Predictive Modeling of Lignocellulosic Content in Crop Straws Using NIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectral Data Processing and Analysis
2.2.1. Spectra Acquisition and Abnormal Sample Elimination
2.2.2. Sample Division
2.2.3. Spectral Preprocessing
2.2.4. Characteristic Wavelength Screening
2.3. Modeling Method
2.4. Model Evaluation
3. Results and Discussion
3.1. Sample Set Division
3.2. Spectral Data Preprocessing
3.2.1. PLS Prediction Models Based on Full Spectrum
3.2.2. SVR Prediction Models Based on Full Spectrum
3.3. Prediction Models for Lignocellulose Based on Characteristic Wavelengths
3.3.1. PLS Prediction Model for Lignocellulose Based on Characteristic Wavelengths
3.3.2. SVR Prediction Model for Cellulose, Hemicellulose, and Lignin Based on Characteristic Wavelength
3.3.3. Comparison of PLS and SVR Model Effects
3.4. Comparison and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, D. Recent Progress in the Catalytic Thermochemical Conversion Process of Biomass for Biofuels. Chem. Eng. J. 2022, 447, 137501. [Google Scholar] [CrossRef]
- Ricciulli, M.O.; Arce, G.L.A.F.; Vieira, E.C.; Ávila, I. Interaction among Lignocellulosic Biomass Components in Thermochemical Processes. Biomass Bioenergy 2024, 182, 107073. [Google Scholar] [CrossRef]
- Müller, J.; Wiedow, D.; Chmit, M.S.; Beuerle, T. Utilization of Biomasses from Landscape Conservation Growths Dominated by Common Ragwort (Jacobaea Vulgaris Gaertn.) for Biomethanization. Plants 2022, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, M.F.; Shaheen, H.; Wu, A.-M. Cell Wall Hemicellulose for Sustainable Industrial Utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Terrett, O.M.; Dupree, P. Covalent Interactions between Lignin and Hemicelluloses in Plant Secondary Cell Walls. Curr. Opin. Biotechnol. 2019, 56, 97–104. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.-D.; Lee, D.-J. Proteomic Researches for Lignocellulose-Degrading Enzymes: A Mini-Review. Bioresour. Technol. 2018, 265, 532–541. [Google Scholar] [CrossRef]
- Najjarzadeh, N.; Matsakas, L.; Rova, U.; Christakopoulos, P. How Carbon Source and Degree of Oligosaccharide Polymerization Affect Production of Cellulase-Degrading Enzymes by Fusarium Oxysporum f. sp. Lycopersici. Front. Microbiol. 2021, 12, 652655. [Google Scholar] [CrossRef]
- Du, B.; Chen, C.; Sun, Y.; Yang, M.; Yu, M.; Liu, B.; Wang, X.; Zhou, J. Unlocking the Response of Lignin Structure by Depolymerization Process Improved Lignin-Based Carbon Nanofibers Preparation and Mechanical Strength. Int. J. Biol. Macromol. 2020, 156, 669–680. [Google Scholar] [CrossRef]
- Wang, D. A Strong Enhancement of Corrosion and Wear Resistance of Polyurethane-Based Coating by Chemically Grafting of Organosolv Lignin. Mater. Today Chem. 2024, 35, 101833. [Google Scholar] [CrossRef]
- Barta-Rajnai, E.; Jakab, E.; Sebestyén, Z.; May, Z.; Barta, Z.; Wang, L.; Skreiberg, Ø.; Grønli, M.; Bozi, J.; Czégény, Z. Comprehensive Compositional Study of Torrefied Wood and Herbaceous Materials by Chemical Analysis and Thermoanalytical Methods. Energy Fuels 2016, 30, 8019–8030. [Google Scholar] [CrossRef]
- Benouadah, N.; Aliouche, D.; Pranovich, A.; Willför, S. Chemical Characterization of Pinus Halepensis Sapwood and Heartwood. Wood Mater. Sci. Eng. 2019, 14, 157–164. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Yang, Y.; Ren, H.; Zhai, H. A Spectroscopic Method for Quantitating Lignin in Lignocellulosic Biomass Based on the Completely Dissolved Solution of Biomass in LiCl/DMSO. Green Chem. 2022, 24, 2212–2221. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Liu, X. A Study of the Reliability and Accuracy of the Real-Time Detection of Forage Maize Quality Using a Home-Built Near-Infrared Spectrometer. Foods 2022, 11, 3490. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Li, J.; Liu, S.; Fan, S. Assessment of Protein Content and Insect Infestation of Maize Seeds Based on On-Line near-Infrared Spectroscopy and Machine Learning. Comput. Electron. Agric. 2023, 211, 107969. [Google Scholar] [CrossRef]
- Liu, J.; Jin, S.; Bao, C.; Sun, Y.; Li, W. Rapid Determination of Lignocellulose in Corn Stover Based on Near-Infrared Reflectance Spectroscopy and Chemometrics Methods. Bioresour. Technol. 2021, 321, 124449. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Deng, X.; Yu, Z.; Liu, Z.; Zhong, H.; Chen, R.; Cai, G.; Zheng, Q.; Liu, X.; Zhong, J.; et al. Auto-Classification of Biomass through Characterization of Their Pyrolysis Behaviors Using Thermogravimetric Analysis with Support Vector Machine Algorithm: Case Study for Tobacco. Biotechnol. Biofuels 2021, 14, 106. [Google Scholar] [CrossRef]
- Xue, J.; Yang, Z.; Han, L.; Liu, Y.; Liu, Y.; Zhou, C. On-Line Measurement of Proximates and Lignocellulose Components of Corn Stover Using NIRS. Appl. Energy 2015, 137, 18–25. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Johnson, L.; Yuan, W.; Pei, Z.; Wang, D. Development of Near-Infrared Spectroscopy Models for Quantitative Determination of Cellulose and Hemicellulose Contents of Big Bluestem. Renew. Energy 2017, 109, 101–109. [Google Scholar] [CrossRef]
- Lestander, T.A.; Rudolfsson, M.; Pommer, L.; Nordin, A. NIR Provides Excellent Predictions of Properties of Biocoal from Torrefaction and Pyrolysis of Biomass. Green Chem. 2014, 16, 4906–4913. [Google Scholar] [CrossRef]
- Triolo, J.M.; Ward, A.J.; Pedersen, L.; Løkke, M.M.; Qu, H.; Sommer, S.G. Near Infrared Reflectance Spectroscopy (NIRS) for Rapid Determination of Biochemical Methane Potential of Plant Biomass. Appl. Energy 2014, 116, 52–57. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Qiu, X.; Zhao, X.; Chen, Z.; Yan, M.; Fang, Z.; Li, Z.; Tu, Z.; Huang, J. Lignin: A Sustainable Photothermal Block for Smart Elastomers†. Green Chem. 2022, 24, 823–836. [Google Scholar] [CrossRef]
- Falcioni, R.; Gonçalves, J.V.; Oliveira, K.M.; Oliveira, C.A.; Demattê, J.A.M.; Antunes, W.C.; Nanni, M.R. Enhancing Pigment Phenotyping and Classification in Lettuce through the Integration of Reflectance Spectroscopy and AI Algorithms. Plants 2023, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.I.; Antunes, C.A.L.; Horta, C.; Pitacas, I.; Gonçalves, J.; Gominho, J.; Gallardo, E.; Anjos, O. Characterization of Mineral Composition and Nutritional Value of Acacia Green Pods. Plants 2023, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, R.; Antunes, W.C.; Demattê, J.A.M.; Nanni, M.R. Reflectance Spectroscopy for the Classification and Prediction of Pigments in Agronomic Crops. Plants 2023, 12, 2347. [Google Scholar] [CrossRef]
- Kang, D.I.; Jeong, H.K.; Park, Y.G.; Jeong, B.R. Flowering and Morphogenesis of Kalanchoe in Response to Quality and Intensity of Night Interruption Light. Plants 2019, 8, 90. [Google Scholar] [CrossRef]
- Gao, J.; Hao, L.; Jiang, R.; Liu, Z.; Tian, L.; Zhao, J.; Ming, W.; Ren, L. Surprisingly Fast Assembly of the MOF Film for Synergetic Antibacterial phototherapeutics††Electronic Supplementary Information (ESI). Green Chem. 2022, 24, 5930–5940. [Google Scholar] [CrossRef]
- Knez, D.; Hanninen, A.M.; Prince, R.C.; Potma, E.O.; Fishman, D.A. Infrared Chemical Imaging through Non-Degenerate Two-Photon Absorption in Silicon-Based Cameras. Light Sci. Appl. 2020, 9, 125. [Google Scholar] [CrossRef]
- Pirutin, S.K.; Jia, S.; Yusipovich, A.I.; Shank, M.A.; Parshina, E.Y.; Rubin, A.B. Vibrational Spectroscopy as a Tool for Bioanalytical and Biomonitoring Studies. Int. J. Mol. Sci. 2023, 24, 6947. [Google Scholar] [CrossRef]
- Rasooli, N.; Farpoor, M.H.; Mahmoodabadi, M.; Esfandiarpour-Boroujeni, I. Vis-NIR Spectroscopy as an Eco-Friendly Method for Monitoring Pedoenvironmental Variations and Pedological Assessments in Lut Watershed, Central Iran. Soil Tillage Res. 2023, 233, 105808. [Google Scholar] [CrossRef]
- A, R.; Mu, X.; He, J. Enhance Tensor RPCA-Based Mahalanobis Distance Method for Hyperspectral Anomaly Detection. IEEE Geosci. Remote Sens. Lett. 2022, 19, 6008305. [Google Scholar] [CrossRef]
- Shaaban-Ali, M.; Momeni, M.; Denault, A. Clinical and Technical Limitations of Cerebral and Somatic Near-Infrared Spectroscopy as an Oxygenation Monitor. J. Cardiothorac. Vasc. Anesth. 2021, 35, 763–779. [Google Scholar] [CrossRef]
- Choi, J.; Shin, J.G.; Kwon, H.-S.; Tak, Y.-O.; Park, H.J.; Ahn, J.-C.; Eom, J.B.; Seo, Y.; Park, J.W.; Choi, Y.; et al. Development of Intraoperative Near-Infrared Fluorescence Imaging System Using a Dual-CMOS Single Camera. Sensors 2022, 22, 5597. [Google Scholar] [CrossRef] [PubMed]
- de Santana, F.B.; de Giuseppe, L.O.; de Souza, A.M.; Poppi, R.J. Removing the Moisture Effect in Soil Organic Matter Determination Using NIR Spectroscopy and PLSR with External Parameter Orthogonalization. Microchem. J. 2019, 145, 1094–1101. [Google Scholar] [CrossRef]
- Park, J.; Choi, D.H.; Jeon, Y.-B.; Nam, Y.; Hong, M.; Park, D.-S. Network Anomaly Detection Based on Probabilistic Analysis. Soft Comput. 2018, 22, 6621–6627. [Google Scholar] [CrossRef]
- Du, Z.; Tian, W.; Tilley, M.; Wang, D.; Zhang, G.; Li, Y. Quantitative Assessment of Wheat Quality Using Near-Infrared Spectroscopy: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2956–3009. [Google Scholar] [CrossRef]
- Vivó-Truyols, G.; Schoenmakers, P.J. Automatic Selection of Optimal Savitzky−Golay Smoothing. Anal. Chem. 2006, 78, 4598–4608. [Google Scholar] [CrossRef]
- Roger, J.-M.; Boulet, J.-C.; Zeaiter, M.; Rutledge, D.N. 3.01—Pre-Processing Methods☆. In Comprehensive Chemometrics, 2nd ed.; Brown, S., Tauler, R., Walczak, B., Eds.; Elsevier: Oxford, UK, 2020; pp. 1–75. ISBN 978-0-444-64166-3. [Google Scholar]
- Fearn, T.; Riccioli, C.; Garrido-Varo, A.; Guerrero-Ginel, J.E. On the Geometry of SNV and MSC. Chemom. Intell. Lab. Syst. 2009, 96, 22–26. [Google Scholar] [CrossRef]
- Visnupriyan, R.; Flanagan, B.M.; Harper, K.J.; Cozzolino, D. Near Infrared Spectroscopy Combined with Chemometrics as Tool to Monitor Starch Hydrolysis. Carbohydr. Polym. 2024, 324, 121469. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Che, Z.; Liao, T.W.; Zhang, Z. Prediction Using Multi-Objective Slime Mould Algorithm Optimized Support Vector Regression Model. Appl. Soft Comput. 2023, 145, 110580. [Google Scholar] [CrossRef]
- Pearson, K. Determination of the Coefficient of Correlation. Science 1909, 30, 23–25. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, Z.; Cheng, Y.; Shi, X.; Qiao, Y. MDL and RMSEP Assessment of Spectral Pretreatments by Adding Different Noises in Calibration/Validation Datasets. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 163, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.; Jiang, Y.; Omar, S.; Wang, J.; Xia, L.; Ren, J. Rapid Measurement of Cellulose, Hemicellulose, and Lignin Content in Sargassum Horneri by Near-Infrared Spectroscopy and Characteristic Variables Selection Methods. Molecules 2022, 27, 335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Han, G.; Gao, S.; Xing, M.; Song, Y.; Jiang, W. Rapid determination of cellulose content in pulp using near infrared modeling technique. Bioresources 2018, 13, 6122–6132. [Google Scholar] [CrossRef]
- Guo, T.; Dai, L.; Yan, B.; Lan, G.; Li, F.; Li, F.; Pan, F.; Wang, F. Measurements of Chemical Compositions in Corn Stover and Wheat Straw by Near-Infrared Reflectance Spectroscopy. Animals 2021, 11, 3328. [Google Scholar] [CrossRef]
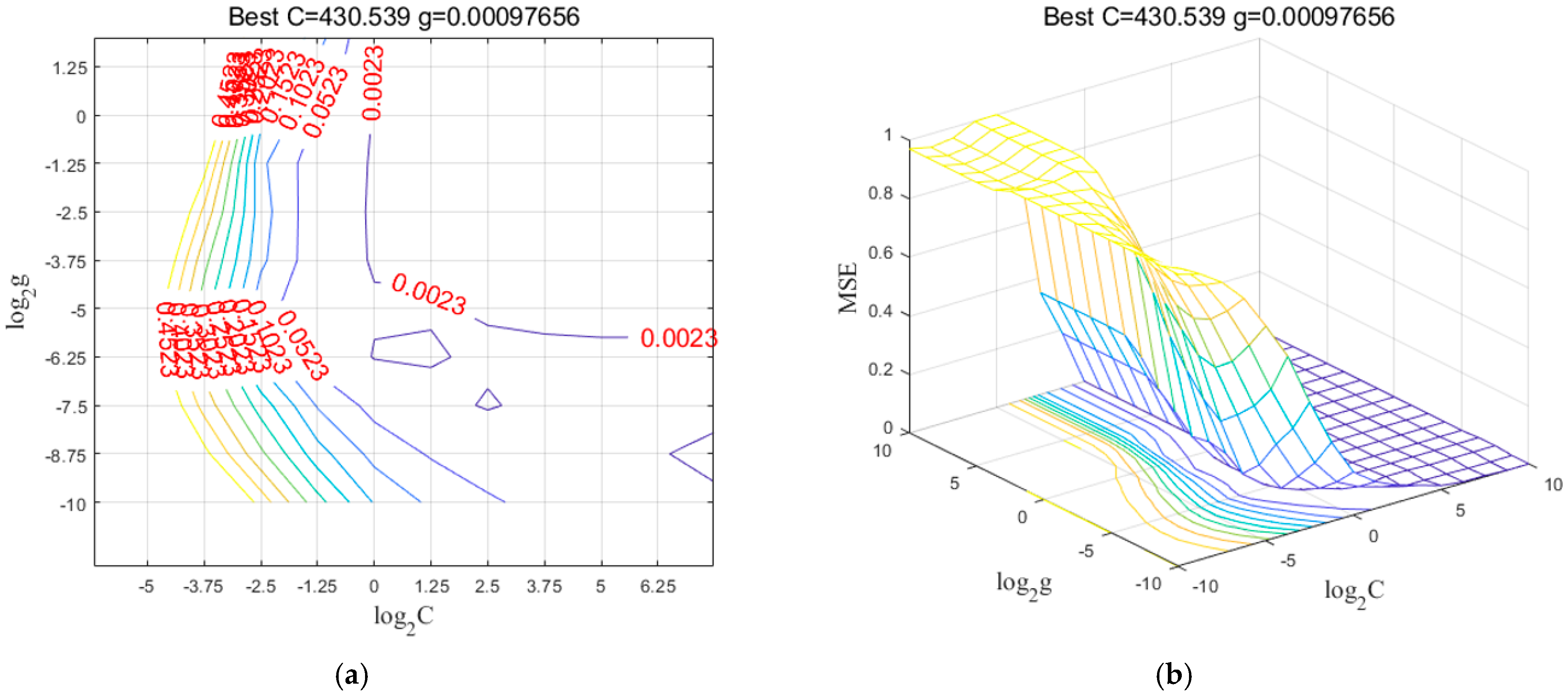
| Sample Set | Sample Size | Max. (%) | Min. (%) | Average (%) | Standard Deviation | |
|---|---|---|---|---|---|---|
| Cellulose | Calibration Set | 96 | 31.32 | 25.85 | 26.01 | 1.78 |
| Prediction Set | 48 | 30.82 | 22.23 | 26.13 | 1.94 | |
| Hemicellulose | Calibration Set | 96 | 30.61 | 15.62 | 19.98 | 2.78 |
| Prediction Set | 48 | 26.66 | 15.04 | 20.56 | 2.81 | |
| Lignin | Calibration Set | 96 | 18.83 | 12.63 | 15.69 | 1.41 |
| Prediction Set | 48 | 17.73 | 13.61 | 15.72 | 1.21 |
| PLS | SVR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method | Factors | R2P | RMSEP | RPD | Penalty Factor c | Kernel Parameter g | R2P | RMSEP | RPD | |
| Cellulose | None | 8 | 0.6214 | 1.2498 | 1.77 | 13.45 | 9.76 × 10−4 | 0.6135 | 1.4582 | 1.51 |
| SNV | 8 | 0.7230 | 1.0850 | 2.03 | 430 | 9.76 × 10−4 | 0.6820 | 1.3010 | 1.69 | |
| MSC | 6 | 0.7850 | 0.9617 | 2.30 | 5.65 | 2.32 × 10−3 | 0.7938 | 1.0521 | 2.09 | |
| FD | 7 | 0.8224 | 0.8136 | 2.70 | 13.45 | 4.10 × 10−4 | 0.7784 | 1.0780 | 2.04 | |
| SD | 6 | 0.8578 | 0.7650 | 2.89 | 13.45 | 4.10 × 10−4 | 0.7832 | 1.0658 | 2.06 | |
| SNV + FD | 8 | 0.5118 | 1.4090 | 1.54 | 13.45 | 4.10 × 10−4 | 0.6379 | 1.4104 | 1.56 | |
| Hemicellulose | None | 11 | 0.5438 | 2.5134 | 1.33 | 13.45 | 9.76 × 10−4 | 0.5428 | 2.3441 | 1.43 |
| SNV | 8 | 0.7077 | 1.9609 | 1.71 | 3.2 | 2.32 × 10−3 | 0.6266 | 2.1647 | 1.55 | |
| MSC | 6 | 0.7465 | 1.7114 | 1.96 | 13.45 | 9.76 × 10−4 | 0.4728 | 2.5117 | 1.34 | |
| FD | 10 | 0.5742 | 2.4354 | 1.38 | 13.45 | 4.10 × 10−4 | 0.5334 | 2.3740 | 1.42 | |
| SD | 10 | 0.6551 | 2.0838 | 1.61 | 13.45 | 4.10 × 10−4 | 0.6329 | 2.1159 | 1.59 | |
| SNV + FD | 9 | 0.5545 | 2.4770 | 1.35 | 2.37 | 2.32 × 10−3 | 0.5360 | 2.2203 | 1.51 | |
| Lignin | None | 9 | 0.5235 | 0.7691 | 1.83 | 13.45 | 9.76 × 10−4 | 0.5457 | 0.8674 | 1.63 |
| SNV | 7 | 0.7635 | 0.6193 | 2.17 | 7.61 | 9.76 × 10−4 | 0.5735 | 0.8448 | 1.67 | |
| MSC | 8 | 0.6863 | 0.6565 | 1.99 | 13.45 | 9.76 × 10−4 | 0.4483 | 0.9736 | 1.45 | |
| FD | 8 | 0.6776 | 0.6699 | 2.10 | 13.45 | 4.10 × 10−4 | 0.3926 | 1.0267 | 1.37 | |
| SD | 7 | 0.5310 | 0.7514 | 1.88 | 13.45 | 1.72 × 10−4 | 0.4324 | 0.9847 | 1.43 | |
| SNV + FD | 7 | 0.4055 | 0.7925 | 1.51 | 5.65 | 4.10 × 10−4 | 0.5186 | 0.8964 | 1.57 | |
| Method | Number of Wavelength Variables | Factors | R2C | RMSEC | R2P | RMSEP | RPD | |
|---|---|---|---|---|---|---|---|---|
| Cellulose | SD | 1556 | 6 | 0.9734 | 0.4290 | 0.8578 | 0.7650 | 2.89 |
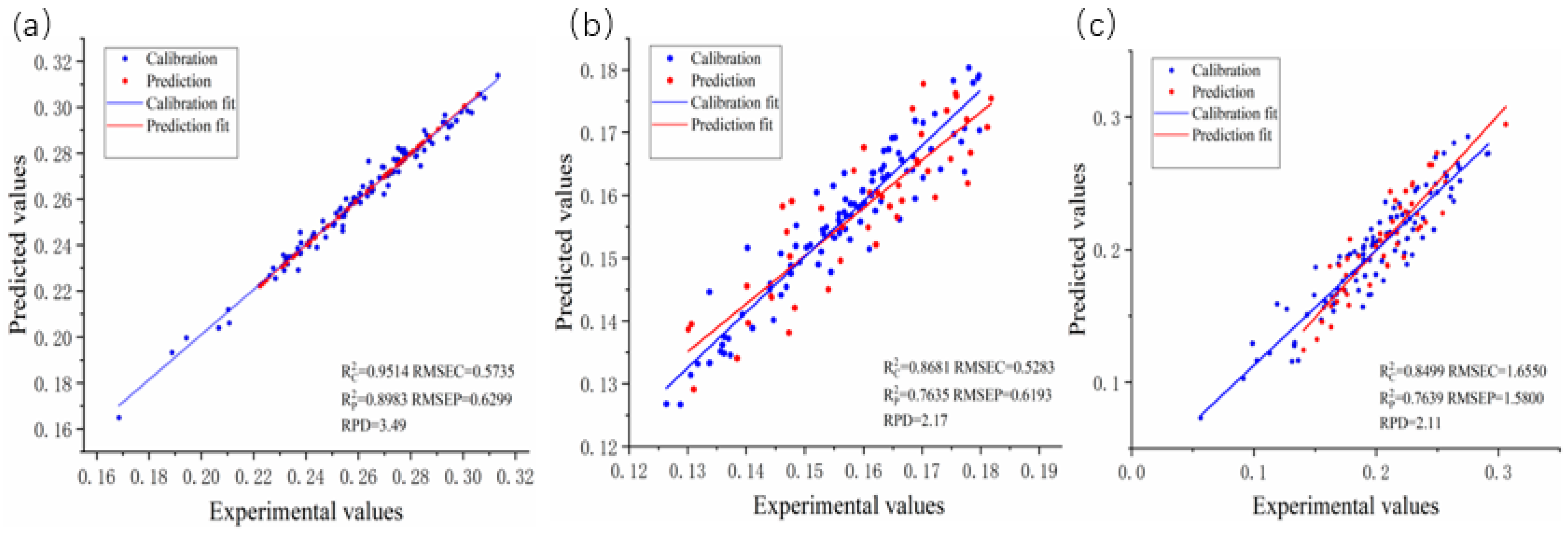
| SD + CARS | 68 | 4 | 0.9514 | 05735 | 0.8983 | 0.6299 | 3.49 | |
| SD + SPA | 9 | 4 | 0.7608 | 1.1713 | 0.4572 | 1.2339 | 1.78 | |
| SD + IVISSA | 948 | 4 | 0.9357 | 0.6552 | 0.8267 | 0.7960 | 2.76 | |
| SD + GA | 123 | 6 | 0.9712 | 0.4455 | 0.8564 | 0.7747 | 2.84 | |
| Hemicellulose | MSC | 1556 | 6 | 0.7804 | 1.9440 | 0.7465 | 1.7114 | 1.96 |
| MSC + CARS | 35 | 7 | 0.8499 | 1.6550 | 0.7639 | 1.5800 | 2.11 | |
| MSC + SPA | 10 | 4 | 0.6304 | 2.3810 | 0.5738 | 1.9712 | 1.65 | |
| MSC + IVISSA | 115 | 6 | 0.8175 | 1.8241 | 0.5554 | 2.2600 | 1.43 | |
| MSC + GA | 1063 | 4 | 0.7521 | 2.0420 | 0.5803 | 2.2072 | 1.47 | |
| Lignin | SNV | 1556 | 7 | 0.8681 | 0.5238 | 0.7635 | 0.6193 | 2.17 |
| SNV + CARS | 52 | 5 | 0.9201 | 0.7643 | 0.6432 | 0.7657 | 1.68 | |
| SNV + SPA | 18 | 5 | 0.1256 | 1.0482 | 0.2414 | 0.8626 | 1.49 | |
| SNV + IVISSA | 973 | 4 | 0.4284 | 0.9255 | 0.3857 | 0.8385 | 1.54 | |
| SNV + GA | 196 | 5 | 0.9514 | 0.2656 | 0.5518 | 0.7233 | 1.77 |
| Method | Number of Wavelength Variables | Penalty Factor c | Kernel Parameter g | R2C | RMSEC | R2P | RMSEP | RPD | |
|---|---|---|---|---|---|---|---|---|---|
| Cellulose | MSC | 1556 | 5.65 | 2.32 × 10−3 | 0.8853 | 0.9139 | 0.7938 | 1.0521 | 2.09 |
| MSC + CARS | 68 | 13.45 | 5.52 × 10−3 | 0.9337 | 0.7535 | 0.8931 | 0.8035 | 3.01 | |
| MSC + SPA | 9 | 5.65 | 1.31 × 10−2 | 0.8255 | 1.1899 | 0.6953 | 1.2677 | 1.76 | |
| MSC + GA | 123 | 13.45 | 5.52 × 10−3 | 0.9701 | 0.4598 | 0.8446 | 0.8948 | 2.53 | |
| MSC + IVISSA | 948 | 13.45 | 4.41 × 10−4 | 0.9294 | 0.7374 | 0.8175 | 0.9746 | 2.29 | |
| Hemicellulose | SD | 1556 | 13.45 | 4.10 × 10−4 | 0.8966 | 1.5181 | 0.6329 | 2.1159 | 1.59 |
| SD + CARS | 35 | 13.45 | 0.17 | 0.8910 | 1.5168 | 0.6552 | 1.9806 | 1.63 | |
| SD + SPA | 10 | 13.45 | 0.42 | 0.8347 | 1.8674 | 0.6446 | 1.9593 | 1.64 | |
| SD + GA | 115 | 1024 | 2.32 × 10−3 | 0.8430 | 0.6099 | 0.4930 | 1.0731 | 1.20 | |
| SD + IVISSA | 1063 | 13.45 | 2.32 × 10−3 | 0.8019 | 2.1266 | 0.5288 | 2.2500 | 1.44 | |
| Lignin | SNV | 1556 | 7.61 | 9.76 × 10−4 | 0.5994 | 0.9762 | 0.5735 | 0.8448 | 1.67 |
| SNV + CARS | 52 | 13.45 | 3.12 × 10−2 | 0.8059 | 0.7118 | 0.6639 | 0.7470 | 1.72 | |
| SNV + SPA | 18 | 2.37 | 7.43 × 10−2 | 0.4991 | 1.089 | 0.6176 | 0.7929 | 1.61 | |
| SNV + GA | 196 | 1024 | 2.32 × 10−3 | 0.8202 | 0.6559 | 0.4278 | 1.5060 | 1.12 | |
| SNV + IVISSA | 973 | 13.45 | 2.32 × 10−3 | 0.6966 | 0.8771 | 0.5667 | 0.8756 | 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhu, Y.; Ren, Y.; Lu, Y.; Yu, C.; Chen, G.; Hong, Y.; Liu, Q. Predictive Modeling of Lignocellulosic Content in Crop Straws Using NIR Spectroscopy. Plants 2025, 14, 1430. https://doi.org/10.3390/plants14101430
Zhao Y, Zhu Y, Ren Y, Lu Y, Yu C, Chen G, Hong Y, Liu Q. Predictive Modeling of Lignocellulosic Content in Crop Straws Using NIR Spectroscopy. Plants. 2025; 14(10):1430. https://doi.org/10.3390/plants14101430
Chicago/Turabian StyleZhao, Yifan, Yingying Zhu, Yumeng Ren, Yu Lu, Chunling Yu, Geng Chen, Yu Hong, and Qian Liu. 2025. "Predictive Modeling of Lignocellulosic Content in Crop Straws Using NIR Spectroscopy" Plants 14, no. 10: 1430. https://doi.org/10.3390/plants14101430
APA StyleZhao, Y., Zhu, Y., Ren, Y., Lu, Y., Yu, C., Chen, G., Hong, Y., & Liu, Q. (2025). Predictive Modeling of Lignocellulosic Content in Crop Straws Using NIR Spectroscopy. Plants, 14(10), 1430. https://doi.org/10.3390/plants14101430





