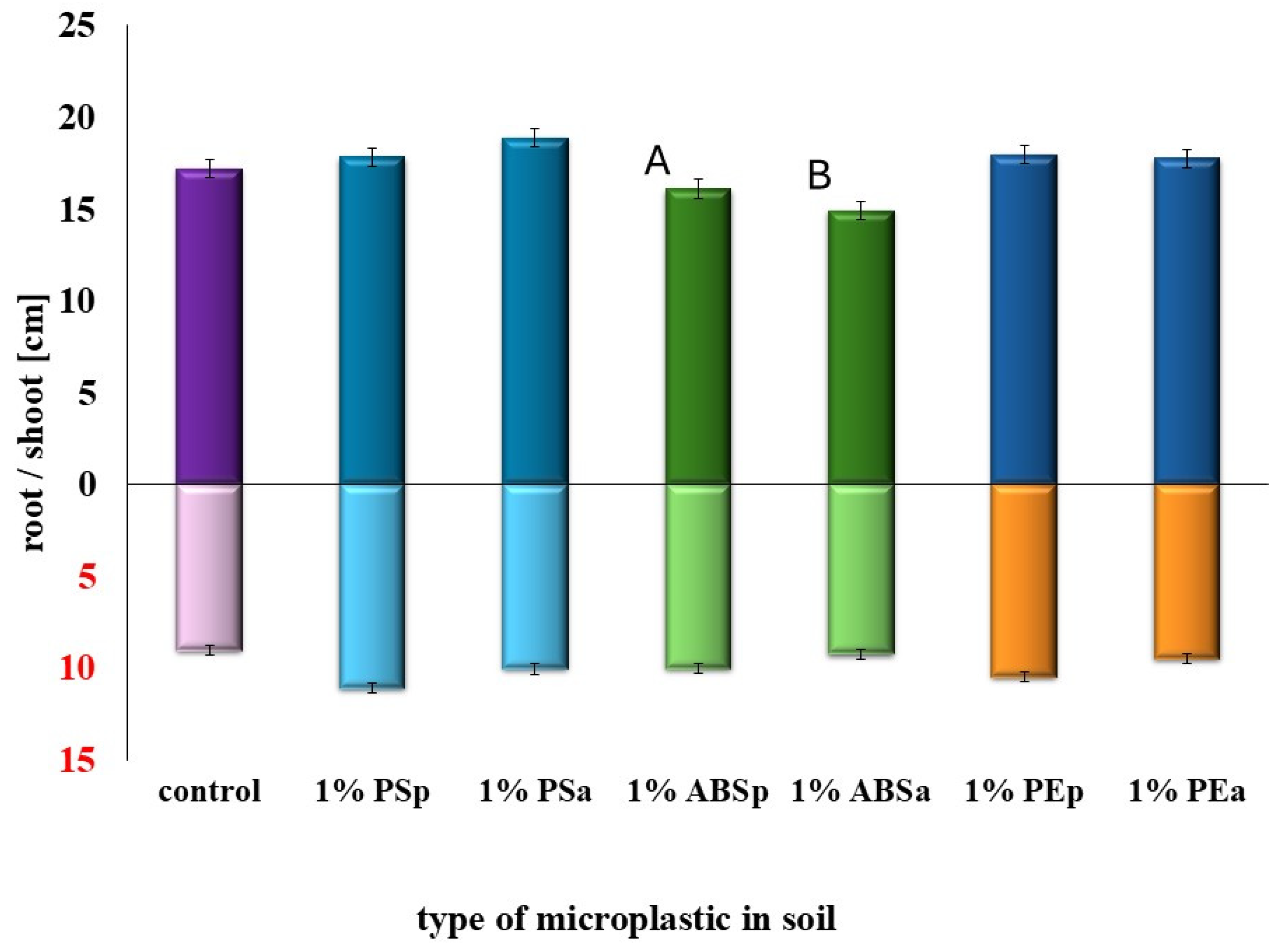

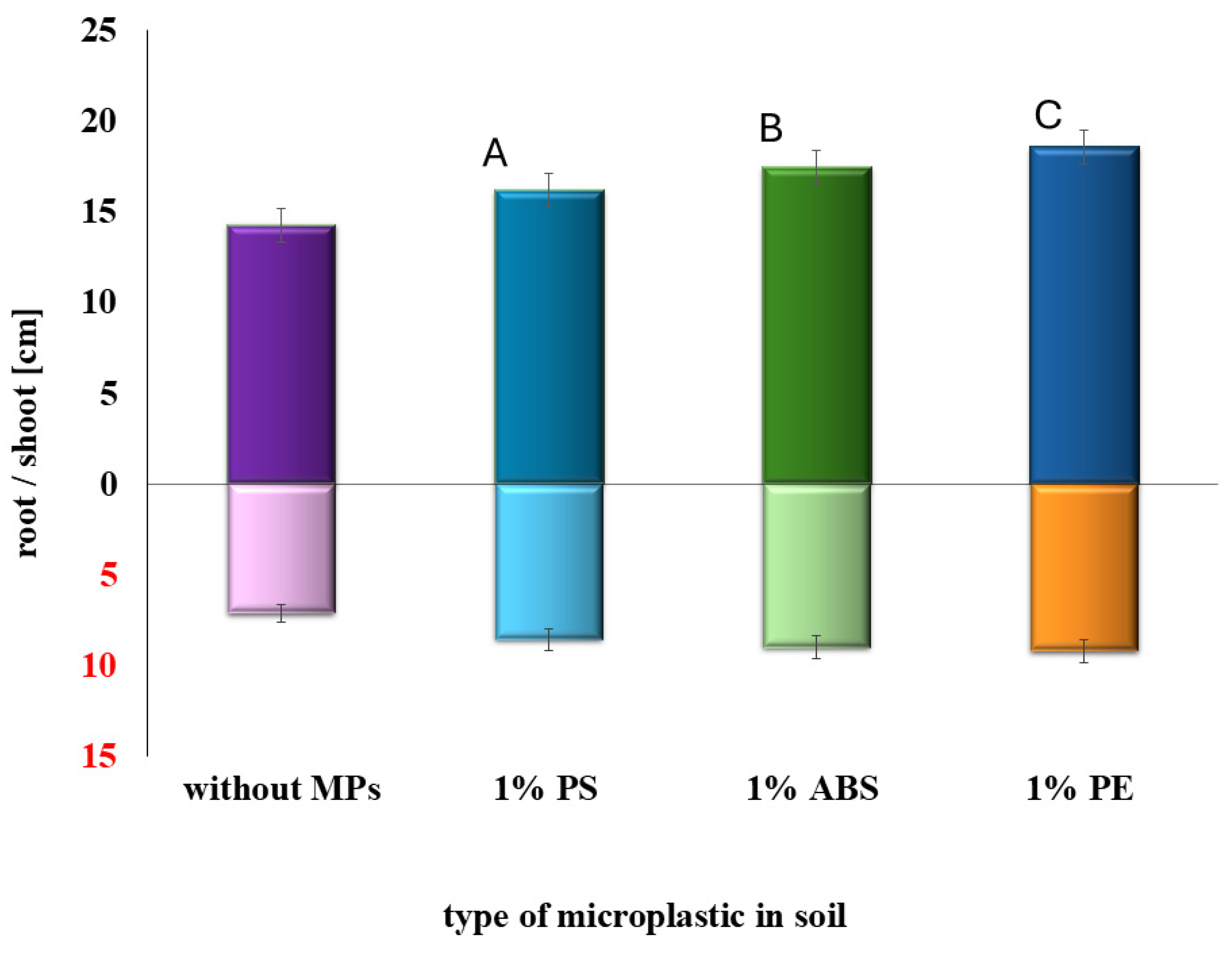
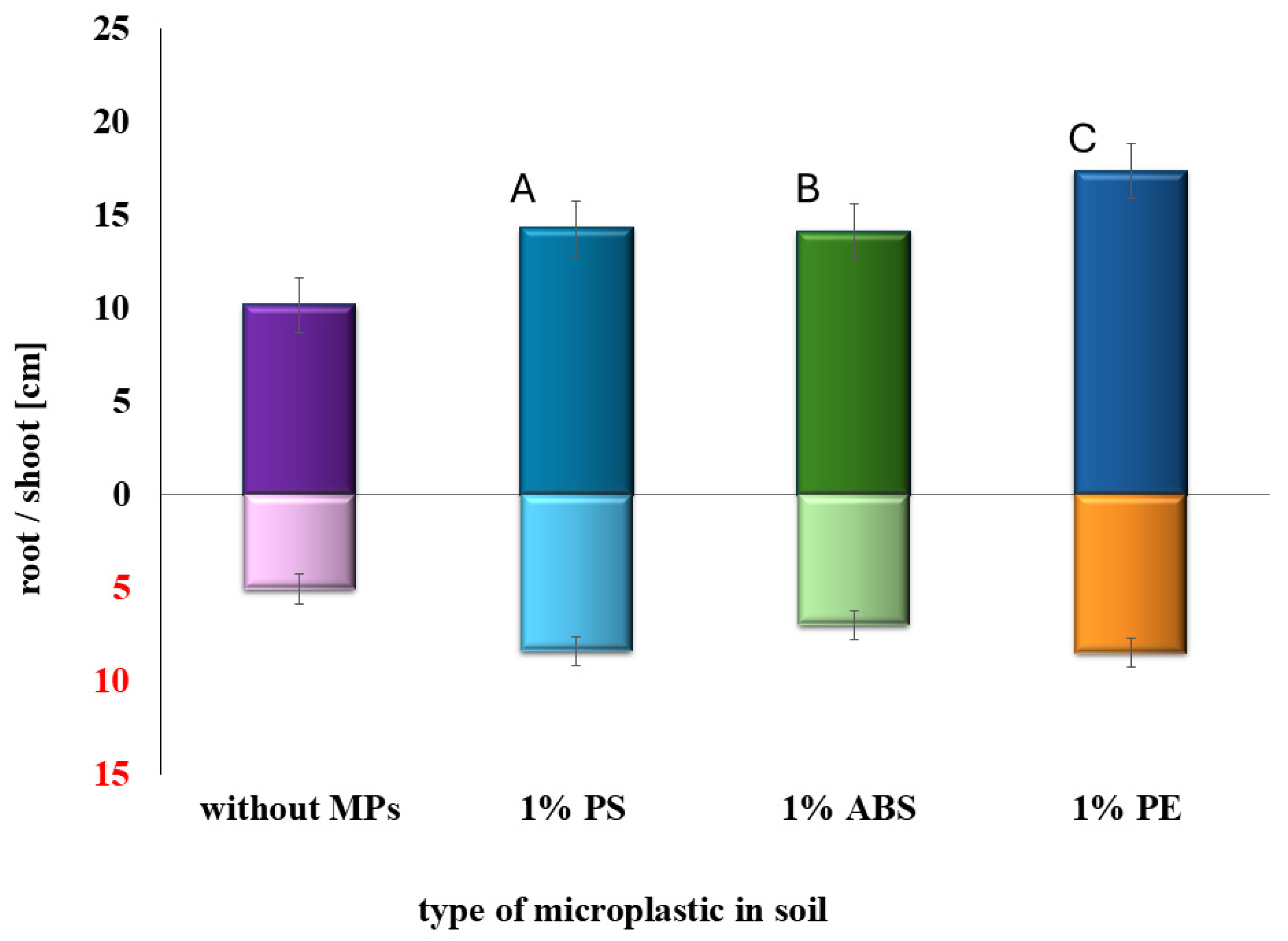
| Type of Soil | Adsorption [%] | Bioavailability [%] | Adsorption [%] | Bioavailability [%] |
|---|---|---|---|---|
| 10 mg/kg Soil (EC25) | 25 mg/kg Soil (EC50) | |||
| OECD | 26.9 ± 0.2 | 88.9 ± 0.5 | 25.1 ± 0.2 | 85.1± 0.6 |
| OECD + ABSp | 23.2 ± 0.4 | 86.3 ± 0.6 | 23.2 ± 0.3 | 94.1 ± 0.9 |
| OECD + ABSa | 28.0 ± 0.9 | 84.4 ± 0.4 | 27.6 ± 0.1 | 90.4 ± 0.5 |
| OECD + PSp | 22.5 ± 0.1 | 99.5 ± 0.3 | 23.4 ± 0.2 | 104.1 ± 0.8 |
| OECD + PSa | 27.3 ± 0.3 | 89.2 ± 0.4 | 29.7 ± 0.3 | 96.8 ± 0.7 |
| OECD + PEp | 21.0 ± 0.5 | 99.4 ± 0.8 | 19.4 ± 0.4 | 101.1 ± 0.3 |
| OECD + PEa | 21.6 ± 0.2 | 88.1 ± 0.9 | 21.1 ± 0.3 | 86.4 ± 0.2 |
| Type of MPs | BET Surface Area [m2/g] | Average Pore Diameter [nm] | Total Pore Volume [cm3/g] | Mean Particle Size [μm] |
|---|---|---|---|---|
| ABSp | 0.2 | 15.3 | 0.001 | 242 |
| ABSa | 0.3 | 14.4 | 0.001 | 242 |
| PSp | 0.2 | 23.1 | 0.001 | 350 |
| PSa | 0.1 | 23.9 | 0.001 | 350 |
| PEp | 0.2 | 18.6 | 0.001 | 500 |
| PEa | 0.2 | 21.0 | 0.001 | 500 |
| Compound | Precursor Ion [M-H]–m/z | Declustering Potential (V) | MRM1 */MRM2 ** Transitions Ion (Precursor Ion m/z → Product Ion m/z) | Collision Energy (V) |
|---|---|---|---|---|
| SMX | 291 | 11 | 291 → 230 291 → 123 | 33 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parus, A.; Lisiecka, N.; Kloziński, A.; Zembrzuska, J. Do Microplastics in Soil Influence the Bioavailability of Sulfamethoxazole to Plants? Plants 2025, 14, 1639. https://doi.org/10.3390/plants14111639
Parus A, Lisiecka N, Kloziński A, Zembrzuska J. Do Microplastics in Soil Influence the Bioavailability of Sulfamethoxazole to Plants? Plants. 2025; 14(11):1639. https://doi.org/10.3390/plants14111639
Chicago/Turabian StyleParus, Anna, Natalia Lisiecka, Arkadiusz Kloziński, and Joanna Zembrzuska. 2025. "Do Microplastics in Soil Influence the Bioavailability of Sulfamethoxazole to Plants?" Plants 14, no. 11: 1639. https://doi.org/10.3390/plants14111639
APA StyleParus, A., Lisiecka, N., Kloziński, A., & Zembrzuska, J. (2025). Do Microplastics in Soil Influence the Bioavailability of Sulfamethoxazole to Plants? Plants, 14(11), 1639. https://doi.org/10.3390/plants14111639










