1. Introduction
Wheat is the second largest stable crop in the world, and maintaining high yields of this grain is thus vital to global food security [
1,
2], while also providing a strong basis for social stability and economic development. Weed control is a key consideration in the context of wheat production, as effective weed control is vital to achieving superior crop yields and quality [
3]. Recent years have been marked by a rise in the harm caused by
Aegilops tauschii (
A. tauschii) as a weed in wheat fields, posing a serious threat to reliable wheat production.
A. tauschii Coss. (2 n = 2 x = 14, DD) is a diploid self-pollinating plant in the
Aegilops genus that grows extensively through the Mediterranean coast of West Asia, the Middle East, Southeastern Europe, and Northern Africa. It is primarily distributed in regions that produce large quantities of wheat situated between 30 and 45 degrees north latitude. Like in the case of many other weeds, such as
Ambrosia artemisiifolia [
4], global climate change has contributed to the overall expansion of the potential distribution range of
A. tauschii [
5]. Both wild natural populations and weed-type populations of
A. tauschii have been documented. The former comprises a natural community distributed throughout natural habitats with high levels of genetic diversity [
6,
7], and it has even been proposed as a valuable genetic resource for use in the context of wheat breeding [
8,
9,
10,
11,
12]. The latter, however, has established itself as a weed found in wheat fields throughout China, Iran, Afghanistan, and Pakistan. While the prolonged use of tribenuron-methyl and other herbicides in China has led to the control of the once-dominant weed species,
A. tauschii and other gramineous species have taken their place as the most common weeds found growing in wheat fields [
13]. Wheat and
A. tauschii grow in a similar niche [
14], with the latter exhibiting similar growth dynamics, emergence timing, and morphological features to those of wheat such that it competes with wheat for access to resources including water, light, and fertilizer. After harvesting,
A. tauschii spikes can easily become intermixed with wheat grains and their removal is challenging, thereby having adverse direct or indirect effects on wheat yield and quality [
15,
16,
17,
18,
19,
20]. Wheat yields in areas with average levels of
A. tauschii growth can reportedly be reduced by 12–15%, with reductions of up to 20–38% in areas with particularly high levels of weed growth and >50% yield reductions in severely infested areas. This issue is further compounded by the strong tillering ability of
A. tauschii [
21], together with its propensity for rapid propagation, ease of dissemination, and status as an alternative host for wheat stripe rust [
11]. Given the challenges associated with manual weeding-based removal of this species and the insufficient availability of effective herbicides,
A. tauschii is widely regarded as one of the most economically harmful and difficult-to-control weeds encountered in wheat fields [
22]. Strategies for effectively controlling the spread of
A. tauschii and associated harm are a major focus of ongoing research. Most farmers elect to spray herbicides on their wheat fields as a weed control strategy [
23]. However, only one compound capable of controlling
A. tauschii in wheat fields without also harming wheat—mesosulfuron-methyl—has been identified to date, and it is readily absorbed through the stems and leaves of these weeds [
24]. As it inhibits acetolactate synthase activity in plants by interacting with a single site in this enzyme, weeds can readily develop resistance to this herbicide through the mutation of a single gene locus. Other agricultural control strategies beyond chemical control have also been employed to control weeds. Prior to sowing, for instance, the leveling of farmland is important to remove weed seeds, while also eliminating those weeds present along field edges to prevent the introduction of their seeds during sowing. Employing agricultural methods for weed control can not only protect the ecological environment, but also achieve effective weed management and enhance overall farming practices. One such method is deep turning rotation [
25]. By burying weed seeds deep into the soil, agricultural workers can significantly reduce the germination and emergence of weeds [
26,
27]. Furthermore, crop rotation can alter the environment through differences in crop management practices, disrupting the favorable growth conditions for weeds and thereby achieving effective control. Therefore, adopting an integrated management strategy that prioritizes agronomical measures and supplements them with chemical control is crucial for improving wheat yield, mitigating the impact of
A. tauschii on wheat growth, and promoting sustainable agricultural development.
Given that the range and severity of A. tauschii infestations continue to increase, this study sought to explore and implement the development of a more robust understanding of the weed’s occurrence and infestation through the examination of trends in population growth, its effects on wheat, and the characteristics that define its emergence. The associations between population growth and agronomical practices relevant to this weed species were also explored at length, with the ultimate goal of disrupting A. tauschii growth habitats as a means of reducing or eliminating its occurrence. This study has laid a theoretical foundation for establishing a comprehensive weed control system for wheat, which is significant for improving the effectiveness of A. tauschii control, enhancing wheat production, and ensuring safety.
3. Discussion
Aegilops tauschii Coss., a highly problematic and invasive weed species, poses increasingly significant challenges to modern agricultural systems worldwide [
28]. This weed shares a substantial ecological niche overlap with wheat, leading to intense and direct competition for essential resources such as sunlight, nutrients, water, and physical growing space. The resulting competition drastically reduces both the yield and quality of wheat crops, thereby posing a serious threat to the stability, sustainability, and security of global wheat production systems [
29]. In this study, we conducted a thorough analysis of the emergence characteristics, population dynamics, and impacts of
A. tauschii on wheat yields. The findings presented herein provide a crucial foundation for comprehending the occurrence and damage patterns of this invasive species and for developing effective strategies to manage and mitigate its impact.
A. tauschii seeds mature in late May, following which their dormancy typically terminates after approximately 18 weeks, coinciding with early October. This timing aligns precisely with the sowing period of winter wheat, resulting in the simultaneous emergence of both
A. tauschii and wheat plants. This synchronization solidifies the role of
A. tauschii as a major and persistent weed in wheat fields. However, conducting experiments with seeds stored at room temperature does not provide an accurate representation of the actual dormancy patterns observed in the field. The results presented in this paper can be regarded as a simulation experiment and serve as a reference for understanding dormancy behavior under field conditions. In our future efforts, we aim to improve the storage conditions of the seeds to closely mimic those encountered in the field. The ability of weed seeds to emerge under varying environmental conditions is a critical determinant of their survival, colonization, and subsequent spread. Previous research has demonstrated that seedling emergence depends heavily on seed reserves, which are often insufficient to support germination when buried in deeper soil layers [
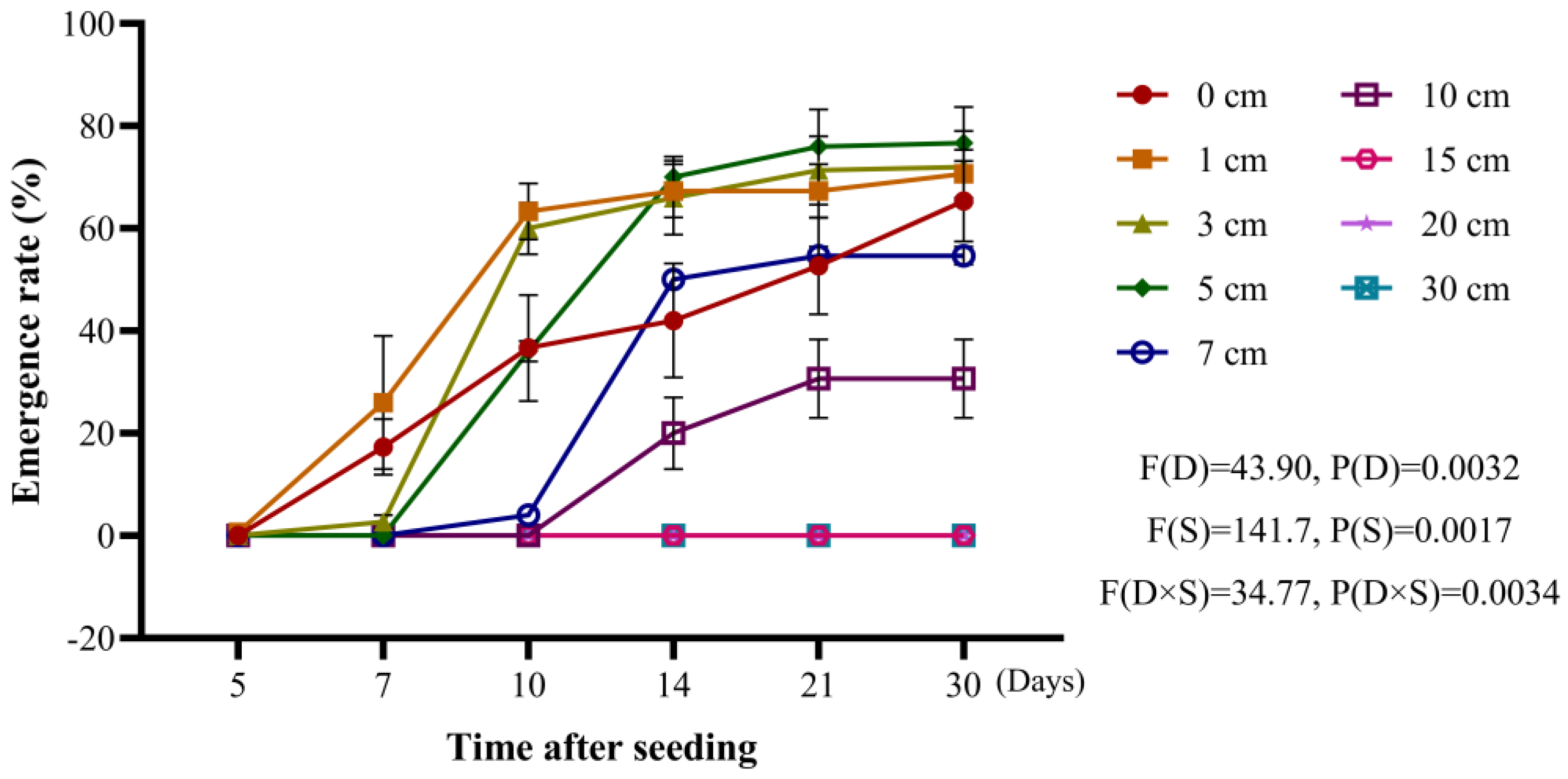
30]. Our study found that the optimal depth for the emergence of
A. tauschii seeds was within the range of 1–5 cm, where emergence rates were at their highest, and the seedlings exhibited greater uniformity. Conversely, deeper planting depths resulted in reduced emergence rates, likely due to nutrient depletion occurring before the coleoptile reached the soil surface or because of sustained seed dormancy at deeper levels. Zhao et al. [
31] reported that ungerminated seeds located in deeper soil layers can establish a persistent seed bank, germinating only under favorable environmental conditions. This “self-rescue” mechanism effectively ensures the long-term survival of the weed species. It is highly plausible that
A. tauschii exhibits similar behavior. Based on these observed emergence characteristics, deep plowing is recommended as an effective strategy to bury
A. tauschii seeds deeper within the soil, thereby reducing their ability to emerge and ultimately lowering the density of
A. tauschii in wheat fields.
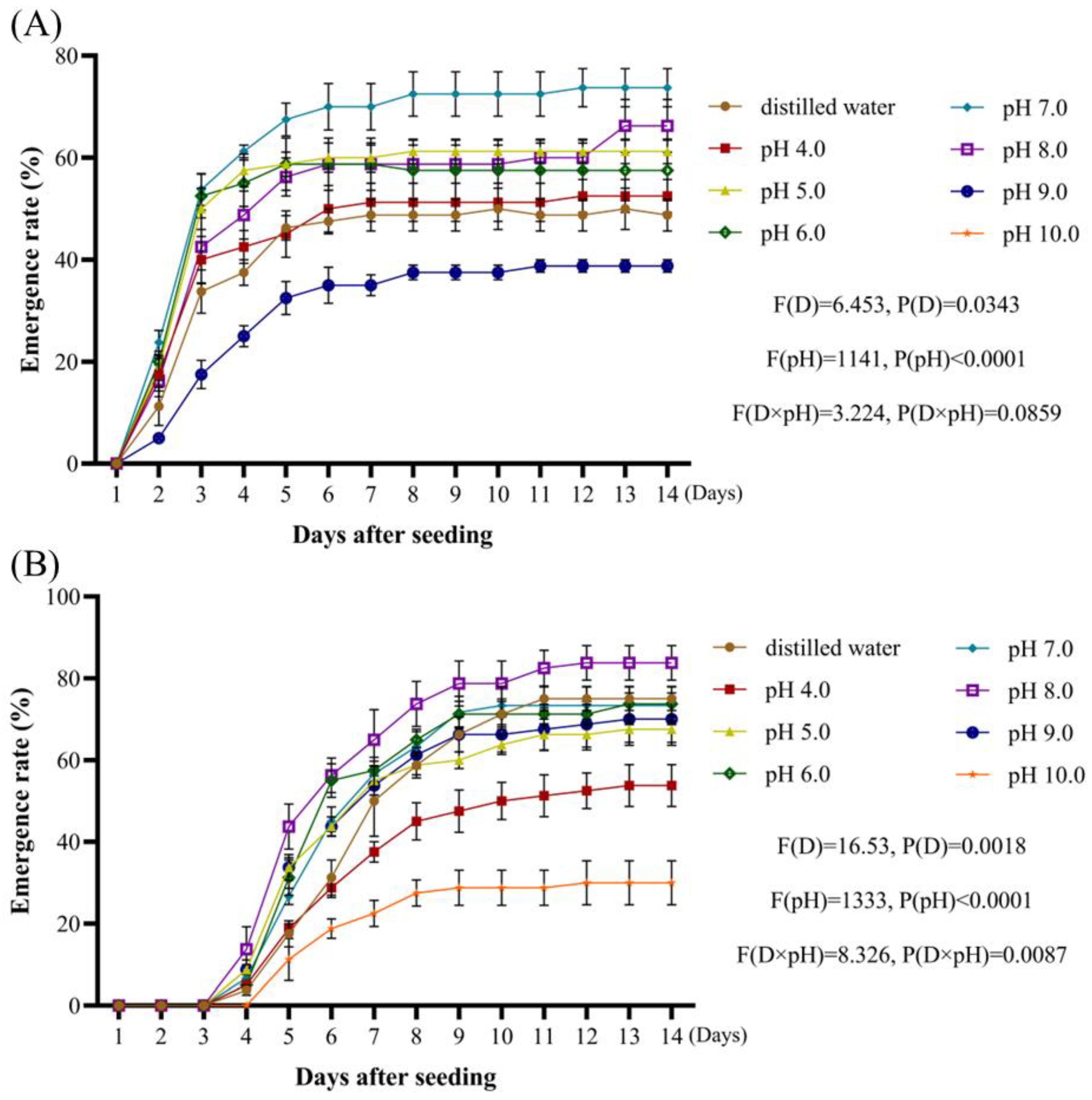
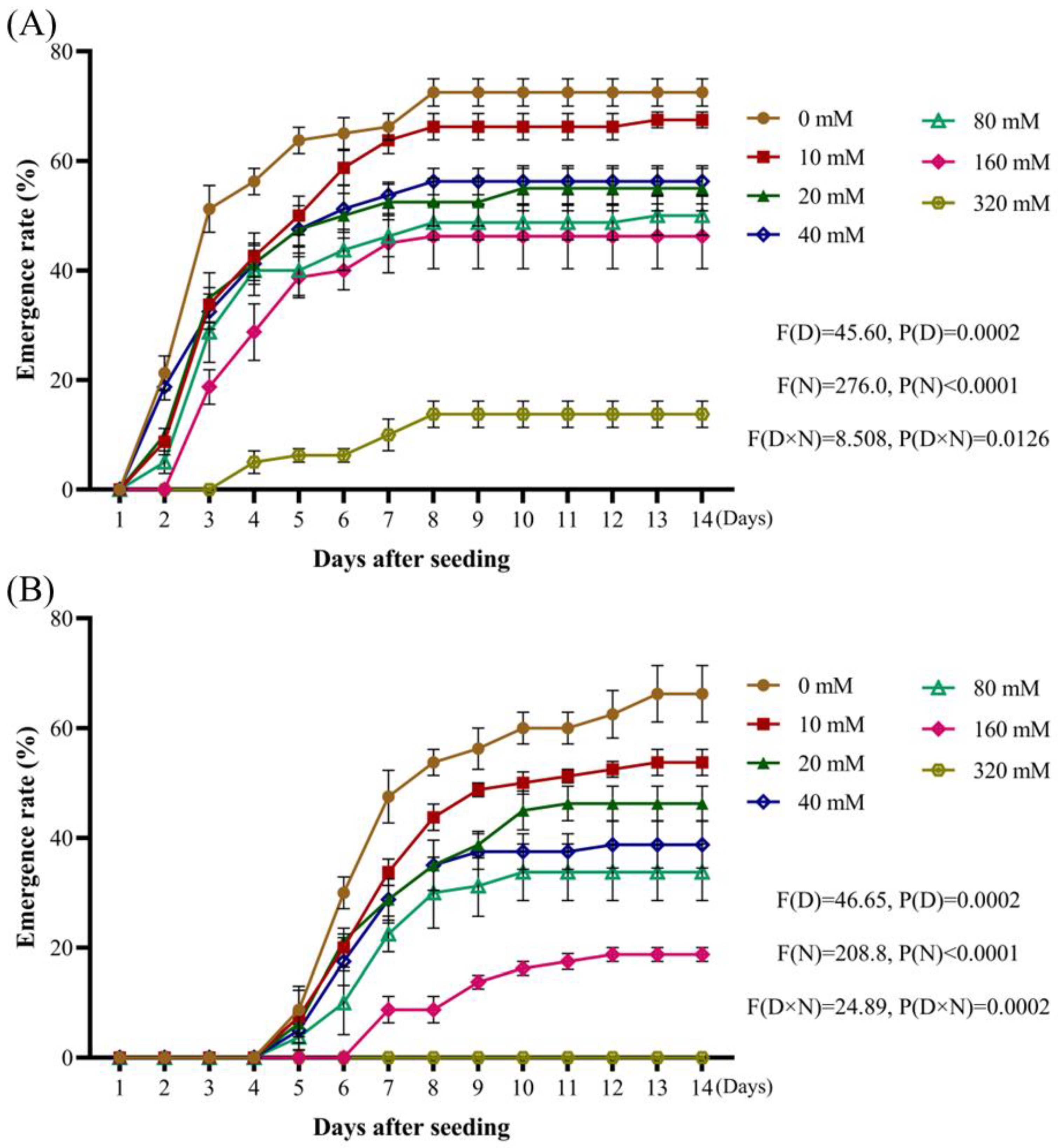
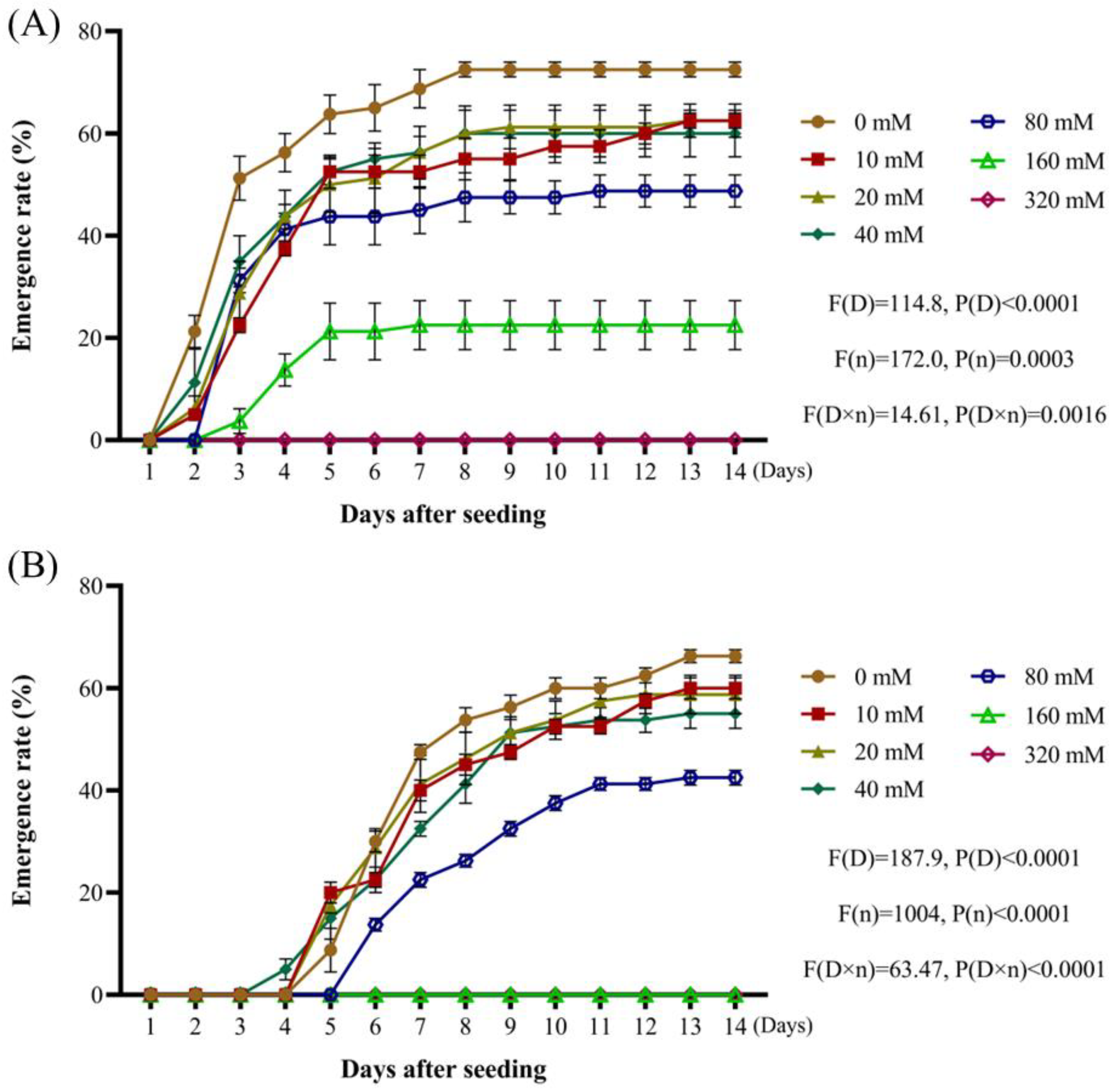
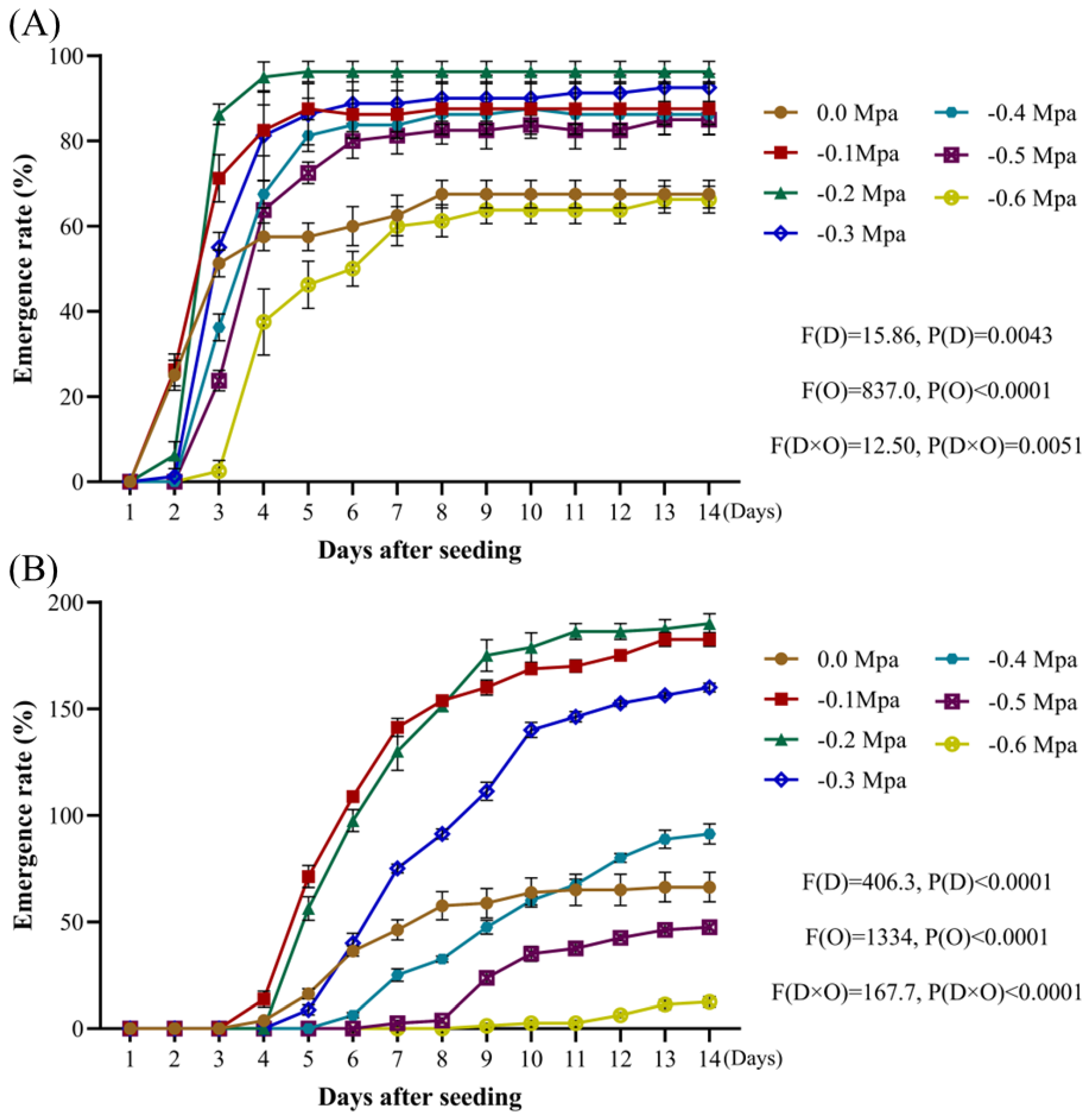
Weed seeds that exhibit tolerance to a wide range of acid-base and salt stress conditions tend to demonstrate enhanced competitiveness under various environmental stressors [
32,
33,
34]. By analyzing the rates of
A. tauschii seed emergence as a function of pH in this study, these seeds were found to possess a remarkably broad pH tolerance range. This adaptability highlights their ecological plasticity, which is a key factor contributing to the rapid and widespread dissemination of
A. tauschii. Increasing salt concentrations resulted in a gradual decline in both radicle emergence and overall seedling emergence rates. Compared to other weed species,
A. tauschii seeds exhibit moderate salt tolerance. When the osmotic potential ranged between −0.4 MPa and −0.1 MPa, the seed emergence rates were significantly enhanced. However, at an osmotic potential of −0.5 MPa and −0.6 MPa, seedling emergence was inhibited, although there was no significant suppression of radicle sprouting, and it was even enhanced in some cases. These findings suggest that mild drought stress conditions can promote the emergence of
A. tauschii, whereas severe drought stress inhibits germination. This ensures that
A. tauschii seeds do not germinate prematurely during humid summer conditions or under excessively dry environments, thereby enhancing their capacity to compete effectively within wheat fields.
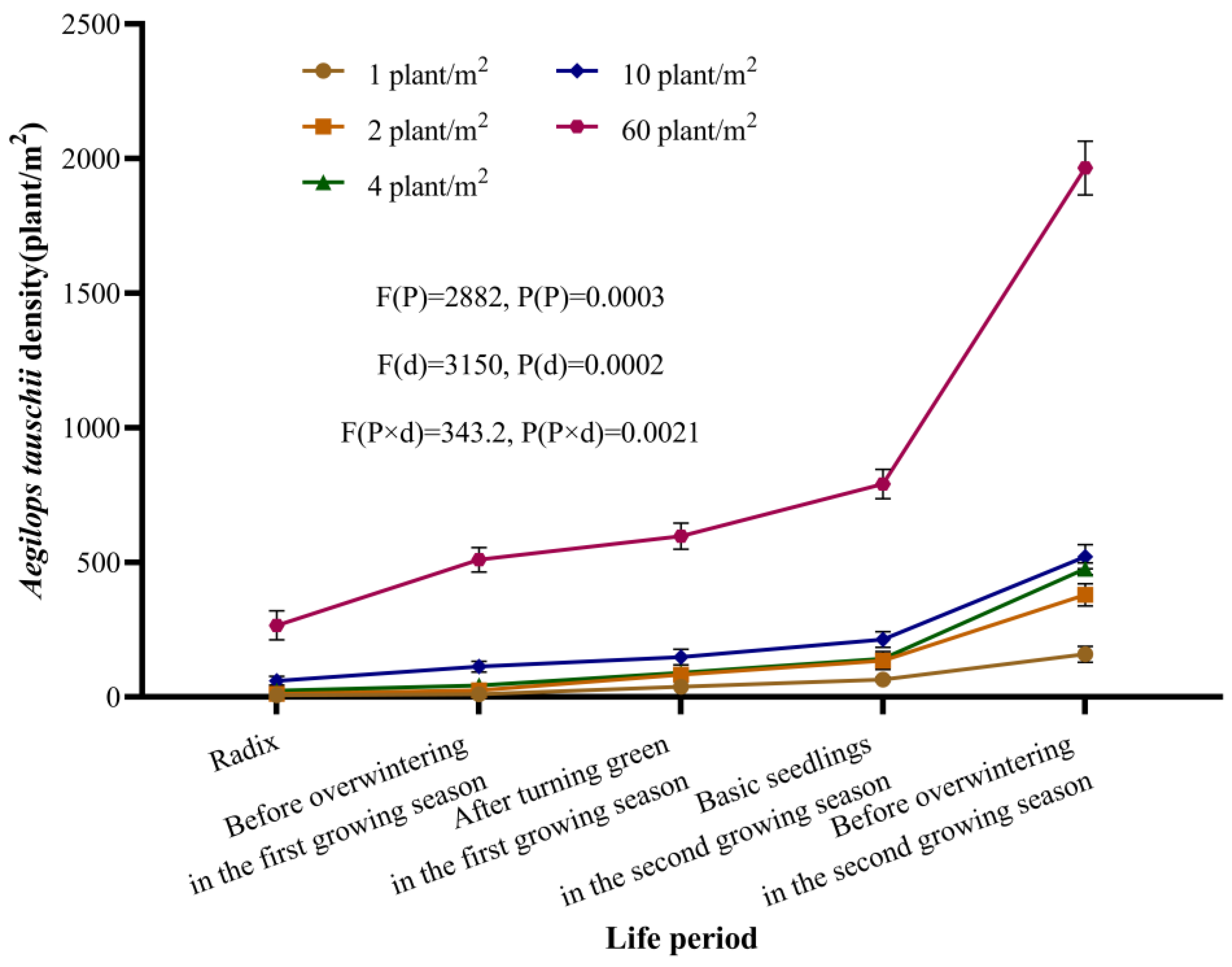
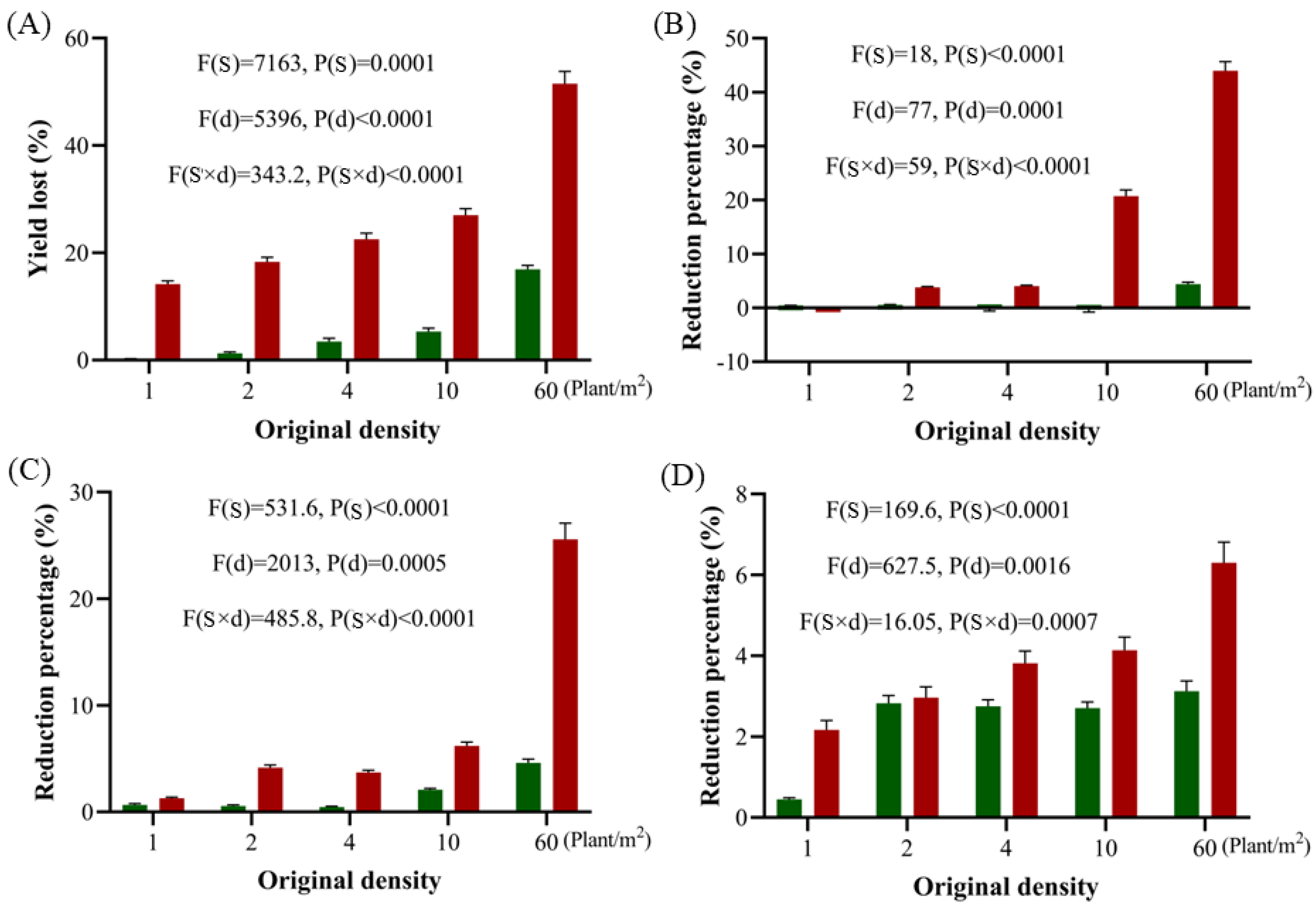
When A. tauschii invades new areas, its initial population size is often small. However, the species is capable of forming dense and extensive populations within a relatively short period if left unchecked. To date, no studies have comprehensively reported the population growth patterns or the dynamic changes in infestation capacity as A. tauschii populations expand. Our multi-year, continuous experiments demonstrated that A. tauschii can establish and rapidly develop dense populations in wheat fields at densities ranging from as low as 1 plant/m2 to as high as 60 plants/m2 if no control measures are implemented. Remarkably, even a single plant per square meter can lead to the establishment of a population due to the species’ robust colonization capabilities. Once colonized, these populations expand rapidly, with initial infestations often being relatively inconspicuous but becoming increasingly evident over time. During the first growing season, the impact of A. tauschii on wheat yield was observed to be relatively minor. However, by the second growing season, wheat yield reductions ranging from 14% to 52% were recorded. Biomass was identified as the most responsive trait in our analyses, reflecting the highly competitive nature of A. tauschii growth. As weed density increases, its inhibitory effects on wheat become increasingly pronounced, primarily through reductions in the number of effective wheat ears and the 1000-grain weight. Consequently, exhaustive efforts are essential to prevent the spread of A. tauschii into new areas. In regions where the weed is already present, it is imperative to implement control measures early in the growing season to curb population growth and minimize the sizes of established infestations.
Based on the observed relationship between A. tauschii density and wheat-yield loss, a yield loss prediction model was developed using probability value analysis. Given the high correlation coefficient of this model, it can serve as a reliable tool for farmers and agricultural researchers to better understand and predict wheat yield losses caused by A. tauschii infestations. Moreover, it offers valuable guidance for devising effective prevention and control strategies tailored to the specific infestation densities encountered in wheat fields.
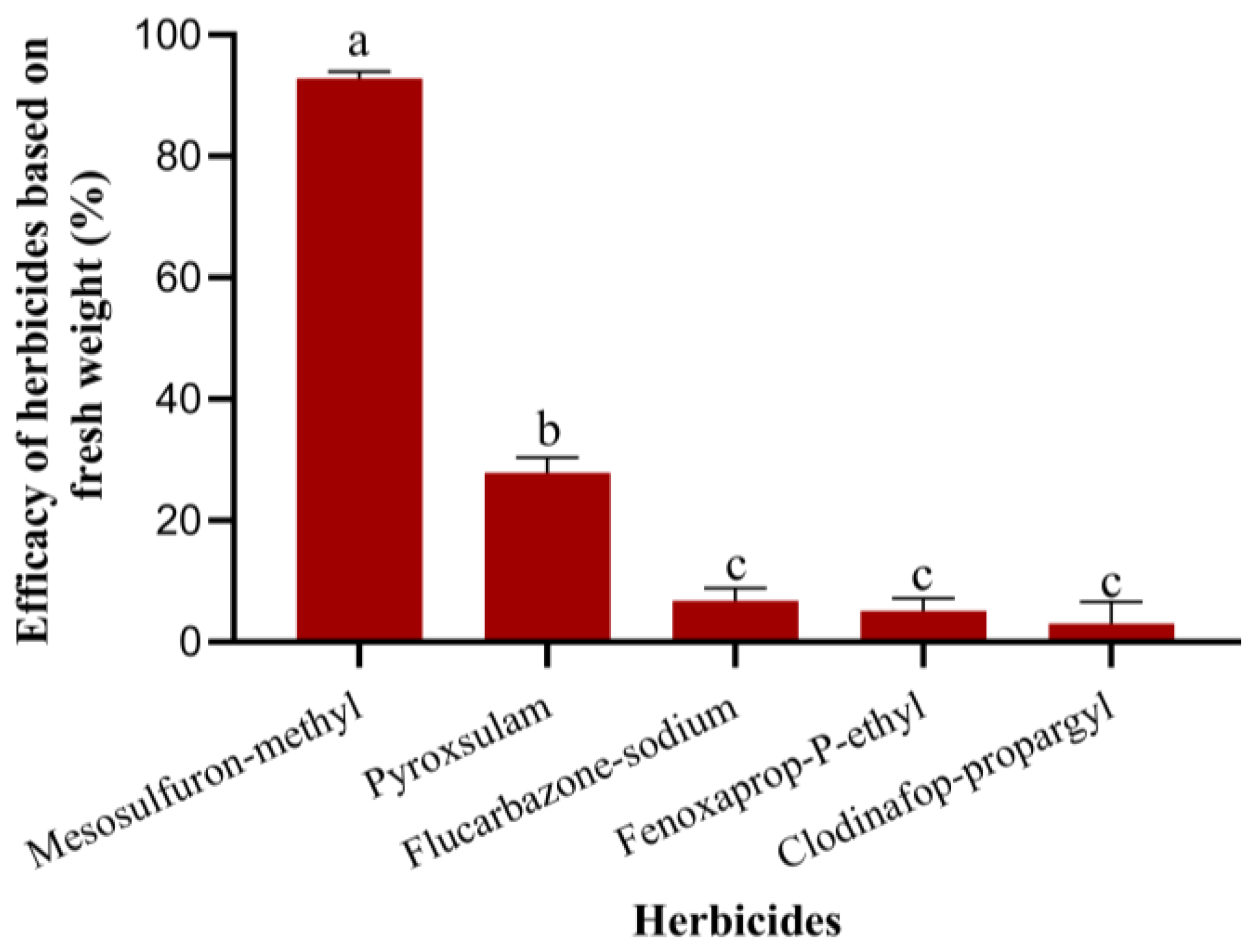
The control of A. tauschii requires a multifaceted, prevention-oriented, and integrated strategy that combines chemical herbicides with agronomic measures to achieve optimal results. Chemical control is widely recognized as a cornerstone of weed management due to its rapid action and high effectiveness in suppressing weed populations. However, controlling A. tauschii using herbicides presents several challenges, including the limited availability of effective options and the need for specific application conditions to ensure efficacy. This study identified mesosulfuron-methyl as the only herbicide effective in managing A. tauschii. This chemical was shown to inhibit the growth of the weed, ultimately leading to plant death. Nevertheless, the limitations of chemical herbicides are becoming increasingly apparent as agricultural practices evolve. The prolonged and repetitive use of herbicides containing specific active ingredients has been shown to result in the development of herbicide resistance in weed species, thereby reducing the long-term effectiveness of these chemicals. Consequently, there is an urgent need for the adoption of more diversified and sustainable cultural control methods to complement chemical approaches. These methods should address the root causes of weed proliferation while mitigating the risk of resistance.
In conventional agricultural systems, extensive farming practices have frequently contributed to the exacerbation of
A. tauschii infestations. In many regions, farmers utilize a two-crop rotation system involving winter wheat and summer corn. After the wheat harvest, corn is often sown directly into the remaining stubble without plowing, leaving a significant portion of
A. tauschii seeds on the soil surface. This practice leads to a gradual accumulation of
A. tauschii seeds over time. Crop rotation, a long-established principle of integrated pest management, offers an effective means of disrupting the growth and reproductive cycles of weeds by redistributing soil nutrients and introducing variability into the growing environment. This variability limits the ability of weeds to adapt and thrive, ultimately reducing their impact on crops [
35,
36]. The present study explored different farming systems and found that, in comparison to the conventional winter wheat–summer corn double-cropping system, three alternative two-year, three-harvest cropping patterns—winter wheat–summer corn–spring soybean, winter wheat–summer corn–spring peanut, and winter wheat–summer corn–spring corn—significantly reduced
A. tauschii densities. These alternative systems introduced different crops into the rotation, thereby altering the soil environment and disrupting the weed’s life cycle. This not only reduced
A. tauschii populations but also altered the field population structure, achieving more effective overall control.
The depth of plowing is another critical factor influencing weed emergence, establishment, and persistence [
37,
38,
39]. Environmental conditions, such as temperature, humidity, and light availability, vary with soil depth and significantly impact seedling emergence rates. When weed seeds are buried in deeper soil layers, their nutrient reserves may be depleted before they can penetrate the soil surface, leading to germination failure [
40]. Laboratory experiments conducted during this study revealed that the optimal depth for
A. tauschii seedling emergence is 1–5 cm. Emergence rates declined sharply when the depth exceeded 5 cm, and no seedlings were observed to emerge from depths greater than 15 cm. In China, shallow rotary tillage remains the predominant plowing method in agricultural systems. This practice buries
A. tauschii seeds in shallow soil layers, creating conditions favorable for their emergence. In contrast, deep plowing, which generally reaches depths of 50–60 cm, buries weed seeds at much greater depths, where emergence is unlikely. Field experiments demonstrated that transitioning from shallow rotary tillage to deep plowing significantly reduced
A. tauschii occurrence. This approach not only eliminated a substantial portion of the existing seed bank but also disrupted the weed’s reproductive cycle, offering a promising agronomic strategy for long-term weed management.
Water availability plays a fundamental role in seed emergence, as it activates critical biological processes, enhances enzyme activity, supplies essential nutrients for seedling growth, regulates temperature, facilitates gas exchange, and promotes early germination [
41]. Controlled watering can be utilized strategically to stimulate the early germination of weed seeds, allowing for their subsequent removal through physical or chemical methods. This technique reduces the potential for future weed emergence and minimizes the competitive pressure on crops. This study clarified the role of timely watering in promoting the germination of
A. tauschii seeds in the soil. When combined with rotary tillage during sowing, the germinated seedlings can be incorporated into the soil, where they decompose, effectively reducing
A. tauschii populations.
Winter wheat, the predominant wheat type grown in China, often requires watering before or after sowing to ensure proper germination. Before sowing, low soil-moisture levels generally inhibit A. tauschii germination. However, watering during the wheat sowing period triggers the germination of these weed seeds. Wheat seedlings typically emerge within seven days of sowing, while A. tauschii emergence continues over a more extended period, spanning from the time of watering until the onset of winter. Delaying wheat sowing narrows the window for A. tauschii emergence, effectively reducing the associated weed-population size. However, it is essential to avoid excessive delays in sowing, as this could negatively impact wheat yields. Field experiments conducted during this study demonstrated the effectiveness of delayed sowing as a control measure. The results showed that appropriate delays significantly reduced A. tauschii emergence, with later sowing dates producing the most favorable outcomes.
4. Materials and Methods
4.1. A. tauschii Seed Dormancy Characterization
Mature
A. tauschii seeds were collected and stored at a room temperature of 25–30 °C. Randomly selected seeds were sown weekly, and, after 14 days, the number of seedlings was recorded to calculate the emergence rate. For cultivation, nutrient soil was placed in a 7 cm diameter plastic culture basin. The soil used was garden organic nutritious soil (Xiao Tie Agriculture Industrial Park of Beijing Agricultural College, Beijing, China), with N, P, and K content ≥ 6%, organic matter ≥ 90%, and a PH value ranging from 5.5 to 6.5. The soil was thoroughly wetted by water infiltration from the bottom, after which seeds were sown at a rate of 20 spikelets per pot with six replicates. The seeds were covered with 1 cm of soil and were incubated in a light-controlled chamber (temperature: 20 °C; 12 h light/dark cycle).
4.2. Evaluation of the Impact of Soil Depth on A. tauschii Seedling Emergence
A. tauschii seeds were sown in custom-made seedling tubes with a height of 50 cm. A base layer of soil was added, followed by sowing 20 spikelets and covering them with soil at depths of 0, 1, 3, 5, 7, 10, 15, 20, and 30 cm. The total soil depth was maintained at 45 cm. Each treatment was repeated four times, and the cultivation conditions were consistent with those detailed in
Section 4.1.
4.3. Evaluation of the Impact of Environmental Factors on A. tauschii Seed Emergence
4.3.1. Main Reagents
These were NaClO (Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China), HCl (Purple Ocean Chemical Factory, Langfang, China), NaOH (Tianjin Fengchuan Chemical Reagent Co., Ltd., Tianjin, China), NaCl (Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China), Na2SO4 (Tianjin Fengchuan Chemical Reagent Co., Ltd., Tianjin, China), and PEG-6000 (Polyethylene glycol 6000, Tianjin Fengchuan Chemical Reagent Co., Ltd., Tianjin, China).
4.3.2. Main Equipment
This comprised a PHS-3C pH meter (INASE Scientific Instrument Co., Ltd., Shanghai, China) and a MGC-250BP-2-type Illumination incubator (Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China).
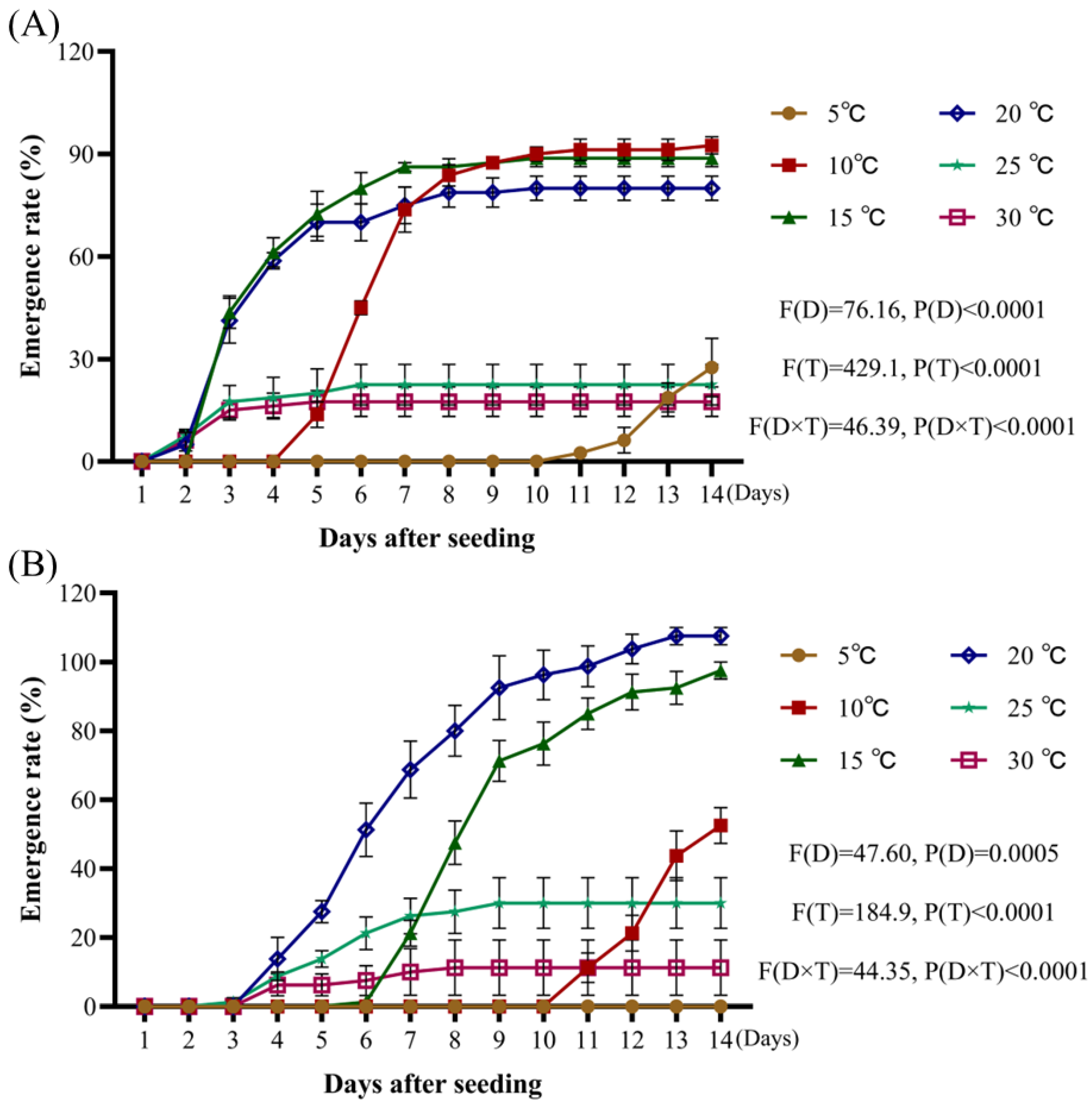
4.3.3. The Impact of Temperature Tests
- A.
Setting of temperature gradient
There were 6 MGC-250BP-2 illuminated incubators which were set to six temperature gradients: 5 °C, 10 °C, 15 °C, 20 °C, 25 °C, and 30 °C, respectively. The illumination was set to a 12 h light/dark cycle;
- B.
Seed processing and cultivation
A. tauschii seeds were immersed in 0.5% NaClO for 30 s, rinsed three times with distilled water, and evenly placed in 9 cm Petri dishes lined with two layers of filter paper. Each dish received 9 mL distilled water to ensure complete wetting. Twenty spikelets were used per dish, with four replicates per treatment. The Petri dishes were changed every 48 h.
4.3.4. The Impact of pH, Salt Concentration, and Osmotic Potential Tests
- A.
Reagent preparation
- a.
Solutions of HCl and NaOH with different pH levels
After preparing 1 mol/L HCl and NaOH solutions, their pH levels were adjusted to 4, 5, 6, 7, 8, 9, and 10 using distilled water;
- b.
Solutions with different NaCl concentrations
After dissolving 4.699 g NaCl in 100 mL of distilled water, the volume was adjusted to 250 mL to produce 320 mmol/L NaCl. Then, the solution was serially diluted by half to produce 20, 40, 80, and 160 mmol/L NaCl solutions;
- c.
Solutions with different Na2SO4 concentrations
After dissolving 11.420 g Na2SO4 in 100 mL of distilled water, the volume was adjusted to 250 mL to produce 320 mmol/L Na2SO4. Then, the solution was serially diluted by half to produce 20, 40, 80, and 160 mmol/L solutions;
- d.
Solutions with different osmotic potential levels
After dissolving 18.128 g, 28.095 g, 36.323 g, 43.103 g, 49.098 g, or 54.533 g of PEG 6000 in 250 mL of distilled water, solutions with osmotic potentials of −0.1, −0.2, −0.3, −0.4, −0.5, and −0.6 MPa were prepared.
- B.
Seed processing and cultivation
The seeds and Petri dishes were treated as described in
Section 4.3.3, except each dish received 9 mL of the test solution. The Petri dishes were incubated under standard conditions (20 °C, 12 h light/dark cycle).
4.3.5. Investigation
Analyses were conducted every 24 h, recording the number of spikelets with radicle or seedling emergence. The radicle emergence criterion was a radicle length > 0.5 cm. The seedling emergence criterion was seedling length > 0.5 cm. This analysis was performed for 14 days.
4.4. Population Growth Dynamics of A. tauschii
Field plot experiments were conducted on the farm of the Plant Protection Institute, Hebei Academy of Agriculture and Forestry Sciences (115°31′39.52″ E, 38°51′51.85″ N) from October 2022 to July 2024. Each plot was 1 m2. The wheat sowing dates were 8 October 2022 and 8 October 2023, which were suitable for the sowing in terms of local production. A. tauschii was inoculated after wheat sowing at densities of 0, 1, 2, 4, 10, and 60 plants/m2 on 8 October 2022 without prior infestations. The winter wheat–summer corn double cropping system was adopted. Before wheat sowing, 60 g/m2 of compound fertilizer (Shandong Enbao Biological Technology Co., Ltd., Qingdao, China, N + P2O5 + K2O ≥ 51%; N:P:K = 18:25:8) was applied, followed by manual soil tillage, ditch digging, and seeding wheat at a rate of 22.5 g/m2. Watering was performed after sowing. After weeds’ head sprouting, each plot was isolated with gauze nets to prevent weed seeds from falling outside the plots. Upon wheat maturation, the wheat ears were harvested. The wheat and A. tauschii straws were crushed and returned to the field before the corn was seeded. There was no abnormal weather that affected the normal growth of the crops during the experiment. The density of A. tauschii was measured before the weeds tillering, before overwintering, and after regrowth for each growing cycle. Data collected over three years defined the population growth dynamics of A. tauschii and its impact on wheat.
4.5. Wheat Yield Loss Calculations
Based on the data from
Section 4.4, after harvest, the wheat yield parameters, including the spike number, grains per spike, 1000-grain weight, and total yield, were assessed. A yield loss model was developed, correlating
A. tauschii density after emergence with wheat yield in each growing cycle.
4.6. Screening for Herbicides to Control A. tauschii
The experimental field was located in Dayang Village, Lianchi District, Baoding City, Hebei Province, China (115°32′55.78″ E, 38°50′47.44″ N). This farmland, similar to the surrounding districts, followed a winter wheat–summer corn double-cropping system. Conventionally, wheat was sown around 8 October every year. After the harvest of corn and before the sowing of wheat, the land was rotary tilled, followed by seeding and fertilization using a seeding machine. The standard fertilization rate was 600 kg/hm2, and the wheat-seeding rate was 225 kg/hm2. Irrigations were carried out after wheat sowing, before overwintering, after turning green, and during the wheat-grain filling stage. Winter wheat was harvested around 10 June. Generally, summer corn was sown on the same day of wheat harvest without tillage, followed by irrigation. Summer corn was harvested around 1 October. Besides A. tauschii, there were Descurainia sophia and a small amount of Capsella bursa-pastoris in the wheat field, which were manually pulled out in the experiment. A. tauschii had the highest occurrence and was evenly distributed.
Field plot experiments (30 m2 per plot) were conducted to evaluate the control effects of several herbicides, including 30 g/L mesosulfuron-methyl OD (Shi ma, Bayer Aktiengesellschaft, Beijing, China), 4% pyroxsulam OD (You xian, Corteva Agriscience, Beijing, China), 70% flucarbazone-sodium WG (Biao hu, Arysta Lifescience North America, Ltd., Shanghai, China), 69 g/L fenoxaprop-p-ethyl EW (Biao ma, Bayer CropScience China Co., Ltd., Hangzhou, China), and 15% clodinafop-propargyl WP (Mai ji, Syngenta, Shanghai, China). A control treatment without herbicide application was included. The herbicides were sprayed 30 days after wheat sowing when the weeds had largely emerged. The sprayer was Knapsack Sprayer Jacto PJB-16 (INTERMAN Corporration Ltd., Rayong, Thailand) with Fan nozzle DEF-06. The spray volume of water was 450 L/hm2. The control effects on A. tauschii fresh weight were assessed the following spring using random sampling with three sampling points per plot. There was no abnormal weather that affected the experiment.
4.7. Control Effects of Different Cultural Methods on A. tauschii
The location of the experimental field and the conventional wheat cultivation methods were the same as described in
Section 4.6.
- A.
Crop rotation
Field experiments tested the control efficacy associated with three two-year, three-crop rotation systems (winter wheat–summer corn–spring bean, winter wheat–summer corn–spring peanut, and winter wheat–summer corn–spring corn) compared to the conventional winter wheat–summer corn two-crop system. Each treatment had a planting area of 1500 m2. For the conventional system, rotary tillage, wheat seeding, fertilization and watering were performed on 9 October 2022. The winter wheat was harvested on 10 June 2023. The following summer corn was sowed on 10 June 2023, and harvested on 4 October 2023. For the three types of two-year, three-crop rotation systems, no seeding was performed after the harvest of summer corn on 9 October 2022. In the second year, watering was performed in the spring, followed by rotary tillage and land preparation. The spring-sown crops were seeded on 25 April 2023 using a seeding machine for both seeding and fertilization and they were harvested on 20 September 2023. A new wheat-growing cycle continued from 8 October 2023 to 12 June 2024, in all the four rotation systems, where A. tauschii density was assessed at nine sampling points before overwintering and after regrowth;
- B.
Deep plowing
A field experiment was conducted with treatments set at 1000 m2 each to assess the effect of plowing depth on controlling A. tauschii. Plowing depths of 5, 10, 15, 20, or 30 cm were evaluated. A rotary tilled field served as the control. Weed samples were taken from nine points in each treatment plot to record the number of A. tauschii plants in wheat fields before winter and after the wheat turned green;
- C.
Watering-induced emergence
A field experiment was carried out in a winter wheat–summer corn rotation system to test the watering-induced emergence on A. tauschii. Corn fields were irrigated 5, 10, 15, 20, and 25 days prior to wheat sowing on 8 October 2023. A non-irrigated field served as the control. After the corn harvest, the soil was tilled before sowing wheat. Samples were collected from nine points per treatment plot to record A. tauschii density in the field before sowing, before winter, and after the wheat turned green.
- D.
Delayed sowing
Field experiments were conducted to study the effect of delayed sowing on A. tauschii. Taking time as a variable, the sowing was divided into five treatments: conventional sowing on 8 October 2023 and sowing delayed by 5, 10, 15, and 20 days, each covering an area of 1000 m2. Samples were collected from nine points per treatment to count A. tauschii plants under different sowing schedules.
4.8. Statistical Analysis
Statistical analysis was performed using SPSS software version 21.0. The characterization of A. tauschii seed dormancy and screening for herbicides to control A. tauschii used one-way analysis of variance (ANOVA), and the mean values of each treatment group were compared using Duncan’s test at p < 0.05. Other experiments employed two-way ANOVA, incorporating main effect terms and their interaction term in the model. If the interaction effect was significant, further analysis was conducted through Simple Effects Analysis to clarify differences among various level combinations. If the main effect was significant but there was no interaction, Duncan’s multiple range test was used for pairwise comparisons between groups, with a significance level set at p < 0.05.