The Phytochemistry and Pharmacology of Onocleaceae Plants: Pentarhizidium orientale, Pentarhizidium intermedium, and Matteuccia struthiopteris—A Review
Abstract
1. Introduction
2. Results and Discussion
2.1. Botany
2.2. Phytochemistry
2.2.1. Flavonoids and Flavonoid Glycosides
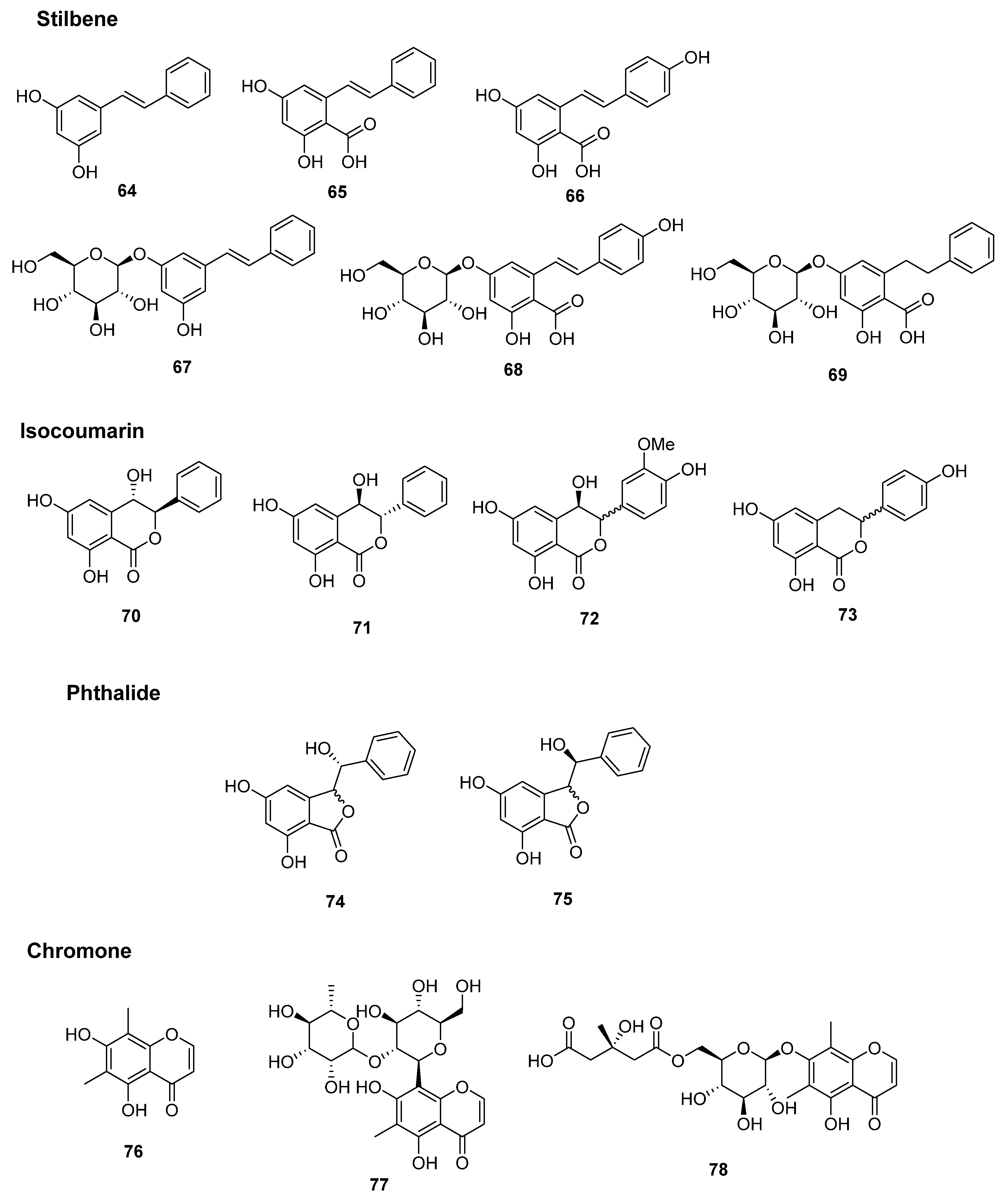
2.2.2. Stilbene Derivatives
2.2.3. Isocoumarins, Phthalides, and Chromone Derivatives
2.2.4. Lignan Glycosides
2.2.5. Isoprenoids Derivatives
2.2.6. Miscellaneous
2.3. Pharmacological Activities
2.3.1. Antiviral Activity
2.3.2. Anti-Inflammatory Activity
2.3.3. α-Glucosidase Inhibitory Activity
2.3.4. Aldose Reductase Inhibition
2.3.5. Antioxidant Activity
2.3.6. Hypoglycemic Activity
3. Conclusions and Future Perspectives
4. Methodology
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhao, J.; Wang, J.-G.; Hu, Y.-P.; Huang, C.-J.; Fang, S.-L.; Wan, Z.-Y.; Li, R.-J.; Yu, H.; He, Z.-R.; Zhou, X.-M. Phylogenetic Inferences and Historical Biogeography of Onocleaceae. Plants 2025, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Plants of the World Online. Available online: https://powo.science.kew.org (accessed on 18 February 2025).
- WFO Plant List. Available online: https://wfoplantlist.org (accessed on 15 March 2025).
- The National Insititute of Biological Resources. Available online: https://species.nibr.go.kr (accessed on 15 April 2025).
- EFloras. Available online: http://www.efloras.org (accessed on 15 April 2025).
- Bergot, B.J.; Baker, F.C.; Lee, E.; Schooley, D.A. Absolute Configuration of Homomevalonate and 3-Hydroxy-3-Ethylglutaryl- and 3-Hydroxy-3-Methylglutaryl CoA, Produced by Cell-Free Extracts of Insect Corpora Allata; Cautionary Note on Prediction of Absolute Stereochemistry Based on Liquid Chromatographic Elution Order of Diastereomeric Derivatives. J. Am. Chem. Soc. 1979, 101, 7432–7434. [Google Scholar]
- Hattori, Y.; Horikawa, K.-H.; Makabe, H.; Hirai, N.; Hirota, M.; Kamo, T. A Refined Method for Determining the Absolute Configuration of the 3-Hydroxy-3-Methylglutaryl Group. Tetrahedron Asymmetry 2007, 18, 1183–1186. [Google Scholar] [CrossRef]
- Mohri, K.; Takemoto, T.; Kondo, Y. Studies on the Constituents of Matteuccia orientalis TREV. Structures of Two New Flavanones, Matteucin and Methoxymatteucin. Yakugaku Zasshi 1982, 102, 310–312. [Google Scholar] [CrossRef]
- Basnet, P.; Kadota, S.; Shimizu, M.; Xu, H.X.; Namba, T. 2’-Hydroxymatteucinol, a New C-Methyl Flavanone Derivative from Matteuccia orientalis; Potent Hypoglycemic Activity in Streptozotocin (STZ)-Induced Diabetic Rat. Chem. Pharm. Bull. 1993, 41, 1790–1795. [Google Scholar] [CrossRef]
- Basnet, P.; Kadota, S.; Hase, K.; Namba, T. Five New C-Methyl Flavonoids, the Potent Aldose Reductase Inhibitors from Matteuccia Orientalis Trev. Chem. Pharm. Bull. 1995, 43, 1558–1564. [Google Scholar] [CrossRef]
- Kadota, S.; Basnet, P.; Hase, K.; Namba, T. Matteuorienate A and B, Two New and Potent Aldose Reductase Inhibitors from Matteuccia orientalis (Hook.) Trev. Chem. Pharm. Bull. 1994, 42, 1712–1714. [Google Scholar] [CrossRef]
- Huh, J.; Ha, T.K.Q.; Kang, K.B.; Kim, K.H.; Oh, W.K.; Kim, J.; Sung, S.H. C-Methylated Flavonoid Glycosides from Pentarhizidium orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J. Nat. Prod. 2017, 80, 2818–2824. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.-J.; Chen, J.-P.; Shi, C.-Y.; Niu, L.-T.; Zhang, X.; Yao, X.-S. C-Methylated Flavanones from the Rhizomes of Matteuccia Intermedia and Their α-Glucosidase Inhibitory Activity. Fitoterapia 2019, 136, 104147. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, L.; Fu, M.-H.; Tu, Y.-Y. Studies on chemical constituents of rhizome of Matteuccia struthiopteris. Zhongguo Zhong Yao Za Zhi 2008, 33, 1703–1705. [Google Scholar]
- Li, B.; Ni, Y.; Zhu, L.-J.; Wu, F.-B.; Yan, F.; Zhang, X.; Yao, X.-S. Flavonoids from Matteuccia Struthiopteris and Their Anti-Influenza Virus (H1N1) Activity. J. Nat. Prod. 2015, 78, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, S.-B.; Yang, L.; Li, Y.-J.; Zhu, X.-X.; Kmoníčková, E.; Zídek, Z.; Fu, M.-H.; Fang, J. Two New C-Methyl Flavanones from the Rhizomes and Frond Bases of Matteuccia struthiopteris. J. Asian Nat. Prod. Res. 2013, 15, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Lim, H.; Chun, S.; Lee, S.B.; Huh, J.; Oh, D.-C.; Hong, S. First Total Synthesis of Gaylussacin and Its Stilbene Derivatives. J. Nat. Prod. 2021, 84, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-J.; Song, Y.; Shao, P.; Zhang, X.; Yao, X.-S. Matteucens I-J, Phenolics from the Rhizomes of Matteuccia orientalis. J. Asian Nat. Prod. Res. 2018, 20, 62–66. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, X.; Li, B.; Jiao, W.-H.; Wu, L.-J.; Yao, X.-S. New Isocourmarin and Phthalide Derivatives from the Rhizomes of Matteuccia orientalis. Chem. Pharm. Bull. 2010, 58, 1650–1654. [Google Scholar] [CrossRef]
- Li, X.; Niu, L.-T.; Meng, W.-T.; Zhu, L.-J.; Zhang, X.; Yao, X.-S. Matteuinterins A-C, Three New Glycosides from the Rhizomes of Matteuccia intermedia. J. Asian Nat. Prod. Res. 2020, 22, 225–232. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Yan, F.; Chen, J.-P.; Zhang, N.; Zhang, X.; Yao, X.-S. 8-O-4′ Neolignan Glycosides from the Aerial Parts of Matteuccia struthiopteris. Chin. Chem. Lett. 2016, 27, 63–65. [Google Scholar] [CrossRef]
- Kimura, T.; Suzuki, M.; Takenaka, M.; Yamagishi, K.; Shinmoto, H. L-O-Caffeoylhomoserine from Matteuccia struthiopteris. Phytochemistry 2004, 65, 423–426. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, L.; Viau, C.; Lu, Y.; Salavati, R.; Basu, N.; Xia, J. MetaboAnalystR 4.0: A Unified LC-MS Workflow for Global Metabolomics. Nat. Commun. 2024, 15, 3675. [Google Scholar] [CrossRef]
- Lee, S.; van Santen, J.A.; Farzaneh, N.; Liu, D.Y.; Pye, C.R.; Baumeister, T.U.H.; Wong, W.R.; Linington, R.G. NP Analyst: An Open Online Platform for Compound Activity Mapping. ACS Cent. Sci. 2022, 8, 223–234. [Google Scholar] [CrossRef]

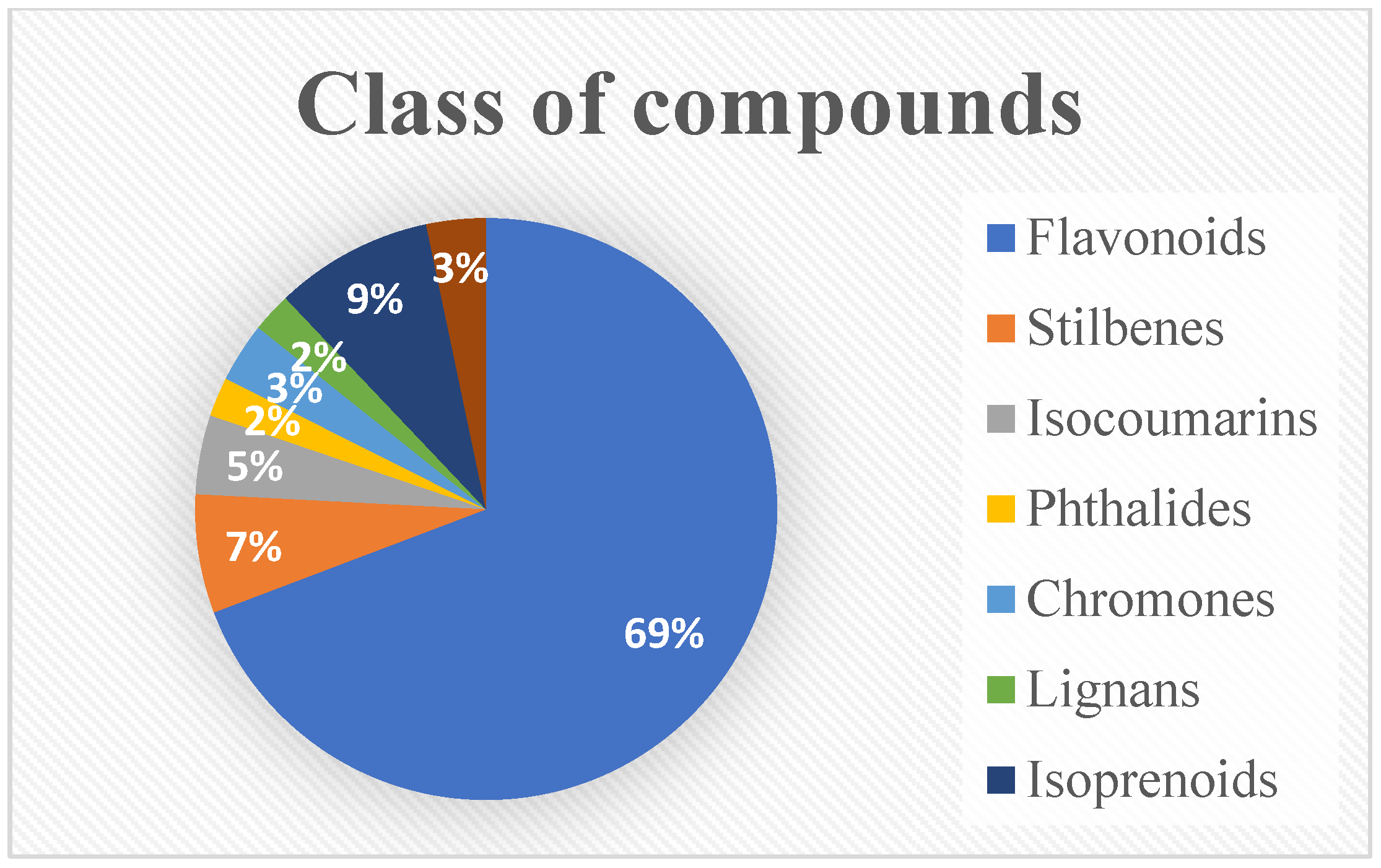
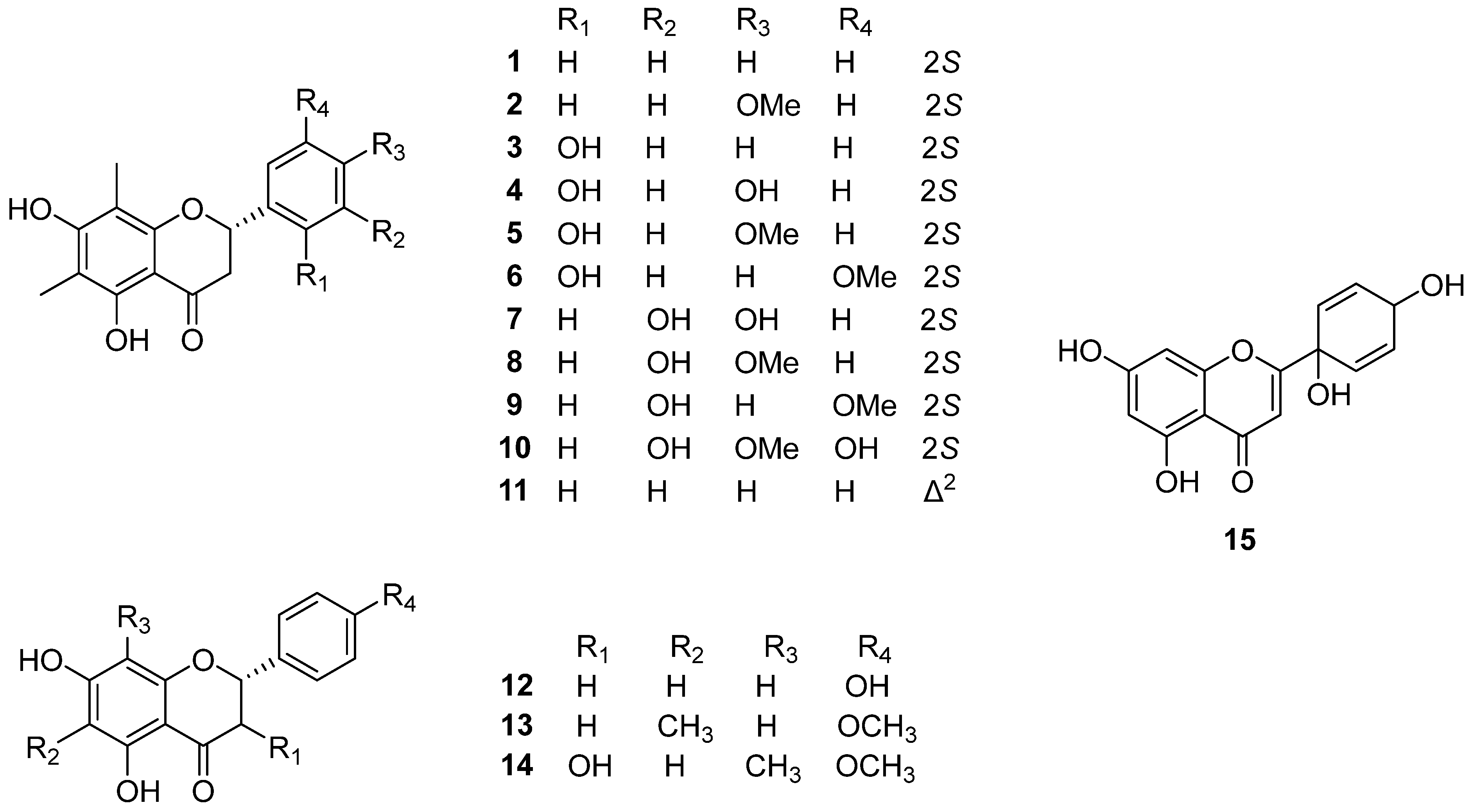
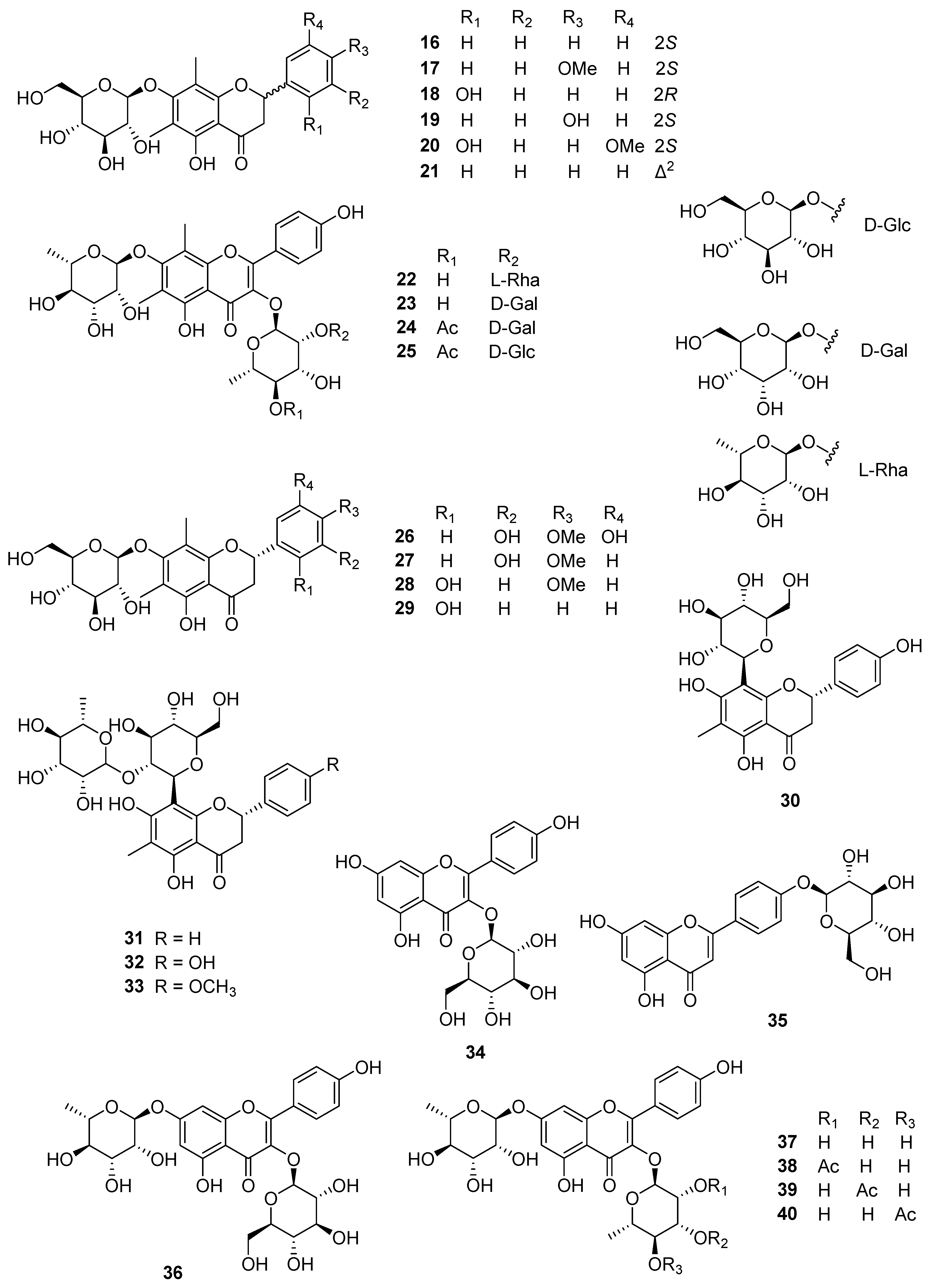
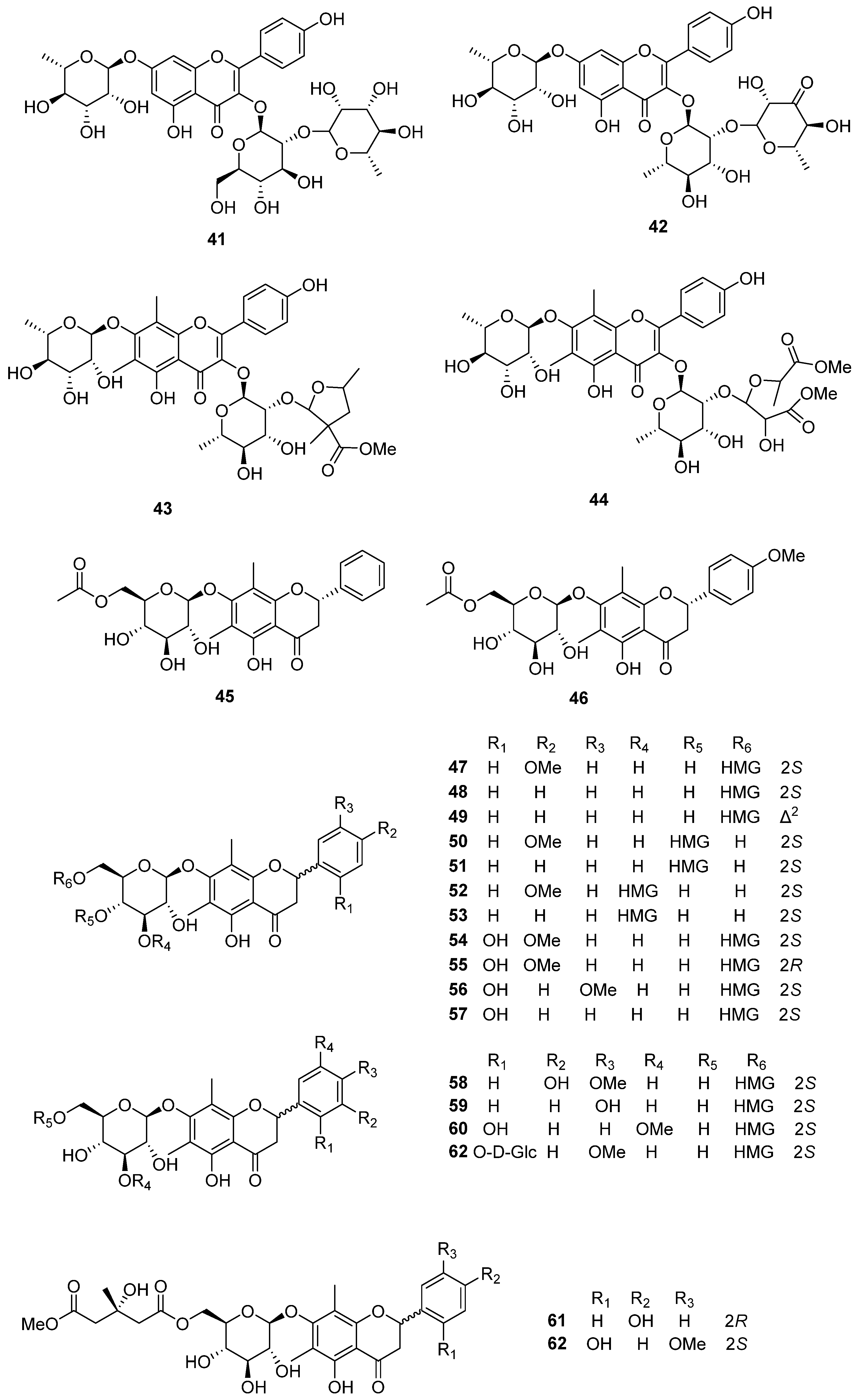

| No. | Compound Name | Part of the Plant | Plant Species | References |
|---|---|---|---|---|
| Flavonoids | ||||
| 1 | Demethoxymatteucinol | rhizome, root | PO | [8,9,10,11,12] |
| rhizome | PI | [13] | ||
| rhizome | MS | [14] | ||
| 2 | Mattuecinol | rhizome, root | PO | [8,9,10,11,12] |
| rhizome | PI | [13] | ||
| rhizome | MS | [14] | ||
| 3 | Matteucin | rhizome, root | PO | [8,12,13] |
| PI | [13] | |||
| 4 | Farrerol | rhizome | PO | [12] |
| PI | [13] | |||
| 5 | 2′-Hydroxymatteucinol | rhizome | PO | [9,10,11] |
| 6 | Methoxymatteucin | rhizome, root | PO | [8,12] |
| PI | [13] | |||
| MS | [16] | |||
| 7 | Cyrtominetin | rhizome | PI | [13] |
| 8 | 3′-Hydroxymatteucinol | rhizome | PI | [13] |
| 9 | 3′-Hydroxy-5′-methoxy 6,8-dimethyl huazhongilexone | rhizome | PO | [12] |
| 10 | Ophiofolius A | rhizome | MS | [15] |
| 11 | Matteuorien | rhizome | PO | [10] |
| MS | [15] | |||
| 12 | Naringenin | rhizome | PO | [12] |
| PO | 5,7-Dihydroxy-4′-methoxy-6-methyl flavanone | rhizome | PI | [13] |
| MS | [16] | |||
| 14 | Demethylmatteucinol | rhizome | PI | [13] |
| 15 | Protoapigenone | rhizome | MS | [15] |
| No | Compound Name | Part of the Plant | Plant Species | References |
|---|---|---|---|---|
| Flavonoid glycosides | ||||
| 16 | Demethoxymatteucinol 7-O-β-D-glucoside | rhizome | PO | [10,12] |
| PI | [13] | |||
| MS | [16] | |||
| 17 | Mattuecinol 7-O-β-D-glucopyranoside | rhizome | PO | [10,12] |
| PI | [13] | |||
| MS | [16] | |||
| 18 | Matteucin 7-O-β-D-glucopyranoside | rhizome | PO | [12] |
| 19 | Farrerol 7-O-β-D-glucopyranoside | rhizome | PI | [13] |
| 20 | Myrciacitrin II | rhizome | PO | [12] |
| PI | [13] | |||
| 21 | Matteuorien 7-O-β-D-glucopyranoside | rhizome | PO | [12] |
| 22 | Matteflavoside A | rhizome | MS | [15] |
| 23 | Matteflavoside B | rhizome | MS | [15] |
| 24 | Matteflavoside C | rhizome | MS | [15] |
| 25 | Matteflavoside D | rhizome | MS | [15] |
| 26 | Matteflavoside G | rhizome | PI | [13] |
| MS | [15] | |||
| 27 | Matteflavoside H | rhizome | PI | [13] |
| 28 | Matteflavoside I | rhizome | PI | [13] |
| 29 | Matteflavoside J | rhizome | PI | [13] |
| 30 | Matteuorienin | rhizome | PO | [12] |
| 31 | Matteuorienin B | rhizome | PO | [12] |
| 32 | Matteuorienin C | rhizome | PO | [12] |
| 33 | Matteuorienin D | rhizome | PO | [12] |
| 34 | Kaempferol-3-O-β-D-glucopyranoside | rhizome | MS | [15] |
| 35 | Apigenin-4′-O-β-D-glucopyranoside | rhizome | MS | [15] |
| 36 | Kaempferol-3-O-β-D-glucopyranosyl-7-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 37 | Kaempferol-3,7-di-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 38 | Kaempferol-3-O-(α-L-2-O-acetyl-Khamnopyranosyl)-7-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 39 | Kaempferol-3-O-(α-L-3-O-acetyl-rhamnopyranosyl)-7-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 40 | Kaempferol-3-O-(α-L-4-O-acetyl-rhamnopyranosyl)-7-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 41 | Kaempferol-3-O-[β-D-glucopyranosyl-(1→2)-α-L-rhamnopyranosyl]-7-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 42 | Kaempferol-3-O-[1,2,4-trihdroxy-3-oxo-5-methyltetrahydropyran-(1→2)-α-L-rhamnopyranosyl]-7-O-α-L-rhamnopyranoside | rhizome | MS | [15] |
| 43 | Matteflavoside E | rhizome | MS | [15] |
| 44 | Matteflavoside F | rhizome | MS | [15] |
| 45 | (2S)-5,7-dihydroxy-6,8-dimethyl-dihydroflavone-7-O-(6″-O-acetyl)-β-D-glucopyranoside | rhizome | MS | [16] |
| 46 | (2S)-5,7-dihydroxy-6,8-dimethyl-4′-methoxydihydroflavone-7-O-(6″-O-acetyl)-β-D-glucopyranoside | rhizome | MS | [16] |
| 47 | Mateuorienate A | rhizome | PO | [10,11,12] |
| PI | [13] | |||
| MS | [14] | |||
| 48 | Mateuorienate B | rhizome | PO | [10,11,12] |
| PI | [13] | |||
| 49 | Mateuorienate C | rhizome | PO | [10,12] |
| 50 | Mateuorienate D | rhizome | PO | [12] |
| PI | [13] | |||
| 51 | Mateuorienate E | rhizome | PO | [12] |
| 52 | Mateuorienate F | rhizome | PO | [12] |
| PI | [13] | |||
| 53 | Mateuorienate G | rhizome | PO | [12] |
| 54 | Mateuorienate H | rhizome | PO | [12] |
| PI | [13] | |||
| 55 | Mateuorienate I | rhizome | PO | [12] |
| PI | [13] | |||
| 56 | Matteuorienate J | rhizome | PO | [12] |
| PI | [13] | |||
| 57 | Matteuorienate K | rhizome | PO | [12] |
| PI | [13] | |||
| 58 | Matteuinterate A | rhizome | PI | [13] |
| 59 | Matteuinterate B | rhizome | PI | [13] |
| 60 | Matteuinterate C | rhizome | PI | [13] |
| 61 | Matteuinterate D | rhizome | PI | [13] |
| 62 | Matteuinterate E | rhizome | PI | [13] |
| 63 | Matteuinterate F | rhizome | PI | [13] |
| No | Compound Name | Part of Plant | Plant | References |
|---|---|---|---|---|
| Stilbene derivatives | ||||
| 64 | Pinosylvin | rhizome | PO | [10] |
| MS | [14] | |||
| 65 | Pinosylvic acid | rhizome | PO | [10,17] |
| 66 | Resveratrolic acid | rhizome | PO | [17] |
| 67 | Gaylussacin (Pinosylvin 3-O-β-D-glucopyranoside) | rhizome | PO | [17] |
| MS | [14] | |||
| 68 | Resveratrolic acid 5-O-β-D-glucopyranoside | rhizome | PO | [17] |
| 69 | Matteucen J | rhizome | PO | [18] |
| Isocoumarins | ||||
| 70 | (-)-matteucen A | rhizome | PO | [19] |
| 71 | (+)-matteucen A | rhizome | PO | [19] |
| 72 | (±)-matteucen B | rhizome | PO | [19] |
| 73 | Thunberginol C | rhizome | MS | [16] |
| Phthalides | ||||
| 74 | (±)-Matteucen C | rhizome | PO | [19] |
| 75 | (±)-Matteucen D | rhizome | PO | [19] |
| Chromone derivatives | ||||
| 76 | Leptorumol | rhizome | PO | [12] |
| 77 | Matteucen I | rhizome | PO | [18] |
| 78 | Matteuinterin B | rhizome | PI | [20] |
| Lignan glycosides | ||||
| 79 | Matteustruthioside A | aerial parts | MS | [21] |
| 80 | Matteustruthioside B | aerial parts | MS | [21] |
| Isoprenoid derivatives | ||||
| 81 | Matteuinterin A | rhizome | PI | [20] |
| 82 | (3S,6S)-6,7-dihydroxy-6,7-dihydrolinalool 3-O-β-D-glucopyranoside | rhizome | PI | [20] |
| 83 | (6R,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one 9-O-β-D-glucopyranoside | rhizome | PI | [20] |
| 84 | (6S,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one 9-O-β-D-glucopyranoside | rhizome | PI | [20] |
| 85 | Byzantionoside B | rhizome | PI | [20] |
| 86 | isodonmegastigmane I | rhizome | PI | [20] |
| 87 | 9ξ-O-β-D-Glucopyranosyloxy-5-megastigmen-4-one | rhizome | PI | [20] |
| 88 | Kankanoside P | rhizome | PI | [20] |
| Miscellaneous | ||||
| 89 | Matteuinterin C | rhizome | PI | [20] |
| 90 | Chlorogenic acid | rhizome | MS | [22] |
| 91 | L-O-caffeoyl-homoserine | rhizome | MS | [22] |
| No | Compounds | Study Model | Dose and/or Concentration | Effects | References |
|---|---|---|---|---|---|
| Antiviral activity | |||||
| 1 | Demethoxymatteucinol | H1N1, A/PR/8/34 H9N2, A/chicken/Korea/01210/2001 | IC50: 30.3 μM/ 31.3 μM EC50: 30.7 μM | neuraminidase inhibitory activity | [12] |
| 2 | Matteucinol | IC50: 25.2 μM/ 27.2 μM EC50: 26.9 μM | |||
| 3 | Matteucin | IC50: 23.9 μM/ 24.1 μM EC50: 22.9 μM | |||
| 6 | Methoxymatteucin | IC50: 24.5 μM/ 24.6 μM EC50: 23.0 μM | |||
| 9 | 3′-hydroxy-5′-methoxy 6,8-dimethyl huazhongilexone | IC50: 24.4 μM/ 23.1 μM EC50: 21.4 μM | |||
| 10 | Ophiofolius A | H1N1 | EC50: 72.8 μM | neuraminidase inhibitory activity | [15] |
| 26 | Matteflavoside G | EC50: 6.8 μM | |||
| 34 | Kaempferol-3-O-β-D-glucopyranoside | EC50: 30.5 μM | |||
| Anti-inflammatory activity | |||||
| 82 | (3S,6S)-6,7-dihydroxy-6,7-dihydrolinalool 3-O-β-D-glucopyranoside | LPS-induced RAW 264.7 murine macrophages | IC50: 17.8 μM | PGE2 production inhibition | [20] |
| 83 | (6R,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one 9-O-β-D-glucopyranoside | IC50: > 50 μM | |||
| 84 | (6S,7E,9R)-9-hydroxy-4,7-megastigmadien-3-one 9-O-β-D-glucopyranoside | IC50: > 50 μM | |||
| 85 | Byzantionoside B | IC50: > 50 μM | |||
| 86 | Isodonmegastigmane I | IC50: > 50 μM | |||
| 87 | 9ξ-O-β-D-Glucopyranosyloxy-5-megastigmen-4-one | IC50: 30.3 μM | |||
| α-Glucosidase activity | |||||
| 2 | Matteucinol | In vitro, Enzyme (0.5 U/mL) from Saccharomyces cerevisiae), substrate: p-NPG | IC50: 28.0 μM | α-Glucosidase inhibition | [13] |
| 3 | Matteucin | IC50: 37.6 μM | |||
| 4 | Farrerol | IC50: 44.1 μM | |||
| 6 | Methoxymatteucin | IC50: 69.7 μM | |||
| 7 | Cyrtominetin | IC50: 12.4 μM | |||
| 8 | 3′-hydroxymatteucinol | IC50: 43.6 μM | |||
| Aldose reductase inhibition | |||||
| 47 | Matteuorienate A | In vitro, Enzyme (eye lens from 5-week-old male Wistar rats) Substrate: glyceraldehyde | IC50: 3.6 μM (in presence of 1% BSA) | Aldose reductase inhibition | [10] |
| 48 | Matteuorienate B | IC50: 3.7 μM (in presence of 1% BSA) | |||
| 49 | Matteuorienate C | IC50: 6.4 μM (in presence of 1% BSA) | |||
| Antioxidant activity | |||||
| 90 | Chlorogenic acid | Chemiluminescence method, DPPH radical degradation method | IC50: 0.31 μM IC50: 0.13 μM | Radical scavenging activity | [22] |
| 91 | L-O-caffeoylhomoserine | IC50: 0.45 μM IC50: 0.30 μM | |||
| Hypoglycemic activity | |||||
| 5 | 2′-hydroxymatteucinol | In vivo, STZ-induced diabetic rats | i.p.: 28.7% (50 mg/kg)/ 38.2% (100 mg/kg) p.o.: 22.0% (25 mg/kg)/ 14.8% (50 mg/kg)/ 27.5% (100 mg/kg) | Reducing blood glucose level | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huh, J. The Phytochemistry and Pharmacology of Onocleaceae Plants: Pentarhizidium orientale, Pentarhizidium intermedium, and Matteuccia struthiopteris—A Review. Plants 2025, 14, 1608. https://doi.org/10.3390/plants14111608
Huh J. The Phytochemistry and Pharmacology of Onocleaceae Plants: Pentarhizidium orientale, Pentarhizidium intermedium, and Matteuccia struthiopteris—A Review. Plants. 2025; 14(11):1608. https://doi.org/10.3390/plants14111608
Chicago/Turabian StyleHuh, Jungmoo. 2025. "The Phytochemistry and Pharmacology of Onocleaceae Plants: Pentarhizidium orientale, Pentarhizidium intermedium, and Matteuccia struthiopteris—A Review" Plants 14, no. 11: 1608. https://doi.org/10.3390/plants14111608
APA StyleHuh, J. (2025). The Phytochemistry and Pharmacology of Onocleaceae Plants: Pentarhizidium orientale, Pentarhizidium intermedium, and Matteuccia struthiopteris—A Review. Plants, 14(11), 1608. https://doi.org/10.3390/plants14111608






