Elevated Growth Temperature Modifies Drought and Shade Responses of Fagus sylvatica Seedlings by Altering Growth, Gas Exchange, Water Relations, and Xylem Function
Abstract
1. Introduction
2. Results
2.1. Changes in Morphology and Biomass Allocation
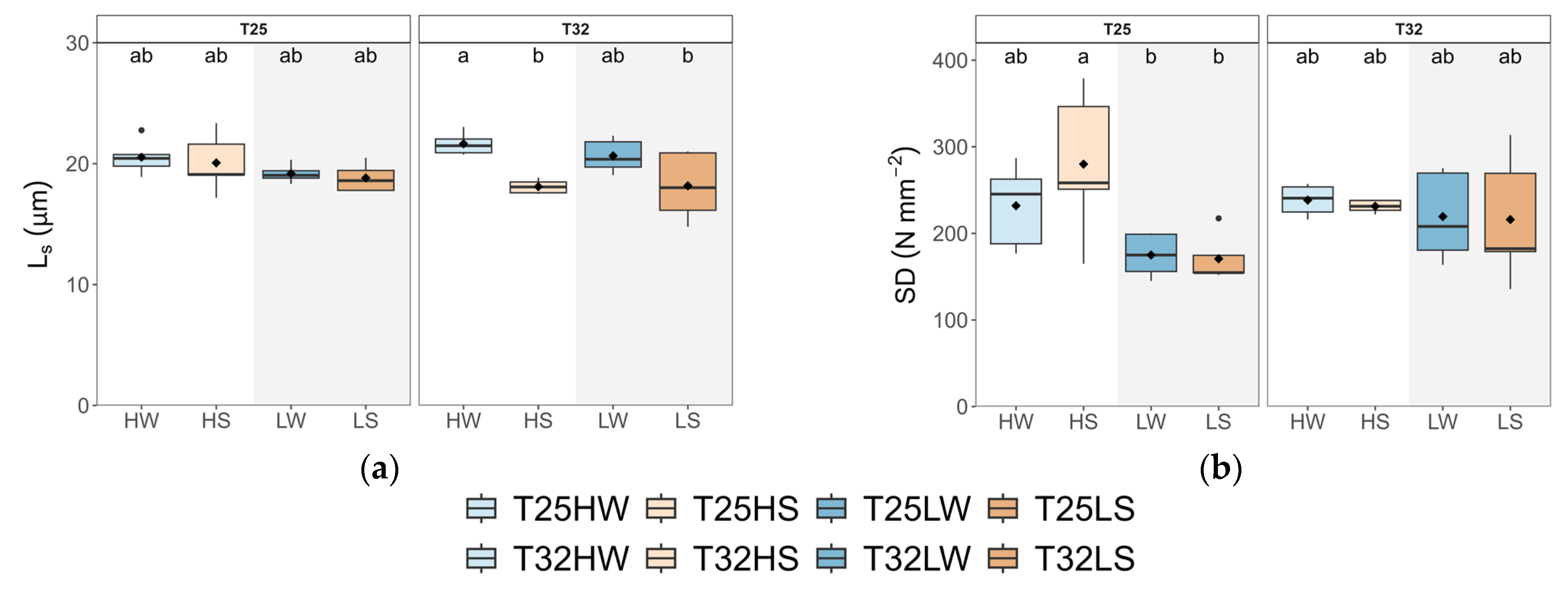
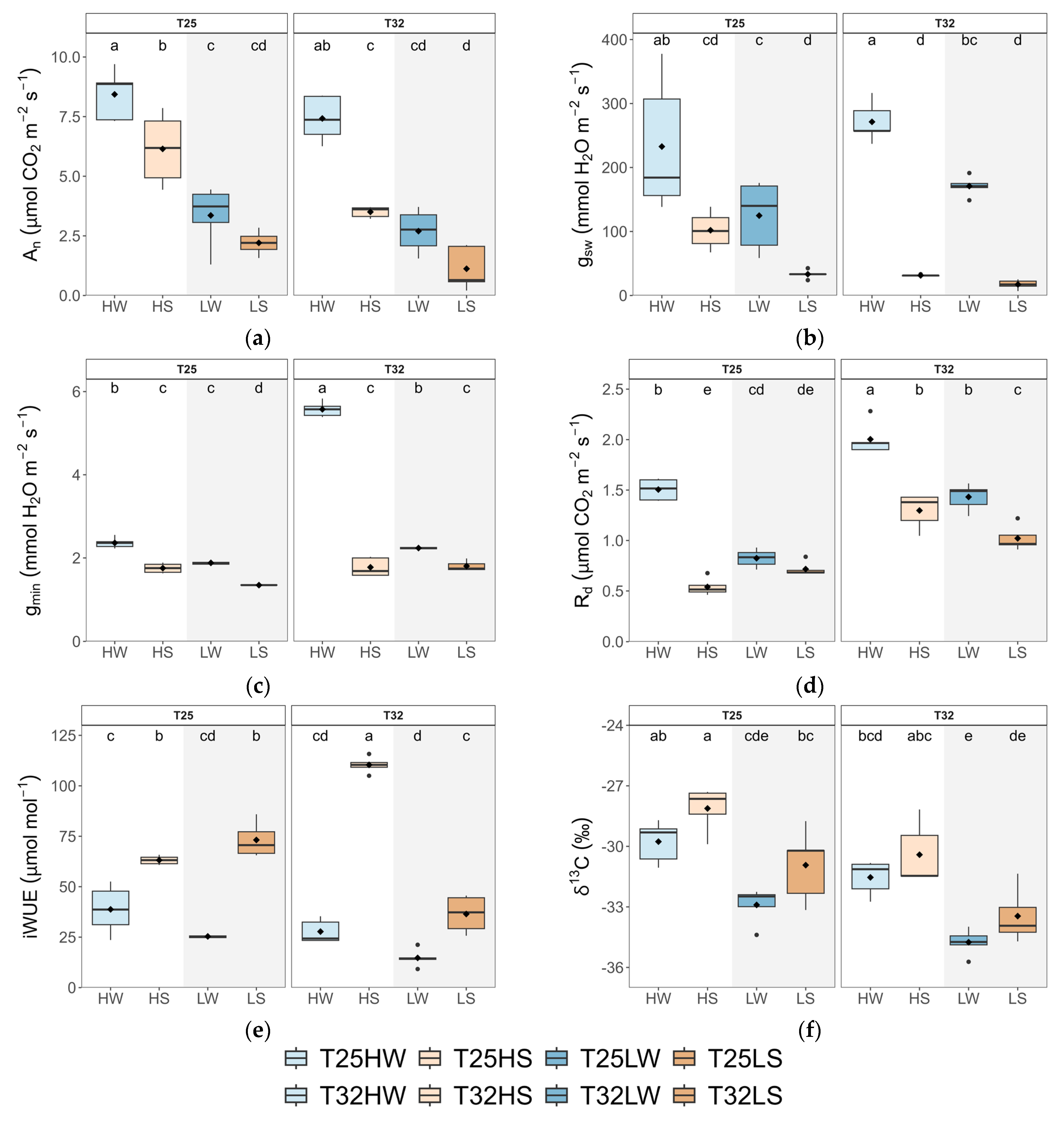
2.2. Leaf Gas Exchange, Water Use Efficiency, and Stomatal Traits
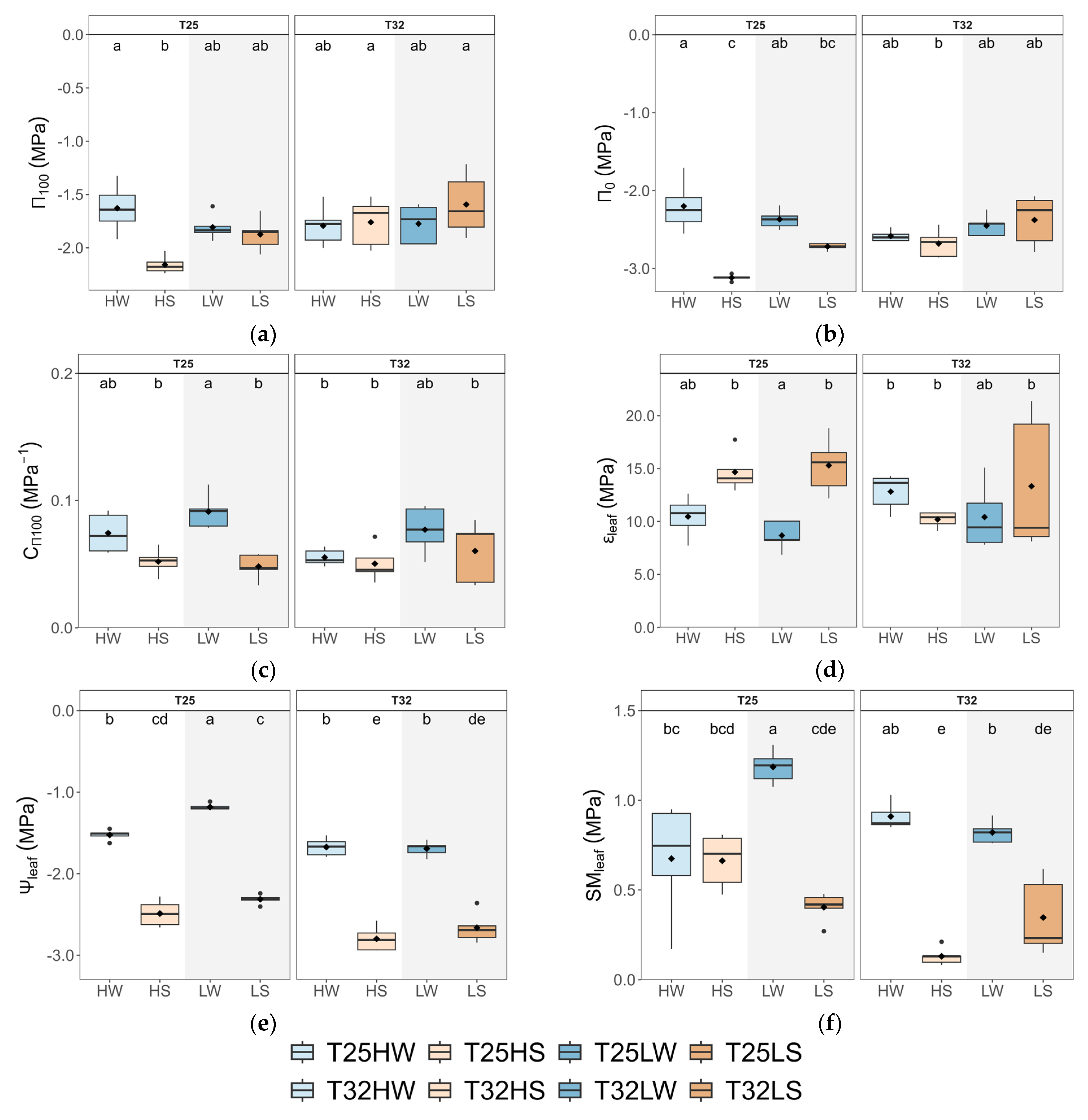
2.3. Leaf Water Relations
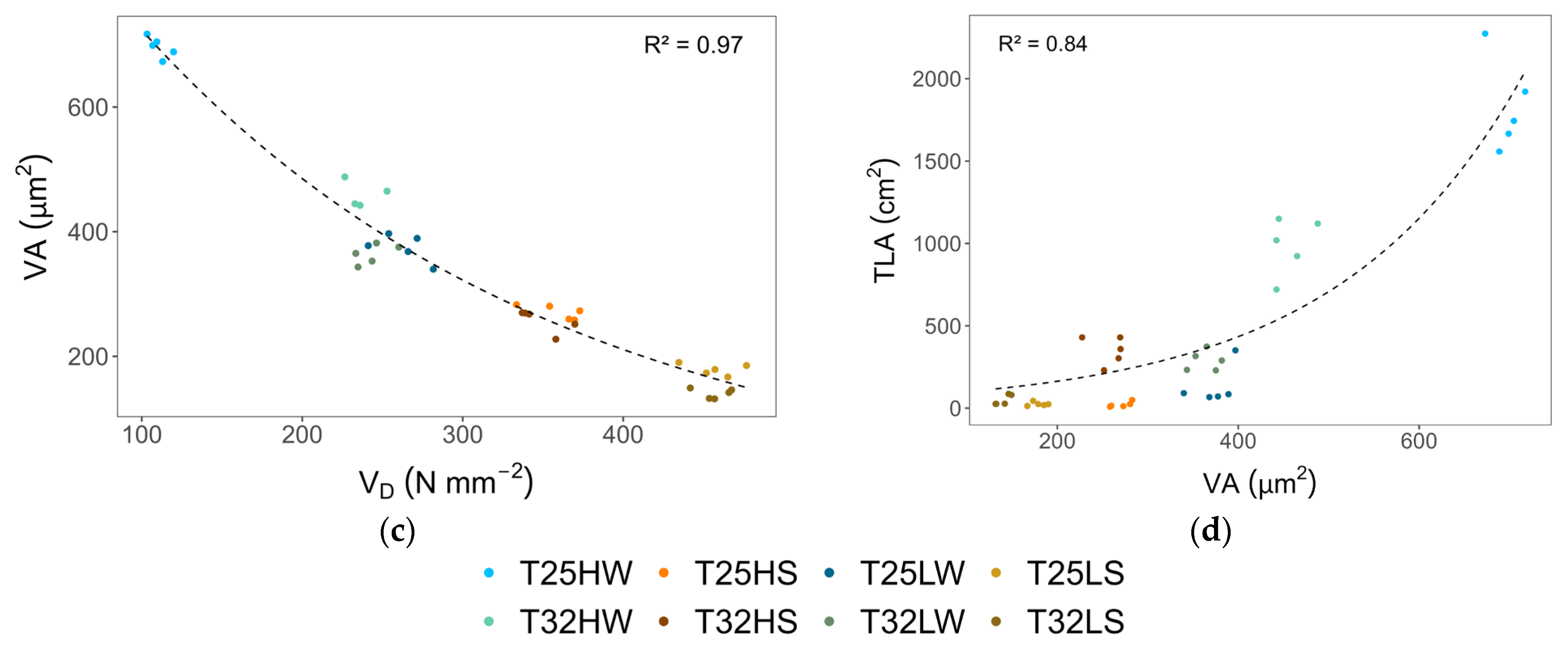
2.4. Hydraulic Traits and Stem Anatomy
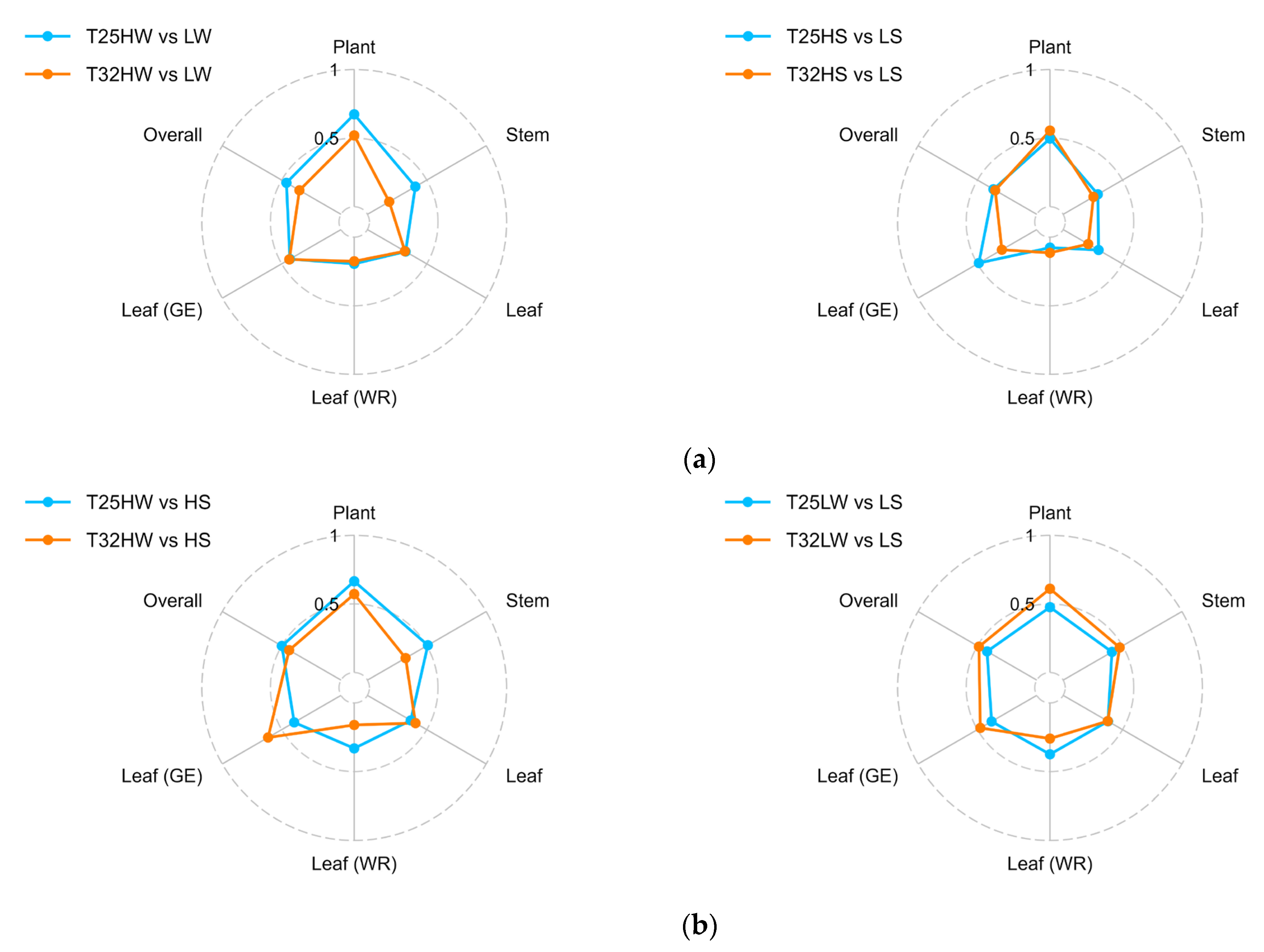
2.5. Plasticity in Response to Temperature, Light, and Water Availability
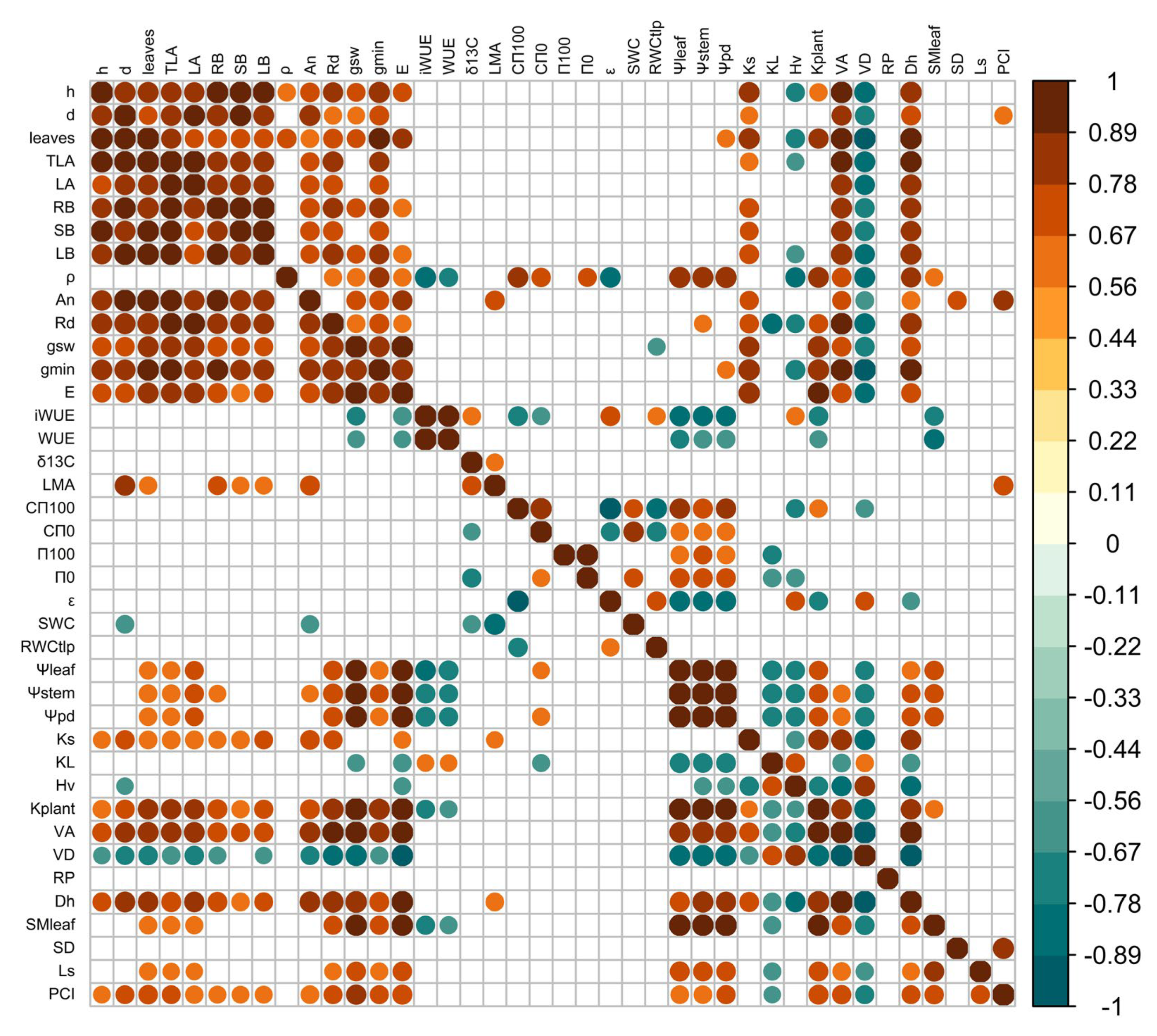
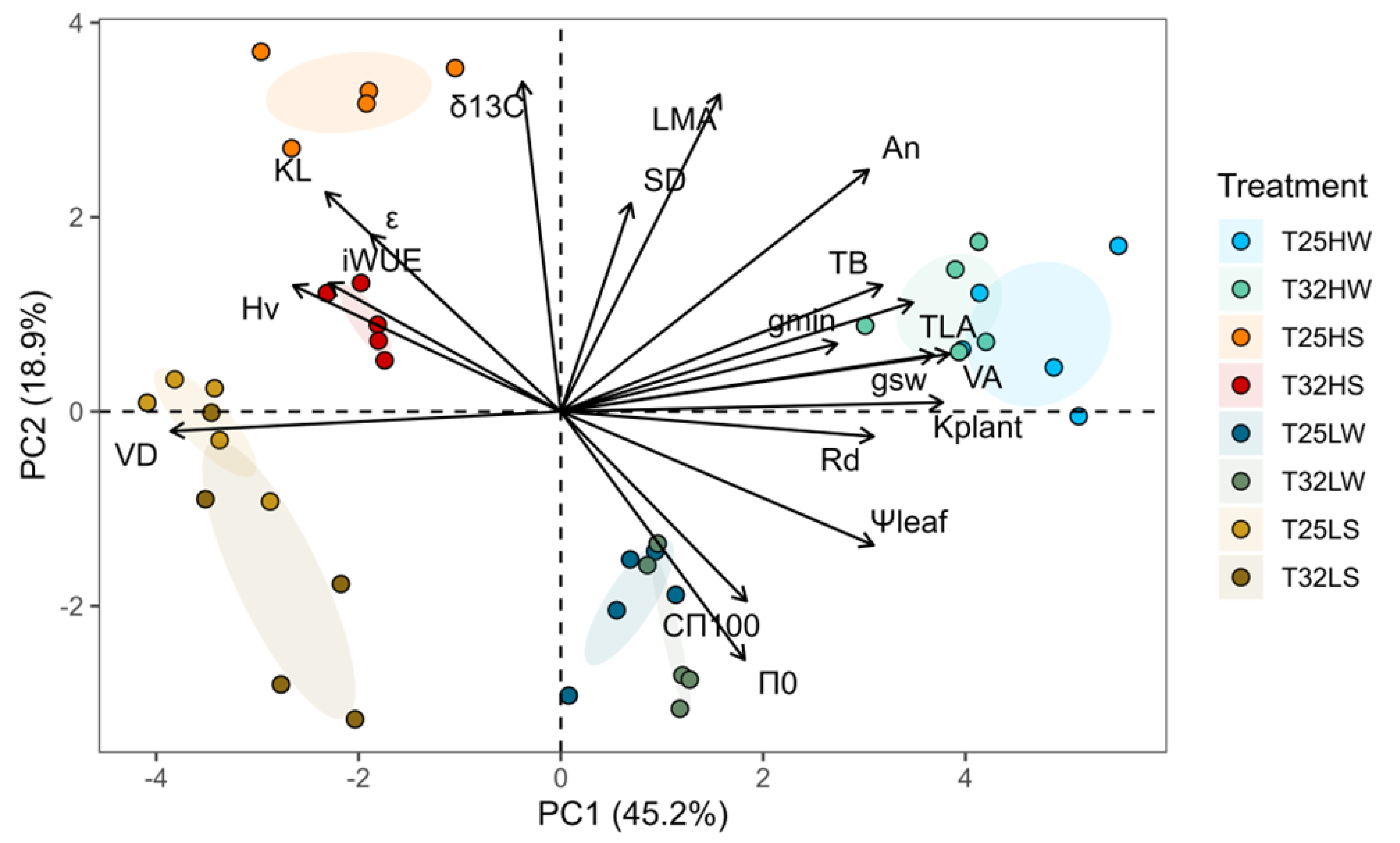
2.6. Correlations Among Traits and Principal Components Analysis (PCA)
3. Discussion
3.1. Impact of Warming on Plant Morphology, Physiology, and Anatomy at High and Low Irradiance
3.2. Impact of Warming and Water Deficit at High and Low Light Intensity
3.3. Phenotypic Plasticity and Regeneration Niche of Beech Seedlings Under Multi-Stress Factors
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
4.2. Growth and Biomass Allocation
4.3. Gas Exchange and Water Use Efficiency
4.4. Stomatal Traits and Carbon Isotope Composition (δ13C)
4.5. Minimum Leaf Conductance or Residual Conductance (gmin)
4.6. Pressure-Volume Curves and Related Traits
4.7. Predawn and Midday Water Potentials
4.8. Hydraulic Conductivity and Huber Value
4.9. Stem Xylem Anatomy
4.10. Trait Variation and Plasticity
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Symbol | Level for PPi | Definition | Values Units |
|---|---|---|---|
| h | Plant | Plant height | (cm) |
| d | Plant | Plant diameter | (mm) |
| leaves | Plant | Number of fully developed leaves | (nº) |
| TLA | Plant | Total plant leaf area | (cm2) |
| LA | Leaf GE relations | Average leaf area | (cm2) |
| RB | Plant | Root biomass | (g) |
| SB | Plant | Shoot biomass | (g) |
| LB | Plant | Leaf biomass | (g) |
| ρ | Plant | Wood density | (g cm−3) |
| An | Leaf GE relations | Leaf net assimilation rate | (μmol CO2 m−2 s−1) |
| Rd | Leaf GE relations | Leaf respiration | (μmol CO2 m−2 s−1) |
| gsw | Leaf GE relations | Leaf stomatal conductance to water vapor | (mol H2O m−2 s−1) |
| gmin | Leaf GE relations | Leaf minimum conductance to water vapor | (mmol H2O m−2 s−1) |
| E | Leaf GE relations | Leaf transpiration | (mol H2O m−2 s−1) |
| iWUE | Not used for PPi | Intrinsic water use efficiency | (μmol CO2 mol−1 H2O) |
| WUE | Not used for PPi | Water use efficiency | (μmol CO2 mol−1 H2O) |
| δ13C | Not used for PPi | Carbon isotope composition | (‰) |
| LMA | Leaf GE relations | Leaf mass per area | (g m−2) |
| SD | Stomatal anatomy | Stomatal density | (nº stomata per mm2) |
| Ls | Stomatal anatomy | Stomatal length | (μm) |
| PCI | Stomatal anatomy | Potential conductance index | (μm2 mm−2 10−4) |
| Ws | Stomatal anatomy | Stomatal complex width | (μm) |
| Pls | Stomatal anatomy | Stomatal pore length | (μm) |
| GWs | Stomatal anatomy | Guard cell width | (μm) |
| CΠ100 | Leaf water relations | Leaf capacitance at full turgor | (MPa−1) |
| CΠ0 | Leaf water relations | Leaf capacitance at turgor loss point | (MPa−1) |
| Π100 | Leaf water relations | Leaf osmotic potential at full turgor | (MPa) |
| Π0 | Leaf water relations | Leaf turgor loss point | (MPa) |
| εleaf | Leaf water relations | Leaf maximum Young’s modulus of elasticity | (MPa) |
| RWC Π0 | Leaf water relations | Relative water content at the turgor loss point | (%) |
| SWC | Leaf water relations | Leaf-saturated water content | (g g−1) |
| Ψleaf | Leaf water relations | Leaf midday water potential | (MPa) |
| Ψstem | Leaf water relations | Stem midday water potential | (MPa) |
| Ψpd | Not used for PPi | Predawn water potential | (MPa) |
| SMleaf | Not used for PPi | Leaf safety margin | (MPa) |
| Anisohydry | Plant | Ratio Ψleaf to Ψpd | (dimensionless) |
| KS | Stem | Hydraulic specific conductivity | (kg m−1 s−1 Mpa−1) |
| KL | Stem | Leaf hydraulic conductivity | (mmol m−1 s−1 Mpa−1) |
| Hv | Plant | Huber value | (m2 m−2) |
| kplant | Plant | Plant hydraulic conductance | (mmol m−2 s−1 Mpa−1) |
| VA | Stem | Average vessel lumen area | (μm2) |
| VD | Stem | Vessel density | (nº vessels per mm2) |
| RP | Stem | Xylem area occupied by radial parenchyma | (%) |
| Dh | Not used for PPi | Hydraulic diameter | (μm) |
| Variables | Tgrowth | Light | Water | Tgrowth × Light | Tgrowth × Water | Light × Water | Tgrowth × Light × Water |
|---|---|---|---|---|---|---|---|
| h | 6.70 * | 297.50 *** | 193.51 *** | 3.16 | 0.76 | 67.01 *** | <0.01 |
| d | 42.33 *** | 520.49 *** | 98.20 *** | 42.15 *** | 1.05 | 29.80 *** | 6.72 * |
| leaves | 100.86 *** | 145.62 *** | 188.08 *** | 7.40 ** | 23.78 *** | 34.68 *** | 2.88 |
| TLA | 9.40 ** | 300.24 *** | 276.75 *** | 39.46 *** | 45.09 *** | 150.84 *** | 60.06 *** |
| LA | 14.39 *** | 89.00 *** | 74.64 *** | 12.26 ** | 0.89 | 26.41 *** | 1.16 |
| RB (abs) | 41.51 *** | 240.44 *** | 149.30 *** | 45.23 *** | 24.45 *** | 109.47 *** | 35.29 *** |
| SB (abs) | 19.35 *** | 145.18 *** | 74.65 *** | 15.00 *** | 5.46 * | 55.17 *** | 6.45 * |
| LB (abs) | 8.48 ** | 267.33 *** | 122.04 *** | 15.91 *** | 18.65 *** | 90.60 *** | 27.50 *** |
| RB (rel) | 0.30 | 0.71 | 9.25 ** | 4.69 * | 5.96 * | 0.02 | 5.29 * |
| SB (rel) | 15.35 *** | 4.59 * | 1.84 | 3.93 | 0.03 | <0.01 | 4.80 * |
| LB (rel) | 25.41 *** | 1.53 | 2.84 | 1.08 | 4.37 * | <0.01 | 1.17 |
| An | 18.99 *** | 169.38 *** | 52.01 *** | 2.37 | 2.77 | 7.88 ** | 0.95 |
| Rd | 195.44 *** | 66.12 *** | 229.84 *** | 3.53 | 0.19 | 67.24 *** | 10.88 ** |
| gsw | 0.01 | 26.59 *** | 119.13 *** | 1.21 | 9.21 ** | 4.94 * | 0.70 |
| gmin | 652.60 *** | 700.90 *** | 1149.90 *** | 232.70 *** | 379.90 *** | 470.70 *** | 432.10 *** |
| E | 0.15 | 50.60 *** | 195.30 *** | 2.74 | 26.13 *** | 2.79 | 2.03 |
| iWUE | 1.02 | 108.38 *** | 390.07 *** | 77.93 *** | 17.56 *** | 18.80 *** | 84.06 *** |
| WUE | 7.26 * | 59.41 *** | 97.25 *** | 38.82 *** | 2.68 | 6.81 * | 44.05 *** |
| LMA | 264.22 *** | 505.64 *** | 166.07 *** | 22.42 *** | 111.74 *** | 7.13 ** | 118.75 *** |
| δ13C | 31.65 *** | 50.34 *** | 11.99 ** | 0.08 | 0.37 | 0.13 | 0.32 |
| SD | 0.59 | 10.49 ** | 0.29 | 4.55 * | 0.77 | 0.62 | 0.82 |
| LS | <0.01 | 2.98 | 11.35 ** | 0.67 | 6.55 * | 0.33 | 0.22 |
| PCI | 1.00 | 27.23 *** | 6.87 * | 7.18 * | 11.39 ** | 0.03 | 2.03 |
| CΠ0 | 1.49 | 5.69 * | 21.60 *** | 1.00 | 5.47 * | 3.01 | 0.22 |
| CΠ100 | 33.79 *** | 22.46 *** | 86.54 *** | 0.49 | 14.93 *** | 22.86 *** | 0.33 |
| Π0 | 5.02 * | 1.43 | 2.46 | 0.11 | 11.08 ** | 6.28 * | 1.67 |
| Π100 | 0.27 | 4.25 * | 16.44 *** | 1.40 | 16.27 *** | 4.50 * | 0.88 |
| εleaf | 0.28 | 0.04 | 9.40 ** | 0.11 | 7.08 * | 4.19 * | 0.66 |
| Ψleaf | 69.36 *** | 29.73 *** | 737.58 *** | 6.85 * | 0.01 | <0.01 | 4.305 * |
| Ψstem | 59.97 *** | 15.49 *** | 710.44 *** | 12.86 ** | 0.07 | 0.35 | 0.75 |
| Ψpd | 46.04 *** | 11.67 ** | 852.71 *** | 1.87 | 0.03 | 3.55 | 4.87 * |
| SMleaf | 13.28 *** | 3.69 | 107.36 *** | 0.41 | 5.42 * | 5.53 * | 29.57 *** |
| Ks | 0.14 | 32.26 *** | 28.46 *** | 0.38 | 0.81 | 0.01 | 0.93 |
| Kl | 90.41 *** | 0.40 | 221.90 *** | 0.85 | 74.61 *** | 27.33 *** | 54.54 *** |
| Hv | 76.66 *** | 15.01 *** | 99.57 *** | 1.45 | 55.79 *** | 1.05 | 6.37 * |
| kplant | 0.39 | 23.98 *** | 256.35 *** | 0.01 | 7.94 ** | 2.54 | 3.85 |
| VD | 48.24 *** | 447.68 *** | 2158.38 *** | 55.35 *** | 42.96 *** | 5.88 * | 87.85 *** |
| VA | 261.49 *** | 893.15 *** | 2680.29 *** | 90.51 *** | 90.14 *** | 111.22 *** | 148.31 *** |
| RP | 2.17 | 0.05 | 0.93 | 3.67 | 3.00 | 0.06 | <0.01 |
| Dh | 245.32 *** | 1165.82 *** | 3405.51 *** | 11.11 ** | 52.28 *** | 14.03 *** | 190.31 *** |
| Group of Variables | Measured Variables | Well-Watered | Water Deficit | ||
|---|---|---|---|---|---|
| T25 | T32 | T25 | T32 | ||
| Response to Light (HW vs. LW) | Response to Light (HW vs. LW) | Response to Light (HS vs. LS) | Response to Light (HS vs. LS) | ||
| Plant level | 10 | 0.67 | 0.52 | 0.50 | 0.56 |
| Stem | 6 | 0.40 | 0.18 | 0.29 | 0.26 |
| Leaf (PV + GE) | 13 | 0.32 | 0.32 | 0.30 | 0.21 |
| Leaf hydric relations | 6 | 0.19 | 0.18 | 0.08 | 0.11 |
| Leaf GE relations | 7 | 0.43 | 0.44 | 0.49 | 0.29 |
| Overall | 29 | 0.46 | 0.35 | 0.37 | 0.35 |
| Group of Variables | Measured Variables | High Light | Low Light | ||
|---|---|---|---|---|---|
| T25 | T32 | T25 | T32 | ||
| Response to Water (HW vs. HS) | Response to Water (HW vs. HS) | Response to Water (LW vs. LS) | Response to Water (LW vs. LS) | ||
| Full plant | 10 | 0.67 | 0.57 | Full plant | 10 |
| Stem | 7 | 0.51 | 0.32 | Stem | 7 |
| Leaf (PV + GE) | 12 | 0.37 | 0.41 | Leaf (PV + GE) | 12 |
| Leaf water relations | 6 | 0.33 | 0.16 | Leaf water relations | 6 |
| Leaf GE relations | 6 | 0.39 | 0.61 | Leaf GE relations | 6 |
| Overall | 29 | 0.50 | 0.44 | Overall | 29 |
| Growth Temperature | T25 | T32 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | HW | HS | LW | LS | HW | HS | LW | LS | |
| Sample Size (n) | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| h | (cm) | 61.9 ± 2.3 | 28.4 ± 1.6 | 18.9 ± 1.2 | 11.3 ± 0.5 | 63.1 ± 3.9 | 27.2 ± 0.8 | 26.2 ± 2.3 | 16.3 ± 1.6 |
| d | (mm) | 12.6 ± 0.2 | 9.0 ± 0.3 | 4.7 ± 0.5 | 4.6 ± 0.2 | 9.4 ± 0.4 | 6.6 ± 0.1 | 5.4 ± 0.3 | 3.8 ± 0.2 |
| leaves | (nº) | 23 ± 2 | 11 ± 1 | 10 ± 2 | 3 ± 0 | 41 ± 3 | 16 ± 1 | 20 ± 1 | 10 ± 0 |
| TLA | (cm2) | 1881.76 ± 113.43 | 214.56 ± 8.03 | 165.04 ± 54.89 | 31.34 ± 8.24 | 986.67 ± 68.17 | 328.22 ± 31.02 | 273.95 ± 26.37 | 86.74 ± 22.89 |
| LA | (cm2) | 36.16 ± 2.02 | 17.88 ± 1.71 | 13.37 ± 1.48 | 9.62 ± 0.42 | 25.41 ± 2.98 | 11.51 ± 1.1 | 13.65 ± 1.37 | 9.28 ± 0.71 |
| RB | (g) | 30.9 ± 3.1 | 8 ± 1.3 | 2.9 ± 0.5 | 1.4 ± 0.1 | 14.5 ± 0.8 | 4.6 ± 0.3 | 2.7 ± 0.6 | 0.7 ± 0.1 |
| SB | (g) | 16.3 ± 0.8 | 2.6 ± 0.6 | 0.5 ± 0 | 0.4 ± 0.1 | 8.6 ± 0.7 | 3.9 ± 0.4 | 1.4 ± 0.2 | 0.5 ± 0.1 |
| LB | (g) | 49.7 ± 4.1 | 8.7 ± 1.2 | 3 ± 0.5 | 1.5 ± 0.1 | 21.2 ± 2.2 | 5.6 ± 0.3 | 4.7 ± 0.6 | 1 ± 0.2 |
| TB | (g) | 95.8 ± 6.5 | 19.3 ± 2.5 | 6.6 ± 1.1 | 4.1 ± 0.8 | 46.9 ± 2.6 | 14.8 ± 1 | 9 ± 1.3 | 2.5 ± 0.5 |
| RB | (%) | 32.4 ± 2.6 | 38.6 ± 1.9 | 43.2 ± 4 | 41.8 ± 1.6 | 32.7 ± 3 | 30.8 ± 0.8 | 28.7 ± 2 | 34.9 ± 2.2 |
| SB | (%) | 15.9 ± 1.1 | 13.1 ± 0.3 | 10.7 ± 2 | 11.7 ± 2.5 | 17.1 ± 1.1 | 24.7 ± 1.2 | 18.1 ± 0.9 | 22.6 ± 3.8 |
| LB | (%) | 50 ± 1.3 | 44.9 ± 1 | 43.8 ± 1.4 | 46.5 ± 1.3 | 45.3 ± 4.1 | 40.3 ± 2 | 55 ± 1 | 42.4 ± 2.7 |
| Ks | (kg m−1 s−1 Mpa−1) | 25.94 ± 4.21 | 14.78 ± 0.92 | 14.56 ± 0.91 | 6.11 ± 1.31 | 22.09 ± 2.58 | 16.85 ± 2.23 | 15.86 ± 1.93 | 7.19 ± 2.61 |
| KL | (mmol m−1 s−1 Mpa−1) | 0.62 ± 0.11 | 5.26 ± 0.15 | 1.72 ± 0.32 | 2.84 ± 0.45 | 0.81 ± 0.12 | 1.37 ± 0.07 | 0.44 ± 0.08 | 1.62 ± 0.23 |
| Kplant | (mmol m−2 s−1 Mpa−1) | 2.46 ± 0.31 | 0.96 ± 0.13 | 1.92 ± 0.29 | 0.34 ± 0.02 | 2.93 ± 0.12 | 0.32 ± 0.01 | 1.96 ± 0.06 | 0.18 ± 0.03 |
| HV | (cm2 m−2) | 7.6 ± 1 | 59.8 ± 6.4 | 21.9 ± 3.8 | 64.5 ± 12.1 | 7.2 ± 0.8 | 9.9 ± 0.9 | 6 ± 0.7 | 26 ± 3.2 |
| LMA | (g m−2) | 82.9 ± 1.4 | 90.2 ± 2.1 | 62.2 ± 1.4 | 49.6 ± 1.5 | 80.1 ± 1.9 | 43.9 ± 1.1 | 49.5 ± 0.7 | 40.5 ± 0.7 |
| An | (μmol m-2 s-1) | 8.4 ± 0.5 | 6.1 ± 0.7 | 3.4 ± 0.6 | 2.2 ± 0.2 | 7.4 ± 0.4 | 3.5 ± 0.1 | 2.7 ± 0.4 | 1.1 ± 0.4 |
| Rl | (μmol m−2 s−1) | 0.75 ± 0.02 | 0.25 ± 0.01 | 0.41 ± 0.02 | 0.35 ± 0.02 | 1.00 ± 0.04 | 0.61 ± 0.04 | 0.72 ± 0.03 | 0.51 ± 0.03 |
| gsw | (mmol m−2 s−1) | 232.7 ± 46.7 | 101.9 ± 13 | 124.8 ± 24 | 33.2 ± 3 | 271.4 ± 14 | 31.3 ± 0.5 | 171 ± 6.8 | 17.2 ± 3.2 |
| gmin | (mmol m−2 s−1) | 2.36 ± 0.06 | 1.75 ± 0.05 | 1.88 ± 0.02 | 1.35 ± 0.01 | 5.57 ± 0.08 | 1.78 ± 0.1 | 2.24 ± 0.01 | 1.81 ± 0.05 |
| E | (mol m−2 s−1) | 3.2 ± 0.4 | 1.8 ± 0.2 | 1.9 ± 0.3 | 0.6 ± 0 | 3.9 ± 0.1 | 0.7 ± 0 | 2.7 ± 0.1 | 0.4 ± 0.1 |
| iWUE | (μmol mol−1) | 38.8 ± 5.3 | 63.1 ± 0.9 | 25.4 ± 0.4 | 73.2 ± 4.7 | 27.8 ± 2.6 | 110.4 ± 1.7 | 14.7 ± 1.9 | 36.5 ± 5 |
| WUE | (μmol mol−1) | 2.7 ± 0.2 | 3.3 ± 0.1 | 1.8 ± 0 | 4 ± 0.3 | 1.9 ± 0.1 | 5.3 ± 0.4 | 1.1 ± 0.2 | 1.5 ± 0.2 |
| δ13C | (‰) | −29.8 ± 0.5 | −27.6 ± 0.7 | −32.3 ± 0.7 | −30.9 ± 0.8 | −31.5 ± 0.4 | −30.4 ± 0.7 | −34.8 ± 0.3 | −33.5 ± 0.6 |






References
- IPCC. IPCC Summary for Policymakers. In Climate Change 2023: Synthesis Report.Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Pineda-García, F.; Paz, H.; Meinzer, F.C. Drought Resistance in Early and Late Secondary Successional Species from a Tropical Dry Forest: The Interplay between Xylem Resistance to Embolism, Sapwood Water Storage and Leaf Shedding. Plant Cell Environ. 2013, 36, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araújo, M.B.; Balaguer, L.; Benito-Garzón, M.; Cornwell, W.; Gianoli, E.; van Kleunen, M.; Naya, D.E.; et al. The Effects of Phenotypic Plasticity and Local Adaptation on Forecasts of Species Range Shifts under Climate Change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Valiente, J.A.; González-Martínez, S.C.; Robledo-Arnuncio, J.J.; Matesanz, S.; Anadon-Rosell, A.; Martínez-Vilalta, J.; López, R.; Cano-Martín, F.J. Genetically Based Trait Coordination and Phenotypic Plasticity of Growth, Gas Exchange, Allometry, and Hydraulics across the Distribution Range of Pinus Pinaster. New Phytol. 2025, 246, 984–1000. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The World-Wide ‘Fast–Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Arroyo, J.I.; Díez, B.; Kempes, C.P.; West, G.B.; Marquet, P.A. A General Theory for Temperature Dependence in Biology. Proc. Natl. Acad. Sci. USA 2022, 119, e2119872119. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Drake, J.E.; Tjoelker, M.G.; López, R.; Pfautsch, S.; Vårhammar, A.; Medlyn, B.E. The Temperature Optima for Tree Seedling Photosynthesis and Growth Depend on Water Inputs. Glob. Change Biol. 2020, 26, 2544–2560. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential Responses to Changes in Growth Temperature between Trees from Different Functional Groups and Biomes: A Review and Synthesis of Data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature Response of Photosynthesis in C3, C4, and CAM Plants: Temperature Acclimation and Temperature Adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Dai, L.; Xu, Y.; Harmens, H.; Duan, H.; Feng, Z.; Hayes, F.; Sharps, K.; Radbourne, A.; Tarvainen, L. Reduced Photosynthetic Thermal Acclimation Capacity under Elevated Ozone in Poplar (Populus tremula) Saplings. Glob. Change Biol. 2021, 27, 2159–2173. [Google Scholar] [CrossRef]
- Atkin, O.K.; Tjoelker, M.G. Thermal Acclimation and the Dynamic Response of Plant Respiration to Temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-Analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation Between Species. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme Heat Increases Stomatal Conductance and Drought-Induced Mortality Risk in Vulnerable Plant Species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The Influence of Leaf Size and Shape on Leaf Thermal Dynamics: Does Theory Hold up under Natural Conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef]
- Crous, K.Y.; Uddling, J.; De Kauwe, M.G. Temperature Responses of Photosynthesis and Respiration in Evergreen Trees from Boreal to Tropical Latitudes. New Phytol. 2022, 234, 353–374. [Google Scholar] [CrossRef]
- Ghannoum, O.; Phillips, N.; Sears, M.; Logan, B.; Lewis, J.; Conroy, J.; Tissue, D. Photosynthetic Responses of Two Eucalypts to Industrial-Age Changes in Atmospheric [CO2] and Temperature: Eucalyptus Photosynthesis in Past and Future Climates. Plant Cell Environ. 2010, 33, 1671–1681. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Tjoelker, M.G.; Aspinwall, M.J.; Battaglia, M.; Cano, F.J.; Carter, K.R.; Cavaleri, M.A.; Cernusak, L.A.; et al. Acclimation and Adaptation Components of the Temperature Dependence of Plant Photosynthesis at the Global Scale. New Phytol. 2019, 222, 768–784. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal Conductance Increases with Rising Temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef]
- Drake, J.E.; Tjoelker, M.G.; Vårhammar, A.; Medlyn, B.E.; Reich, P.B.; Leigh, A.; Pfautsch, S.; Blackman, C.J.; López, R.; Aspinwall, M.J.; et al. Trees Tolerate an Extreme Heatwave via Sustained Transpirational Cooling and Increased Leaf Thermal Tolerance. Glob. Change Biol. 2018, 24, 2390–2402. [Google Scholar] [CrossRef]
- Diao, H.; Cernusak, L.A.; Saurer, M.; Gessler, A.; Siegwolf, R.T.W.; Lehmann, M.M. Uncoupling of Stomatal Conductance and Photosynthesis at High Temperatures: Mechanistic Insights from Online Stable Isotope Techniques. New Phytol. 2024, 241, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.C.; Schuler, P.; Lehmann, M.M.; Mas, E.; Mekarni, L.; Pivovaroff, A.L.; Turberg, P.; Grossiord, C. Increasing Temperature and Vapour Pressure Deficit Lead to Hydraulic Damages in the Absence of Soil Drought. Plant Cell Environ. 2022, 45, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive Oxygen Species and Temperature Stresses: A Delicate Balance between Signaling and Destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The Role of Stomata in Sensing and Driving Environmental Change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of Tree Mortality under Drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Mantova, M.; Cochard, H.; Burlett, R.; Delzon, S.; King, A.; Rodriguez-Dominguez, C.M.; Ahmed, M.A.; Trueba, S.; Torres-Ruiz, J.M. On the Path from Xylem Hydraulic Failure to Downstream Cell Death. New Phytol. 2023, 237, 793–806. [Google Scholar] [CrossRef]
- Grossiord, C.; Sevanto, S.; Borrego, I.; Chan, A.M.; Collins, A.D.; Dickman, L.T.; Hudson, P.J.; McBranch, N.; Michaletz, S.T.; Pockman, W.T.; et al. Tree Water Dynamics in a Drying and Warming World. Plant Cell Environ. 2017, 40, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Way, D.A.; Domec, J.-C.; Jackson, R.B. Elevated Growth Temperatures Alter Hydraulic Characteristics in Trembling Aspen (Populus tremuloides) Seedlings: Implications for Tree Drought Tolerance. Plant Cell Environ. 2013, 36, 103–115. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Aranda, I.; Cadahía, E.; Fernández de Simón, B. Specific Leaf Metabolic Changes That Underlie Adjustment of Osmotic Potential in Response to Drought by Four Quercus Species. Tree Physiol. 2021, 41, 728–743. [Google Scholar] [CrossRef]
- Maherali, H.; DeLucia, E.H. Xylem Conductivity and Vulnerability to Cavitation of Ponderosa Pine Growing in Contrasting Climates. Tree Physiol. 2000, 20, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.S.; Montagu, K.D.; Conroy, J.P. Changes in Wood Density of Eucalyptus Camaldulensis Due to Temperature—The Physiological Link between Water Viscosity and Wood Anatomy. For. Ecol. Manag. 2004, 193, 157–165. [Google Scholar] [CrossRef]
- Thomas, D.S.; Montagu, K.D.; Conroy, J.P. Temperature Effects on Wood Anatomy, Wood Density, Photosynthesis and Biomass Partitioning of Eucalyptus Grandis Seedlings. Tree Physiol. 2007, 27, 251–260. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Atkin, O.K.; Robson, T.M.; Zaragoza-Castells, J.; Gil, L.; Aranda, I. Thermal Acclimation of Leaf Dark Respiration of Beech Seedlings Experiencing Summer Drought in High and Low Light Environments. Tree Physiol. 2010, 30, 214–224. [Google Scholar] [CrossRef]
- Osonubi, O.; Davies, W.J. The Influence of Water Stress on the Photosynthetic Performance and Stomatal Behaviour of Tree Seedlings Subjected to Variation in Temperature and Irradiance. Oecologia 1980, 45, 3–10. [Google Scholar] [CrossRef]
- Cano, F.J.; Sánchez-Gómez, D.; Rodríguez-Calcerrada, J.; Warren, C.R.; Gil, L.; Aranda, I. Effects of Drought on Mesophyll Conductance and Photosynthetic Limitations at Different Tree Canopy Layers. Plant Cell Environ. 2013, 36, 1961–1980. [Google Scholar] [CrossRef]
- Cano, F.J.; López, R.; Warren, C.R. Implications of the Mesophyll Conductance to CO2 for Photosynthesis and Water-Use Efficiency during Long-Term Water Stress and Recovery in Two Contrasting Eucalyptus Species. Plant Cell Environ. 2014, 37, 2470–2490. [Google Scholar] [CrossRef]
- Bartlett, M.K.; Scoffoni, C.; Ardy, R.; Zhang, Y.; Sun, S.; Cao, K.; Sack, L. Rapid Determination of Comparative Drought Tolerance Traits: Using an Osmometer to Predict Turgor Loss Point. Methods Ecol. Evol. 2012, 3, 880–888. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Elevated [CO2] Negatively Impacts C4 Photosynthesis under Heat and Water Stress without Penalizing Biomass. J. Exp. Bot. 2023, 74, 2875–2890. [Google Scholar] [CrossRef]
- López, R.; Cano, F.J.; Martin-StPaul, N.K.; Cochard, H.; Choat, B. Coordination of Stem and Leaf Traits Define Different Strategies to Regulate Water Loss and Tolerance Ranges to Aridity. New Phytol. 2021, 230, 497–509. [Google Scholar] [CrossRef]
- Duursma, R.A.; Blackman, C.J.; Lopéz, R.; Martin-StPaul, N.K.; Cochard, H.; Medlyn, B.E. On the Minimum Leaf Conductance: Its Role in Models of Plant Water Use, and Ecological and Environmental Controls. New Phytol. 2019, 221, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, C.; D’Odorico, P.; Buchmann, N. Light and VPD Gradients Drive Foliar Nitrogen Partitioning and Photosynthesis in the Canopy of European Beech and Silver Fir. Oecologia 2020, 192, 323–339. [Google Scholar] [CrossRef]
- Aranda, I.; Robson, T.; Rodríguez-Calcerrada, J.; Valladares, F. Limited Capacity to Cope with Excessive Light in the Open and with Seasonal Drought in the Shade in Mediterranean Ilex Aquifolium Populations. Trees 2008, 22, 375–384. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J.A. Physiological Responses of Fagus sylvatica L. Seedlings under Pinus sylvestris L. and Quercus pyrenaica Willd. Overstories. For. Ecol. Manag. 2002, 162, 153–164. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Gebre, G.M.; Shirshac, T.L. Osmotic Potential of Several Hardwood Species as Affected by Manipulation of Throughfall Precipitation in an Upland Oak Forest during a Dry Year. Tree Physiol. 1998, 18, 291–298. [Google Scholar] [CrossRef]
- Aranda, I.; Bergasa, L.F.; Gil, L.; Pardos, J.A. Effects of Relative Irradiance on the Leaf Structure of Fagus sylvatica L. Seedlings Planted in the Understory of a Pinus sylvestris L. Stand after Thinning. Ann. For. Sci. 2001, 58, 673–680. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Pardos, J.A.; Gil, L.; Aranda, I. Ability to Avoid Water Stress in Seedlings of Two Oak Species Is Lower in a Dense Forest Understory than in a Medium Canopy Gap. For. Ecol. Manag. 2008, 255, 421–430. [Google Scholar] [CrossRef]
- Weston, D.J.; Bauerle, W.L. Inhibition and Acclimation of C3 Photosynthesis to Moderate Heat: A Perspective from Thermally Contrasting Genotypes of Acer Rubrum (Red maple). Tree Physiol. 2007, 27, 1083–1092. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant Responses to Multifactorial Stress Combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef]
- Seversike, T.M.; Sermons, S.M.; Sinclair, T.R.; Carter, T.E., Jr.; Rufty, T.W. Temperature Interactions with Transpiration Response to Vapor Pressure Deficit among Cultivated and Wild Soybean Genotypes. Physiol. Plant. 2013, 148, 62–73. [Google Scholar] [CrossRef]
- Koehler, T.; Wankmüller, F.J.P.; Sadok, W.; Carminati, A. Transpiration Response to Soil Drying versus Increasing Vapor Pressure Deficit in Crops: Physical and Physiological Mechanisms and Key Plant Traits. J. Exp. Bot. 2023, 74, 4789–4807. [Google Scholar] [CrossRef]
- Mills, C.; Bartlett, M.; Buckley, T. The Poorly-Explored Stomatal Response to Temperature at Constant Evaporative Demand. Plant Cell Environ. 2024, 47, 3428–3446. [Google Scholar] [CrossRef]
- Duan, H.; Amthor, J.S.; Duursma, R.A.; O’Grady, A.P.; Choat, B.; Tissue, D.T. Carbon Dynamics of Eucalypt Seedlings Exposed to Progressive Drought in Elevated [CO2] and Elevated Temperature. Tree Physiol. 2013, 33, 779–792. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Peláez-Vico, M.Á.; Sinha, R.; Pascual, L.S.; Mittler, R. The Impact of Multifactorial Stress Combination on Plants, Crops, and Ecosystems: How Should We Prepare for What Comes Next? Plant J. 2024, 117, 1800–1814. [Google Scholar] [CrossRef]
- Wertin, T.M.; McGuire, M.A.; Teskey, R.O. Higher Growth Temperatures Decreased Net Carbon Assimilation and Biomass Accumulation of Northern Red Oak Seedlings near the Southern Limit of the Species Range. Tree Physiol. 2011, 31, 1277–1288. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Rice, K.; Rich, R.L.; Stefanski, A.; Hobbie, S.E.; Montgomery, R.A. Geographic Range Predicts Photosynthetic and Growth Response to Warming in Co-Occurring Tree Species. Nat. Clim. Chang. 2015, 5, 148–152. [Google Scholar] [CrossRef]
- Van Gelder, H.A.; Poorter, L.; Sterck, F.J. Wood Mechanics, Allometry, and Life-History Variation in a Tropical Rain Forest Tree Community. New Phytol. 2006, 171, 367–378. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in Wood Density and Structure Are Linked to Prevention of Xylem Implosion by Negative Pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Sperry, J. Evolution of Water Transport and Xylem Structure. Int. J. Plant Sci. 2003, 164, S115–S127. [Google Scholar] [CrossRef]
- Cochard, H.; Martin, R.; Gross, P.; Bogeat-Triboulot, M.B. Temperature Effects on Hydraulic Conductance and Water Relations of Quercus robur L. J. Exp. Bot. 2000, 51, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Gates, D.M. Transpiration and Leaf Temperature. Annu. Rev. Plant Biol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Blasini, D.E.; Koepke, D.F.; Bush, S.E.; Allan, G.J.; Gehring, C.A.; Whitham, T.G.; Day, T.A.; Hultine, K.R. Tradeoffs between Leaf Cooling and Hydraulic Safety in a Dominant Arid Land Riparian Tree Species. Plant Cell Environ. 2022, 45, 1664–1681. [Google Scholar] [CrossRef]
- Mencuccini, M.; Rosas, T.; Rowland, L.; Choat, B.; Cornelissen, H.; Jansen, S.; Kramer, K.; Lapenis, A.; Manzoni, S.; Niinemets, Ü.; et al. Leaf Economics and Plant Hydraulics Drive Leaf: Wood Area Ratios. New Phytol. 2019, 224, 1544–1556. [Google Scholar] [CrossRef]
- Šigut, L.; Holišová, P.; Klem, K.; Šprtová, M.; Calfapietra, C.; Marek, M.V.; Špunda, V.; Urban, O. Does Long-Term Cultivation of Saplings under Elevated CO2 Concentration Influence Their Photosynthetic Response to Temperature? Ann. Bot. 2015, 116, 929–939. [Google Scholar] [CrossRef]
- Ameye, M.; Wertin, T.M.; Bauweraerts, I.; McGuire, M.A.; Teskey, R.O.; Steppe, K. The Effect of Induced Heat Waves on Pinus taeda and Quercus rubra Seedlings in Ambient and Elevated CO2 Atmospheres. New Phytol. 2012, 196, 448–461. [Google Scholar] [CrossRef]
- Duan, H.; O’Grady, A.P.; Duursma, R.A.; Choat, B.; Huang, G.; Smith, R.A.; Jiang, Y.; Tissue, D.T. Drought Responses of Two Gymnosperm Species with Contrasting Stomatal Regulation Strategies under Elevated [CO2] and Temperature. Tree Physiol. 2015, 35, 756–770. [Google Scholar] [CrossRef]
- Ogren, W.L. Photorespiration: Pathways, Regulation, and Modification. Annu. Rev. Plant Biol. 1984, 35, 415–442. [Google Scholar] [CrossRef]
- Garen, J.C.; Michaletz, S.T. Temperature Governs the Relative Contributions of Cuticle and Stomata to Leaf Minimum Conductance. New Phytol. 2025, 245, 1911–1923. [Google Scholar] [CrossRef]
- Cochard, H.; Lemoine, D.; Dreyer, E. The Effects of Acclimation to Sunlight on the Xylem Vulnerability to Embolism in Fagus sylvatica L. Plant Cell Environ. 1999, 22, 101–108. [Google Scholar] [CrossRef]
- Watson-Lazowski, A.; Cano, F.J.; Kim, M.; Benning, U.; Koller, F.; George-Jaeggli, B.; Cruickshank, A.; Mace, E.; Jordan, D.; Pernice, M.; et al. Multi-Omic Profiles of Sorghum genotypes with Contrasting Heat Tolerance Connect Pathways Related to Thermotolerance. J. Exp. Bot. 2024, erae506. [Google Scholar] [CrossRef] [PubMed]
- Hacke, U.; Sauter, J.J. Vulnerability of Xylem to Embolism in Relation to Leaf Water Potential and Stomatal Conductance in Fagus sylvatica f. Purpurea and Populus Balsamifera. J. Exp. Bot. 1995, 46, 1177–1183. [Google Scholar] [CrossRef]
- Aranda, I.; Cano, F.J.; Gascó, A.; Cochard, H.; Nardini, A.; Mancha, J.A.; López, R.; Sánchez-Gómez, D. Variation in Photosynthetic Performance and Hydraulic Architecture across European Beech (Fagus sylvatica L.) Populations Supports the Case for Local Adaptation to Water Stress. Tree Physiol. 2015, 35, 34–46. [Google Scholar] [CrossRef]
- Likhachev, E. Dependence of Water Viscosity on Temperature and Pressure. Tech. Phys. 2003, 48, 514–515. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Cano, F.; Valbuena, M.; Gil, L.; Aranda, I. Functional Performance of Oak Seedlings Naturally Regenerated across Microhabitats of Distinct Overstorey Canopy Closure. New For. 2009, 39, 245–259. [Google Scholar] [CrossRef]
- Aranda, I.; Gil, L.; Pardos, J. Seasonal Water Relations of Three Broadleaved Species (Fagus sylvatica L., Quercus petraea (Mattuschka) Liebl. and Quercus pyrenaica Willd.) in a Mixed Stand in the Centre of the Iberian Peninsula. For. Ecol. Manag. 1996, 84, 219–229. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and Let Live: Leaf Senescence Contributes to Plant Survival under Drought Stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Fanourakis, D.; Heuvelink, E.; Carvalho, S.M.P. A Comprehensive Analysis of the Physiological and Anatomical Components Involved in Higher Water Loss Rates after Leaf Development at High Humidity. J. Plant Physiol. 2013, 170, 890–898. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Steiner, U.K. The Fitness Costs of Developmental Canalization and Plasticity. J. Evol. Biol. 2009, 22, 852–860. [Google Scholar] [CrossRef]
- Grime, J.P.; Mackey, J.M.L. The Role of Plasticity in Resource Capture by Plants. Evol. Ecol. 2002, 16, 299–307. [Google Scholar] [CrossRef]
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer Associates Incorporated: Sunderland, UK, 1998; ISBN 9780878937998. [Google Scholar]
- Robson, T.; Rodríguez-Calcerrada, J.; Sánchez-Gómez, D.; Aranda, I. Summer Drought Impedes Beech Seedling Performance More in a Sub-Mediterranean Forest Understory than in Small Gaps. Tree Physiol. 2009, 29, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Fotelli, M.; Rudolph, P.; Rennenberg, H.; Gessler, A. Irradiance and Temperature Affect the Competitive Interference of Blackberry on the Physiology of European Beech Seedlings. New Phytol. 2005, 165, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Gil, L.; Pardos, J. Water Relations and Gas Exchange in Fagus sylvatica L. and Quercus petraea (Mattuschka) Liebl. In a Mixed Stand at Their Southern Limit of Distribution in Europe. Trees 2000, 14, 344–352. [Google Scholar] [CrossRef]
- Mas, E.; Cochard, H.; Deluigi, J.; Didion-Gency, M.; Martin-StPaul, N.; Morcillo, L.; Valladares, F.; Vilagrosa, A.; Grossiord, C. Interactions between Beech and Oak Seedlings Can Modify the Effects of Hotter Droughts and the Onset of Hydraulic Failure. New Phytol. 2024, 241, 1021–1034. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Warren, C.R.; Aranda, I.; Cano, F.J. Responses to Water Stress of Gas Exchange and Metabolites in Eucalyptus and Acacia spp. Plant Cell Environ. 2011, 34, 1609–1629. [Google Scholar] [CrossRef]
- Holland, N.; Richardson, A. Stomatal Length Correlates with Elevation of Growth in Four Temperate Species†. J. Sustain. For. 2009, 28, 63–73. [Google Scholar] [CrossRef]
- Araus, J.L.; Febrero, A.; Vendrell, P. Epidermal Conductance in Different Parts of Durum Wheat Grown under Mediterranean Conditions: The Role of Epicuticular Waxes and Stomata. Plant Cell Environ. 1991, 14, 545–558. [Google Scholar] [CrossRef]
- Robichaux, R.H. Variation in the Tissue Water Relations of Two Sympatric Hawaiian Dubautia Species and Their Natural Hybrid. Oecologia 1984, 65, 75–81. [Google Scholar] [CrossRef]
- Tyree, M.T.; Hammel, H.T. The Measurement of the Turgor Pressure and the Water Relations of Plants by the Pressure-Bomb Technique. J. Exp. Bot. 1972, 23, 267–282. [Google Scholar] [CrossRef]
- Dreyer, E.; Bousquet, F.; Ducrey, M. Use of Pressure Volume Curves in Water Relation Analysis on Woody Shoots: Influence of Rehydration and Comparison of Four European Oak Species. Ann. For. Sci. 1990, 47, 285–297. [Google Scholar] [CrossRef]
- Kubiske, M.E.; Abrams, M.D. Pressure-Volume Relationships in Non-Rehydrated Tissue at Various Water Deficits. Plant Cell Environ. 1990, 13, 995–1000. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Stomatal Protection against Hydraulic Failure: A Comparison of Coexisting Ferns and Angiosperms. New Phytol. 2004, 162, 663–670. [Google Scholar] [CrossRef]
- Al-Salman, Y.; Cano, F.J.; Mace, E.; Jordan, D.; Groszmann, M.; Ghannoum, O. High Water Use Efficiency Due to Maintenance of Photosynthetic Capacity in Sorghum under Water Stress. J. Exp. Bot. 2024, 75, 6778–6795. [Google Scholar] [CrossRef]
- López, R.; Nolf, M.; Duursma, R.A.; Badel, E.; Flavel, R.J.; Cochard, H.; Choat, B. Mitigating the Open Vessel Artefact in Centrifuge-Based Measurement of Embolism Resistance. Tree Physiol. 2019, 39, 143–155. [Google Scholar] [CrossRef]
- Robson, T.M.; Sánchez-Gómez, D.; Cano, F.J.; Aranda, I. Variation in Functional Leaf Traits among Beech Provenances during a Spanish Summer Reflects the Differences in Their Origin. Tree Genet. Genomes 2012, 8, 1111–1121. [Google Scholar] [CrossRef]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to Quantify Conduits in Wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef]
- Valladares, F.; Sánchez-Gómez, D.; Zavala, M.A. Quantitative Estimation of Phenotypic Plasticity: Bridging the Gap between the Evolutionary Concept and Its Ecological Applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio, F.; Aranda, I.; López, R.; Cano, F.J. Elevated Growth Temperature Modifies Drought and Shade Responses of Fagus sylvatica Seedlings by Altering Growth, Gas Exchange, Water Relations, and Xylem Function. Plants 2025, 14, 1525. https://doi.org/10.3390/plants14101525
Rubio F, Aranda I, López R, Cano FJ. Elevated Growth Temperature Modifies Drought and Shade Responses of Fagus sylvatica Seedlings by Altering Growth, Gas Exchange, Water Relations, and Xylem Function. Plants. 2025; 14(10):1525. https://doi.org/10.3390/plants14101525
Chicago/Turabian StyleRubio, Faustino, Ismael Aranda, Rosana López, and Francisco Javier Cano. 2025. "Elevated Growth Temperature Modifies Drought and Shade Responses of Fagus sylvatica Seedlings by Altering Growth, Gas Exchange, Water Relations, and Xylem Function" Plants 14, no. 10: 1525. https://doi.org/10.3390/plants14101525
APA StyleRubio, F., Aranda, I., López, R., & Cano, F. J. (2025). Elevated Growth Temperature Modifies Drought and Shade Responses of Fagus sylvatica Seedlings by Altering Growth, Gas Exchange, Water Relations, and Xylem Function. Plants, 14(10), 1525. https://doi.org/10.3390/plants14101525





.png)



