Allocation Patterns and Strategies of Carbon, Nitrogen, and Phosphorus Densities in Three Typical Desert Plants
Abstract
1. Introduction
2. Results
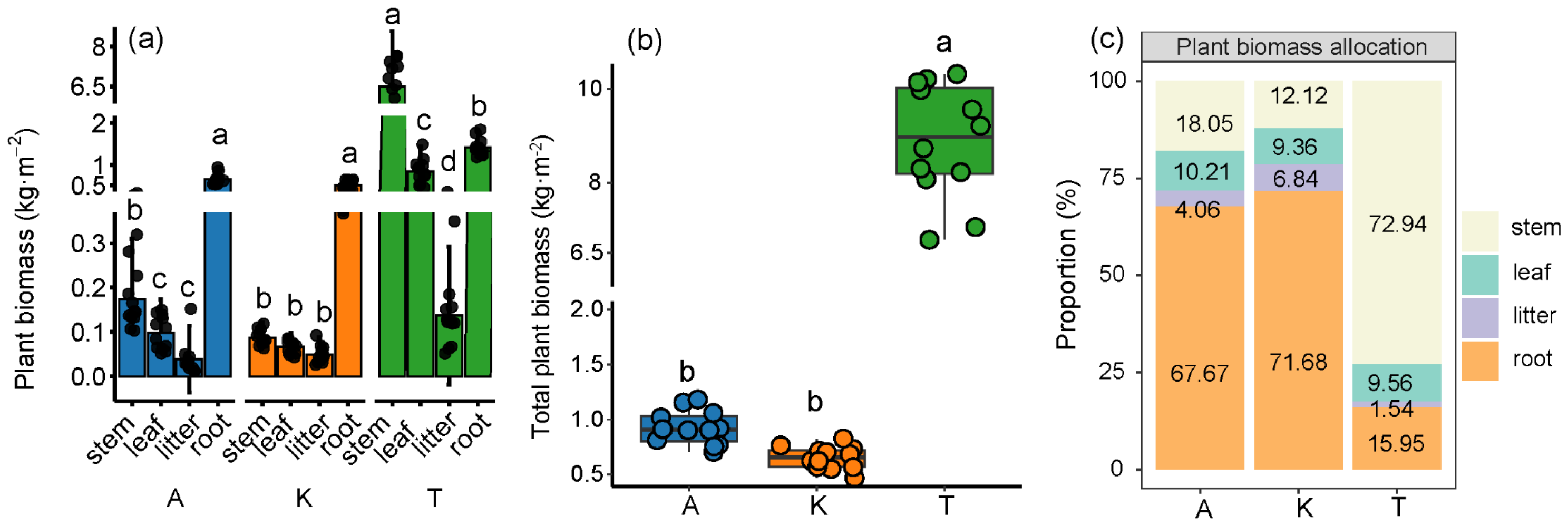
2.1. Allocation of Biomass in Different Plant Components
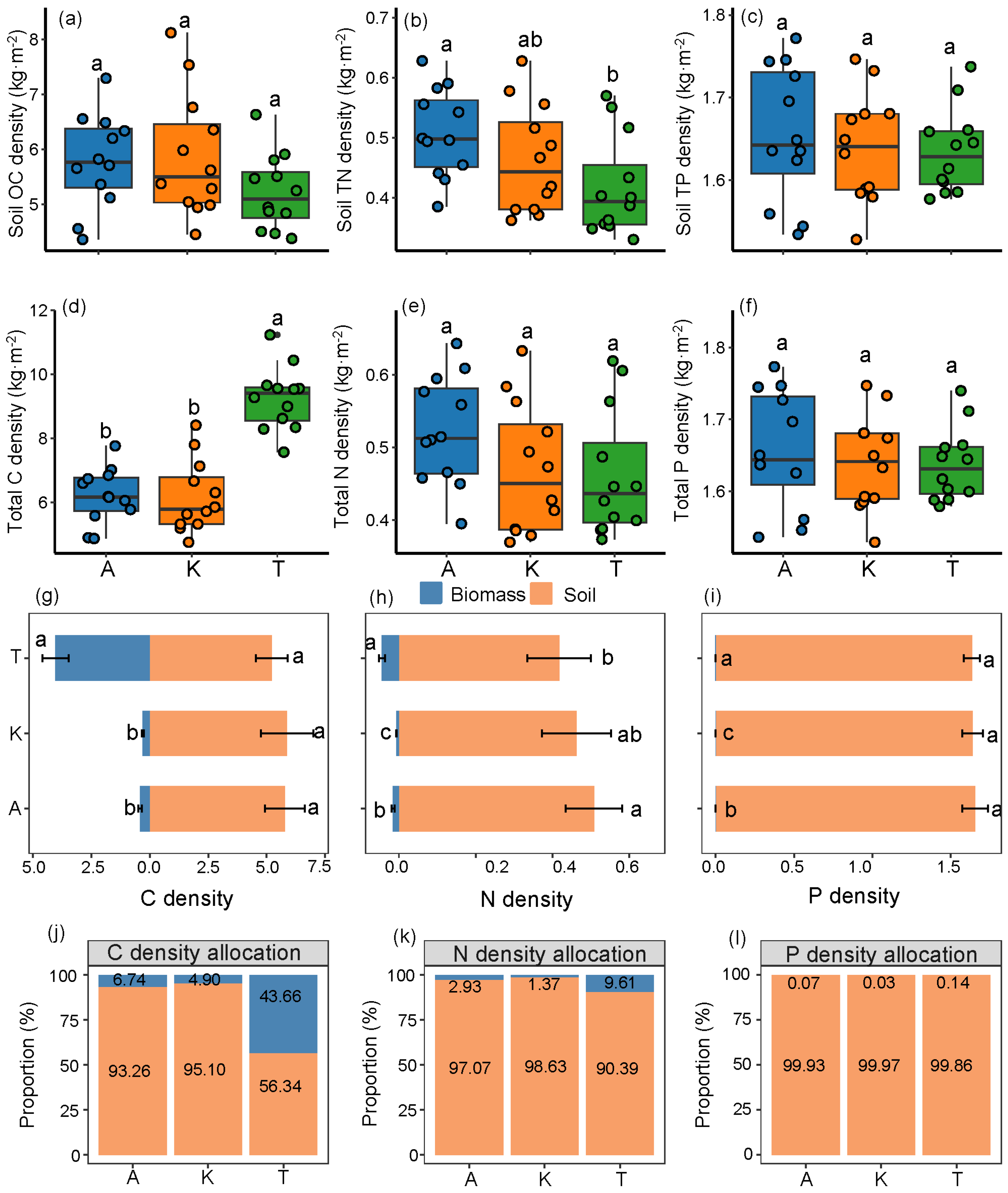
2.2. Carbon, Nitrogen, and Phosphorus Densities in Different Plant Components
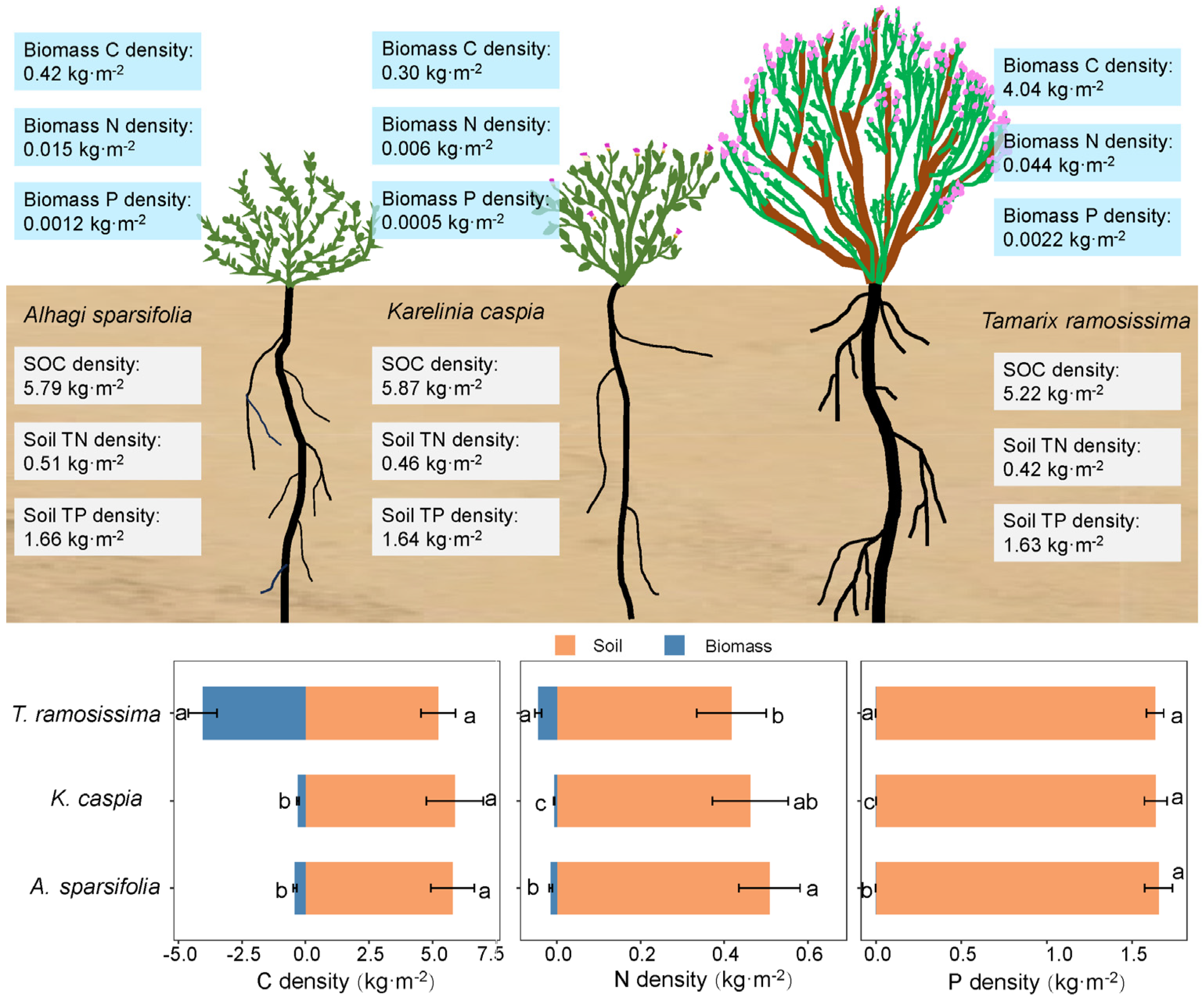
2.3. Distribution of Soil and Total C-N-P Densities
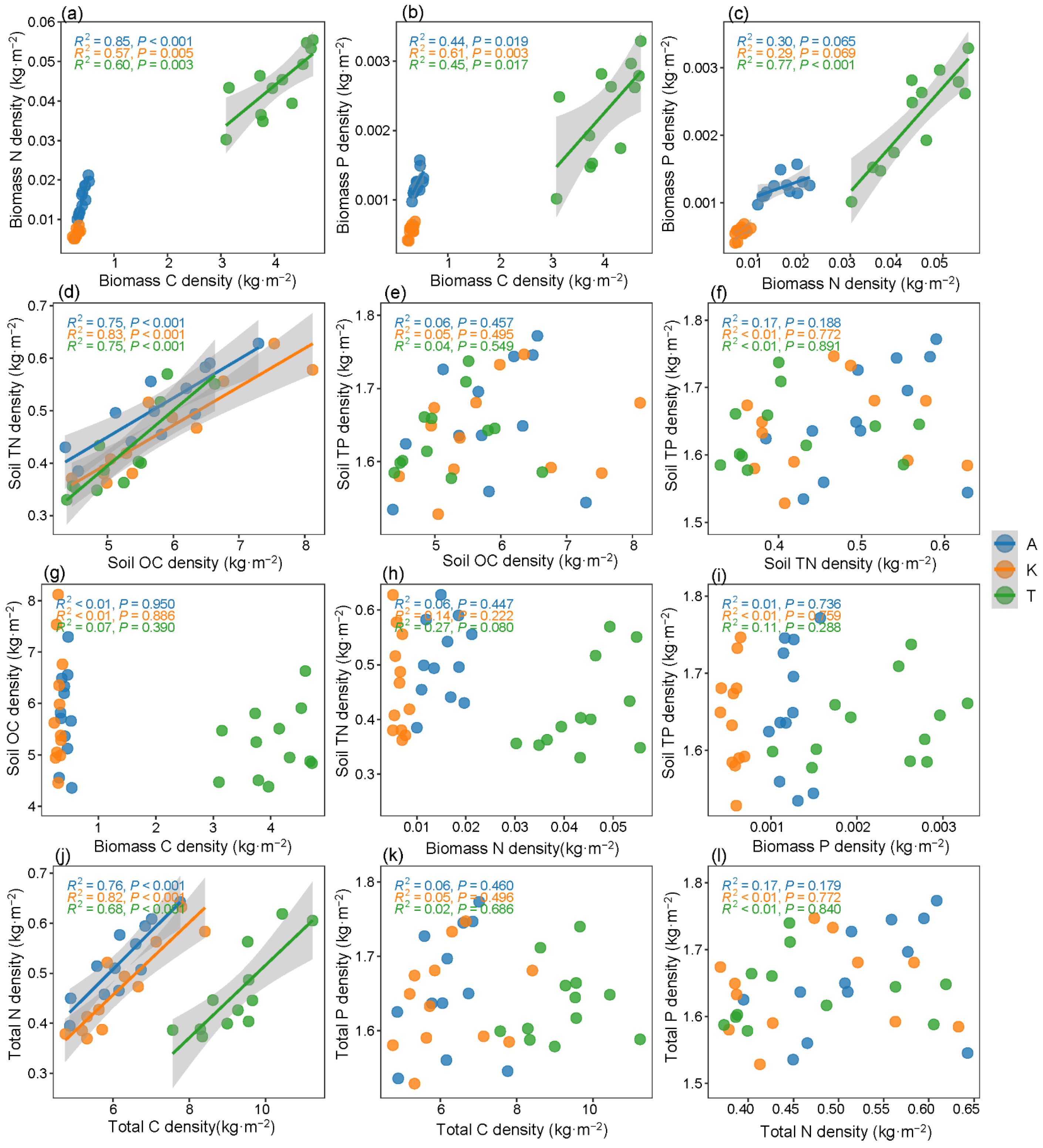
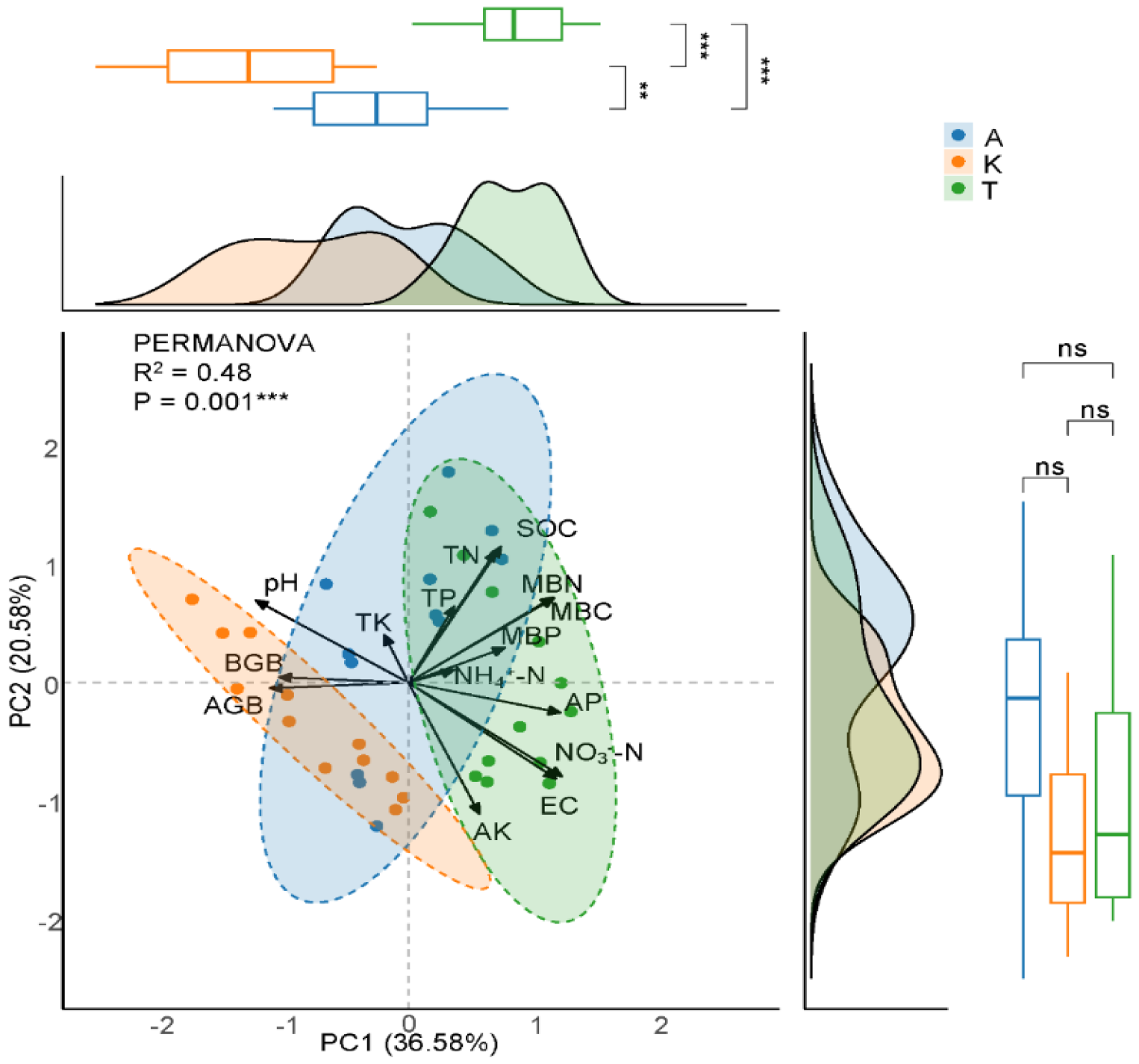
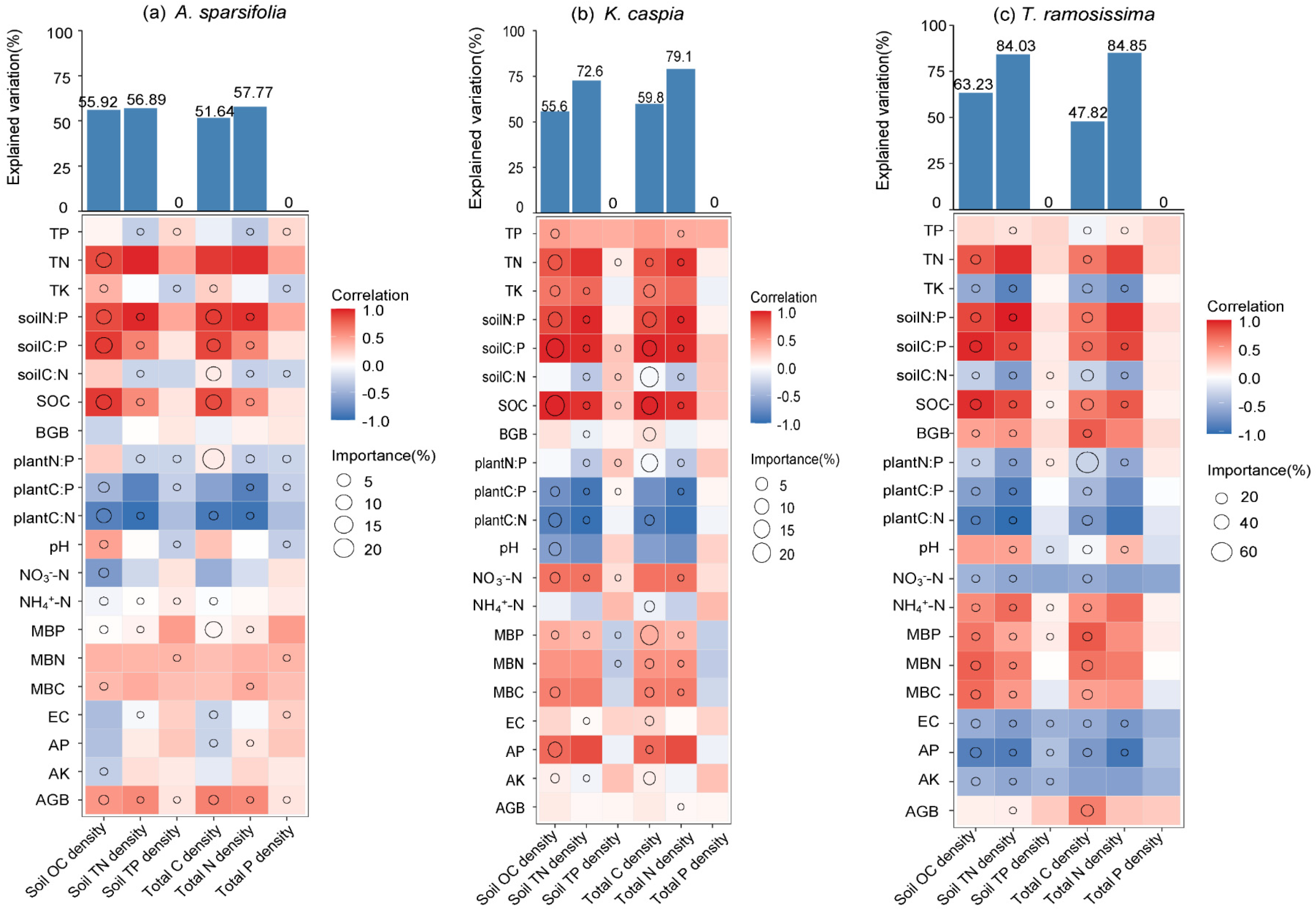
2.4. Correlation and Driving Factors of C-N-P Densities
3. Discussion
3.1. Allocation of C-N-P Densities Among Plants of Different Plant Life Forms
3.2. Allocation of Total C-N-P Densities Among Plants of Different Life Forms
3.3. Drivers of Total C-N-P Densities Allocation
4. Materials and Methods
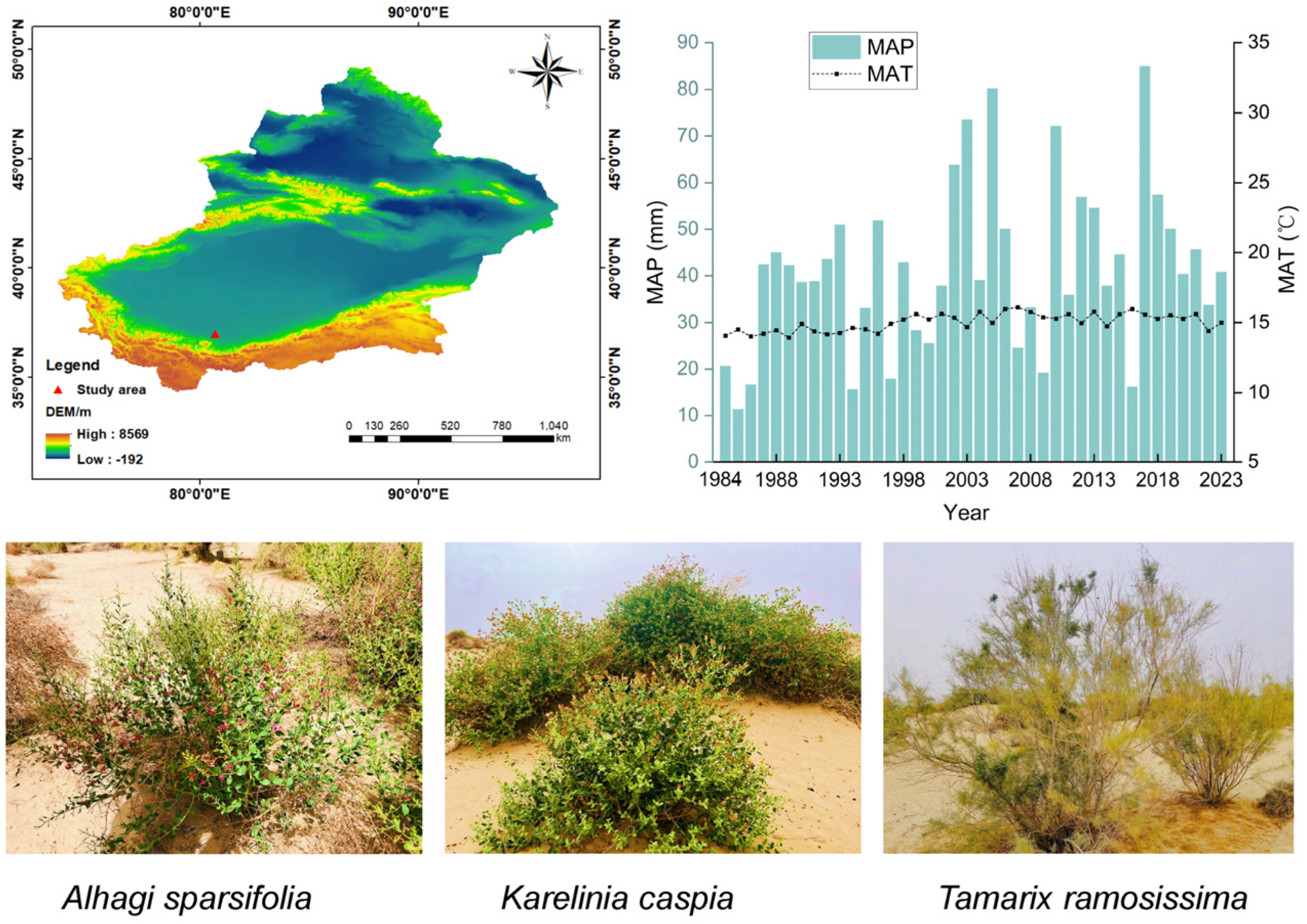
4.1. Study Area
4.2. Experimental Design and Samples Collocation
4.3. Plant C-N-P Analysis
4.4. Soil Physicochemical and Microbial Biomass Analysis
4.5. Data Calculations
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burrell, A.L.; Evans, J.P.; De Kauwe, M.G. Anthropogenic climate change has driven over 5 million km2 of drylands towards desertification. Nat. Commun. 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Graciano, C.; Sardans, J.; Zeng, F.J.; Hughes, A.C.; Ahmed, Z.; Ullah, A.; Ali, S.; Gao, Y.J.; Peñuelas, J. Plant root mechanisms and their effects on carbon and nutrient accumulation in desert ecosystems under changes in land use and climate. New Phytol. 2024, 242, 916–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Su, Y.Z.; Yang, R. Biomass and nutrient allocation strategies in a desert ecosystem in the Hexi Corridor, northwest China. J. Plant Res. 2017, 130, 699–708. [Google Scholar] [CrossRef]
- Jaman, M.S.; Wu, H.H.; Yu, Q.; Tan, Q.Q.; Zhang, Y.L.; Dam, Q.K.; Muraina, T.O.; Xu, C.; Jing, M.H.; Jia, X.T.; et al. Contrasting responses of plant above and belowground biomass carbon pools to extreme drought in six grasslands spanning an aridity gradient. Plant Soil 2022, 473, 167–180. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. The Role of Plants in the Effects of Global Change on Nutrient Availability and Stoichiometry in the Plant-Soil System. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef]
- Xu, J.; Lei, L.; Zeng, L.X.; Ni, Y.Y.; Jian, Z.J.; Deng, X.X.; Xiao, W.F. The Responses of C Allocation of New Needle and Fine Root Affected the Phosphorus Adaptation of Pinus massoniana Seedlings. J. Soil Sci. Plant Nutr. 2024, 24, 295–307. [Google Scholar] [CrossRef]
- Sardans, J.; Grau, O.; Chen, H.Y.H.; Janssens, I.A.; Ciais, P.; Piao, S.L.; Peñuelas, J. Changes in nutrient concentrations of leaves and roots in response to global change factors. Glob. Change Biol. 2017, 23, 3849–3856. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Zeng, Y.L.; Fang, X.; Xiang, W.H.; Deng, X.W.; Peng, C.H. Stoichiometric and nutrient resorption characteristics of dominant tree species in subtropical Chinese forests. Ecol. Evol. 2017, 7, 11033–11043. [Google Scholar] [CrossRef]
- Ren, C.J.; Chen, J.; Deng, J.; Zhao, F.Z.; Han, X.H.; Yang, G.H.; Tong, X.G.; Feng, Y.Z.; Shelton, S.; Ren, G.X. Response of microbial diversity to C:N:P stoichiometry in fine root and microbial biomass following afforestation. Biol. Fertil. Soils 2017, 53, 457–468. [Google Scholar] [CrossRef]
- Guo, Y.P.; Yan, Z.B.; Gheyret, G.; Zhou, G.Y.; Xie, Z.Q.; Tang, Z.Y. The community-level scaling relationship between leaf nitrogen and phosphorus changes with plant growth, climate and nutrient limitation. J. Ecol. 2020, 108, 1276–1286. [Google Scholar] [CrossRef]
- Yang, X.; Yan, X.H.; Guo, Q.; Ghanizadeh, H.; Li, M.H.; Tuo, H.H.; Wen, Z.M.; Li, W. Effects of Different Management Practices on Plant Community and Soil Properties in a Restored Grassland. J. Soil Sci. Plant Nutr. 2022, 22, 3811–3821. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Xu, W.T.; Zhou, G.Y.; Bai, Y.F.; Li, J.X.; Tang, X.L.; Chen, D.M.; Liu, Q.; Ma, W.H.; Xiong, G.M.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef]
- Fortunel, C.; Fine, P.V.A.; Baraloto, C. Leaf, stem and root tissue strategies across 758 Neotropical tree species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Zhang, J.H.; He, N.P.; Liu, C.C.; Xu, L.; Chen, Z.; Li, Y.; Wang, R.M.; Yu, G.R.; Sun, W.; Xiao, C.W.; et al. Variation and evolution of C:N ratio among different organs enable plants to adapt to N-limited environments. Glob. Change Biol. 2020, 26, 2534–2543. [Google Scholar] [CrossRef]
- Meng, H.H.; Yin, B.F.; Tao, Y.; Zhou, X.B.; Zang, Y.X.; Zhang, Y.M. Stoichiometric patterns of assimilative branches of four dominant shrubs and the drivers in a Central Asian desert. Environ. Exp. Bot. 2024, 219, 14. [Google Scholar] [CrossRef]
- Bai, W.M.; Zhou, M.; Fang, Y.; Zhang, W.H. Differences in spatial and temporal root lifespan of three Stipa grasslands in northern China. Biogeochemistry 2017, 132, 293–306. [Google Scholar] [CrossRef]
- Sardans, J.; Janssens, I.A.; Alonso, R.; Veresoglou, S.D.; Rillig, M.C.; Sanders, T.G.M.; Carnicer, J.; Filella, I.; Farré-Armengol, G.; Peñuelas, J. Foliar elemental composition of European forest tree species associated with evolutionary traits and present environmental and competitive conditions. Glob. Ecol. Biogeogr. 2015, 24, 240–255. [Google Scholar] [CrossRef]
- Poorter, L.; McDonald, I.; Alarcón, A.; Fichtler, E.; Licona, J.C.; Peña-Claros, M.; Sterck, F.; Villegas, Z.; Sass-Klaassen, U. The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol. 2010, 185, 481–492. [Google Scholar] [CrossRef]
- Khan, A.; Liu, X.D.; Waseem, M.; Qi, S.H.; Ghimire, S.; Hasan, M.M.; Fang, X.W. Divergent Nitrogen, Phosphorus, and Carbon Concentrations among Growth Forms, Plant Organs, and Soils across Three Different Desert Ecosystems. Forests 2024, 15, 607. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, Q.W.; Li, K.H.; Gong, Y.M.; Liu, Y.Y.; Han, W.X. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 8. [Google Scholar] [CrossRef]
- Xiao, L.; Bi, Y.L.; Du, S.Z.; Wang, Y.; Guo, C.; Christie, P. Response of ecological stoichiometry and stoichiometric homeostasis in the plant-litter-soil system to re-vegetation type in arid mining subsidence areas. J. Arid. Environ. 2021, 184, 9. [Google Scholar] [CrossRef]
- Imada, S.; Taniguchi, T.; Acharya, K.; Yamanaka, N. Vertical distribution of fine roots of Tamarix ramosissima in an arid region of southern Nevada. J. Arid. Environ. 2013, 92, 46–52. [Google Scholar] [CrossRef]
- Li, W.Q.; Shen, F.X.; Liu, Y.J.; Li, Z.W.; Jiang, J.F.; Li, Q.; Zheng, H.; Wang, X.; Wu, J. Soil depth determine the ecological stoichiometry of soil aggregates after returning ancient rice terraces to forest. Catena 2022, 219, 10. [Google Scholar] [CrossRef]
- Guo, Y.P.; Jiang, M.W.; Liu, Q.; Xie, Z.Q.; Tang, Z.Y. Climate and vegetation together control the vertical distribution of soil carbon, nitrogen and phosphorus in shrublands in China. Plant Soil 2020, 456, 15–26. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.Y.; Guo, A.X.; Yue, P.; Guo, X.X.; Lv, P.; Zhao, S.L.; Zuo, X.A. Species diversity is a strong predictor of ecosystem multifunctionality under altered precipitation in desert steppes. Ecol. Indic. 2022, 137, 10. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, A.X.; Li, X.Y.; Yue, P.; Zhao, S.L.; Lv, P.; Zuo, X.A. Multi-trait functional diversity predicts ecosystem multifunctionality under nitrogen addition in a desert steppe. Plant Soil 2023, 491, 33–44. [Google Scholar] [CrossRef]
- Dibar, D.T.; Zhang, K.; Yuan, S.Q.; Zhang, J.Y.; Zhou, Z.Z.; Ye, X.X. Ecological stoichiometric characteristics of Carbon (C), Nitrogen (N) and Phosphorus (P) in leaf, root, stem, and soil in four wetland plants communities in Shengjin Lake, China. PLoS ONE 2020, 15, 18. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; He, X.Z.; Hou, F.J. Different effects of sheep excrement type and supply level on plant and soil C:N:P stoichiometry in a typical steppe on the loess plateau. Plant Soil 2021, 462, 45–58. [Google Scholar] [CrossRef]
- Yan, Y.; Tian, L.L.; Du, Z.Y.; Chang, S.X.; Cai, Y.J. Carbon, nitrogen and phosphorus stocks differ among vegetation patch types in a degraded alpine steppe. J. Soils Sediments 2019, 19, 1809–1819. [Google Scholar] [CrossRef]
- Wang, W.C.; Peng, Y.Y.; Chen, Y.Z.; Lei, S.L.; Wang, X.J.; Farooq, T.H.; Liang, X.C.; Zhang, C.; Yan, W.D.; Chen, X.Y. Ecological Stoichiometry and Stock Distribution of C, N, and P in Three Forest Types in a Karst Region of China. Plants 2023, 12, 2503. [Google Scholar] [CrossRef]
- Wu, H.F.; Hu, B.A.; Ma, Y.; Shi, W.K.; Cheng, X.Q.; Kang, F.F.; Han, H.R. Vegetation C-N-P accumulation and allocation patterns at the community level in early restored plantations in the loess hilly-gully region. For. Ecosyst. 2023, 10, 100132. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.R.; Wang, Q.F.; Wang, R.L.; Zhang, J.H.; Liu, C.C.; He, N.P. Conservative allocation strategy of multiple nutrients among major plant organs: From species to community. J. Ecol. 2020, 108, 267–278. [Google Scholar] [CrossRef]
- Ma, X.Z.; Wang, X.P. Biomass partitioning and allometric relations of the Reaumuria soongorica shrub in Alxa steppe desert in NW China. For. Ecol. Manag. 2020, 468, 10. [Google Scholar] [CrossRef]
- Liu, H.Y.; Lin, L.; Wang, H.; Zhang, Z.H.; Shangguan, Z.J.; Feng, X.J.; He, J.S. Simulating warmer and drier climate increases root production but decreases root decomposition in an alpine grassland on the Tibetan plateau. Plant Soil 2021, 458, 59–73. [Google Scholar] [CrossRef]
- Peng, Y.F.; Yang, Y.H. Allometric biomass partitioning under nitrogen enrichment: Evidence from manipulative experiments around the world. Sci. Rep. 2016, 6, 7. [Google Scholar] [CrossRef]
- Yan, Z.B.; Li, P.; Chen, Y.H.; Han, W.X.; Fang, J.Y. Nutrient allocation strategies of woody plants: An approach from the scaling of nitrogen and phosphorus between twig stems and leaves. Sci. Rep. 2016, 6, 9. [Google Scholar] [CrossRef]
- Yang, Z.J.; Midmore, D.J. Modelling plant resource allocation and growth partitioning in response to environmental heterogeneity. Ecol. Model. 2005, 181, 59–77. [Google Scholar] [CrossRef]
- Liu, J.G.; Gou, X.H.; Zhang, F.; Bian, R.; Yin, D.C. Spatial patterns in the C:N:P stoichiometry in Qinghai spruce and the soil across the Qilian Mountains, China. Catena 2021, 196, 10. [Google Scholar] [CrossRef]
- Zhang, J.H.; He, N.P.; Liu, C.C.; Xu, L.; Yu, Q.; Yu, G.R. Allocation strategies for nitrogen and phosphorus in forest plants. Oikos 2018, 127, 1506–1514. [Google Scholar] [CrossRef]
- Tuo, D.F.; Gao, G.Y.; Chang, R.Y.; Li, Z.S.; Ma, Y.; Wang, S.; Wang, C.; Fu, B.J. Effects of revegetation and precipitation gradient on soil carbon and nitrogen variations in deep profiles on the Loess Plateau of China. Sci. Total Environ. 2018, 626, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.T.; Zhao, H.L.; Zhao, X.Y.; Drake, S. Facilitative effects of shrubs in shifting sand on soil macro-faunal community in Horgin Sand Land of Inner Mongolia, Northern China. Eur. J. Soil Biol. 2011, 47, 316–321. [Google Scholar] [CrossRef]
- She, W.W.; Bai, Y.X.; Zhang, Y.Q.; Qin, S.G.; Liu, Z.; Wu, B. Plasticity in Meristem Allocation as an Adaptive Strategy of a Desert Shrub under Contrasting Environments. Front. Plant Sci. 2017, 8, 9. [Google Scholar] [CrossRef]
- Yao, X.Y.; Hui, D.F.; Xing, S.; Zhang, Q.C.; Chen, J.W.; Li, Z.H.; Xu, Y.; Deng, Q. Mixed plantations with N-fixing tree species maintain ecosystem C:N:P stoichiometry: Implication for sustainable production. Soil Biol. Biochem. 2024, 191, 10. [Google Scholar] [CrossRef]
- Dissanayaka, D.; Maruyama, H.; Masuda, G.; Wasaki, J. Interspecific facilitation of P acquisition in intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil 2015, 390, 223–236. [Google Scholar] [CrossRef]
- Gong, Y.M.; Hu, Y.K.; Fang, F.; Liu, Y.Y.; Li, K.H.; Zhang, G.M. Carbon storage and vertical distribution in three shrubland communities in Gurbantunggut Desert, Uygur Autonomous Region of Xinjiang, Northwest China. Chin. Geogr. Sci. 2012, 22, 541–549. [Google Scholar] [CrossRef]
- Hasibeder, R.; Fuchslueger, L.; Richter, A.; Bahn, M. Summer drought alters carbon allocation to roots and root respiration in mountain grassland. New Phytol. 2015, 205, 1117–1127. [Google Scholar] [CrossRef]
- Perez-Quezada, J.F.; Pérez, C.A.; Brito, C.E.; Fuentes, J.P.; Gaxiola, A.; Aguilera-Riquelme, D.; Lopatin, J. Biotic and abiotic drivers of carbon, nitrogen and phosphorus stocks in a temperate rainforest. For. Ecol. Manag. 2021, 494, 14. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Lu, Y.; Guo, H.; Guo, X.; Zhou, X.; Zhang, Y. C:N:P stoichiometric characteristics and drivers of surface soil in the drylands of China. Environ. Earth Sci. 2024, 83, 270. [Google Scholar] [CrossRef]
- Nianpeng, H.E.; Xingguo, H.A.N.; Guirui, Y.U. Carbon and nitrogen sequestration rate in long-term fenced grasslands in Inner Mongolia, China. Acta Ecol. Sin. 2011, 31, 4270–4276. [Google Scholar]
- Liu, S.S.; Shen, H.H.; Zhao, X.; Zhou, L.H.; Li, H.; Xu, L.C.; Xing, A.J.; Fang, J.Y. Estimation of plot-level soil carbon stocks in China’s forests using intensive soil sampling. Geoderma 2019, 348, 107–114. [Google Scholar] [CrossRef]
- Li, J.W.; Wang, K.B.; Shangguan, Z.P.; Deng, L. Coupling and decoupling of soil carbon, nitrogen and phosphorus stocks following grazing exclusion in temperate grasslands. Catena 2022, 211, 10. [Google Scholar] [CrossRef]
- Yang, J.; Wu, F.Z.; Ni, X.Y.; Yue, K.; Wei, X.Y.; Zhang, X.Y.; Zhang, X.Y. Responses of Soil Nitrogen Pools to Litter Input Under Nitrogen Addition: A Meta-Analysis. J. Geophys. Res.-Biogeosci. 2024, 129, 12. [Google Scholar] [CrossRef]
- Gao, Y.J.; Zeng, F.J. Patterns of carbon, nitrogen, and phosphorus stoichiometry of three life-form desert plants and responses to soil and microbial biomass factors in a hyper-arid desert ecosystem. Environ. Sci. Pollut. Res. 2023, 30, 43962–43974. [Google Scholar] [CrossRef]
- Li, J.W.; Liu, Y.L.; Hai, X.Y.; Shangguan, Z.P.; Deng, L. Dynamics of soil microbial C:N:P stoichiometry and its driving mechanisms following natural vegetation restoration after farmland abandonment. Sci. Total Environ. 2019, 693, 11. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, Z.; Liu, Y.; Wu, G.L. Carbon accumulation by Pinus sylvestris forest plantations after different periods of afforestation in a semiarid sandy ecosystem. Land Degrad. Dev. 2021, 32, 2094–2104. [Google Scholar] [CrossRef]
- Song, C.; Zeng, X.Y.; Duan, P.; Li, Y.X.; Shan, R.Q.J.; Yang, P.; Shi, G.X.; Zhou, H.K.; Wang, F.P.; Yao, B.Q. Different impacts of artificial thicketization of Caragana korshinskii and Hippophae rhamnoides on soil organic carbon in alpine grasslands of the Qinghai-Tibetan Plateau. Restor. Ecol. 2024, 32, 10. [Google Scholar] [CrossRef]
- Gao, X.L.; Liu, X.Q.; Ma, L.N.; Wang, R.Z. Root vertical distributions of two Artemisia species and their relationships with soil resources in the Hunshandake desert, China. Ecol. Evol. 2020, 10, 3112–3119. [Google Scholar] [CrossRef]
- Ma, L.L.; Bai, S.H.; Chen, J.; Lucas-Borja, M.E.; Yue, C.; Deng, L.; Zheng, Y.X.; Bell, S.M.; Hu, Z.H. Tree species identity drives the vertical distribution of soil carbon and nutrient concentrations in the Loess Plateau, China. Plant Soil 2024, 501, 89–105. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.M.; Qu, M.J.; Shao, S.; Li, J.W. Soil fungal guilds as important integrators linking plant richness and carbon, nitrogen and phosphorus stocks in oasis-desert ecosystems. Soil Biol. Biochem. 2023, 177, 11. [Google Scholar] [CrossRef]
- Yang, D.X.; Song, L.; Jin, G.Z. The soil C:N:P stoichiometry is more sensitive than the leaf C:N:P stoichiometry to nitrogen addition: A four-year nitrogen addition experiment in a Pinus koraiensis plantation. Plant Soil 2019, 442, 183–198. [Google Scholar] [CrossRef]
- Wang, S.Q.; Yu, G.R. Ecological stoichiometry characteristics of ecosystem carbon, nitrogen and phosphorus elements. Acta Ecol. Sin. 2008, 28, 3937–3947. [Google Scholar]
- Luo, G.W.; Xue, C.; Jiang, Q.H.; Xiao, Y.; Zhang, F.G.; Guo, S.W.; Shen, Q.R.; Ling, N. Soil Carbon, Nitrogen, and Phosphorus Cycling Microbial Populations and Their Resistance to Global Change Depend on Soil C:N:P Stoichiometry. mSystems 2020, 5, 15. [Google Scholar] [CrossRef]
- Li, J.Q.; Nie, M.; Pendall, E. Soil physico-chemical properties are more important than microbial diversity and enzyme activity in controlling carbon and nitrogen stocks near Sydney, Australia. Geoderma 2020, 366, 9. [Google Scholar] [CrossRef]
- Lu, J.N.; Feng, S.; Wang, S.K.; Zhang, B.L.; Ning, Z.Y.; Wang, R.X.; Chen, X.P.; Yu, L.L.; Zhao, H.S.; Lan, D.M.; et al. Patterns and driving mechanism of soil organic carbon, nitrogen, and phosphorus stoichiometry across northern China’s desert-grassland transition zone. Catena 2023, 220, 11. [Google Scholar] [CrossRef]
- Chen, L.L.; Wang, K.X.; Baoyin, T. Effects of grazing and mowing on vertical distribution of soil nutrients and their stoichiometry (C: N: P) in a semi-arid grassland of North China. Catena 2021, 206, 8. [Google Scholar] [CrossRef]
- Yang, H.T.; Wang, Z.R.; Li, X.J.; Gao, Y.H. Vegetation restoration drives the dynamics and distribution of nitrogen and phosphorous pools in a temperate desert soil-plant system. J. Environ. Manag. 2019, 245, 200–209. [Google Scholar] [CrossRef]
- Huang, L.; Hu, H.; Bao, W.K.; Hu, B.; Liu, J.; Li, F.L. Shifting soil nutrient stoichiometry with soil of variable rock fragment contents and different vegetation types. Catena 2023, 220, 10. [Google Scholar] [CrossRef]
- Wang, X.G.; Lü, X.T.; Han, X.G. Responses of nutrient concentrations and stoichiometry of senesced leaves in dominant plants to nitrogen addition and prescribed burning in a temperate steppe. Ecol. Eng. 2014, 70, 154–161. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Zhong, Q.L.; Cheng, D.L.; Huang, Z.Q.; Xu, C.B.; Yu, H.; Xiao, S.H. Carbon dynamics in three subtropical forest ecosystems in China. Environ. Sci. Pollut. Res. 2020, 27, 15552–15564. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.P.; Song, Z.L.; Van Zwieten, L.; Guo, L.D.; Yu, C.X.; Wang, W.Q.; Li, Q.; Hartley, I.P.; Yang, Y.H.; Liu, H.Y.; et al. Storage, patterns and influencing factors for soil organic carbon in coastal wetlands of China. Glob. Change Biol. 2022, 28, 6065–6085. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Tariq, A.; Mu, Z.; Zhang, Z.; Graciano, C.; Cong, M.; Dong, X.; Sardans, J.; Al-Bakre, D.A.; Penuelas, J.; et al. Allocation Patterns and Strategies of Carbon, Nitrogen, and Phosphorus Densities in Three Typical Desert Plants. Plants 2025, 14, 1595. https://doi.org/10.3390/plants14111595
Zhao G, Tariq A, Mu Z, Zhang Z, Graciano C, Cong M, Dong X, Sardans J, Al-Bakre DA, Penuelas J, et al. Allocation Patterns and Strategies of Carbon, Nitrogen, and Phosphorus Densities in Three Typical Desert Plants. Plants. 2025; 14(11):1595. https://doi.org/10.3390/plants14111595
Chicago/Turabian StyleZhao, Guangxing, Akash Tariq, Zhaobin Mu, Zhihao Zhang, Corina Graciano, Mengfei Cong, Xinping Dong, Jordi Sardans, Dhafer A. Al-Bakre, Josep Penuelas, and et al. 2025. "Allocation Patterns and Strategies of Carbon, Nitrogen, and Phosphorus Densities in Three Typical Desert Plants" Plants 14, no. 11: 1595. https://doi.org/10.3390/plants14111595
APA StyleZhao, G., Tariq, A., Mu, Z., Zhang, Z., Graciano, C., Cong, M., Dong, X., Sardans, J., Al-Bakre, D. A., Penuelas, J., & Zeng, F. (2025). Allocation Patterns and Strategies of Carbon, Nitrogen, and Phosphorus Densities in Three Typical Desert Plants. Plants, 14(11), 1595. https://doi.org/10.3390/plants14111595






