Abstract
The red seaweed Asparagopsis taxiformis (Bonnemaisoniaceae, Rhodophyta) produces a bioactive natural product, bromoform, which, when fed to ruminant livestock, can eradicate methane emissions. However, to cultivate enough A. taxiformis to produce a yield that would have a meaningful impact on global greenhouse gas emissions, we need to advance our current understanding of the biology of this seaweed species. Here, we used both a domesticated diploid tetrasporophyte (>1.5 years in culture) and wild samples to establish a high-quality draft nuclear genome for A. taxiformis (lineage 6 based upon phylogenetic analyses using the cox2-3 spacer). The constructed nuclear genome is 142 Mb in size (including 70.67% repeat regions) and was determined to encode for approximately 10,474 protein-coding genes, including those associated with secondary metabolism, photosynthesis, and defence. To obtain information regarding molecular differences between cultured and wild tetrasporophytes, we further explored differential gene expression relating to their different growth environments. Cultured tetrasporophytes, which contained a relatively higher level of bromoform compared to wild tetrasporophytes, demonstrated an enrichment of regulatory factors, such as protein kinases and transcription factors, whereas wild tetrasporophytes were enriched for the expression of defence and stress-related genes. Wild tetrasporophytes also expressed a relatively high level of novel secretory genes encoding proteins with von Willebrand factor A protein domains (named rhodophyte VWAs). Gene expression was further confirmed by proteomic investigation of cultured tetrasporophytes, resulting in the identification of over 400 proteins, including rhodophyte VWAs, and numerous enzymes and phycobiliproteins, which will facilitate future functional characterisation of this species. In summary, as the most comprehensive genomic resource for any Asparagopsis species, this resource for lineage 6 provides a novel avenue for seaweed researchers to interrogate genomic information, which will greatly assist in expediating production of Asparagopsis to meet demand by both aquaculture and agriculture, and to do so with economic and environmental sustainability.
1. Introduction
Our ability to identify and harness the potential of biodiversity to meet the environmental challenges posed by agriculture is critical for future food production and security and, while in parallel, mitigate the effects of climate change [1,2]. This notion is particularly pertinent for ruminant livestock, including cattle and sheep, that collectively “belch” the methane produced by their gut microbes, with the produced methane accounting for more than 6% of global greenhouse gas emissions [3]. Methane has a global warming potential 28 times that of carbon dioxide over a 100-year period, and in Australia, around 15% of total greenhouse gas emissions come from agriculture, of which two-thirds is accounted for by the methane produced by ruminants [4]. Therefore, reducing methane emissions from agriculture represents one of the most significant challenges for the sustainability of extant agricultural systems, not just in Australia, but globally.
Recently, the use of seaweed (marine macroalgae) as feed additives for cattle and sheep was investigated [5], with this research demonstrating that one species of tropical-to-warm-temperate red seaweed, Asparagopsis taxiformis (Delile) Trevisan, can largely eliminate enteric methanogenesis in cattle rumen fluid when added in dried form at 2% of the organic matter. This effect was almost exclusively related to one halogenated natural product known as bromoform or tribromomethane [5]. The research was based on the natural product knowledge of the genus A. taxiformis and its evaluation against other marine and freshwater macroalgae [6]. While many seaweeds and microalgae release de novo bromoform [7], A. taxiformis is unique in its capacity to store high concentrations of bromoform (and other halogenated compounds) in specialised gland cells, reaching up to 4% of dry weight. This contrasts with other red algae like Laurencia and Delisea, which store halogenated metabolites in similar distinct cellular structures (e.g., corps en cerise), yet lack comparable bromoform accumulation [8]. This natural source of bromoform is so concentrated that the glands are a prominent feature of A. taxiformis cells under light microscopy, with no other seaweed or plant species known to localise or accumulate bromoform in its tissues to this extent [8].
With regulations against the manufacture, import and export of some of the most effective synthetic additives for mitigating methane emissions [9], local researchers and industry have instead heavily invested in finding natural alternatives [10]. Many seaweeds other than the species of Asparagopsis, and more specifically, the natural products contained within them, have antimicrobial activity. Moreover, while some seaweed additives can alter the ruminant microbiome [11], and others can also reduce methane production; none are comparable to A. taxiformis with activity at <2% feed dry matter, leading to undetectable methane production [5]. A. taxiformis is the only seaweed that can be used as a high-efficacy feed additive for ruminants because it specifically targets the archaea portion of the gut microbiome without impacting fermentation [5].
International interest in the use of a natural feed additive in feedlots, including New Zealand, the United States, and countries in Europe, has shifted the question from “what additive?” to “how can we produce A. taxiformis at scale?” There needs to be significant investment in infrastructure and knowledge to meet the global demand for this seaweed feed additive and to retain the efficacy of the biomass under large-scale production. Controlled, large-scale aquaculture is the only means to deliver a product of consistent quality and quantity (i.e., a minimum content of bromoform that is free from impurities). However, we know that the content of the halogenated natural products in natural populations of seaweeds is variable, with many direct and indirect effects influencing the production of such products, including biosynthetic pathway variation [12], genetic and environmental components [13], through to ecological interactions with microbes [14,15]. For seaweeds such as A. taxiformis with a triphasic diplohaplontic heteromorphic life cycle (i.e., dioecious haploid gametophyte, diploid carposporophyte, and diploid tetrasporophyte phases), there are also life cycle differences in halogenated natural product production [16]. The biological and ecological variation in the production of natural products needs to be understood and addressed before companies can sensibly invest in commercialising the production of A. taxiformis.
By understanding the biosynthetic pathways of bromoform and other halogenated natural products in A. taxiformis and evaluating differential gene expression across the life cycle, critical information can be gained regarding the biology and ecology of the production of bromoform in A. taxiformis under different culture conditions and environments. To date, two draft genomes of A. taxiformis (unknown lineages) have been used to identify a bromoform-producing haloperoxidase gene cluster [termed the marine bromoform biosynthesis (mbb) locus], leading to their recombinant production for functional analysis [17]. Genetic information is critical for fast-tracking the development of this new aquaculture species and for setting the parameters for what is possible within this biological system and guiding investment in research and natural product development. The availability of a high-quality genome can provide the foundation resource to support such multi-level investigations [18], the benefits of which have been demonstrated in other red seaweeds, including Chondrus crispus Stackhouse, Gracilariopsis chorda (Holmes) ohmi, Porphyra umbilicalis Kützing, Pyropia haitanensis (T.J. Chang and B.F. Zheng) N.Kikuchi and M.Miyata [2,11,18,19]. For A. taxiformis, genomic sequencing has already been useful for the elucidation of a bromoform biosynthesis locus [11,17], although limited genomic features were reported in this instance. The addition of transcriptomic and proteomic information enables an in-depth multi-omic assessment of this red seaweed species. For example, the interaction between A. taxiformis and the coral Astroides calycularis (Pallas, 1766) was explored based on the microbiome and metabolomic data [20]. In other red seaweeds, a transcriptomic-derived protein database for Eucheuma denticulatum enabled the proteomic characterisation of extracellular proteins following soluble protein extraction [21]. In the red seaweed Pyropia haitanensis, a genome-derived protein database was used to document the proteins associated with molecular responses to various forms of environmental stress [22].
In this study, we present a draft assembly of the A. taxiformis (lineage 6) nuclear genome, sequenced from a cultured tetrasporophyte that was collected and domesticated from Queensland, Australia, where the first demonstration of the anti-methanogenic effects of the seaweed was conducted [6]. Here, we go on to report the genome features of A. taxiformis in comparison to the nuclear genomes of other red seaweed species, which may assist researchers in fundamental and applied investigations related to climate change mitigation, as well as agricultural and aquacultural production. The robustness of this resource was provided by the use of a multi-omic comparative investigative approach of both cultured and wild tetrasporophytes.
2. Results and Discussion
2.1. Phylogeny of A. taxiformis and Description of the Sample Used for Genomic Analysis
Our phylogenetic analysis of the cox2-cox3 spacer, commonly used in intra-specific diversity studies in Asparagopsis, indicated that the sporophyte sample used in the genome sequencing (2019; submitted as NCBI GenBank accession number OP779373) is a member of lineage 6 (L6) of A. taxiformis [23] (Figure S1). This lineage is common in tropical Australia, including coastal regions of Queensland and Western Australia. Our sample is part of a strain designated the Great Barrier Reef strain [23], the strain used in feeding trials in Australia [24,25]. The genetic diversity of Asparagopsis, both A. taxiformis and A. armata, indicates that multiple distinct lineages are found in both species, which prove there may be many cryptic species within these morphological forms, although formal taxonomic changes have not been made [23,26]. While this tropical Australian lineage is the best studied in relation to its anti-methanogenic effects in livestock so far, the monophyly of all other taxonomically related A. taxiformis lineages, and including A. armata, suggests that the genomic information presented here will serve as an important resource and reference for future comparative studies on all other Asparagopsis genus species.
2.2. Genomic Features of Asparagopsis taxiformis (L6)
Here, we present the first draft genome for the red seaweed A. taxiformis (L6), which was determined to have a total size of 142 megabases (Mb). This high-quality nuclear genome for A. taxiformis provides the basis for subsequent multi-omics exploration of this lineage. The assembled genome of A. taxiformis (L6) contained 3308 contigs and a median contig length of 55,006 base pairs (bp) (Table 1). By comparison to the only other reported A. taxiformis assemblies [17], this assembly had a larger total assembly size (142 Mb vs. 75 Mb and 47 Mb), higher average contig length, and higher median contig length (N50). Based on the 255 eukaryotic BUSCO groups, we found 208 complete BUSCOs (81.6% complete genes), among which there were 183 complete single-copy BUSCOs. In addition, there were only 11 fragmented BUSCOs and 36 absent BUSCOs. Therefore, A. taxiformis (L6) had a better overall assembly compared to A. taxiformis Guam (GCA_018397955.1) and A. taxiformis California (GCA_018397975.1) (File S1a).

Table 1.
Overall statistics for A. taxiformis (L6) genome assembly.
Approximately 70.87% of the A. taxiformis (L6) assembly was interspersed repeats, including 20.71% retroelements, 33.48% DNA transposons, and 0.21% rolling circle sequences, and the remaining 16.47% of identified repeats were grouped as unclassified repeats. The conserved gene structures collected from BUSCO were used for training in AUGUSTUS and predicted 10,863 putative genes in A. taxiformis (L6) genome (File S1b). These genes were further cleaned from microbial contaminants, which resulted in a final number of 10,474 A. taxiformis genes. In addition, we generated full-length genomes for the mitochondria (26,034 bp) and plastid (176,936 bp). By using sequence-based searching against our mitochondrial and plastid genomes, we predicted 50 mitochondrial genes and 227 plastid genes, respectively (File S1c). Among the 50 mitochondrial genes, we found 2 ribosomal RNA (rRNA) genes and 22 different transfer RNA (tRNA) genes.
We explored the comparative nuclear genome size and the number of protein-coding genes across eight red seaweeds with whole genome data available (Figure 1A). Asparagopsis taxiformis (L6) was revealed to have the largest genome at 142.472 Mb for the eight red seaweeds assessed, yet it ranked third in terms of the total number of protein-coding genes. For comparison, the Porphyra umbilicalis genome is predicted to encode 13,125 putative genes and to have a total genome size of 87.889 Mb [1]. In total, the A. taxiformis (L6) genome contained 6737 single exon (monoexonic) genes (64.32%), with monoexonic genes a commonly reported feature of seaweed protein-coding sequences. For example, in the red seaweed C. crispus, genes are remarkably compact, containing only 1.32 exons on average (i.e., many fewer than other organisms of similar genome size), and most genes (88%) are monoexonic [24].
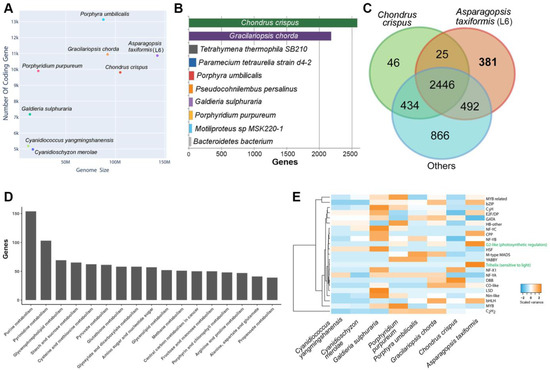
Figure 1.
The Asparagopsis taxiformis (Lineage 6) draft genome and comparative functional overview. (A) Comparison of genome size and the number of protein-coding genes among eight red algal species with completed genome and proteome data. (B) The species distribution based on best-matched BLASTp (2.9.0) search results. (C) Shared Pfam entries among A. taxiformis, C. crispus, and the remaining six red algal genomes. (D) Number of genes associated with metabolism KEGG pathways (50 or more genes). (E) Interspecies comparison of transcription factors, where green font represents relatively large expansion in A. taxiformis.
2.3. Comparative and Functional Features of the A. taxiformis (L6) Genome
To provide a well-annotated genomics resource, we utilised a BLAST2GO pipeline, which is the most efficient approach to associate genes with proteins of known function. Of the 10,474 genes, there were 8409 genes (80.03%) with homologous matches to the NCBI non-redundant (NR) database based on an E-value of 0.00001. The similarity for each BLAST result was defined by division of the exact number of matches over the length of the matched sequence. The majority of matches had over 60% similarity (Figure S2A), and the species distribution showed that the highest identity of A. taxiformis genes was with C. crispus (2583 genes), followed by G. chorda (2182 genes) (Figure 1B), reflecting their evolutionary history at the order level [27]. Among the genes with NR homologues, 6950 genes (66.35%) were further mapped to gene ontology (GO) terms. By examining the top 10 GO terms, we found the most abundant categories consisted of cytosol and membrane genes (cellular components), peptide transport and protein phosphorylation encoding genes (biological processes), and protein and ATP binding encoding genes (molecular function) (Figure S3B).
Of the 10,474 A. taxiformis (L6) genes, 9074 genes contained a protein functional site and domain (assigned by InterPro annotations), while 5931 genes (56.63%) had Pfam protein family/domain mapping. By comparing the Pfam annotation among seven other red algae genomes (same species as Figure 1A), we found that A. taxiformis shared 2471 Pfam domains with C. crispus (supporting our BLAST analysis) and 2938 with the other red algae species (Figure 1C). Of interest, 381 A. taxiformis unique domains could be placed into categories of functional domains, protein families, and repeats based on InterPro annotations (Figure S2C). The most prominent of these, which included 186 A. taxiformis genes, was associated with the tryptophan-aspartic acid 40 (WD40) repeat functional domain: a domain superfamily that encompasses members of the beta-propeller domain family that are known to form interaction scaffolds in protein complexes [28]. Mapping A. taxiformis genes to the KEGG database showed that 5059 genes (48.3%) had KEGG orthologues, of which 1067 genes were categorised into “metabolism”. Of those with 40 or more genes, we found purine metabolism and pyrimidine metabolism contained over 80 A. taxiformis genes (Figure 1D), a finding that suggests that nucleotide hydrolysis, potentially as part of biomolecule production, is of high functional importance in A. taxiformis.
As a key regulator of gene expression, transcription factors (TFs) are central to all developmental processes of an organism. To explore the presence of TF families in the A. taxiformis genome, and for comparison to other red algae with sequenced genomes, we identified 145 TF families (File S1d) and performed a comparative genome-wide analysis of TFs in the seaweeds (Figure 1E). Similar to other seaweeds, TFs belonging to C2H2, bZIP, MYB-related, and bHLH families have the highest number of family members in A. taxiformis. Some TFs are located close to each other in the genome, where, for example, two MYB-related TF encoding loci were determined to be positioned immediately adjacent to each other. Such proximity of genes encoding members of the same gene family strongly infers that they are the product of a gene duplication event as part of the species-specific evolution of the A. taxiformis nuclear genome. In plants, MYB TFs are generally associated with plant development and the response of a plant to either biotic and abiotic stress [29]. The identification of such high numbers of MYB domain containing TFs infers that those members of this TF gene family potentially mediate similar responses to changes to the external environment in A. taxiformis. Interestingly, a single trihelix TF (TTF) was identified in A. taxiformis (L6), while no equivalent TTFs could be identified in the other seven red seaweed species assessed. A TTF protein has a highly conserved three-helix structure (helix-loop-helix-loop-helix) and has been shown to be responsive to light [30]; therefore, identification of a TTF only in A. taxiformis infers that this TF also occupies an important role in the ability of A. taxiformis to respond to changes to its external growth environmental.
Ortholog analysis was performed on eight red seaweed genome-derived proteome datasets (72,277 proteins) that had been subjected to all-against-all BLASTp analysis (E-value of 0.00001). This analysis clustered 56,813 proteins (78.6%) into 9841 orthologous groups, which were used to construct a species tree (Figure 2A,B). Among the orthologous groups, 2430 (11,578 proteins) were species-specific. Interestingly, 15,148 putative gene duplication events were identified among the 8 genomes, all of which are traced back to the node of the species tree on which these duplications occurred. For A. taxiformis (L6), there were 1359 non-terminal duplications. These were mapped to 1233 Pfam domains, and the number of genes in each protein family from each species was aligned with the species tree.
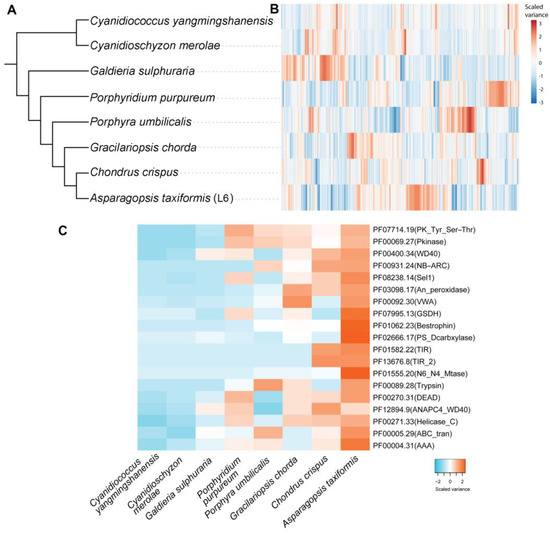
Figure 2.
Comparative Pfam genomics of Asparagopsis taxiformis (L6) and seven other red seaweeds. (A) Phylogenetic tree constructed based on all the orthologous sequences inferred from OrthoFinder. (B) Heatmap showing the clustering of Pfam families based on the gene abundance in each species. Each row is a Pfam domain. (C) The top 20 Pfam domains that underwent a gene duplication event in A. taxiformis following branching from Chondrus crispus. The clustering in the heatmap was based on the number of genes for each Pfam entry (row) in the corresponding species (column).
To better understand lineage-specific gene duplications, we mapped 4829 A. taxiformis genes suspected to be the product of duplication events to Pfam annotations to provide a comprehensive survey of gene duplication in A. taxiformis (e.g., polyploidy). Then we prioritised the Pfam domains by the number of duplicated genes (Figure 2C). Gene duplications often generate new gene copies, which may be under less selection pressure than the template gene. Therefore, these duplicated genes may acquire new expression domains and/or differing function, which forms one of the major driving forces in genome evolution [31]. Potentially the most striking feature was the 66 genes, which were determined to encode a protein product that harbours a N6_N4_Mtase domain in A. taxiformis (L6), compared to six, five, and four such genes in C. crispus, P. umbilicalis and G. chorda, respectively (Figure 2C). In terms of metabolic enzyme duplication, we identified 25 glucose/sorbosone dehydrogenase (GSDH) genes in the A. taxiformis (L6) genome, with this gene number again expanded in A. taxiformis from the number of GSDH genes identified in P. purpureum (1 gene), P. umbilicalis (5 genes), G. chorda (6 genes), and C. crispus (5 genes). In plants, GSDH is important for ascorbic acid metabolism to generate ascorbate precursors [32]. The phosphatidylserine decarboxylases (EC:4.1.1.65, PS_Dcarbxylase), which are known to catalyse the reaction: phosphatidyl-L-serine <=> phosphatidylethanolamine + CO2 [33], are prominent in A. taxiformis (L6), with 15 family members identified, compared to the other analysed red seaweeds that have a combined total of 9 phosphatidylserine decarboxylase genes. In summary, these duplicated genes which encode for enzymes known to function in biosynthesis pathways may provide clues to explore gene innovations in A. taxiformis (L6) required for the biosynthesis of ascorbate precursors and aminophospholipids.
2.4. A. taxiformis (L6) Cultured and Wild Sporophyte: An Integrative Omics Analysis
The availability of a high-quality draft genome provides the first comprehensive resource for the integrative omics-based analysis of A. taxiformis to determine how environmental conditions alter the bromoform content of sporophytes, as well as to link how changes to the expression of the genes, which encode the proteins of this biosynthesis pathway, differ between lab and wild cultivated A. taxiformis. Cultured A. taxiformis (L6) tetrasporophytes were maintained in largely sterile water at a constant temperature, light intensity, and salt level, whereas wild tetrasporophytes are exposed to constantly changing abiotic (e.g., conditions of temperature, light intensity, salt, and nutrient fluctuations) and biotic conditions (e.g., rabbitfish [34]); therefore, the genes associated with defence and stress resistance are likely to have significantly different levels of expression. In addition, tetrasporophytes cultured in the laboratory setting have not been exposed to the growth conditions that would promote their transition into reproductive tetrasporophytes, an artificial bottleneck to their developmental progression, which too would likely induce large gene expression changes in these two distinct populations of A. taxiformis.
Differences in gene expression were therefore next investigated between cultured and wild tetrasporophytes of A. taxiformis. Overall, cultured tetrasporophyte total gene expression exhibited stronger clustering based on principal component analysis (PCA), a finding that readily highlighted the greater variation in gene expression in the wild samples (Figure S3). This may reflect the greater variability in growth conditions, such as temperature and light, that the wild sporophyte samples would have been exposed to compared to the controlled conditions of cultured tetrasporophytes. Of those genes expressed, 399 and 257 genes were identified as significantly differentially expressed (FDR p < 0.05, >±4 log2 fold-change) between cultured and wild tetrasporophytes, respectively (Figure 3A). In cultured tetrasporophytes, there was a relative enrichment of genes whose sequences aligned to enzymes (e.g., kinases, serine carboxypeptidases, and cytochrome P450s) and regulatory factors (e.g., zinc finger proteins and translation initiation factors) (Figure 3B). In addition, cyclin (AtaL6 9753), which encodes a protein well known for complexing with cyclin-dependent kinase (CDK-cyclin complexes) to regulate the progression of a cell through the cell cycle [25], was notably absent in cultured tetrasporophytes yet was highly expressed in wild tetrasporophytes (File S1e). This finding aligns well with our observed suppression of cultured sporophyte progression through to the reproductive stage of their development. We expect that more in-depth molecular comparative analysis of non-reproductive and reproductive (with sporangia) tetrasporophytes, including changes in CDK-cyclin complex coupling, could provide the necessary knowledge to manipulate sporophyte transitions in seaweeds, including A. taxiformis (L6), an area of study that should form a high-priority item of A. taxiformis research by our group in the future.
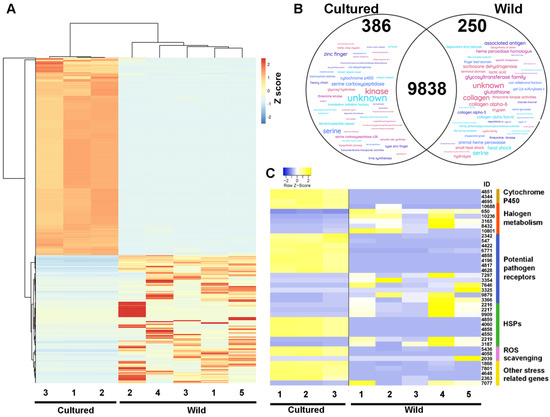
Figure 3.
Summary of differential gene expression between A. taxiformis (L6) cultured and wild tetrasporophytes. (A) Heatmap with hierarchical clustering showing relative gene expression between cultured and wild tetrasporophytes. (B) Venn diagram shows those genes with greater than four-fold higher expression in cultured or wild tetrasporophytes and highlights (Word Cloud) their proposed function based on relative abundance of annotation. (C) Heatmap showing relative expression of genes significantly different between cultured and wild tetrasporophytes that are associated with stress and defence.
In wild tetrasporophytes, there was a relative enrichment of genes that were annotated as haloperoxidases, homologues of animal heme peroxidase-like proteins and heat shock proteins (HSPs), which fit within designated categories (based on C. crispus gene annotations [24]) related to defence and stress-resistance genes (Table 2 and Figure 3C). Therefore, the relative abundance of these transcripts in wild tetrasporophytes formed an interesting finding. The HSP family (including, but not limited to, HSP20, HSP70, and HSP100) are well known for their role in facilitating protein folding, assembly, translocation, and degradation during normal cellular homeostasis [35]. However, they additionally have the ability to direct the stabilisation of proteins and to assist their refolding under stress conditions [24]. We identified that several stress-responsive HSPs were upregulated in wild tetrasporophytes, specifically those members subcategorized within the HSP20 family (molecular weights ~20 kDa). This finding aligns well with research in the red seaweed Pyropia yezoensis, where small-sized HSPs were upregulated during high temperature, oxidative stress, or altered copper levels [36].

Table 2.
Summary of A. taxiformis (L6) genes involved in defence-related and stress-related processes. Number of corresponding genes significantly upregulated (>4-fold) in cultured or wild is shown. For more details, see File S2.
Halogenating peroxidases, or haloperoxidases (HPOs), are enzymes that oxidatively activate halides to the corresponding hypohalites at the expense of peroxides and are categorised into two groups, including (1) heme-dependent or (2) vanadium-dependent haloperoxides, which differ with respect to the prosthetic group and consequently in their catalytic mechanisms [37]. In seaweeds, numerous vanadium haloperoxidases (VHPOs) exist, while vanadium-dependent bromoperoxidases (VBPOs) have been of most interest due to their production of brominated compounds (e.g., bromine), which are assumed to be used in chemical-mediated defences in seaweeds [8]. Knowledge of how brominated compounds are biosynthesised could be used to improve their production [17]. We found that A. taxiformis (L6) tetrasporophytes maintained in culture produced higher levels of bromoform (18.02 ± 2.3 mg/g of dried algae) when compared to those in the wild (4.4 ± 0.96 mg/g of dried algae) (Figure 4A). A total of 21 genes identified in the A. taxiformis (L6) genome returned significant (e-value > 10−3) similarities with type 2 phosphatidic acid phosphatases (PAP2)/VHPOs. Phylogenetic analysis supported three predominant gene clusters, of which, 15 genes had at least some detectable level of expression in cultured or wild tetrasporophytes (Figure 4B). Only the VHPO gene, AtaL6 10688, showed a significant difference, being higher in cultured tetrasporophytes than in wild tetrasporophytes. The AtaL6 9003 gene was determined to have the highest overall expression of the 15 active VHPO genes identified via RNA-seq, but its level of expression was comparable between cultured and wild samples. The AtaL6 9003 gene corresponds to a VBPO previously described as mbb1 of the mbb locus [17]. The mbb locus, known to consist of three VBPO and one NADPH-oxidase (NOX) gene, was identified in A. taxiformis (L6), with VBPO AtaL6 8990 identified to be proximal to mbb1 (Figure 4C). A fifth gene within the mbb locus, denoted as mbb2.5 (AtaL6 9001), was predicted and further confirmed to be present in the A. taxiformis California and Guam genomes (GenBank MN966723 and MN893468, respectively) (Figure 4D) [17]. Conservation of mbb locus genes between the sequenced A. taxiformis genomes indicated significant intra-specific variability for these genes of this locus (e.g., mbb1 returned 96.56–97.74% sequence identity across the three genomes). It is possible that the variability of the mbb locus genes could result from neither the California nor Guam genomes being derived directly from the A. taxiformis Lineage 6 genome, although this cannot be ascertained from the data presented in [6]. Similarly, Lineage 6 of A. taxiformis has not been confirmed from California or Guam. In comparison, sequence analysis of the three VBPOs demonstrated that these proteins are moderately conserved and have similar lengths of between 581 and 590 amino acid residues. The AtaL6 VBPOs all featured a PAP2 domain, which is situated between the early-300 to the mid-500 amino acid residue positions and covered short regions of both high and low sequence conservation. In addition, all three AtaVBPOs contained eight highly conserved PAP2 active site residues, including a histidine (H) residue, to suggest that each AtaVBPO can form a covalent bond to the vanadate cofactor [38].
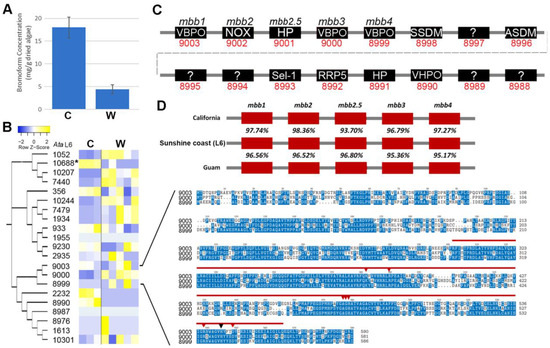
Figure 4.
Comparative sequence analysis of PAP2/VHPOs in A. taxiformis (L6). (A) Bromoform concentration measured in the tetrasporophytes of cultured (C) and wild (W) A. taxiformis. Values correspond to mean % dry weight ± standard error (n = 3). (B) Phylogeny of vBPO genes and their relative level of gene expression (TPM log2) in cultured and wild A. taxiformis sporophytes. An asterisk (*) indicates significantly different (p < 0.05) and >4 FDR fold-change. Multiple sequence alignment shows VBPO protein sequences. The PAP2 domain is highlighted by the bold red line, and the red arrowheads point to active site residues. The black arrowhead denotes a histidine proposed to covalently bond to the vanadate cofactor [34]. (C) Schematic showing genomic organization of contig 5424 containing the mbb gene cluster in A. taxiformis (L6). This gene cluster includes a novel fifth gene within the mbb locus (AtaL6 9001) of uncharacterised function. VBPO, vanadium-dependent bromoperoxidase; NOX, NADPH oxidase; HP, hypothetical protein; SSDM, site-specific DNA-methyltransferase; ?, unknown function; ASDM, adenine-specific DNA methyltransferase; VHPO, vanadium haloperoxidase-like; Sel1, Sel1-repeat containing protein; RRP5, rRNA biogenesis protein 5. (D) Comparison of A. taxiformis (L6) mbb locus to those of the other two A. taxiformis lineages with available genome sequence data.
An in silico secretome analysis of the A. taxiformis (L6) genome-derived proteins, using conventional protein secretion pathway predictions [39], found that 332 proteins were likely secreted from cells (File S1f). This included an expanded family of proteins annotated as “collagen-alpha-like” proteins (~43; 12.95% of total proteins secreted), several of which were enriched (Figure 5) and demonstrated significantly higher levels of gene expression in wild sporophytes (Figure 5A). These proteins all shared the features of a signal peptide and at least one von Willebrand factor type A (VWA) domain with spatially conserved cysteine residues; a structure model indicated similar sequence identity (16.29%) to a proximal thread matrix protein-1 (PDB # 4cn9.2.A) found in the mussel byssus (Figure 5B and Figure S4) [40]. The proximal thread matrix protein 1 can bind collagen, thereby influencing the thread mechanical characteristics such as flexibility [41]. VWA domain-containing proteins are often associated with protein–protein interactions [42]. BLASTp similarity searches demonstrated some conservation (up to 62%) with predicted proteins from the red seaweeds G. chorda and G. domingensis (Figure 5C). Based on the aforementioned evidence, we suggest that these secreted VWA domain-containing proteins in A. taxiformis (L6), herein named rhodophyte VWAs, may help regulate the flexibility of the seaweed and/or mediate substrate attachment. It is important to note here that the cultured tetrasporophytes used in this study were not attached to a substrate. A secreted liquid mucilage is critical for seaweed bioadhesion, allowing for cell wall adhesion to a substrate, then for the cell wall to irreversibly harden post adhesion [43].
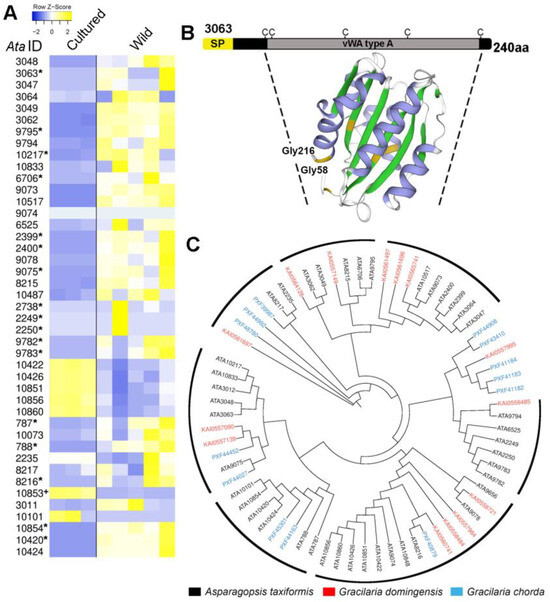
Figure 5.
Identification and comparative analysis of Asparagopsis taxiformis (L6) rhodophyte VWA domain-containing proteins. (A) List of rhodophyte VWAs and their relative levels of gene expression (TPM log2) in cultured and wild tetrasporophytes. The asterisks (*) and the plus symbol (+) indicate significantly higher expression (p < 0.05) in wild and cultured samples, respectively, and with >4 FDR fold-change. (B) Schematic showing organisation of AtaL6 3063 and its basic protein structure. SP, signal peptide; vWA type A, vWA type A domain. (C) Phylogenetic tree of rhodophyte VWAs identified in A. taxiformis (L6), G. chorda, and G. domingensis. Seven clusters that include A. taxiformis (L6) are shown.
2.5. A. taxiformis Tetrasporophyte Proteomics
The comparative gene expression analysis provided protein targets for further investigation, and their isolation (as native proteins) from seaweeds would enable such analysis, including structural and functional characterisation. For example, the A. taxiformis (L6) PAP2/VHPOs, animal heme peroxidase-like, and rhodophyte VWA proteins were of particular interest so their isolation would help for future functional characterisation to provide a more detailed understanding into their role in brominated compound biosynthesis and stress and/or defence responses. The A. taxiformis (L6) genome provided an excellent opportunity for proteomic investigation of this seaweed, with specific interest to isolate the secretome proteins stated above. Of the proteins predicted from the genome, 421 proteins were identified following tetrasporophyte extraction (via the use of Tris and Urea) and proteomic analysis (Figure 6 and File S3), of which 25 were predicted to be secreted, including a VHPO (encoded by AtaL6 1934), an animal heme peroxidase-like (Ata3164) and a rhodophyte VWA protein (AtaL6 10422). Besides putative secreted proteins, 45% of the extracted proteins consisted of enzymes (e.g., lipoxygenase, fructose-1,6-biphosphate aldolase, and peroxiredoxin), 4% phycoproteins (e.g., allophycocyanin beta 18 subunit, phycoerythrin gamma 31 kDa subunit, and phycobilisome linker polypeptides) and 44% that were either a hypothetical protein or a protein of unknown function.
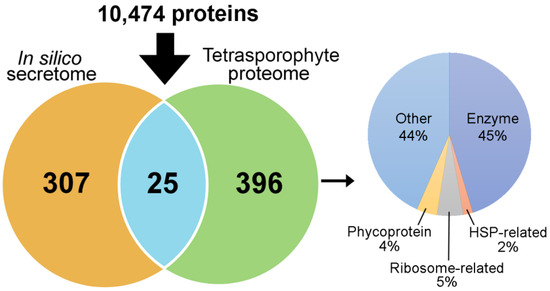
Figure 6.
Venn showing in silico secretome of A. taxiformis (L6) genome-derived proteins (based on SignalP and TMHMM predictions) and tetrasporophyte identified proteins, also with distribution of functional categories.
This tetrasporophyte proteomic investigation of A. taxiformis (L6) will complement other experimental approaches to protein-based production in A. taxiformis (and other seaweeds), such as production of recombinant proteins (e.g., [17]) and gene/protein knockdowns. In addition, proteins that were annotated to enzymes were highly represented in the proteome; seaweed enzymes show prebiotic and nutraceutical potential, primarily through applications that develop bioactive compounds from seaweeds, as well as assist in the extraction and hydrolysis of macromolecules [44]. Also, phycobiliproteins (water-soluble pigments) are highly sought after as natural pigments of food, cosmetics, dyes, and other industries [45], so their purification and identification via a genomic resource database will assist the development of approaches for large-scale purification.
3. Materials and Methods
3.1. Asparagopsis taxiformis Tissue Collection
Triplicates of wild A. taxiformis tetrasporophytes were collected both on 24 February and 18 March 2021 from rocky reefs at Moffat Beach, Queensland, Australia (26°47′21.0″ S 153°08′37.5″ E), at a water depth of between 1 to 3 metres (m). Samples were collected with nitrile disposable gloves, rinsed with sterile seawater (SSW), placed in sterile centrifuge tubes, snap-frozen in a Taylor–Wharton CP300 Cryogenic Liquid Nitrogen (LN2) Shipper, and then stored in a laboratory freezer at −80 °C prior to use for DNA extraction. Additionally, a fresh portion of these samples were fixed in 4.0% paraformaldehyde in SSW for microscopic analysis.
Cultured A. taxiformis tetrasporophytes were grown vegetatively from tetrasporophytes collected at Moffat Beach (30 July 2019) and maintained under standard indoor cultivation conditions of a day/night light cycle of 16 hours (h) of light and 8 h of dark and a constant temperature of 22 °C. The tetrasporophytes were cultivated in aerated 2.0 litre (L) flasks with fortnightly SSW changes, along with the addition of F/8 enrichment medium. Healthy filaments were isolated at a small scale in 5 to 30 millilitre (mL) Petri dishes, and these were maintained with regular SSW exchanges (enriched with F/8) until April 2020. At this time, the cultured filaments were upscaled to 280 mL inverted culture vessels (“hatchers”) at a density of 1.0 grams per litre (g/L). Once upscaled to hatchers, cultures were maintained with weekly SSW changes (i.e., filtered and autoclaved SSW supplemented with 0.5 mL/L of F/4 medium and 448 micrograms per litre (µg/L) of germanium dioxide (GeO2) to prevent diatom growth. Cultured tetrasporophytes were then subsampled for microbial analysis on 24 February 2021 prior to processing for genomic analysis. Five subsamples were obtained from distinct culture vessels and snap-frozen in LN2 prior to microbial DNA extractions; then, fresh fragments were fixed in 4.0% paraformaldehyde in SSW for microscopic analysis.
3.2. Library Construction and Sequencing
Asparagopsis taxiformis cultured tetrasporophytes (2019 November collection, average temperature 23 °C) were selected as the most appropriate material for genome sequencing based on minimal microbial contamination (see section above). This sample of clonally propagated filaments was subsequently analysed using DNA barcoding with the Cox2-3 spacer to confirm the cultured sample was A. taxiformis lineage 6 (L6), Great Barrier Reef strain [23], as were additional cultures collected and domesticated from the same site in 2018 and 2021. DNA extraction, amplification, and phylogenetic analysis followed procedures described previously [23,26].
For genomic DNA sample preparation, approximately (~) 20 g (g) fresh weight of a single-cultured sporophyte was harvested from hatcher vessels (28 January 2021) and snap-frozen in LN2. An approximately 5.0 g sample was shipped in dry ice to BGI, Hong Kong. Following nuclear DNA extraction via the standard protocols of BGI Genomics, a hybrid sequencing strategy was used for genome sequencing via combining both long reads from PacBio sequencing and short reads from BGI DNBSeq sequencing. Isolated genomic DNA was subjected to short-read library preparation with standard paired-end protocols. In brief, 1.0 micrograms (µg) of genomic DNA was randomly fragmented by Covaris, followed by fragment selection by Agencourt AMPure XP-Medium kit to an average fragment length of 300 to 400 base pairs (bp). Selected fragments were repaired and adenylated at their 3′ terminus, and then adaptors were ligated to the ends of each 3′ adenylated fragment. Each product was then amplified by PCR and purified using the Agencourt AMPure XP-Medium kit. The purified double-stranded PCR products were heat denatured into single strands, and these were then circularized by the splint oligo sequence. The single-strand circle DNA (ssCir DNA) were formatted as the final library and qualified by quality control. Subsequently, libraries with insert lengths of up to 370 bp were next constructed and sequenced using a MGISEQ-2000. As part of this sequencing process, each ssCir DNA molecule formed a “DNA nanoball (DNB)”, which contained more than 300 copies of each insert through rolling-cycle replication. The DNBs were loaded into the patterned nanoarray by using high-density DNA nanochip technology. Finally, 150 bp paired-end reads were obtained by combinatorial Probe-Anchor Synthesis.
To sequence longer reads, the high molecular weight DNA fraction of the A. taxiformis nuclear genome was sheared into fragments of approximately 20 kb using a Covaris g-tube (part No. 520079; KBiosciences, Maple Park, UK). The preparation and sequencing of the SMRTbell DNA library were performed according to the manufacturer’s protocols (Pacific Biosciences, Menlo Park, CA, USA). The SMRTbell library with 20 kb insert size was generated with the BluePippin system (Sage Scientific, Beverly, MA, USA). One lane was loaded and run on the Sequel System, which utilises single molecule, real-time (SMRT) sequencing with fluorescently labelled nucleotides.
3.3. Genome Assembly and Functional Evaluation
The DNBSeq generated a total of ~4.6 gigabases (Gb) of genomic data, which was composed of 31,146,070 raw reads. By filtering out low-quality reads, unknown nucleotides, adapters, and duplicated reads, a total of 29,530,652 clean reads with a GC content of ~44% were obtained. For pair-end reads, the k-mer frequencies (k = 17) were computed via the Jellyfish v2.2.10 dataset [46]. Finally, the A. taxiformis nuclear genome size was estimated based on the 17-mer depth distribution. In total, the PacBio sequencing approach generated ~11.8 Gb of data, which consisted of 1,137,468 polymerase reads. With >80x PacBio data, PacBio reads were firstly corrected and assembled using Canu (v1.7.1) [47]. Contigs were then polished using Pilon based on the short reads from DNBSeq [48]. The raw data from all sequencing approaches reported here was then submitted to the NCBI Bioproject and SRA database under a Bioproject accession number PRJNA809757. By using a sequence-based BLAST search approach, the published A. taxiformis mitochondria genome (NCBI accession number KJ398158.1) was compared to the assembly constructed here. The complete mitochondrial genome of A. taxiformis and the genes it encodes were then predicted. By running the same procedure on a complete A. taxiformis plastid genome (NCBI accession number NC_031148.1), we identified the putative plastid genes in our assembly.
Based on the assembly, overall statistics were calculated for GC content and median contig length. A Benchmarking Universal Single-Copy Orthologs (BUSCO 5.2.2) analysis was then performed to evaluate the accuracy and completeness of our assembly [49]. In general, BUSCO focused on core genes based on evolutionarily informed expectations of gene content of those conserved sequences. BUSCO relied on the Eukaryota_odb10 dataset from the OrthoDB database, which was used to construct single-copy gene sets of several large evolutionary branches. Via this approach, the BUSCO metric (% complete genes) can be used as a functionally complementary tool to the other technical metrics performed, such as median contig length N50.
3.4. Repeat Masking and Transposable Elements
Before we explored the functional fragments in the genome, specifically the protein-coding regions of the A. taxiformis nuclear genome, we examined the repetitive regions present in the assembly. By using RepeatModeler2 [50], a de novo repeat library was built. The repeat library, including known self-mobilizing transposable elements from other species, was then subjected to assessment by RepeatMasker (v 4.1.2-p1) [51] to find and mask homologous repeats in the assembled genome using RMBLAST (v 2.10.0+) as the default search engine. The total length of repetitive sequences was 100,684,772 bp, accounting for 70.67% of the assembled genome.
3.5. Gene Prediction and Functional Annotation
Since the BUSCO output is a set of highly conserved genes in our assembly that are single copy in most eukaryotic species, these sequences were used as a training dataset for the gene prediction tool, AUGUSTUS [52]. Briefly, AUGUSTUS (3.4.0) used the summarized features, such as the length distribution of single exons and the distribution of the number of exons per gene, to optimise gene prediction. By inputting the assembly after repeat masking, we are able to predict the protein-coding regions [52]. In total, the AUGUSTUS prediction identified 10,863 genes. Based on the predicted gene sequences and the translated protein sequences, we then ran the BLAST2GO pipeline to annotate and predict the putative function of each identified gene [53]. In brief, BLASTp was used to search the NCBI non-redundant protein databases [54] and Kyoto Encyclopedia of Genes and Genomes (KEGG) [55] with a threshold of E-value less than 1E−5. The top five BLASTp alignment results were used to extract the functional annotations. In addition to the BLASTp approach, the InterProScan tool was also utilised to identify all possible functional domains of each of the 10,863 proteins. To annotate the protein family information, HMMer was used to search all the Pfam protein family Supplementary Files [56].
3.6. Genome Decontamination
Since seaweeds, including A. taxiformis, coexist in symbiosis with their associated microbiome, any genomic data may contain microbial genomic contamination. In order to present a microbial contaminant-free genome, the predicted protein-coding genes were subjected to an in silico clean-up pipeline. Initially, the nucleotide sequences of the 10,863 protein-coding genes were cleaned using the FCS-GX tool [57] that searches for potential microbial contaminants using BLASTn against the NCBI non-redundant nucleotide database. However, it is important to note here that the majority of seaweed genomes currently deposited into this database have only been partially constructed to the scaffold level. When comparing gene sequences against considerably longer scaffolds, the resultant E-value may be low due to poor scaffold coverage. In our case, this resulted in several false positive results (i.e., a nucleotide sequence was falsely denoted as a contaminant). To prevent this, the translated open reading frames of those genes denoted as contaminants were subjected to further analysis using DIAMOND BLASTp [58] against a manually curated database, which contained all the reviewed Swiss–Prot amino acid sequences, as well as both reviewed and unreviewed (TrEMBL) amino acid sequences originating from algal groups rhodophyta, phaeophyceae, and chlorophyta [59] Following this analysis, only those genes that had a confident (E-value < 0.00001) top BLAST hit against a microbial subject (i.e., bacteria, archaea, and viruses) were classified as contaminants (n = 389) and removed (File S1g). Following this refinement, 10,474 genes were confidently predicted to be encoded by the A. taxiformis nuclear genome (File S1h).
3.7. Comparative Genome Analysis
To explore the common and unique functions of the A. taxiformis genes identified in our assembly, we first downloaded seven high-quality representative genomes and proteomes of red algae (Cyanidiococcus yangmingshanensis, Cyanidioschyzon merolae, Galdieria sulphuraria, Porphyridium purpureum, Porphyra umbilicalis, Gracilariopsis chorda, and Chondrus crispus) from NCBI based on the RefSeq category as a representative genome (File S1i). A comparative proteome analysis was then performed using OrthoFinder2. This provided a genome-wide comparison by grouping orthologous proteins. In brief, eight proteome sequences were used as input for OrthoFinder2 [60] to predict the orthologous relationship. The output from OrthoFinder2 includes the phylogenetic trees for thousands of detected orthologous groups. In OrthoFinder, these sequences were concatenated into a “supergene” and used for multiple sequence alignment by MAFFT [61]. Then, the consensus phylogenetic information from the orthologous groups was inferred by STAG algorithm [62]. The final unrooted species tree was built by FastTree using the maximum likelihood method [63]. Based on the species tree, the gene duplication events that were satisfied by the duplication-loss-coalescent model were extracted at each branch of the species tree. To obtain the basic functional information, we ran HMMer and extracted all the Pfam domain information for each of the seven proteomes [56]. For data analysis and visualisation, all statistical analyses were generated by R language. The basic bar plots were generated using the Excel chart function. The overlapping relationship of Pfam annotations and the heatmaps of the comparative genomics analyses were generated using in-house R scripts.
3.8. Targeted Gene Annotation
To predict transcription factors, we downloaded all known transcription factors from plant genomes in PlantTFDB version 4 [64] and then mapped our proteome to these. Finally, all the conserved transcription factor families were assigned based on the best match from BLAST output. Genome annotations were then used to target our investigation onto genes associated with (1) cell structure and regulation, (2) defence-related and stress genes, and (3) secreted proteins. The identification of genes in these groups was performed by using homologues identified in C. crispus [24] as BLASTp searches in the A. taxiformis (L6) genome (E-value cutoff 10−3). The mbb locus (involved in halogenated natural product synthesis) was identified through BLASTp for each mbb gene (GenBank MN966723). ExPASy Translate tool was used to identify the presence of open reading frames and introns across the mbb locus. The SignalP (Version 5.0) [65] and TMHMM (Version 2.0c) [66] tools were used to predict secreted peptides and proteins with a transmembrane domain. Multiple sequence alignments of the protein and nucleotide sequences were generated using Clustal Omega [67] in a ClustalW output format. NCBI Conserved Domains [68] were used to locate the domain and active site residues. Clustal Omega was utilised to determine nucleotide identity. Predicted structure homology modelling was performed using the SWISS-MODEL workspace [69].
3.9. Cultured and Wild Sporophyte Samples for Comparative Bromoform Analysis and RNA-Seq Gene Quantification
Cultured tetrasporophyte (filamentous, non-reproductive) of A. taxiformis (L6) were collected as described above. Wild tetrasporophytes (filamentous, non-reproductive) of A. taxiformis (L6) were also collected from Moffat Beach, Queensland, from subtidal depths of ~0.5 to 3.0 m (July 2020) for comparisons to the cultured strains. Wild sporophyte samples were transported in seawater to the laboratory of the University of the Sunshine Coast. Within 1 h, the samples were cleaned with sterile seawater to remove visible mud, epiphytes, and epibionts. All samples were then immediately snap-frozen in 1.5 mL microfuge tubes in LN2 and stored at −80 °C until use.
For gas chromatography-mass spectrometry (GC-MS) quantification of bromoform, bromoform was extracted from cultured and wild sporophytes (n = 3) by solid-liquid extraction of 0.02 g of dried, milled biomass into 4.0 mL of methanol, which contained a naphthalene internal standard, using the previously described method [70]. Samples were stored for 3 days at 4 °C prior to filtration and GC-MS analysis. Chromatography was performed on a Perkin Elmer Clarus 580 GC tandem Perkin Elmer Clarus SQ8S MS using a DB-5 column (Perkin Elmer Elite-5MS, 30.0 m × 0.25 mm × 0.25 µm). Injections (1.0 µL) were introduced to a 280 °C injection port with a 50:1 split ratio. The gas chromatography parameters were as follows: 40 °C for 1 min, increased to 250 °C at a rate of 20 °C/min, and immediately followed by a 30 second (s) equilibration time. The carrier gas used was helium, with a flow rate of 1.0 mL/min. The mass spectrometer analysed a mass range from 50 to 340 (m/z), from 3.0 to 12.0 min at 70 eV. Quantitation was conducted by referencing the TIC integrated by TurboMass software against analytical standard calibration curves. Final bromoform concentration did not account for the moisture content of biomass (typically ~3–5% under laboratory conditions).
For genome-guided RNA-seq quantification for cultured and wild tetrasporophytes, total RNA was extracted from the experimental samples (n = 8; 3 × cultured samples and 5 × wild samples), using a RNeasy Plant Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s protocol with the following modifications: 82 ± 8 mg of tissue was ground into a fine powder under LN2 before further lysis using Buffer RLT supplemented with 1.0% (v/v) β-mercaptoethanol. The extracted total RNA was treated with a DNA-freeTM DNA Removal Kit (Invitrogen, Carlsbad, CA, USA) to reduce genomic DNA contamination for cultured tetrasporophytes. RNA quality was measured using a NanoDrop2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) and the concentration and integrity verified using an Agilent RNA 6000 Nano assay run on an Agilent 2100 Bioanalyzer (Agilent, Lexington, MA, USA).
RNA samples (n = 8) were either sent on dry ice or stabilised for transport at ambient temperature by drying in GenTegra RNA tubes (GenTegra, Pleasanton, CA, USA) on a SpeedVac concentrator (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Whole transcriptome sequence analysis by RNA-seq was performed by Novogene (Singapore) using Next-Generation Illumina sequencing, according to their standardised workflow. This process involved construction of cDNA libraries before sequencing of cDNA fragments using an Illumina NovaSeq 6000 System (raw sequence reads have been deposited in the NCBI GenBank PRJNA809757). Differential gene expression between cultured and wild tetrasporophyte RNA-seq data was mapped against the A. taxiformis (L6) genome using the CLC Genomics Workbench (Ver. 21.0.3) with default parameters. Transcript abundances were converted to transcripts per million (TPM) for each gene. Genes with significant upregulation or downregulation were identified using a significance cutoff set at a false discovery rate (FDR) p-value of <0.05. Differentially expressed genes (DEGs) underwent Gene Ontology (GO) annotation obtained using Blast2Go (E-value < 1.0E−3), InterProScan, and GO Mapping [71] performed on OmicsBox software, (Ver. 3.1). Venn diagrams were constructed to compare the total number of expressed genes between each replicate for the cultured and wild samples using the tool, Venn Diagram Maker (Good Calculators). A heatmap of DEGs from all of the cultured and wild replicates was constructed using ClustVis, utilising Z-Scores as input data [72].
3.10. Asparagopsis taxiformis Sporophyte Proteomics
Cultured tetrasporophyte samples (as described above) were snap-frozen and manually powdered with a mortar and pestle in LN2. For a single extraction, approximately 100 mg of the milled sample was homogenised in 20 mM Tris-HCl pH 7.4 buffer containing protease inhibitor (Merck, Bayswater, VIC, Australia) and then briefly vortexed and centrifuged at 4000× g for 25 min at 4 °C. The resulting supernatants and pelleted material were then extracted separately.
Initial supernatants were then further extracted by non-acetone and acetone procedures. For extractions without acetone, each supernatant was collected and processed through an Amicon Ultra-4 centrifugal filter unit (3 kDa cutoff; Millipore Corporation, Billerica, MA, USA) at 4000× g for 40 min (4 °C); then >3 kDa and <3 kDa samples were lyophilized in a vacuum concentrator (Express SpeedVac Concentrator SC250, Thermo Fisher Scientific). For extractions with acetone, the filtered supernatants were mixed with a pre-chilled (−20 °C) 100% acetone (volume = 4 times that of each protein sample) and stored overnight at −20 °C to allow for precipitation. Following centrifugation at 4000× g for 25 min at 4 °C, supernatants were decanted, and the protein pellets were air-dried to evaporate the acetone. Protein pellets were resuspended in lysis buffer (6.0 M urea), briefly vortexed, and then centrifuged at 4000× g for 25 min at 4 °C. As for the without acetone samples, the supernatant was collected and centrifuged through an Amicon Ultra-4 centrifugal filter unit (3 kDa cutoff); then >3 kDa and <3 kDa samples were lyophilised in a vacuum concentrator.
Lyophilised protein samples were resuspended in 0.1% (v/v) trifluoroacetic acid (TFA) in Milli-Q water and then processed by solid-phase extraction using a C18 Sep-Pak cartridges (500 mg sorbent per cartridge, 55 to 105 µm particle size; Waters), following the manufacturer’s instructions, to ensure complete removal of salts from all samples. Eluates were lyophilised in a vacuum concentrator (Express SpeedVac Concentrator SC250, Thermo Fisher Scientific) and stored at −20 °C prior to analysis. Samples extracted with acetone were trypsin-digested as previously described [73], following resuspension in 6.0 M urea. Samples were analysed by ultra-high-performance liquid chromatography (uHPLC) followed by mass spectrometry (MS) to identify proteins as per the previously described procedure [73]. A 6.0 µL aliquot of each sample was injected onto a 50 mm × 300 µm C18 trap column (Agilent Technologies, Mulgrave, VIC, Australia) and de-salted for 5 min using 0.1% (v/v) formic acid (aq) at 30 µL/min. The trap column was then placed in line with a 150 mm × 75 µm 300SBC18, 3.5 µm nano HPLC column (Agilent Technologies, Australia) for LC-MS analysis. Peptides were eluted at a flow rate of 300 nanolitres per min (nL/min) using a linear gradient of 1.0% to 40% solvent B (solvent A = 0.1% formic acid (v/v) in Milli-Q water; solvent B = acetonitrile: 0.1% formic acid (aq) 90:10) over 35 min followed by a steeper gradient from 40% to 80% solvent B over 5 min. The gradient was held at 80% solvent B for 5 min to wash the column and then returned to 1.0% solvent B for equilibration prior to injection of the next sample. The ion-spray voltage was set to 2400 V, declustering potential (DP) 100 V, curtain gas flow 25, nebuliser gas 1 (GS1) 12, and interface heater at 150 °C. The mass spectrometer acquired 500 ms full scan TOF-MS data, followed by 20 × 50 ms full-scan product ion data in an Information Dependent Acquisition (IDA) mode. Full-scan TOF-MS data was acquired over the mass range 350–1800 m/z and for product ion MS/MS 100–1800 m/z. Ions observed in the TOF-MS scan exceeding a threshold of 100 counts and a charge state of +2 to +5 were set to trigger the acquisition of product ion MS/MS spectra of the resultant 20 most intense ions. The data was acquired and processed using Analyst TF 1.5.1 software (ABSCIEX, Toronto, ON, Canada).
Proteins were analysed using PEAKS v7.0 (Bioinformatic Solutions Inc., Toronto, ON, Canada) against the protein database built from the reference A. taxiformis (L6) genome-derived protein database. De novo sequencing of proteins, database searches, and characterisation of specific post-translational modifications were used to analyse the raw data; FDR was set to ≤1.0%, and [−10 × log(p)] was calculated accordingly, where p is the probability that an observed match is a random event. The PEAKS used the following parameters: (i) precursor ion mass tolerance, 0.1 Da; (ii) fragment ion mass tolerance, 0.1 Da (the error tolerance); (iii) tryptic enzyme specificity with two missed cleavages allowed; (iv) monoisotopic precursor mass and fragment ion mass; (v) a fixed modification of cysteine carbamidomethylation; and (vi) variable modifications including lysine acetylation, deamidation on asparagine and glutamine, oxidation of methionine, and conversion of glutamic acid and glutamine to pyroglutamate. MS raw data was deposited into the PRIDE database under accession number PXD035669.
4. Conclusions
Our research has generated a high-quality reference genome for A. taxiformis (lineage 6) and identified substantial differences in gene expression patterns between tetrasporophytes collected from natural habitats versus those maintained in laboratory cultures. These differences were especially pronounced in genes associated with stress response mechanisms and defensive functions. Wild sporophytes exhibited upregulation of genes associated with heat shock proteins (HSPs), haloperoxidases, and oxidative stress responses, reflecting their adaptation to fluctuating environmental conditions. In contrast, cultured sporophytes showed enrichment in regulatory factors like kinases and TFs, likely due to their stable, controlled growth environment. Notably, the cyclin gene (AtaL6_9753), critical for cell cycle progression, was highly expressed in wild sporophytes but absent in cultured ones, suggesting suppressed reproductive development under laboratory conditions.
Our results also provided novel insights into the conservation and variability of the bromoform biosynthesis (mbb) locus across A. taxiformis lineages. The mbb locus, comprising VBPOs and a NOX gene, was identified in lineage 6, with AtaL6_9003 (mbb1) showing high expression in both wild and cultured tetrasporophytes. Comparative analysis revealed sequence variability in mbb genes among different A. taxiformis genomes (e.g., California and Guam lineages), suggesting lineage-specific adaptations in bromoform production. Thus, our study supports evidence of a biosynthetic pathway for bromoform in Asparagopsis that is driven by the VBPO proteins [74]. Besides VBPOs, our genomic analysis identified 21 genes with significant similarities to type 2 phosphatidic acid phosphatases (PAP2)/vanadium haloperoxidases (VHPOs), of which 15 showed detectable expression in tetrasporophytes. Our findings that cultured tetrasporophytes produced four times higher levels of bromoform than wild specimens further support the connection between these enzymes and bromoform biosynthesis, although additional research is needed to explicitly link these genes to the specific biochemical steps outlined in prior work. This enhanced understanding of the genetic and enzymatic mechanisms driving bromoform production in Asparagopsis could be leveraged to improve the production of these valuable brominated compounds.
Additionally, proteomic analysis uncovered novel secretory proteins, including rhodophyte von Willebrand factor A (VWA) domain-containing proteins, which may play a role in gland cell function and halogenated compound storage. These findings enhance our understanding of A. taxiformis biology and provide a foundation for optimising its cultivation for methane mitigation in livestock.
The genome sequencing of A. taxiformis (L6) has greatly enhanced the genetic resources available for this much-lauded seaweed, most well-known for its potential in mitigating methane production in ruminants and facilitating further comparisons between lineages. However, the potential applications of all lineages of A. taxiformis in agriculture and aquaculture are diversifying, with new research and development relating to enhancing fish production and boosting immunity [75], novel antimicrobial and biotechnology uses [76], as well as environmental applications with respect to understanding and utilising A. taxiformis where it has become a pest and invasive species [77]. More specifically, A. taxiformis is an invasive species within Europe and the Mediterranean Sea [78], whose impact on the resident ecosystem and coastal biodiversity is still debatable. For instance, the spread of Asparagopsis affects the composition, variability, and relative abundance of the associated macrofauna [79]. A recent microbiome and metabolomic-based study also indicated that the interaction between A. taxiformis and the coral Astroides calycularis could induce metabolic changes in both species. In another ecological example, Asparagopsis is associated with the distribution and abundance of peracarid crustaceans [80]. Therefore, future genomics-driven chemical ecology studies might provide insight to estimate system responses to macroalgal invasions based on the cascading effects of observed epifaunal assemblage changes.
The original commercialisation of bioactives from Asparagopsis related to extraction of antimicrobials for use in cosmetic and human care products was patented in the 1990s [81]. In addition, A. taxiformis has demonstrated potent antioxidant and cytotoxic activity, indicating its potential use of derived products as an anti-aging drug [82]. Moreover, it is a rich source of bioactive compounds such as iodine, lipids, carrageenans, and cellulose [75,83]. Finally, the A. taxiformis (L6) genome allows for more robust comparative evolutionary comparisons and assessment of genome innovations in seaweed.
To facilitate precision genetic manipulation of this seaweed for large-scale aquaculture production, we have shared our high-quality draft genome assembly, which includes well-annotated gene and protein sequences, which can be used for future in-depth research of the red seaweeds. This could include the use of modern molecular techniques such as CRISPR-catalysed mutagenesis for the targeted manipulation of specific biosynthesis pathways (e.g., the bromoform biosynthesis pathway) in A. taxiformis. Furthermore, due to the significant variability between A. taxiformis lineages, there also exists the potential for the application of advanced genomic tools such as Nanopore technology to further characterise both the DNA and RNA landscapes of individual lineages. Similarly, our transcriptomic comparison of gene expression, including the expression of TF encoding genes, in cultured and wild A. taxiformis (L6) tetrasporophytes revealed considerable differences in the level of expression of individual genes in these two samples originating from dramatically different growth environments. In plants, including the genetic model plant species Arabidopsis thaliana (Arabidopsis), small non-coding RNAs (sRNAs), including the microRNA (miRNA) class of sRNA, have been demonstrated to act as master regulators of TF gene expression as part of the adaptive or defensive response of a plant to environment stress or invading pathogens, respectively [84,85,86]. Thus, the availability of a genome sequence will enable the future identification of A. taxiformis-specific miRNAs involved in stress responses and/or pathogen defence, and these novel miRNA species could potentially be used as the basis of the development of artificial miRNA (amiRNA) technology for use in A. taxiformis, and other red seaweed species, for highly specific gene expression manipulation [87,88]. At present, across the globe, significant investment has led to the farm-level demonstration of the utility of A. taxiformis as an aquacultural species. Therefore, the timely delivery of this database resource could further identify A. taxiformis (L6) as an aquacultural species of importance, and via the functional characterisation of genes associated with bromoform synthesis, stress responses, pathogen defence, and reproduction, new and exciting avenues for marker-assisted seaweed breeding will be identified and used in the future to generate novel A. taxiformis lineages that meet the demands of the consumer market.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14101523/s1, File S1: (a) Overall statistics for Asparagopsis genomes. (b) Genome annotation, including gene ID, protein description, length, and sequence. (c) Summary of mitochondrial and plastid genes predicted from their genomes. (d) Transcription factors in Asparagopsis taxiformis (L6). (e) Differential gene expression between cultured and wild sporophytes. (f) In silico secretome. (g) List of the taxonomy IDs for all Asparagopsis taxiformis (L6) genomic sequences classified through the miniKraken database. Yellow, genes which were excluded from final genome. (h) Genome annotation, including gene ID, protein description, length and sequence. (i) Summary of red algae genomes used for comparative analysis; File S2. List of Asparagopsis taxiformis (L6) genes involved in defence and stress; File S3. Sporophyte proteome; Figure S1: Phylogenetic analysis of A. taxiformis used in this study, based on cox2-cox3 spacer; Figure S2: Summary of (A) A. taxiformis (L6) gene BLAST match identity, (B) number of genes for the top ten most abundant gene ontology terms in cellular component, biological process, and molecular function, and (C) summary of A. taxiformis (L6) gene InterPro annotations; Figure S3: Principal component scatter plot for RNA-seq of culture (C) and wild (W) sporophyte; Figure S4: SWISS-MODEL structure predication for protein encoded by A. taxiformis (L6) gene 3063, using a proximal thread matrix protein (PDB # 4cn9.2.A) as template.
Author Contributions
M.Z. carried out the analyses and developed the database. Z.P., J.W. and A.W.-T. undertook sample collection and preparation for sequencing. Z.P. and T.W. performed proteomics analysis. T.L. carried out in silico secretome analysis of samples. L.M. and C.O. helped to characterise gene families. C.P. and T.L. performed genomic contamination analysis. D.H. performed bromoform concentration analysis. G.C.Z. performed analysis of strain. M.Z., A.H.C., N.S., E.S., A.L.E., G.C.Z., N.A.P. and S.F.C. conceived the idea and helped write the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Australian Research Council Discovery Project (DP200103013).
Data Availability Statement
Raw data of all genome sequencing was submitted to NCBI BioProject and SRA database under a BioProject accession number PRJNA809757. Phylogenetic identification data was submitted under GenBank accession number OP779373. Mass spectrometry peptide raw data was deposited into the PRIDE database under accession number PXD035669. The genome assembly can also be accessed via University of the Sunshine Coast at https://doi.org/10.25907/00708.
Acknowledgments
This research was supported by computational resources provided by the Australian Government through QCIF under the National Computational Merit Allocation Scheme. Storage in this research was supported by the use of the Nectar Research Cloud, a collaborative Australian research platform supported by the NCRIS-funded Australian Research Data Commons (ARDC). We acknowledge the traditional owners of the land and sea in which this work was done, the Kabi Kabi/Gubbi Gubbi people in the area now known as the Sunshine Coast, Queensland, Australia.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GO | Gene ontology |
| HSP | Heat shock protein |
| L6 | Lineage 6 |
| NOX | NADPH-oxidase |
| NR | Non-redundant |
| PAP2 | Type 2 phosphatidic acid phosphatases |
| rRNA | Ribosomal RNA |
| SSW | Sterile seawater |
| TF | Transcription factor |
| TTF | Trihelix transcription factor |
| VBPO | Vanadium-dependent bromoperoxidase |
| VHPO | Vanadium haloperoxidases |
| VWA | von Willebrand factor type A |
References
- Brawley, S.H.; Blouin, N.A.; Ficko-Blean, E.; Wheeler, G.L.; Lohr, M.; Goodson, H.V.; Jenkins, J.W.; Blaby-Haas, C.E.; Helliwell, K.E.; Chan, C.X.; et al. Insights into the red algae and eukaryotic evolution from the genome of Porphyra umbilicalis (Bangiophyceae, Rhodophyta). Proc. Natl. Acad. Sci. USA 2017, 114, E6361–E6370. [Google Scholar] [CrossRef]
- Barrows, G.; Sexton, S.; Zilberman, D. Agricultural biotechnology: The promise and prospects of genetically modified crops. J. Econ. Perspect. 2014, 28, 99–120. [Google Scholar]
- Climate Change 2014—Synthesis Report; IPCC: Geneva, Switzerland, 2015; p. 151.
- National Greenhouse Gas Inventory; Center for Global Environmental Research, Earth System Division, National Institute for Environmental Studies: Tsukuba, Japan, 2025.
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 2016, 28, 1443–1452. [Google Scholar]
- Machado, L.; Magnusson, M.; Paul, N.A.; de Nys, R.; Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 2014, 9, e85289. [Google Scholar]
- Fenical, W. Natural products chemistry in the marine environment. Science 1982, 215, 923–928. [Google Scholar] [CrossRef]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Chemical defence against bacteria in the red alga Asparagopsis armata: Linking structure with function. Mol. Ecol. Prog. Ser. 2006, 306, 87–101. [Google Scholar]
- Tomkins, N.; Colegate, S.; Hunter, R.A. A bromochloromethane formulation reduces enteric methanogenesis in cattle fed grain-based diets. Anim. Prod. Sci. 2009, 49, 1053–1058. [Google Scholar]
- Davison, T.; Hill, J.; Savage, S. Reducing Emissions from Livestock Research Program; Meat & Livestock Australia Ltd.: Sydney, Australia, 2012. [Google Scholar]
- Zhou, M.; Hünerberg, M.; Chen, Y.; Reuter, T.; McAllister, T.A.; Evans, F.; Critchley, A.T.; Guan, L.L. Air-dried brown seaweed, Ascophyllum nodosum, alters the rumen microbiome in a manner that changes rumen fermentation profiles and lowers the prevalence of foodborne pathogens. mSphere 2018, 3, e00017-18. [Google Scholar]
- Manley, S.L. Phytogenesis of halomethanes: A product ofselection or a metabolic accident? Biogeochemistry 2002, 60, 163–180. [Google Scholar]
- Mata, L.; Lawton, R.J.; Magnusson, M.; Andreakis, N.; de Nys, R.; Paul, N.A. Within-species and temperature-related variation in the growth and natural products of the red alga Asparagopsis taxiformis. J. Appl. Phycol. 2017, 29, 1437–1447. [Google Scholar]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar]
- Wahl, M.; Goecke, F.; Labes, A.; Dobretsov, S.; Weinberger, F.J. The second skin: Ecological role of epibiotic biofilms on marine organisms. Front. Microbiol. 2012, 3, 292. [Google Scholar]
- Vergés, A.; Paul, N.A.; Steinberg, P.D. Sex and life-history stage alter herbivore responses to a chemically defended red alga. Ecology 2008, 89, 1334–1343. [Google Scholar]
- Thapa, H.R.; Lin, Z.; Yi, D.; Smith, J.E.; Schmidt, E.W.; Agarwal, V. Genetic and Biochemical Reconstitution of Bromoform Biosynthesis in Asparagopsis Lends Insights into Seaweed Reactive Oxygen Species Enzymology. ACS Chem. Biol. 2020, 15, 1662–1670. [Google Scholar] [CrossRef]
- Hollants, J.; Leliaert, F.; De Clerck, O.; Willems, A. What we can learn from sushi: A review on seaweed–bacterial associations. FEMS Microbiol. Ecol. 2013, 83, 1–16. [Google Scholar]
- Kinley, R.; Fredeen, A.H. In vitro evaluation of feeding North Atlantic stormtoss seaweeds on ruminal digestion. J. Appl. Phycol. 2015, 27, 2387–2393. [Google Scholar]
- Greff, S.; Aires, T.; Serrao, E.A.; Engelen, A.H.; Thomas, O.P.; Perez, T. The interaction between the proliferating macroalga Asparagopsis taxiformis and the coral Astroides calycularis induces changes in microbiome and metabolomic fingerprints. Sci. Rep. 2017, 7, 42625. [Google Scholar] [CrossRef]
- Gregersen, S.; Pertseva, M.; Marcatili, P.; LøvstadHoldt, S.; Jacobsen, C.; García-Morenod, P.J.; BechHansen, E.; ToftOvergaard, M. Proteomic characterization of pilot scale hot-water extracts from the industrial carrageenan red seaweed Eucheuma denticulatum. Algal Res. 2022, 62, 102619. [Google Scholar]
- Wang, D.; You, W.; Chen, N.; Cao, M.; Tang, X.; Guan, X.; Qu, W.; Chen, R.; Mao, Y.; Poetsch, A. Comparative Quantitative Proteomics Reveals the Desiccation Stress Responses of the Intertidal Seaweed NEOPORPHYRA haitanensis. J. Phycol. 2020, 56, 1664–1675. [Google Scholar]
- Andreakis, N.; Costello, P.; Zanolla, M.; Saunders, G.W.; Mata, L. Endemic or introduced? Phylogeography of Asparagopsis (Florideophyceae) in Australia reveals multiple introductions and a new mitochondrial lineage. J. Phycol. 2016, 52, 141–147. [Google Scholar] [CrossRef]
- Collén, J.; Porcel, B.; Carré, W.; Ball, S.G.; Chaparro, C.; Tonon, T.; Barbeyron, T.; Michel, G.; Noel, B.; Valentin, K.; et al. Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc. Natl. Acad. Sci. USA 2013, 110, 5247–5252. [Google Scholar]
- Johnson, D.G.; Walker, C.L. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar]
- Preuss, M.; Nelson, W.A.; D’Archino, R. Cryptic diversity and phylogeographic patterns in the Asparagopsis armata species complex (Bonnemaisoniales, Rhodophyta) from New Zealand. Phycologia 2022, 61, 89–96. [Google Scholar] [CrossRef]
- Verbruggen, H.; Maggs, C.A.; Saunders, G.W.; Le Gall, L.; Yoon, H.S.; De Clerck, O. Data mining approach identifies research priorities and data requirements for resolving the red algal tree of life. BMC Evol. Biol. 2010, 10, 16. [Google Scholar] [CrossRef]
- Xu, C.; Min, J. Structure and function of WD40 domain proteins. Protein Cell. 2011, 2, 202–214. [Google Scholar]
- Ambawat, S.; Sharma, P.; Yadav, N.R.; Yadav, R.C. MYB transcription factor genes as regulators for plant responses: An overview. Physiol. Mol. Biol. Plants 2013, 19, 307–321. [Google Scholar] [CrossRef]
- Cheng, X.; Xiong, R.; Yan, H.; Gao, Y.; Liu, H.; Wu, M.; Xiang, Y. The trihelix family of transcription factors: Functional and evolutionary analysis in Moso bamboo (Phyllostachys edulis). BMC Plant Biol. 2019, 19, 154. [Google Scholar] [CrossRef]
- Rensing, S.A. Gene duplication as a driver of plant morphogenetic evolution. Curr. Opin. Plant Biol. 2014, 17, 43–48. [Google Scholar] [CrossRef]
- Pallanca, J.E.; Smirnoff, N. Ascorbic acid metabolism in pea seedlings. A comparison of D-glucosone, L-sorbosone, and L-galactono-1,4-lactone as ascorbate precursors. Plant Physiol. 1999, 120, 453–462. [Google Scholar] [CrossRef]
- Trotter, P.J.; Pedretti, J.; Yates, R.; Voelker, D.R. Phosphatidylserine decarboxylase 2 of Saccharomyces cerevisiae. Cloning and mapping of the gene, heterologous expression, and creation of the null allele. J. Biol. Chem. 1995, 270, 6071–6080. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Paul, N.A.; Rimmer, M.A. Seaweed dietary supplements enhance the innate immune response of the mottled rabbitfish, Siganus fuscescens. Fish. Shellfish. Immunol. 2021, 113, 176–184. [Google Scholar]
- Park, C.J.; Seo, Y.S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar]
- Uji, T.; Gondaira, Y.; Fukuda, S.; Mizuta, H.; Saga, N. Characterization and expression profiles of small heat shock proteins in the marine red alga Pyropia yezoensis. Cell Stress and Chaperones 2019, 24, 223–233. [Google Scholar]
- Höfler, G.T.; But, A.; Hollmann, F. Haloperoxidases as catalysts in organic synthesis. Org. Biomol. Chem. 2019, 17, 9267–9274. [Google Scholar]
- Isupov, M.N.; Dalby, A.R.; Brindley, A.A.; Izumi, Y.; Tanabe, T.; Murshudov, G.N.; Littlechild, J.A. Crystal structure of dodecameric vanadium-dependent bromoperoxidase from the red algae Corallina officinalis. J. Mol. Biol. 2000, 299, 1035–1049. [Google Scholar] [CrossRef]
- Wang, X.; Chung, K.P.; Lin, W.; Jiang, L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2017, 69, 21–37. [Google Scholar]
- Suhre, M.H.; Scheibel, T.; Steegborn, C.; Gertz, M. Crystallization and preliminary X-ray diffraction analysis of proximal thread matrix protein 1 (PTMP1) from Mytilus galloprovincialis. Acta Crystallogr. F Struct. Biol. Commun. 2014, 70, 769–772. [Google Scholar] [CrossRef]
- Waite, J.H.; Lichtenegger, H.C.; Stucky, G.D.; Hansma, P. Exploring molecular and mechanical gradients in structural bioscaffolds. Biochemistry 2004, 43, 7653–7662. [Google Scholar] [CrossRef]
- Whittaker, C.A.; Hynes, R.O. Distribution and evolution of von Willebrand/integrin A domains: Widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell. 2002, 13, 3369–3387. [Google Scholar]
- Kerrison, P.D.; Stanley, M.S.; De Smet, D.; Buyle, G.; Hughes, A.D. Holding (not so) fast: Surface chemistry constrains kelp bioadhesion. Eur. J. Phycol. 2019, 54, 291–299. [Google Scholar]
- Charoensiddhi, S.; Abraham, R.E.; Su, P.; Zhang, W. Seaweed and seaweed-derived metabolites as prebiotics. Adv. Food Nutr. Res. 2020, 91, 97–156. [Google Scholar]
- Sonani, R.R.; Rastogi, R.P.; Patel, R.; Madamwar, D. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100–109. [Google Scholar]
- Marcais, G.; Kingsford, C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 2011, 27, 764–770. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Flynn, J.M.; Hubley, R.; Goubert, C.; Rosen, J.; Clark, A.G.; Feschotte, C.; Smit, A.F. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl. Acad. Sci. USA 2020, 117, 9451–9457. [Google Scholar] [CrossRef]
- Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinform. 2004, 4, 4–10. [Google Scholar] [CrossRef]
- Stanke, M.; Keller, O.; Gunduz, I.; Hayes, A.; Waack, S.; Morgenstern, B. AUGUSTUS: Ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006, 34, W435–W439. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genom. 2008, 2008, 619832. [Google Scholar] [CrossRef]
- Coordinators, N.R. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016, 44, D7–D19. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Mistry, J.; Finn, R.D.; Eddy, S.R.; Bateman, A.; Punta, M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013, 41, e121. [Google Scholar] [CrossRef]
- Astashyn, A.; Tvedte, E.S.; Sweeney, D.; Sapojnikov, V.; Bouk, N.; Joukov, V.; Mozes, E.; Strope, P.K.; Sylla, P.M.; Wagner, L.; et al. Rapid and sensitive detection of genome contamination at scale with FCS-GX. Genome Biol. 2024, 25, 60. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef]
- Junker, V.; Contrino, S.; Fleischmann, W.; Hermjakob, H.; Lang, F.; Magrane, M.; Martin, M.J.; Mitaritonna, N.; O’Donovan, C.; Apweiler, R. The role SWISS-PROT and TrEMBL play in the genome research environment. J. Biotechnol. 2000, 78, 221–234. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. STAG: Species Tree Inference from All Genes. bioRxiv 2018. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sonderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar]
- Romanazzi, D.; Sanchez-Garcia, C.; Svenson, J.; Mata, L.; Pes, K.; Hayman, C.M.; Wheeler, T.T.; Magnusson, M. Rapid Analytical Method for the Quantification of Bromoform in the Red Seaweeds Asparagopsis armata and Asparagopsis taxiformis Using Gas Chromatography–Mass Spectrometry. ACS Agric. Sci. Technol. 2021, 1, 436–442. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Hall, M.R.; Kocot, K.M.; Baughman, K.W.; Fernandez-Valverde, S.L.; Gauthier, M.E.; Hatleberg, W.L.; Krishnan, A.; McDougall, C.; Motti, C.A.; Shoguchi, E.J.N. The crown-of-thorns starfish genome as a guide for biocontrol of this coral reef pest. Nature 2017, 544, 231–234. [Google Scholar]
- Kupper, F.C.; Leblanc, C.; Meyer-Klaucke, W.; Potin, P.; Feiters, M.C. Different speciation for bromine in brown and red algae, revealed by in vivo X-ray absorption spectroscopic studies. J. Phycol. 2014, 50, 652–664. [Google Scholar] [CrossRef]
- Thépot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Félix, R.; Dias, P.; Félix, C.; Cerqueira, T.; Andrade, P.B.; Valentão, P.; Lemos, M.F. The biotechnological potential of Asparagopsis armata: What is known of its chemical composition, bioactivities and current market? Algal Res. 2021, 60, 102534. [Google Scholar]
- Pinteus, S.; Lemos, M.F.; Alves, C.; Silva, J.; Pedrosa, R. The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci. Total Environ. 2021, 750, 141372. [Google Scholar]
- Streftaris, N.; Zenetos, A. Alien Marine Species in the Mediterranean—The 100 ‘Worst Invasives’ and their Impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Florido, M.; Ros, M.; González-Romero, P.; Guerra-García, J.M. Impoverished mobile epifaunal assemblages associated with the invasive macroalga Asparagopsis taxiformis in the Mediterranean Sea. Mar. Environ. Res. 2018, 141, 44–52. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Ros, M.; Izquierdo, D.; Soler-Hurtado, M.M. The invasive Asparagopsis armata versus the native Corallina elongata: Differences in associated peracarid assemblages. J. Exp. Mar. Biol. Ecol. 2012, 416–417, 121–128. [Google Scholar] [CrossRef]
- Moigne, J.Y. Use of Algae Extracts as Antibacterial and/or Antifungal Agent and Composition Containing Same. WIPO WO1998010656A1, 19 March 1998. [Google Scholar]
- Neethu, P.V.; Suthindhiran, K.; Jayasri, M.A. Antioxidant and Antiproliferative Activity of Asparagopsis taxiformis. Pharmacogn. Res. 2017, 9, 238–246. [Google Scholar]
- Nunes, N.; Valente, S.; Ferraz, S.; Barreto, M.C.; Pinheiro de Carvalho, M.A.A. Nutraceutical potential of Asparagopsis taxiformis (Delile) Trevisan extracts and assessment of a downstream purification strategy. Heliyon 2018, 4, e00957. [Google Scholar] [CrossRef]
- Pegler, J.L.; Nguyen, D.Q.; Oultram, J.M.J.; Grof, C.P.L.; Eamens, A.L. Molecular Manipulation of the miR396 and miR399 Expression Modules Alters the Response of Arabidopsis thaliana to Phosphate Stress. Plants 2021, 10, 2570. [Google Scholar] [CrossRef]
- Litholdo, C.G., Jr.; Eamens, A.L.; Waterhouse, P.M. The phenotypic and molecular assessment of the non-conserved Arabidopsis MICRORNA163/S-ADENOSYL-METHYLTRANSFERASE regulatory module during biotic stress. Mol. Genet. Genom. 2018, 293, 503–523. [Google Scholar] [CrossRef]
- Chow, H.T.; Ng, D.W. Regulation of miR163 and its targets in defense against Pseudomonas syringae in Arabidopsis thaliana. Sci. Rep. 2017, 7, 46433. [Google Scholar] [CrossRef]
- Eamens, A.L.; McHale, M.; Waterhouse, P.M. The use of artificial microRNA technology to control gene expression in Arabidopsis thaliana. Methods Mol. Biol. 2014, 1062, 211–224. [Google Scholar] [CrossRef]
- McHale, M.; Eamens, A.L.; Finnegan, E.J.; Waterhouse, P.M. A 22-nt artificial microRNA mediates widespread RNA silencing in Arabidopsis. Plant J. 2013, 76, 519–529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).