Evaluation of Saponin-Rich Callus from Saponaria officinalis L. as a Novel Scrub Material with Significant Exfoliating and Anti-Inflammatory Effects
Abstract
1. Introduction
2. Results
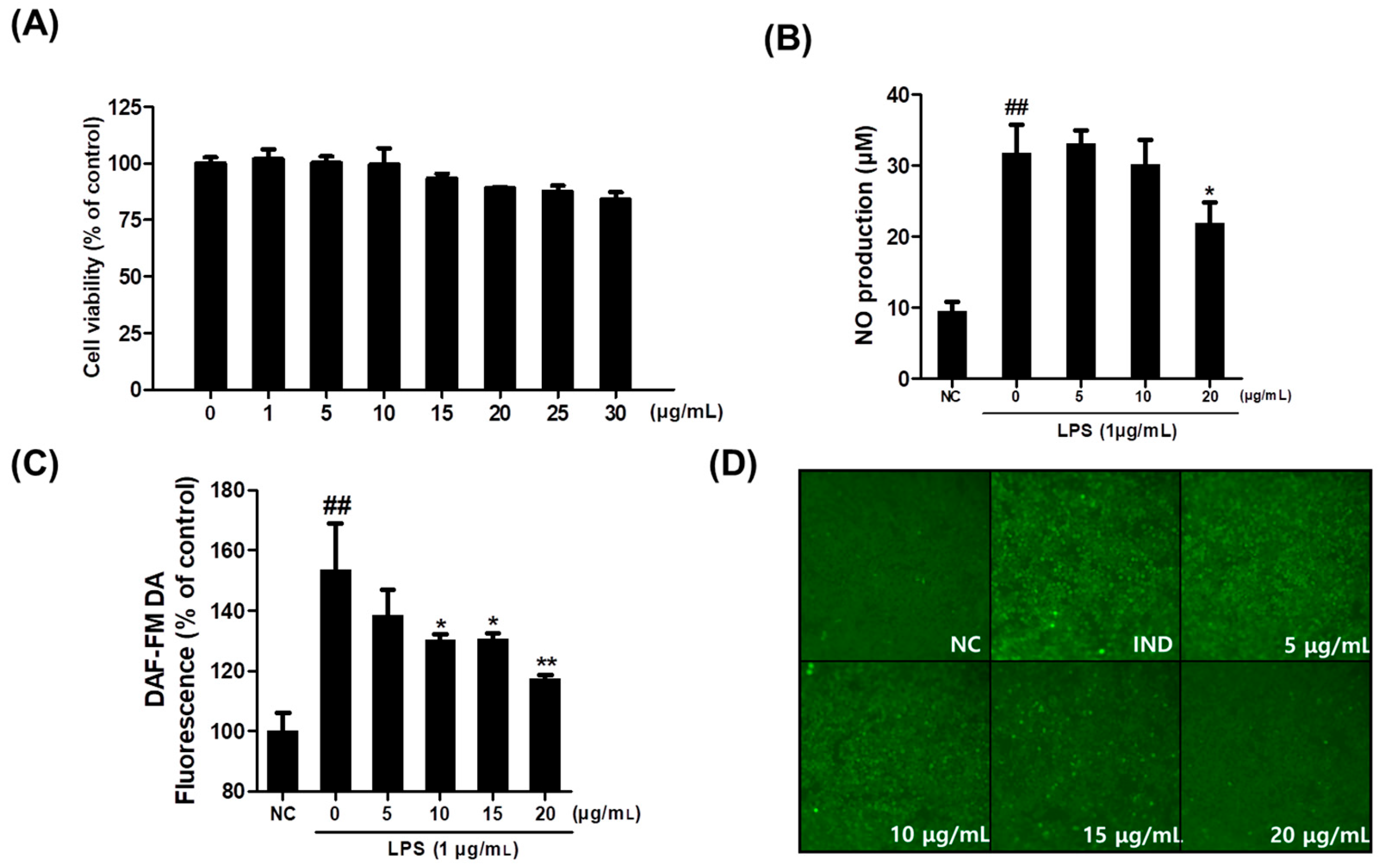
2.1. Effect of Saponaria officinalis L. Callus Extract (SCE) on RAW264.7 Cell Viability
2.2. Effect of Saponaria officinalis L. Callus Extract (SCE) on Intracellular or Extracellular NO Levels
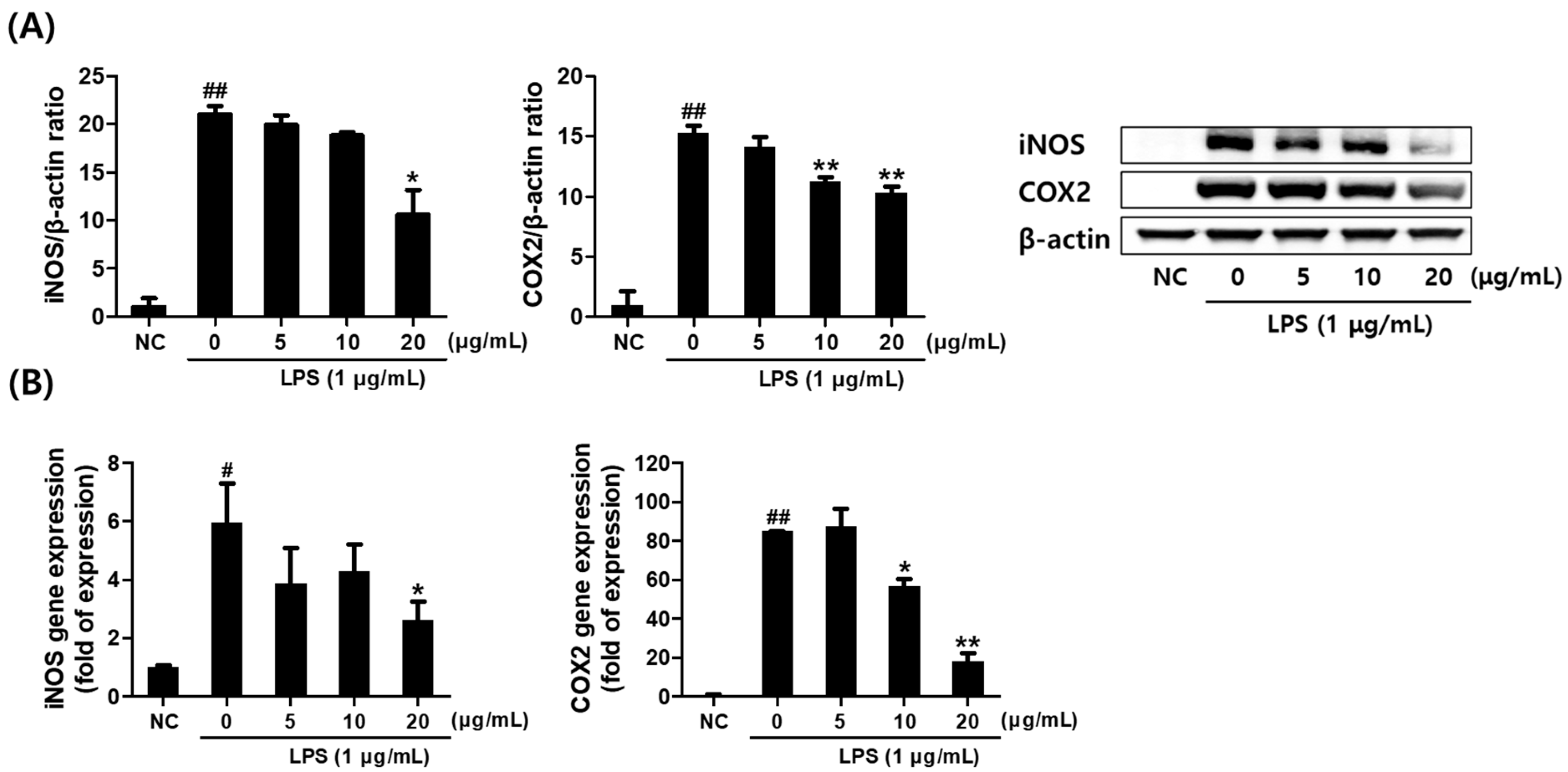
2.3. Effect of Saponaria officinalis L. Callus Extract (SCE) on the Inflammatory Mediator Levels in LPS-Stimulated RAW264.7 Cells
2.4. Effect of Saponaria officinalis L. Callus Extract (SCE) on the Pro-Inflammatory Cytokine Levels in LPS-Stimulated RAW264.7 Cells
2.5. Profiling and Identification of Major Compounds in Callus Extract by High-Performance Liquid Chromatography (HPLC)
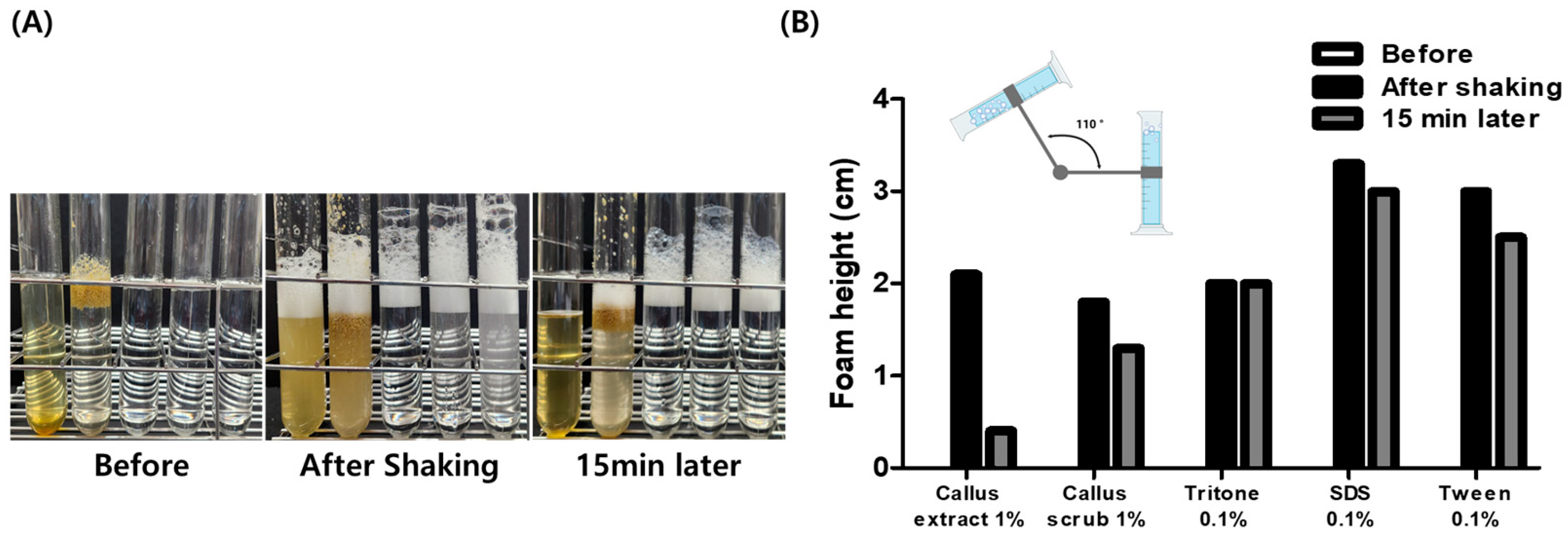
2.6. Foaming Ability and Stability Test of Callus Extract and Scrub by Bartsch Test
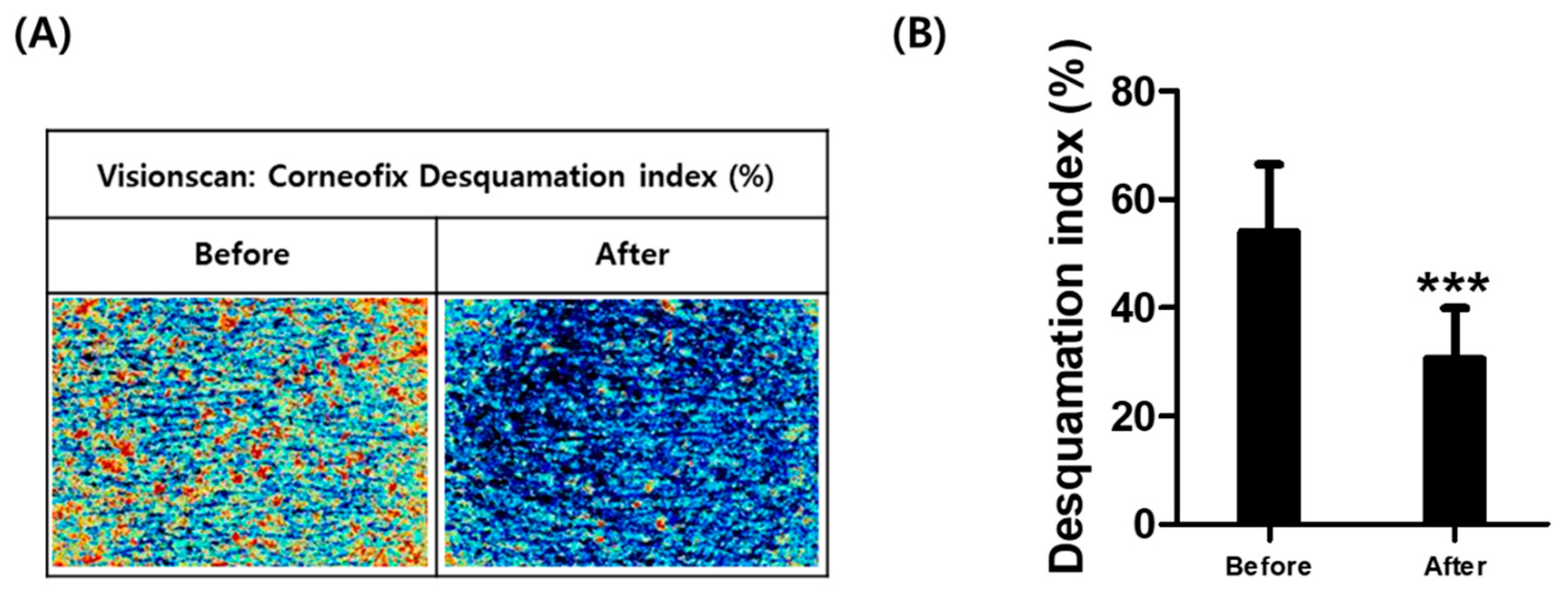
2.7. Clinical Tests of Saponaria officinalis L. Callus (SC) Scrub for Skin Irritability and Exfoliatiory Effect
3. Discussion
4. Materials and Methods
4.1. Chemicals
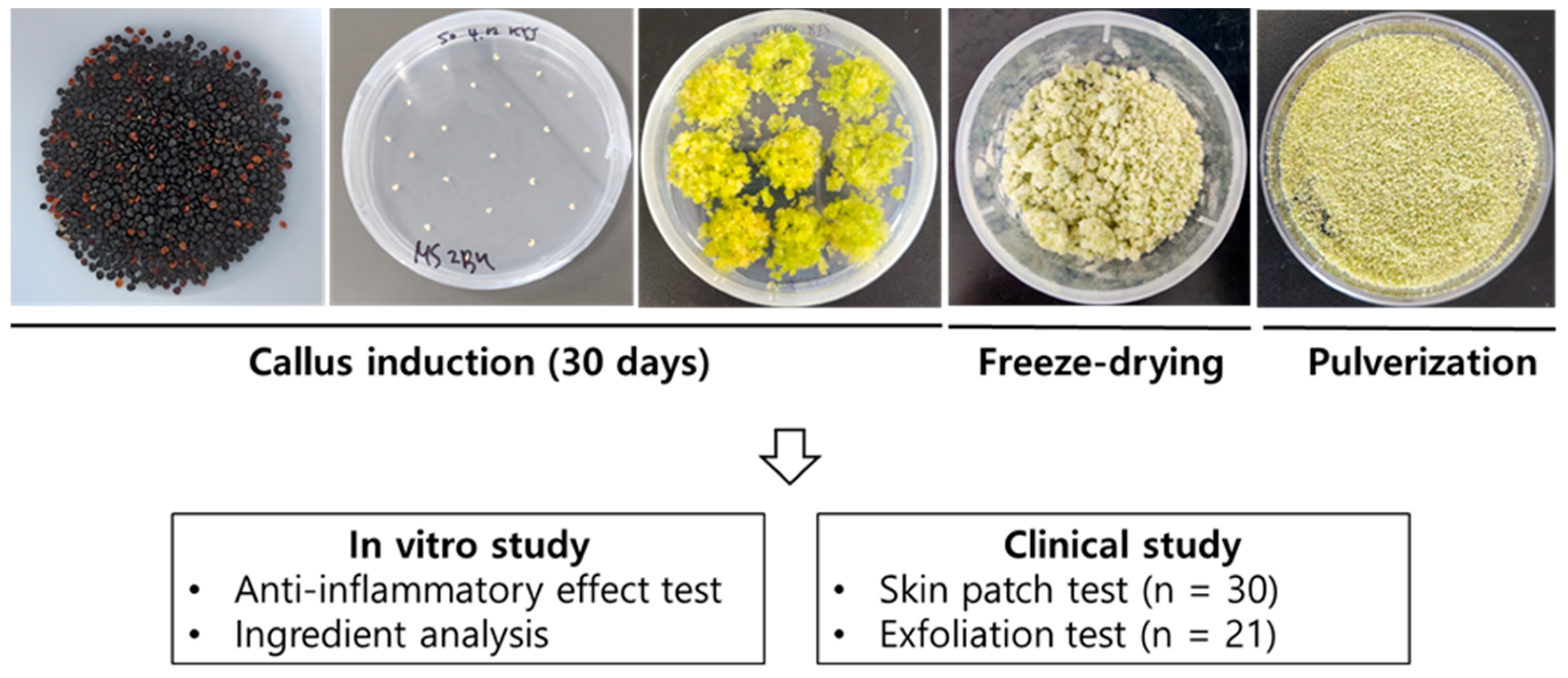
4.2. Seed Sterilization and Callus Induction
4.3. Preparation of Callus Induction Medium
4.4. Preparation of Callus Scrub and Extract
4.5. Cell Culture and Treatment
4.6. Cell Viability Assay
4.7. Measurement of Extracellular and Intracellular NO Levels
4.8. Western Blotting
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. ELISA Analysis
4.11. HPLC Fingerprinting Analysis
4.12. Foaming Ability and Stability Studies
4.13. Skin Irritation Test
4.14. Exfoliation Test
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCO | Stratum corneum |
| UV | Ultraviolet |
| TEWL | Transepidermal water loss |
| SC | Saponaria officinalis callus |
| SCE | Saponaria officinalis callus extract |
| LPS | Lipopolysaccharide |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| MS | Murashige and Skoog |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| PPFD | Photosynthetic Photon Flux Density |
| DMSO | Dimethyl sulfoxide |
| KCLB | Korea Cell Line Bank |
| OD | Optical density |
| DPBS | Dulbecco’s phosphate-buffered saline |
| RIPA | Radioimmunoprecipitation assay |
| BCA | Bicinchoninic acid |
| BSA | Bovine serum albumin |
| HRP | Horseradish peroxidase |
| SDS | Sodium dodecyl sulfate |
| ICDRG | International Contact Dermatitis Research Group |
| DI | Desquamation index |
| ANOVA | One-way analysis of variance |
| SD | Standard deviation |
| IRB | Institutional Review Board |
| HPLC | High-performance liquid chromatography |
References
- Fukuda, K.; Ito, Y.; Furuichi, Y.; Matsui, T.; Horikawa, H.; Miyano, T.; Okada, T.; van Logtestijn, M.; Tanaka, R.J.; Miyawaki, A. Three stepwise pH progressions in stratum corneum for homeostatic maintenance of the skin. Nat. Commun. 2024, 15, 4062. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K. New method of measurement of epidermal turnover in humans. Cosmetics 2017, 4, 47. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Jiao, Q.; Yue, L.; Zhi, L.; Qi, Y.; Yang, J.; Zhou, C.; Jia, Y. Studies on stratum corneum metabolism: Function, molecular mechanism and influencing factors. J. Cosmet. Dermatol. 2022, 21, 3256–3264. [Google Scholar] [CrossRef]
- Carlomagno, F.; Roveda, G.; Michelotti, A.; Ruggeri, F.; Tursi, F. Anti-skin-aging effect of a treatment with a cosmetic product and a food supplement based on a new hyaluronan: A randomized clinical study in healthy women. Cosmetics 2022, 9, 54. [Google Scholar] [CrossRef]
- Andriana, N.; Sitorus, M.L.; Nurhaliza, J.; Ulzannah, N. Antioxidant-Rich Pedada Fruit Scrub Innovation (Natural Beauty Secrets From The Coast). Proceeding Int. Conf. Bus. Econ. 2024, 2, 330–339. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef]
- Yang, G.; Seok, J.K.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci. 2020, 21, 2867. [Google Scholar] [CrossRef]
- Ahn, J.-Y.; Lim, D.-W.; Park, S.Y.; Lee, J.-H. Sinhyotaklisan alleviates inflammation in LPS-activated macrophages by modulating the heme oxygenase pathway. J. Ethnopharmacol. 2025, 344, 119548. [Google Scholar] [CrossRef]
- Hänel, K.H.; Cornelissen, C.; Lüscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Kobayashi, T.; Naik, S.; Nagao, K. Choreographing immunity in the skin epithelial barrier. Immunity 2019, 50, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Packianathan, N.; Kandasamy, R. Skin care with herbal exfoliants. Funct. Plant Sci. Biotechnol. 2011, 5, 94–97. [Google Scholar]
- Salem, Y.; Rajha, H.N.; Sunoqrot, S.; Hammad, A.M.; Castangia, I.; Manconi, M.; Manca, M.L.; Al Lababidi, D.; Touma, J.A.; Maroun, R.G. Exhausted grape seed residues as a valuable source of antioxidant molecules for the formulation of biocompatible cosmetic scrubs. Molecules 2023, 28, 5049. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Bikiaris, N.; Nikolaidis, N.F.; Barmpalexis, P. Microplastics (MPs) in Cosmetics: A Review on Their Presence in Personal-Care, Cosmetic, and Cleaning Products (PCCPs) and Sustainable Alternatives from Biobased and Biodegradable Polymers. Cosmetics 2024, 11, 145. [Google Scholar] [CrossRef]
- Ogorzałek, M.; Klimaszewska, E.; Małysa, A.; Czerwonka, D.; Tomasiuk, R. Research on Waterless Cosmetics in the Form of Scrub Bars Based on Natural Exfoliants. Appl. Sci. 2024, 14, 11329. [Google Scholar] [CrossRef]
- Misra, V.; Shrivastava, A. Expanding Horizon of Sugar Application: Skin Care and Cosmetics. Sugar Sugar Deriv. Chang. Consum. Prefer. 2020, 195–205. [Google Scholar]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Sák, M.; Dokupilová, I.; Mihálik, D.; Lakatošová, J.; Gubišová, M.; Kraic, J. Elicitation phenolic compounds in cell culture of Vitis vinifera L. by Phaeomoniella chlamydospora. Nova Biotechnol. Chim. 2014, 13, 162–171. [Google Scholar] [CrossRef][Green Version]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. Biotechnol. Hairy Root systems 2013, 134, 55–89. [Google Scholar]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Yu, G.-R.; Kim, D.-H.; Kim, H.; Lim, D.-W. Evaluation of Cannabis sativa L. Callus Extract as a Novel Cosmetic Ingredient with Dual Anti-Inflammatory and Antioxidant Effects. Plants 2025, 14, 1148. [Google Scholar] [CrossRef]
- Chandra, S.; Rawat, D.S.; Bhatt, A. Phytochemistry and pharmacological activities of Saponaria officinalis L.: A review. Not. Sci. Biol. 2021, 13, 10809. [Google Scholar] [CrossRef]
- Jurek, I.; Szuplewska, A.; Chudy, M.; Wojciechowski, K. Soapwort (Saponaria officinalis L.) Extract vs. Synthetic Surfactants—Effect on Skin-Mimetic Models. Molecules 2021, 26, 5628. [Google Scholar] [CrossRef]
- Charalambous, D.; Christoforou, M.; Christou, K.; Christou, M.; Ververis, A.; Andreou, M.; Christodoulou, K.; Koutsoulidou, A.; Papachrysostomou, C.; Pantelidou, M. Saponin and Phenolic Composition and Assessment of Biological Activities of Saponaria officinalis L. Root Extracts. Plants 2024, 13, 1982. [Google Scholar] [CrossRef]
- Ashurova, L.; Khurramov, A.; Bobakulov, K.M.; Akramov, D.K.; Ramazonov, N.S.; Ashirov, O.; Sasmakov, S.; Azimova, S.S.; Abdullaev, N. Structure of a New Saponin from Saponaria officinalis growing in Uzbekistan. Chem. Nat. Compd. 2023, 59, 323–326. [Google Scholar] [CrossRef]
- Min, S.-Y.; Park, C.-H.; Yu, H.-W.; Park, Y.-J. Anti-inflammatory and anti-allergic effects of saponarin and its impact on signaling pathways of RAW 264.7, RBL-2H3, and HaCaT Cells. Int. J. Mol. Sci. 2021, 22, 8431. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Krasteva, I.; Zdraveva, P.; Konstantinov, S.; Ionkova, I. Antidiabetic and antioxidant effects of saponarin from Gypsophila trichotoma on streptozotocin-induced diabetic normotensive and hypertensive rats. Phytomedicine 2016, 23, 483–490. [Google Scholar] [CrossRef]
- Yu, G.R.; Lim, D.W.; Karunarathne, W.A.H.M.; Kim, G.Y.; Kim, H.; Kim, J.E.; Park, W.H. A non-polar fraction of Saponaria officinalis L. acted as a TLR4/MD2 complex antagonist and inhibited TLR4/MyD88 signaling in vitro and in vivo. FASEB J. 2022, 36, e22387. [Google Scholar] [CrossRef]
- Alépée, N.; Adriaens, E.; Abo, T.; Magby, J.; Mewes, K.; Giusti, A. Development of a Defined Approach for Eye hazard identification of chemicals having surfactant properties according to the three UN GHS categories. Toxicology Vitr. 2023, 89, 105576. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J. Cell viability assays: Introduction. Mamm. Cell Viability Methods Protoc. 2011, 740, 1–6. [Google Scholar]
- Abcam. Cell Viability Assays. Available online: https://www.abcam.com/en-us/technical-resources/guides/cell-health-guide/cell-viability-assays#:~:text=Briefly%2C%20cell%20viability%20is%20the,get%20washed%20away%20during%20trypsinizing (accessed on 14 May 2025).
- Hernandez-Soriano, A.I.; Martínez-Salvador, C.; Alvarez-Zeferino, J.C.; Vázquez-Morillas, A.; Mesa-Jurado, M.A. Microplastics in cosmetics and personal care products. In Toxic Effects of Micro- and Nanoplastics: Environment, Food and Human Health; Wiley: Hoboken, NJ, USA, 2024; pp. 215–251. [Google Scholar]
- Ju, S.; Shin, G.; Lee, M.; Koo, J.M.; Jeon, H.; Ok, Y.S.; Hwang, D.S.; Hwang, S.Y.; Oh, D.X.; Park, J. Biodegradable chito-beads replacing non-biodegradable microplastics for cosmetics. Green Chem. 2021, 23, 6953–6965. [Google Scholar] [CrossRef]
- Raafat, K.; Al Haj, M. Modulators of diabetic neuropathy and inflammation from Saponaria officinalis: Isolation of active phytochemicals and potential mechanisms of action. J. Tradit. Complement. Med. 2023, 13, 226–235. [Google Scholar] [CrossRef]
- Jang, A.R.; Kim, H.J.; Kim, K.S.; Park, E.K. Development of a Natural Surfactant from Extracts of Saponaria officinalis L. KSBB J. 2013, 28, 202–207. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Morello, A.P.; Mays, D.A. Early inflammatory processes in the skin. Curr. Mol. Med. 2013, 13, 1250–1269. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The immune functions of keratinocytes in skin wound healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Zhang, Y.; Heinemann, N.; Rademacher, F.; Darvin, M.E.; Raab, C.; Keck, C.M.; Vollert, H.; Fluhr, J.W.; Gläser, R.; Harder, J. Skin care product rich in antioxidants and anti-inflammatory natural compounds reduces itching and inflammation in the skin of atopic dermatitis patients. Antioxidants 2022, 11, 1071. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Morris, S.A.; Ananthapadmanabhan, K.P.; Kasting, G.B. Anionic surfactant–induced changes in skin permeability. J. Pharm. Sci. 2019, 108, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yu, F.; Wang, C.; Han, Z.; Liu, S.; Chen, D.; Liu, D.; Meng, X.; He, X.; Huang, Z. The impacts of sodium lauroyl sarcosinate in facial cleanser on facial skin microbiome and lipidome. J. Cosmet. Dermatol. 2024, 23, 1351–1359. [Google Scholar] [CrossRef]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S. Compartmentalized control of skin immunity by resident commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Oliveira, A.L.S.; Santos Pedrosa, S.; Pintado, M.; Pinto-Ribeiro, I.; Madureira, A.R. Skin microbiota and the cosmetic industry. Microb. Ecol. 2023, 86, 86–96. [Google Scholar] [CrossRef]
- Mim, M.F.; Sikder, M.H.; Chowdhury, M.Z.H.; Zinan, N.; Islam, S.M.N. The dynamic relationship between skin microbiomes and personal care products: A comprehensive review. Heliyon 2024, 10, e34549. [Google Scholar] [CrossRef]
- Karnwal, A.; Shrivastava, S.; Al-Tawaha, A.R.M.S.; Kumar, G.; Singh, R.; Kumar, A.; Mohan, A.; Yogita; Malik, T. Microbial biosurfactant as an alternate to chemical surfactants for application in cosmetics industries in personal and skin care products: A critical review. BioMed Res. Int. 2023, 2023, 2375223. [Google Scholar] [CrossRef]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R. Green surfactants (biosurfactants): A petroleum-free substitute for Sustainability—Comparison, applications, market, and future prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef]
- Bezerra, K.G.; Silva, I.G.; Almeida, F.C.; Rufino, R.D.; Sarubbo, L.A. Plant-derived biosurfactants: Extraction, characteristics and properties for application in cosmetics. Biocatal. Agric. Biotechnol. 2021, 34, 102036. [Google Scholar] [CrossRef]
- Cho, W.K.; Kim, S.-Y.; Jang, S.J.; Lee, S.; Kim, H.-I.; Kim, E.; Lee, J.H.; Choi, S.S.; Moh, S.H. Comparative analysis of water extracts from roselle (Hibiscus sabdariffa L.) plants and callus cells: Constituents, effects on human skin cells, and transcriptome profiles. Int. J. Mol. Sci. 2023, 24, 10853. [Google Scholar] [CrossRef]
- Tcholakova, S.; Petkova, B. Bubble size and foamability: Role of surfactants and hydrodynamic conditions. Curr. Opin. Colloid Interface Sci. 2024, 72, 101824. [Google Scholar] [CrossRef]



| Symbol | Score | Morphology | Assessment |
|---|---|---|---|
| - | 0 | No reaction | Negative reaction |
| ± | 0.5 | Faint erythema only | Doubtful reaction |
| + | 1 | Erythema, Infiltration, Possibly papules | Weak positive reaction |
| ++ | 2 | Erythema, Infiltration, Papules, Vesicles | Strong positive reaction |
| +++ | 3 | Intense erythema, Infiltration, Coalescing Vesicles | Extreme positive reaction |
| * | IR | Various morphologies, e.g., soap effect, bulla, necrosis | Irritant reaction of different types |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.-R.; Kim, D.-H.; Kim, H.; Lim, D.-W. Evaluation of Saponin-Rich Callus from Saponaria officinalis L. as a Novel Scrub Material with Significant Exfoliating and Anti-Inflammatory Effects. Plants 2025, 14, 1535. https://doi.org/10.3390/plants14101535
Yu G-R, Kim D-H, Kim H, Lim D-W. Evaluation of Saponin-Rich Callus from Saponaria officinalis L. as a Novel Scrub Material with Significant Exfoliating and Anti-Inflammatory Effects. Plants. 2025; 14(10):1535. https://doi.org/10.3390/plants14101535
Chicago/Turabian StyleYu, Ga-Ram, Da-Hoon Kim, Hyuck Kim, and Dong-Woo Lim. 2025. "Evaluation of Saponin-Rich Callus from Saponaria officinalis L. as a Novel Scrub Material with Significant Exfoliating and Anti-Inflammatory Effects" Plants 14, no. 10: 1535. https://doi.org/10.3390/plants14101535
APA StyleYu, G.-R., Kim, D.-H., Kim, H., & Lim, D.-W. (2025). Evaluation of Saponin-Rich Callus from Saponaria officinalis L. as a Novel Scrub Material with Significant Exfoliating and Anti-Inflammatory Effects. Plants, 14(10), 1535. https://doi.org/10.3390/plants14101535






