Current Findings on Allium Species with Melanogenesis Inhibitory Activity
Abstract
1. Introduction
2. Methodology
3. Melanogenesis
3.1. Melanin Structure and Functions
3.2. Melanin Biosynthesis: The Tyrosinase Enzyme
4. Tyrosinase Inhibitors from Plants
5. Allium spp. Extracts with Tyrosinase Inhibitory Potential
6. Isolated Compounds from Allium Species with Inhibitory Effects on Melanogenesis
7. Negative Outcomes
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| B16 | murine melanoma cell line |
| DPO | diphenol oxidase |
| KAE | kojic acid equivalent |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| MEHQ | hydroquinone mono methyl ether |
| MPO | monophenol oxidase |
| PPOs | plant polyphenol oxidase |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROS | reactive oxygen species |
| TIA | tyrosinase inhibitory activity |
| Tyr | tyrosinase |
| Tyrp1 | tyrosinase-related protein 1 |
| Tyrp2 | tyrosinase-related protein 2 |
| UVB | ultraviolet B |
| α-MSH | α-melanocyte-stimulating hormone |
References
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A Comprehensive Review of Its Mechanism. Biochim. Biophys. Acta BBA-Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to Identify Inhibitors of Melanin Biosynthesis via the Quality Control of Tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef]
- Casanola-Martin, G.M.; Le-Thi-Thu, H.; Marrero-Ponce, Y.; Castillo-Garit, J.A.; Torrens, F.; Rescigno, A.; Abad, C.; Khan, M.T.H. Tyrosinase Enzyme: 1. An Overview on a Pharmacological Target. Curr. Top. Med. Chem. 2014, 14, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- d′Ischia, M.; Wakamatsu, K.; Cicoira, F.; Di Mauro, E.; Garcia-Borron, J.C.; Commo, S.; Galván, I.; Ghanem, G.; Kenzo, K.; Meredith, P.; et al. Melanins and Melanogenesis: From Pigment Cells to Human Health and Technological Applications. Pigment Cell Melanoma Res. 2015, 28, 520–544. [Google Scholar] [CrossRef]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem.–Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Jennifer, C.; Stephie, C.M.; Abhishri, S.B.; Shalini, B.U. A review on skin whitening property of plant extracts. Int. J. Pharma Bio Sci. 2012, 3, 332–347. [Google Scholar]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Zaborowski, M.K.; Długosz, A.; Błaszak, B.; Szulc, J.; Leis, K. The Role of Quercetin as a Plant-Derived Bioactive Agent in Preventive Medicine and Treatment in Skin Disorders. Molecules 2024, 29, 3206. [Google Scholar] [CrossRef]
- Harris, Z.; Donovan, M.G.; Branco, G.M.; Limesand, K.H.; Burd, R. Quercetin as an Emerging Anti-Melanoma Agent: A Four-Focus Area Therapeutic Development Strategy. Front. Nutr. 2016, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Ismail, I.S. Cosmetic Potential of Southeast Asian Herbs: An Overview. Phytochem. Rev. 2015, 14, 419–428. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of Medicinal Herbs for Anti-Tyrosinase Potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Burlando, B.; Clericuzio, M.; Cornara, L. Moraceae Plants with Tyrosinase Inhibitory Activity: A Review. Mini Rev. Med. Chem. 2017, 17, 108–121. [Google Scholar] [CrossRef]
- Ekanayaka, E.; Silva, W. A Review on Tyrosinase Inhibition Potential of Plant Extracts for Skin Whitening. Vavuniya J. Sci. 2023, 2, 1–7. [Google Scholar] [CrossRef]
- Opperman, L.; De Kock, M.; Klaasen, J.; Rahiman, F. Tyrosinase and Melanogenesis Inhibition by Indigenous African Plants: A Review. Cosmetics 2020, 7, 60. [Google Scholar] [CrossRef]
- Muddathir, A.M.; Yamauchi, K.; Batubara, I.; Mohieldin, E.A.M.; Mitsunaga, T. Anti-Tyrosinase, Total Phenolic Content and Antioxidant Activity of Selected Sudanese Medicinal Plants. S. Afr. J. Bot. 2017, 109, 9–15. [Google Scholar] [CrossRef]
- Zaidi, K.; Ali, S.; Ali, A.; Naaz, I. Natural Tyrosinase Inhibitors: Role of Herbals in the Treatment of Hyperpigmentary Disorders. Mini-Rev. Med. Chem. 2019, 19, 796–808. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Hanley, A.B. The Genus Allium—Part 1. Crit. Rev. Food Sci. Nutr. 1985, 22, 199–271. [Google Scholar] [CrossRef]
- Bisen, P.S.; Emerald, M. Nutritional and Therapeutic Potential of Garlic and Onion (Allium sp.). Curr. Nutr. Food Sci. 2016, 12, 190–199. [Google Scholar] [CrossRef]
- Najeebullah, S.; Shinwari, Z.K.; Jan, S.A.; Khan, I.; Ali, M. Ethno Medicinal and Phytochemical Properties of Genus Allium: A Review of Recent Advances. Pak. J. Bot. 2021, 53, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Teotia, D.; Agrawal, A.; Goyal, H.; Jain, P.; Singh, V.; Verma, Y.; Perveen, K.; Bukhari, N.A.; Chandra, A.; Malik, V. Pharmacophylogeny of Genus Allium L. J. King Saud Univ.-Sci. 2024, 36, 103330. [Google Scholar] [CrossRef]
- Liao, N.; Hu, Z.; Miao, J.; Hu, X.; Lyu, X.; Fang, H.; Zhou, Y.-M.; Mahmoud, A.; Deng, G.; Meng, Y.-Q.; et al. Chromosome-Level Genome Assembly of Bunching Onion Illuminates Genome Evolution and Flavor Formation in Allium Crops. Nat. Commun. 2022, 13, 6690. [Google Scholar] [CrossRef] [PubMed]
- de Vahl, E.; Svanberg, I. Traditional Uses and Practices of Edible Cultivated Allium Species (Fam. Amaryllidaceae) in Sweden. J. Ethnobiol. Ethnomedicine 2022, 18, 14. [Google Scholar] [CrossRef]
- Ma, Z.; Bussmann, R.W.; He, H.; Cui, N.; Wang, Q.; Xu, Z.; Liu, B. Traditional Utilization and Management of Wild Allium Plants in Inner Mongolia. Ethnobot. Res. Appl. 2019, 18, 1–14. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Fritsch, R.M. Ornamental Alliums. In Allium Crop Science: Recent Advances; CABI Books: Wallingford, UK, 2002; pp. 459–491. ISBN 978-0-85199-510-6. [Google Scholar]
- Lanzotti, V. Bioactive Polar Natural Compounds from Garlic and Onions. Phytochem. Rev. 2012, 11, 179–196. [Google Scholar] [CrossRef]
- Ivanova, M.I.; Baikov, A.A.; Gins, E.M.; Gins, V.K.; Kashleva, A.I.; Gins, M.S.; Motyleva, S.M.; Pivovarov, V.F.; Smurova, N.V. Assessment of Phytochemicals in Allium Species: A Systematic Review. SABRAO J. Breed. Genet. 2024, 56, 1049–1059. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Ojha, S.; Kalasz, H.; Adeghate, E. Chemical Constituents and Medicinal Properties of Allium Species. Mol. Cell. Biochem. 2021, 476, 4301–4321. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.-D.; Kim, S.-K. Allium Flavonols: Health Benefits, Molecular Targets, and Bioavailability. Antioxidants 2020, 9, 888. [Google Scholar] [CrossRef]
- Elattar, M.M.; Darwish, R.S.; Hammoda, H.M.; Dawood, H.M. An Ethnopharmacological, Phytochemical, and Pharmacological Overview of Onion (Allium cepa L.). J. Ethnopharmacol. 2024, 324, 117779. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic Role of Functional Components in Alliums for Preventive Chronic Disease in Human Being. Evid Based Complement Alternat. 2017, 2017, 9402849. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.; Ayati, Z.; Eghbali, S.; Emami, S.A.; Tayarani-Najaran, Z. Health Benefits of Allium spp. in Metabolic Syndrome: A Review. S. Afr. J. Bot. 2024, 167, 217–255. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef]
- Chung, M.-Y.; Hwang, J.-T.; Park, S.-H. Antiobesity Effects of Onion (Allium cepa) in Subjects with Obesity: Systematic Review and Meta-Analysis. Food Sci. Nutr. 2023, 11, 4409–4418. [Google Scholar] [CrossRef]
- Hosseini, A.; Hosseinzadeh, H. A Review on the Effects of Allium sativum (Garlic) in Metabolic Syndrome. J. Endocrinol. Investig. 2015, 38, 1147–1157. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Di Gioia, F.; Polyzos, N.; Tzortzakis, N. Natural Antioxidants, Health Effects and Bioactive Properties of Wild Allium Species. Curr. Pharm. Des. 2020, 26, 1816–1837. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Mnayer, D.; Tabanelli, G.; Stojanović-radić, Z.Z.; Sharifi-Rad, M.; Yousaf, Z.; Vallone, L.; Setzer, W.N.; Iriti, M. Plants of the Genus Allium as Antibacterial Agents: From Tradition to Pharmacy. Cell. Mol. Biol. 2016, 62, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Packia Lekshmi, N.; Viveka, S.; Jeeva, S.; Raja Brindha, J. Antimicrobial Spectrum of Allium Species—A Review. Indian J. Sci. 2015, 15, 1–5. [Google Scholar]
- Feknous, N.; Boumendjel, M.; Leblab, F.Z. Updated Insights on the Antimicrobial Activities of Allium Genus (A Review). Russ. J. Bioorganic Chem. 2024, 50, 806–823. [Google Scholar] [CrossRef]
- Lanzotti, V.; Scala, F.; Bonanomi, G. Compounds from Allium Species with Cytotoxic and Antimicrobial Activity. Phytochem. Rev. 2014, 13, 769–791. [Google Scholar] [CrossRef]
- De Greef, D.; Barton, E.M.; Sandberg, E.N.; Croley, C.R.; Pumarol, J.; Wong, T.L.; Das, N.; Bishayee, A. Anticancer Potential of Garlic and Its Bioactive Constituents: A Systematic and Comprehensive Review. Semin. Cancer Biol. 2021, 73, 219–264. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Arif Jahan, A.A.; Bari, M.S.; Khandokar, L.; Mahmud, M.H.; Junaid, M.; Chowdhury, M.S.; Khan, M.F.; Seidel, V.; Haque, M.A. Allium Vegetables: Traditional Uses, Phytoconstituents, and Beneficial Effects in Inflammation and Cancer. Crit. Rev. Food Sci. Nutr. 2023, 63, 6580–6614. [Google Scholar] [CrossRef]
- Asemani, Y.; Zamani, N.; Bayat, M.; Amirghofran, Z. Allium Vegetables for Possible Future of Cancer Treatment. Phytother. Res. 2019, 33, 3019–3039. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Ghosh, S.; Bhattacharjee, S. Allium Vegetables in Cancer Prevention: An Overview. Asian Pac. J. Cancer Prev. 2004, 5, 237–245. [Google Scholar]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and Onions: Their Cancer Prevention Properties. Cancer Prev. Res. (Phila Pa.) 2015, 8, 181–189. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ammirato, S.; Felicetti, A.M.; Rogano, D.; Linzalone, R.; Corvello, V. Digitalising the Systematic Literature Review Process: The MySLR Platform. Knowl. Manag. Res. Pract. 2023, 21, 777–794. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Piña-Oviedo, S.; Ortiz-Hidalgo, C.; Ayala, A.G. Human Colors-The Rainbow Garden of Pathology: What Gives Normal and Pathologic Tissues Their Color? Arch. Pathol. Lab. Med. 2017, 141, 445–462. [Google Scholar] [CrossRef]
- Bento-Lopes, L.; Cabaço, L.C.; Charneca, J.; Neto, M.V.; Seabra, M.C.; Barral, D.C. Melanin’s Journey from Melanocytes to Keratinocytes: Uncovering the Molecular Mechanisms of Melanin Transfer and Processing. Int. J. Mol. Sci. 2023, 24, 11289. [Google Scholar] [CrossRef]
- Ramsden, C.A.; Riley, P.A. Tyrosinase: The Four Oxidation States of the Active Site and Their Relevance to Enzymatic Activation, Oxidation and Inactivation. Bioorg. Med. Chem. 2014, 22, 2388–2395. [Google Scholar] [CrossRef]
- Olivares, C.; Solano, F. New Insights into the Active Site Structure and Catalytic Mechanism of Tyrosinase and Its Related Proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The Biochemistry of Melanogenesis: An Insight into the Function and Mechanism of Melanogenesis-Related Proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Nesterov, A.; Zhao, J.; Jia, Q. Natural Tyrosinase Inhibitors for Skin Hyperpigmentation. Drugs Future 2008, 33, 945. [Google Scholar] [CrossRef]
- Seo, S.-Y.; Sharma, V.K.; Sharma, N. Mushroom Tyrosinase: Recent Prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef]
- Emir, C.; Coban, G.; Emir, A. Metabolomics Profiling, Biological Activities, and Molecular Docking Studies of Elephant Garlic (Allium ampeloprasum L.). Process Biochem. 2022, 116, 49–59. [Google Scholar] [CrossRef]
- Phetmanee, T.; Wunnakup, T.; Lukkunaprasit, T.; Madaka, F.; Settharaksa, S.; Kamkaen, N.; Vipunnqeun, N.; Charoenchai, L. Anti-Tyrosinase and Anti-Melanogenic Potential of Shallots (Allium ascalonicum) from Various Cultivation Sites in Thailand. Thai J. Pharm. Sci. TJPS 2020, 44, 107–116. [Google Scholar] [CrossRef]
- Emir, C.; Emir, A. Chemical Composition and Inhibitory Potentials of Key-Enzymes Linked to Neurodegenerative Diseases of Wild Garlic: Allium atrovioleceum Boiss. Indian J. Tradit. Knowl. 2022, 21, 332–340. [Google Scholar] [CrossRef]
- Rocchetti, G.; Zhang, L.; Bocchi, S.; Giuberti, G.; Ak, G.; Elbasan, F.; Yıldıztugay, E.; Ceylan, R.; Picot-Allain, M.C.N.; Mahomoodally, M.F.; et al. The Functional Potential of Nine Allium Species Related to Their Untargeted Phytochemical Characterization, Antioxidant Capacity and Enzyme Inhibitory Ability. Food Chem. 2022, 368, 130782. [Google Scholar] [CrossRef]
- Arung, E.T.; Furuta, S.; Ishikawa, H.; Kusuma, I.W.; Shimizu, K.; Kondo, R. Anti-Melanogenesis Properties of Quercetin- and Its Derivative-Rich Extract from Allium cepa. Food Chem. 2011, 124, 1024–1028. [Google Scholar] [CrossRef]
- Jeong, E.J.; Jegal, J.; Jung, Y.-S.; Chung, K.W.; Chung, H.Y.; Yang, M.H. Fermented Onions Extract Inhibits Tyrosinase and Collagenase-1 Activities as a Potential New Anti–Photoaging Agent. Nat. Prod. Commun. 2017, 12, 1934578X1701200711. [Google Scholar] [CrossRef]
- Nile, A.; Gansukh, E.; Park, G.-S.; Kim, D.-H.; Hariram Nile, S. Novel Insights on the Multi-Functional Properties of Flavonol Glucosides from Red Onion (Allium cepa L.) Solid Waste–In Vitro and in Silico Approach. Food Chem. 2021, 335, 127650. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Mihaylova, D.; Lante, A. Valorization of Onion Extracts as Anti-Browning Agents. Food Sci. Appl. Biotechnol. 2020, 3, 16–21. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, T.; Zhou, F.; Xiao, X.; Ding, X.; He, H.; Rang, J.; Quan, M.; Wang, T.; Zuo, M.; et al. Anticancer Activity of Saponins from Allium chinense against the B16 Melanoma and 4T1 Breast Carcinoma Cell. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 725023. [Google Scholar] [CrossRef] [PubMed]
- Özel, H.B.; Baş Topcu, K.S.; Dere, S.; Genç, N.; Kisa, D. In Vitro and in Silico Based Assessment of Biological Activity of Endemic Allium Species: LC-MS/MS Analysis of Onions. Food Biosci. 2024, 59, 104209. [Google Scholar] [CrossRef]
- Kadyrbayeva, G.; Zagórska, J.; Grzegorczyk, A.; Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Ludwiczuk, A.; Czech, K.; Kumar, M.; Koch, W.; Malm, A.; et al. The Phenolic Compounds Profile and Cosmeceutical Significance of Two Kazakh Species of Onions: Allium galanthum and A. turkestanicum. Molecules 2021, 26, 5491. [Google Scholar] [CrossRef]
- Kısa, D.; Kaya, Z.; İmamoğlu, R.; Genç, N.; Taslimi, P.; Taskin-Tok, T. Assessment of Antimicrobial and Enzymes Inhibition Effects of Allium kastambulense with in Silico Studies: Analysis of Its Phenolic Compounds and Flavonoid Contents. Arab. J. Chem. 2022, 15, 103810. [Google Scholar] [CrossRef]
- Yagi, S.; Nilofar; Zengin, G.; Yildiztugay, E.; Caprioli, G.; Piatti, D.; Menghini, L.; Ferrante, C.; Di Simone, S.C.; Chiavaroli, A.; et al. Exploring for HPLC-MS/MS Profiles and Biological Activities of Different Extracts from Allium lycaonicum Siehe Ex Hayek from Turkey Flora. Foods 2023, 12, 4507. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yıldırım, H. Characterization of Phenolic Profile by LC-ESI-MS/MS and Enzyme Inhibitory Activities of Two Wild Edible Garlic: Allium nigrum L. and Allium subhirsutum L. J. Food Biochem. 2020, 44, e13165. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C. Chemical Profiles and Biological Properties of Methanol Extracts of Allium pallens L. from Different Localities in Turkey. Arch. Biol. Sci. 2020, 72, 193–201. [Google Scholar] [CrossRef]
- Emir, A.; Emir, C.; Yildirim, H. Chemical and Biological Comparison of Different Parts of two Allium species: Allium paniculatum L. subsp. villosulum (Hal.) Stearn and Allium paniculatum L. subsp. paniculatum L. Chem. Pap. 2021, 75, 411–419. [Google Scholar] [CrossRef]
- Somman, A.; Siwarungson, N. Comparison Of Antioxidant Activity And Tyrosinase Inhibition In Fresh And Processed White Radish, Garlic And Ginger. Food Measure 2015, 9, 369–374. [Google Scholar] [CrossRef]
- Samdavid Thanapaul, R.J.R.; Nambur, C.K.; Giriraj, K. Development of Multi-Herbal Formulation with Enhanced Antimicrobial, Antioxidant, Cytotoxic, and Antiaging Properties. J. Indian Chem. Soc. 2024, 101, 101402. [Google Scholar] [CrossRef]
- Mollica, A.; Zengin, G.; Locatelli, M.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multidirectional Investigations on Different Parts of Allium Scorodoprasum L. Subsp. Rotundum (L.) Stearn: Phenolic Components, in Vitro Biological, and in Silico Propensities. Food Res. Int. 2018, 108, 641–649. [Google Scholar] [CrossRef]
- Emiṙ, C.; Emiṙ, A. A Comparative Study of Phenolic Profiles and Biological Activities of Allium sphaerocephalon L. subsp. Sphaerocephalon L. and Allium sphaerocephalon L. subsp. Trachypus (Boiss. Et Spruner) K. Richter. J. Res. Pharm. 2020, 24, 893–900. [Google Scholar] [CrossRef]
- Emir, C.; Emir, A. Phytochemical Analyses with LC-MS/MS and in Vitro Enzyme Inhibitory Activities of an Endemic Species “Allium Stylosum O. Schwarz” (Amaryllidaceae). S. Afr. J. Bot. 2021, 136, 70–75. [Google Scholar] [CrossRef]
- Nikkhahi, M.; Souri, E.; Sarkhail, P.; Baeeri, M.; Mohammadhosseini, N. Evaluation of Anti-Tyrosinase Activity of Allium ursinum Extracts and Their Metal Complexes. Acta Sci. Pol. Technol. Aliment. 2018, 17, 219–226. [Google Scholar] [CrossRef]
- Sathya, R.; Rasane, P.; Singh, J.; Kaur, S.; Bakshi, M.; Gunjal, M.; Kaur, J.; Sharma, K.; Sachan, S.; Singh, A.; et al. Strategic Advances in the Management of Browning in Fruits and Vegetables. Food Bioprocess Technol. 2024, 17, 325–350. [Google Scholar] [CrossRef]
- Emiṙ, C.; Emiṙ, A. Chemical Analysis and Enzyme Inhibitory Activities of Essential Oil Obtained from Allium Proponticum subsp. Proponticum, an Endemic Species. J. Res. Pharm. 2022, 26, 574–580. [Google Scholar] [CrossRef]
- Bito, T.; Koseki, K.; Moriguchi, T.; Sasaki, Y.; Yabuta, Y.; Ichiyanagi, T.; Watanabe, F. Cycloalliin Inhibits Melanin Biosynthesis in B16 Mouse Melanoma Cells. Food Sci. Technol. Res. 2018, 24, 627–633. [Google Scholar] [CrossRef]
- Chu, H.-L.; Wang, B.-S.; Duh, P.-D. Effects of Selected Organo-Sulfur Compounds on Melanin Formation. J. Agric. Food Chem. 2009, 57, 7072–7077. [Google Scholar] [CrossRef]
- Arung, E.T.; Wijaya Kusuma, I.; Shimizu, K.; Kondo, R. Tyrosinase Inhibitory Effect of Quercetin 4’-O-β-D-Glucopyranoside from Dried Skin of Red Onion (Allium cepa). Nat. Prod. Res. 2011, 25, 256–263. [Google Scholar] [CrossRef]
- Arung, E.T.; Furuta, S.; Ishikawa, H.; Tanaka, H.; Shimizu, K.; Kondo, R. Melanin Biosynthesis Inhibitory and Antioxidant Activities of Quercetin-3’-O-Beta-D-Glucoside Isolated from Allium cepa. Z. Naturforschung C J. Biosci. 2011, 66, 209–214. [Google Scholar] [CrossRef]
- Kim, Y.N.; Lee, J.S.; Ock, K.J.; Jeong, E.J. Spirostane-Type Steroidal Saponin from Allium hookeri Roots with Mushroom Tyrosinase Inhibitory Activity. J. Korean Magn. Reson. Soc. 2019, 23, 87–92. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Z.-R.; Chen, P.; Yang-Li; Deng, W.-R.; Wang, Y.-Q.; Li, H.-Y. Effect of the Tyrosinase Inhibitor (S)-N-Trans-Feruloyloctopamine from Garlic Skin on Tyrosinase Gene Expression and Melanine Accumulation in Melanoma Cells. Bioorg. Med. Chem. Lett. 2015, 25, 1476–1478. [Google Scholar] [CrossRef]
- Woo, K.S.; Hwang, I.G.; Kim, H.Y.; Lee, S.H.; Jeong, H.S. Physiological Activities of Thiacremonone Produced in High Temperature and High Pressure Treated Garlic. Prev. Nutr. Food Sci. 2016, 21, 68–72. [Google Scholar] [CrossRef][Green Version]
- Lee, H.J.; Suh, H.J.; Han, S.H.; Hong, J.; Choi, H.-S. Optimization of Extraction of Cycloalliin from Garlic (Allium sativum L.) by Using Principal Components Analysis. Prev. Nutr. Food Sci. 2016, 21, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Mizuno, I.; Yoshida, J.; Ide, N.; Ushijima, M.; Kodera, Y.; Hayama, M.; Ono, K. Pharmacokinetics of Cycloalliin, an Organosulfur Compound Found in Garlic and Onion, in Rats. J. Agric. Food Chem. 2006, 54, 9811–9819. [Google Scholar] [CrossRef]
- Charoenchai, L.; Luprasong, C.; Meksuriyen, D. Characterization of Some Organosulfur Compounds in Shallot Bulbs. Thai J. Pharm. 2018, 42, 9–13. [Google Scholar]
- Schulz, H.; Krüger, H.; Liebmann, J.; Peterka, H. Distribution of Volatile Sulfur Compounds in an Interspecific Hybrid between Onion (Allium cepa L.) and Leek (Allium porrum L.). J. Agric. Food Chem. 1998, 46, 5220–5224. [Google Scholar] [CrossRef]
- Chung, M.-S. Volatile Compounds of the Cultivated Dumebuchu (Allium senescens L. var. Senescens). Food Sci. Biotechnol. 2010, 19, 1679–1682. [Google Scholar] [CrossRef]
- Moldovan, C.; Nicolescu, A.; Frumuzachi, O.; Rocchetti, G.; Lucini, L.; Mocan, A.; Crișan, G. Ultrasound-Assisted Sustainable Extraction of Bioactive Phytochemicals in Shallot (Allium ascalonicum L.) Peel: A DoE and Metabolomics Combined Approach. Sustain. Chem. Pharm. 2024, 41, 101729. [Google Scholar] [CrossRef]
- Moldovan, C.; Frumuzachi, O.; Babotă, M.; Pinela, J.; Barros, L.; Rocchetti, G.; López, V.; Lucini, L.; Crișan, G.; Mocan, A. Untargeted Phytochemical Profiling and Biological Activity of Small Yellow Onion (Allium flavum L.) from Different Regions of Romania. Food Chem. 2023, 426, 136503. [Google Scholar] [CrossRef]
- Nordin, F.N.M.; Aziz, A.; Zakaria, Z.; Wan Mohamed Radzi, C.W.J. A Systematic Review on the Skin Whitening Products and Their Ingredients for Safety, Health Risk, and the Halal Status. J. Cosmet. Dermatol. 2021, 20, 1050–1060. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential Application of Natural Bioactive Compounds as Skin-Whitening Agents: A Review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Manap, A.S.A.; Lum, Y.K.; Ong, L.H.; Tang, Y.Q.; Gew, L.T.; Chia, A.Y.Y. Perspective approaches on melanogenesis inhibition. Dermatol. Sin. 2021, 39, 1–12. [Google Scholar] [CrossRef]
- Desmedt, B.; Courselle, P.; De Beer, J.O.; Rogiers, V.; Grosber, M.; Deconinck, E.; De Paepe, K. Overview of skin whitening agents with an insight into the illegal cosmetic market in Europe. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 943–950. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Škufca, P.; Kristl, A.; Roškar, R. Quality control of retinoids in commercial cosmetic products. J. Cosmet. Dermatol. 2021, 20, 1166–1175. [Google Scholar] [CrossRef]
- Zilles, J.C.; Dos Santos, F.L.; Kulkamp-Guerreiro, I.C.; Contri, R.V. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022, 31, 1500–1521. [Google Scholar] [CrossRef] [PubMed]
- David, S.R.; Baharulnizam, N.B.; Rajabalaya, R. A Review on Biological Assays of Red Algae Marine Compounds: An Insight into Skin Whitening Activities. J. Herb. Med. 2022, 35, 100585. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, Y.I.; Lee, S.K.; Kim, J.E.; Chung, M.H. Aloesin inhibits hyperpigmentation induced by UV radiation. Clin. Exp. Dermatol. 2002, 27, 513–515. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the diverse biological effects of glabridin. Drug Des. Devel. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A. Purification and Characterization of Melanogenic Enzyme Tyrosinase from Button Mushroom. Enzyme Res. 2014, 2014, 120739. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tejedor, D.; Palomo, J.M. Efficient Purification of a Highly Active H-Subunit of Tyrosinase from Agaricus bisporus. Protein Expr. Purif. 2018, 145, 64–70. [Google Scholar] [CrossRef]
- Pazyar, N.; Feily, A. Garlic in dermatology. Dermatol. Rep. 2011, 3, e4. [Google Scholar] [CrossRef]
- Draelos, Z.D. The ability of onion extract gel to improve the cosmetic appearance of postsurgical scars. J. Cosmet. Dermatol. 2008, 7, 101–104. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Al-Obaidi, H.K. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J. Dermatol. 2002, 29, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; De Luca, M.; Toma, C.C.; Grande, F.; Occhiuzzi, M.A.; Caruso, R.; Conforti, F.; Statti, G. Enhancing the nitric oxide inhibitory activity using a combination of plant essential oils and mixture design approach. Heliyon 2024, 10, e31080. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, M.M.; Sun, Y.; Wang, L.F.; Wang, H.; Zhang, Y.J.; Li, H.Y.; Zhuang, P.W.; Yang, Z. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef]
- Siridechakorn, I.; Pimpa, J.; Choodej, S.; Ngamrojanavanich, N.; Pudhom, K. Synergistic impact of arbutin and kaempferol-7-O-α-L-rhamnopyranoside from Nephelium lappaceum L. on whitening efficacy and stability of cosmetic formulations. Sci. Rep. 2023, 13, 22004. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, A.; Pérez-Martínez, M.; Pena-Rodríguez, E.; Gómez-Escalante, S.; Luis, G.S.L.; González, M.C. Depigmenting topical therapy based on a synergistic combination of compounds targeting the key pathways involved in melasma pathophysiology. Exp. Dermatol. 2023, 32, 611–619. [Google Scholar] [CrossRef]
- Ha, A.C.; Le, T.M. Improvement of anti-tyrosinase activity in potential skin whitening products by combining herbal extracts and reducing their tannin content by collagen fibre adsorption. S. Afr. J. Bot. 2023, 155, 118–126. [Google Scholar] [CrossRef]
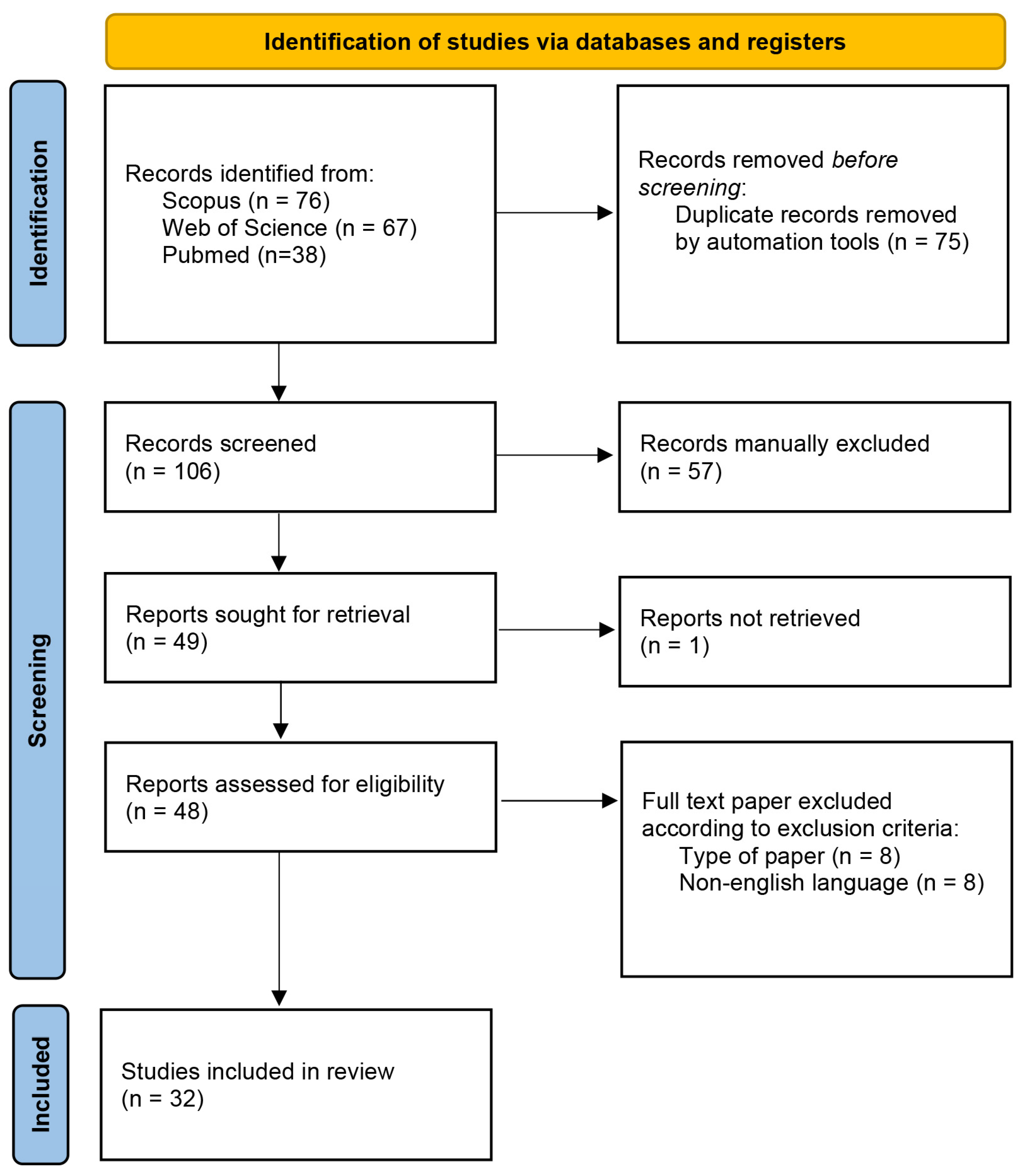





| Species | Plant Part | Extract | In Vitro Model | Results | Ref. |
|---|---|---|---|---|---|
| A. ampeloprasum L. | Flowers, leaves, bulbs | MeOH extract | Mushroom tyrosinase | Enzyme inhibitory potential with IC50 values = 207.85, 313.40, and 348.10 μg/mL | [58] |
| A. ascalonicum L. | Shallots from 14 cultivation sites in Thailand | Aqueous and EtOH extracts | Mushroom tyrosinase and B16-F10 melanoma cells |
At the concentration of 1 mg/mL, the extracts showed about 10–15% tyrosinase inhibition. An optimized shallot extract from fresh shallots decreased melanin synthesis in B16F10 cells in a concentration-dependent manner without affecting cell viability. | [59] |
| A. atrovioleceum Boiss. | Bulb, stem, flower | MeOH extract | Mushroom tyrosinase | The most effective sample, collected in Kemalpaşa, İzmir, Turkey, caused enzyme inhibition, with IC50 values equal to 62.53, 67.40, and 78.83 μg/mL for the different extracts. | [60] |
| Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with MeOH extracts (43.86 and 43.44 mg KAE/g for the aerial parts and bulb, respectively) showing higher activity compared to the water extracts | [61] | |
| A. cappadocicum Boiss. and Balansa | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with MeOH extracts (48.63 and 49.51 mg KAE/g for the aerial parts and bulb, respectively) showing higher activity compared to the water extracts | [61] |
| A. cepa L. | Dried skin | MeOH extract | B16 mouse melanoma cells | Concentration-dependent inhibition of the melanin production | [62] |
| n.s. | Onions were autoclaved and fermented with Saccharomyces cerevisiae and then extracted with MeOH | Murine melanoma B16F10 cells | The treatment at a concentration of 100 μg/mL for 24 h decreased the protein level of cellular tyrosinase to 65.82%. | [63] | |
| Outer dry skins and basal and apical trimmings of red onions bulb | 80% MeOH extract then partitioned with different solvents | Mushroom tyrosinase | Effective TIA. The 80% aqueous methanol extract was the most effective sample (IC50 = 38.9 μg/mL), followed by 80% aqueous ethanol and diethyl ether fractions (IC50 = 40.8 and 48.3 μg/mL). | [64] | |
| Inner layers of white, yellow, and red cultivars and Borettane onions | Juices and distillates | Mushroom tyrosinase | White onion distillate, red onion juice, and yellow onion juice showed the best TIA, with inhibition values equal to 41%, 37%, and 37%, respectively. | [65] | |
| A. chinense G.Don | Bulb | Saponin fraction isolated from a 60% EtOH extract | B16 cells | Inhibition of the tyrosinase activity and decreased melanin biosynthesis | [66] |
| A. eldivanense Özhatay | Aerial parts | MeOH extract | Mushroom tyrosinase | Promising inhibitory effect, with IC50 = 11.87 µg/mL | [67] |
| A. galanthum Kar. and Kir. | Bulb and chives | Absolute ethanol, 70% ethanol, 50% ethanol, and water extracts obtained by ultrasound-assisted maceration. Diethyl ether extracts | Mushroom tyrosinase and murine tyrosinase (B16F10 cells) | The 50% ethanol and 75% ethanol extracts from the chives significantly inhibited murine tyrosinase as tested on B16F10 cells. The most active murine tyrosinase inhibitor was the diethyl ether extract from the bulb (which induced 82.65% inhibition of mushroom tyrosinase and decreased the activity of murine tyrosinase by 54% at 100 μg/mL). | [68] |
| A. goekyigitii Ekim, H.Duman and Güner | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with MeOH extracts (51.17 and 49.70 mg KAE/g for the aerial parts and bulb, respectively) showing higher activity compared to the water extracts | [61] |
| A. hirtovaginatum Kunth | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with MeOH extracts (49.53 and 46.50 mg KAE/g for the aerial parts and bulb, respectively) showing higher activity than the extracts obtained through infusion | [61] |
| A. ilgazense Özhatay | Aerial parts | MeOH extract | Mushroom tyrosinase | Inhibitory effect with an IC50 value equal to 64 µg/mL | [67] |
| A. isauricum Hub.-Mor. and Wendelbo | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with values ranging from 17.25 to 52.84 mg KAE/g | [61] |
| A. kastambulense Bosse | Aerial parts | Methanol/chloroform 4:1 extract | Mushroom tyrosinase | Inhibitory effects with IC50 = 59.17 µg/mL | [69] |
| A. lycaonicum Siehe ex Hayek | Aerial parts, bulbs | n-Hexane, methanol, and water extracts (maceration and soxhlet) | Mushroom tyrosinase | The extracts showed inhibitory properties, with the methanolic extracts being the most effective samples (values ranging from 132.39 to 139.95 mg KAE/g). | [70] |
| A. nigrum L. | Bulbs, aerial parts | MeOH extract | Mushroom tyrosinase | Good inhibitory potential, with IC50 = 22.31 and 51.66 μg/mL | [71] |
| A. olympicum Boiss. | Aerial parts | MeOH extract | Mushroom tyrosinase | Inhibitory effect with an IC50 value equal to 321 µg/mL | [67] |
| A. pallens L. | Bulb, stem, flower | MeOH extract | Mushroom tyrosinase | TIA, with IC50 values equal to 54.58, 96.65 and 138.43 μg/mL | [72] |
| A. paniculatum L. | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | The MeOH extracts showed TIA, with values equal to 52.87 and 53.17 mg KAE/g for the aerial parts and bulb, respectively. A lower activity was observed for the extracts obtained through infusion (6.35 and 3.02 mg KAE/g) | [61] |
| A. paniculatum L. subsp. paniculatum L. | Bulb, stem, flower | MeOH extract | Mushroom tyrosinase | TIA was detected for the flower and bulb samples (IC50 = 73.82 and 139.41 μg/mL, respectively). The stem sample was not effective. | [73] |
| A. paniculatum L. subsp. villosulum (Hal.) Stearn | Bulb, stem, flower | MeOH extract | Mushroom tyrosinase | All the extracts were effective, with IC50 values ranging from 49.16 to 114.25 μg/mL | [73] |
| A. peroninianum Azn. | Aerial parts | MeOH extract | Mushroom tyrosinase | Inhibitory effect with an IC50 value equal to 128 µg/mL | [67] |
| A. proponticum Stearn Et N. Özhatay subsp. proponticum Stearn Et N. Özhatay | Flowers | Essential oil | Mushroom tyrosinase | TIA, with IC50 = 38.22 μg/mL | [41] |
| A. sativum L. | n.s. | 80% MeOH extract, garlic processed-form (syrup) | Mushroom tyrosinase | Inhibitory activity was reported (from 90.88% inhibition to higher values per 100 g) | [74] |
| Bulb | Multi-herbal formulation also containing Coriandrum sativum L., Curcuma longa L., Mentha piperita L., Piper nigrum L., Syzygium aromaticum (L.) Merr. and L.M. Perry, Syzygium cumini (L.) Skeels, Trigonella foenum-graecum L., and Murraya koenigii (L.) Spreng. | Mushroom tyrosinase | Inhibitory potential on tyrosinase enzyme, with IC50 = 252.87 μg/mL. | [75] | |
| A. scabriflorum Boiss. | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with MeOH extracts (44.89 and 43.73 mg KAE/g for the aerial parts and bulb, respectively) showing higher activity compared to the water extracts | [61] |
| A. scorodoprasum L. subsp. rotundum (L.) Stearn | Flower, bulb, stem | MeOH extract | Mushroom tyrosinase | The flower extract showed the highest inhibitory potential on tyrosinase enzyme (55.21 mg KAE/g extract). | [76] |
| A. sphaerocephalon L. subsp. sphaerocephalon L. | Bulb, stem, flower | MeOH extract | Mushroom tyrosinase | TIA, with IC50 = 65.94, 179.42 and 204.71 μg/mL | [77] |
| A. sphaerocephalon L. subsp. trachypus (Boiss. Et Spruner) K. Richter | Bulb and stem | MeOH extract | Mushroom tyrosinase | Tyrosinase inhibition (IC50 values = 262.50 and 315.88 μg/mL) | [77] |
| A. stylosum O. Schwarz | Dried bulbs, leaves, flowers | MeOH extract | Mushroom tyrosinase | IC50 values equal to 49.87, 75.97, and 170.35 μg/mL were obtained for the most effective samples, collected in Bayramli, Izmir, Turkey. | [78] |
| A. subhirsutum L. | Bulbs, aerial parts | MeOH extract | Mushroom tyrosinase | TIA, with IC50 = 49.21 and 63.77 μg/mL | [71] |
| A. trachycoleum Wendelbo | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | TIA, with MeOH extracts (51.23 and 48.70 mg KAE/g for the aerial parts and bulb, respectively) showing higher activity compared to the water infusion extracts | [61] |
| A. ursinum L. | Leaves | Water, 70% EtOH, absolute EtOH extracts | Mushroom tyrosinase | The 70% ethanol extract showed the highest activity (IC50 = 0.392 mg/mL) | [79] |
| A. vineale L. | Aerial parts, bulb | MeOH extract, water infusion | Mushroom tyrosinase | The MeOH extracts showed the highest inhibitory properties (49.67 and 48.41 mg KAE/g for the aerial parts and bulb, respectively) | [61] |
| Compound | Class of Compounds | Investigated Allium Species | In Vitro Model | Results | Ref. |
|---|---|---|---|---|---|
| Cycloalliin (1) | Sulfur compound | - | B16 mouse melanoma cells | Reduced α-MSH -induced melanin levels and both protein and mRNA levels of tyrosinase in B16 cells at 3.8 μM | [82] |
| Mushroom tyrosinase | Weak inhibition of mushroom tyrosinase | [82] | |||
| Diallyl disulfide (2) | Sulfur compound | - | B16 mouse melanoma cells | At a concentration of 500 μM, inhibition of melanin formation (15.61%) and tyrosinase activity (24.35%) | [83] |
| Dimethyl disulfide (3) | Sulfur compound | - | Mushroom tyrosinase | Inhibitory activity, with IC50 value equal to 6.5 mM | [83] |
| B16 mouse melanoma cells | At a concentration of 500 μM, inhibition of melanin formation (40.57%) and tyrosinase activity (20.77%) | [83] | |||
| 2,5-dimethylthiophene (4) | Sulfur compound | - | B16 mouse melanoma cells | At a concentration of 500 μM, inhibition of melanin formation (15.61%) and tyrosinase activity (35.77%) | [83] |
| Propyl disulfide (5) | Sulfur compound | - | B16 mouse melanoma cells | At a concentration of 500 μM, inhibition of melanin formation (14.62%) and tyrosinase activity (24.79%) | [83] |
| 1-Propylmercaptan (6) | Sulfur compound | - | Mushroom tyrosinase | Inhibitory activity, with IC50 = 0.5 mM | [83] |
| B16 mouse melanoma cells | At a concentration of 500 μM, inhibition of melanin formation (24.15%) and tyrosinase activity (46.89%) | [83] | |||
| Quercetin (7) | Flavonoid | A. cepa L. (dried skin extract) | B16 mouse melanoma cells | Inhibition of the melanin production (IC50 = 26.5 μM) | [62] |
| Quercetin 4′-O-β-glucoside (syn. quercetin 4′-O-β-D-glucopyranoside; syn. Spiraeoside) (8) | Flavonoid | A. cepa L. (dried skin extract) | B16 mouse melanoma cells | Inhibition of the melanin production (IC50 = 131 μM) | [62] |
| Mushroom tyrosinase | Inhibition of mushroom tyrosinase (IC50 values = 4.3 μM and 52.7 μM using L-tyrosine and L-DOPA as substrates, respectively) | [84] | |||
| Quercetin-3′-O-β-D-glucoside (syn. Isoquercitrin) (9) | Flavonoid | A. cepa L. (dried skin extract) | B16 mouse melanoma cells | Inhibition of the melanin production (IC50 = 38.8 μM) | [85] |
| Mushroom tyrosinase | Inhibition of mushroom tyrosinase (IC50 values equal to 6.5 μM and 48.5 μM using L-tyrosine and L-dihydroxyphenylalanine as substrates, respectively) | [85] | |||
| Quercetin-3, 4′-O-diglucoside (10) | Flavonoid | A. cepa L. | Mushroom tyrosinase | Inhibition of tyrosinase enzyme (IC50 = 12.6 μM) | [64] |
| (3β, 22R, 25S)-spirost-5-en-3yl O-6-deoxy-α-L-mannopyranosyl-( 1→4)-O-6-deoxy-α-L-mannopyranosyl-(1→4)- O-[6-deoxy-α-L-mannopyranosyl-(1→2)]-β-D-gluco pyranoside (11) | Spirostane-type steroidal saponin | A. hookeri Thwaite (root extract) | Mushroom tyrosinase | Inhibitory activity on mushroom tyrosinase with IC50 value = 248.7 μM | [86] |
| (S)-N-trans-Feruloyloctopamine (12) | Phenolic compound | garlic skin | B16F10 cells | Decreased α-MSH induced cellular melanin content. Real-time PCR and Western blot analyses demonstrated that it down-regulates mRNA and protein expression levels of tyrosinase, leading to a lower melanin content | [87] |
| Thiacremonone (2,4-dihydroxy-2,5-dimethyl-thiophene- 3-one) (13) | alpha-hydroxy ketone | Heated garlic (A. sativum L.) juice treated at 130 °C for 2 h | Mushroom tyrosinase | Inhibition of tyrosinase enzyme, with IC50 = 101.931 μg/mL | [88] |
| Species | Plant Part | Extract | In Vitro Model | Results | Ref. |
|---|---|---|---|---|---|
| A. cepa L. | Flesh | MeOH extract (maceration) | B16 mouse melanoma cells | Any effect was detected on melanin production even at concentrations up to 250 and 500 μg/mL | [62] |
| A. ascalonicum L. | Peel | Hydroalcoholic extract (ethanol 70%) | Mushroom tyrosinase | No activity was observed | [94] |
| A. flavum L. | Stems, flowers | Hydroalcoholic extracts (ethanol 70%) | Mushroom tyrosinase | Any tyrosinase inhibitory activity was detected | [95] |
| A. turkestanicum Regel. | Bulb | Water extracts obtained by ultrasound-assisted maceration | murine tyrosinase (B16F10 cells) | The extract increased the activity of murine tyrosinase | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrelli, M.; Argentieri, M.P.; Musolino, V.; Lupia, C.; Toma, C.-C.; Conforti, F.; Mollace, V.; Statti, G. Current Findings on Allium Species with Melanogenesis Inhibitory Activity. Plants 2025, 14, 1635. https://doi.org/10.3390/plants14111635
Marrelli M, Argentieri MP, Musolino V, Lupia C, Toma C-C, Conforti F, Mollace V, Statti G. Current Findings on Allium Species with Melanogenesis Inhibitory Activity. Plants. 2025; 14(11):1635. https://doi.org/10.3390/plants14111635
Chicago/Turabian StyleMarrelli, Mariangela, Maria Pia Argentieri, Vincenzo Musolino, Carmine Lupia, Claudia-Crina Toma, Filomena Conforti, Vincenzo Mollace, and Giancarlo Statti. 2025. "Current Findings on Allium Species with Melanogenesis Inhibitory Activity" Plants 14, no. 11: 1635. https://doi.org/10.3390/plants14111635
APA StyleMarrelli, M., Argentieri, M. P., Musolino, V., Lupia, C., Toma, C.-C., Conforti, F., Mollace, V., & Statti, G. (2025). Current Findings on Allium Species with Melanogenesis Inhibitory Activity. Plants, 14(11), 1635. https://doi.org/10.3390/plants14111635










