Nutritional, Phytochemical, and Biological Characterization of Peel, Pulp, and Seed Powder from the Fruits of Berberis mikuna and Berberis burruyacuensis: Potential as a Functional Ingredient
Abstract
1. Introduction
2. Results and Discussion
2.1. Chromatic Parameters of Berberis Powder
2.2. Nutritional and Phytochemical Composition of the Pulp, Peel, and Seed Powders of B. mikuna and B. burruyacuensis
2.2.1. Nutritional Composition of Berberis Seed, Pulp, and Peel Powders
2.2.2. Phytochemical Composition
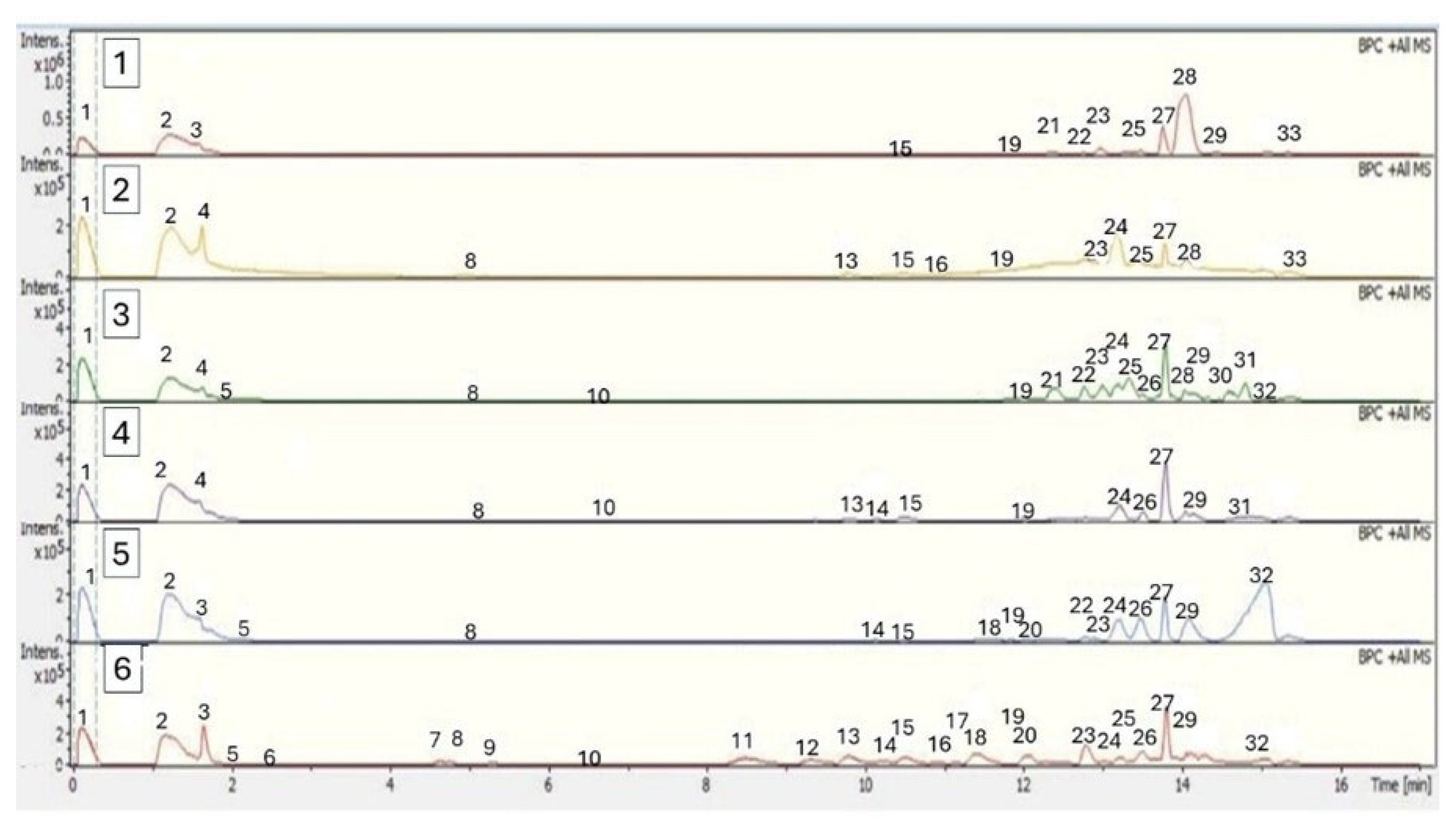
2.2.3. Identification of Phytochemicals Using UHPLC-MS
2.3. Functional Properties of Berberis burruyacuensis and Berberis mikuna Seed, Pulp, and Skin Powders
2.3.1. Antioxidant Activity
2.3.2. Effect of Berberis Fruit Powder Extracts on Enzymes Involved in the Development of Metabolic Syndrome
2.4. Toxicity Analysis of Berberis burruyacuensis and Berberis mikuna Seed, Pulp, and Skin Powders
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Quality Parameters of Fruits
3.4. Nutritional Analysis
3.5. Functional Phytochemicals
3.5.1. Extract Preparation
3.5.2. Total Polyphenols and Flavonoids
3.5.3. Condensed Tannins and Pigments
3.5.4. Identification of Phytochemicals Using UHPLC-PDA-ESI-QT-MS/MS
3.6. Antioxidant Activity of Polyphenolic Extracts
3.6.1. ABTS●+ Scavenging Assay
3.6.2. Hydrogen Peroxide Scavenging Assay
3.7. Antihyperglycemic and Antihyperlipidemic Activity of Berberis Extracts
3.7.1. α-Glucosidase Inhibition
3.7.2. α-Amylase Inhibition Assay
3.7.3. Lipase Inhibition Assay
3.8. Toxicity Assessment
3.8.1. Acute Toxicity Assay
3.8.2. Mutagenicity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahrendt, L.W.A. Berberis and Mahonia: A taxonomic revision. Bot. J. Linn. 1961, 57, 1–410. [Google Scholar] [CrossRef]
- Job, M.M. Los berberis de la región de Nahuel Huapi. Rev. Mus. Plata 1942, 5, 21–72. [Google Scholar]
- Orsi, M.C. Berberidaceae. In Flora Patagónica; Correa, M.N., Ed.; INTA: Buenos Aires, Argentina, 1984; pp. 325–348. [Google Scholar]
- IBODA. Flora del Cono Sur Catálogo de Plantas Vasculares. 2019. Available online: http://www.darwin.edu.ar/Proyectos/FloraArgentina/Generos.asp (accessed on 28 April 2025).
- Zuloaga, F.O.; Morrone, O.; Belgrano, M.J. Catalogue of the Vascular Plants of the Southern Cone (Argentina, Southern Brazil, Chile, Paraguay and Uruguay). Volume 2: Dicotyledoneae: Acanthaceae-Fabaceae (Abarema-Schizolobium); Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; pp. 985–2286. [Google Scholar]
- Ayarde, H.; Bulacio, E. Una nueva especie de Berberis (Berberidaceae) de las montañas del noroeste de Argentina y sur de Bolivia. Bol. Soc. Argent. Bot. 2015, 50, 595–600. [Google Scholar] [CrossRef]
- Gori, M.; Biricolti, S.; Pedrazzani, S.; Giordani, E.; Papini, A.; Dantur, O.R.; Arena, M.E.; Radice, S. Berberis burruyacuensis OR Dantur, S. Radice, E. Giordani, A. Papini sp. nov.(Berberidaceae): A new species. Genet. Resour. Crop Evol. 2021, 68, 1799–1808. [Google Scholar] [CrossRef]
- Pedrazzani, S.; Scali, E.; Radice, S.; Giordani, E. WARMIPURA: Recovery of ancestral techniques for dyeing wool and natural fibers in North-West Argentina. J. Univ. Int. Dev. Coop. 2020, 4, 43–52. [Google Scholar]
- Shaffer, J.E. Inotropic and chronotropic activity of berberine on isolated guinea pig atria. J. Cardiovasc. Pharmacol. 1985, 7, 307–315. [Google Scholar] [CrossRef]
- Fajardo Morales, V. Estudio químico de las especies chilenas del género Berberis. Rev. Lat. Quím. 1987, 18, 46–50. [Google Scholar]
- Fajardo Morales, V.; Podestá, F.; Urzúa, A. Reseña de los alcaloides encontrados en el género Berberis de Chile. Rev. Lat. Quím. 1986, 16, 141–156. [Google Scholar]
- Küpeli, E.; Koşar, M.; Yeşilada, E.; Başer, K.H.C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef]
- Andola, H.C.; Rawal, R.S.; Rawat, M.S.M.; Bhatt, I.D.; Purohit, V.K. Variations of berberine contents in Berberis pseudumbellata: A high value medicinal shrub of west Himalaya, India. Med. Plants 2010, 2, 111–115. [Google Scholar] [CrossRef]
- Mokhber-Dezfuli, N.; Saeidnia, S.; Reza Gohari, A.; Kurepaz-Mahmoodabadi, M. Phytochemistry and Pharmacology of Berberis species. Pharmacogn. Rev. 2014, 8, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Bisht, A.; Devkota, H.P.; Ullah, H.; Khan, H.; Pandey, A.; Bhatt, I.D.; Echeverría, J. Phytopharmacology and clinical updates of Berberis species against diabetes and other metabolic diseases. Front. Pharmacol. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Radice, S.; Dantur, G.; Ceribelli, A.; Arena, M.E. Berberís mikuna Job., una especie frutal nativa de Tucumán con potencial nutracéutico y tintóreo. Agriscientia 2021, 38, 41–60. [Google Scholar] [CrossRef]
- Cazar Villacís, I.M. Análisis Físico-Químico Para la Determinación de la Calidad de Las Frutas. 2016. Available online: https://repositorio.puce.edu.ec/handle/123456789/20896 (accessed on 12 March 2025).
- Vega, E.N.; González-Zamorano, L.; Cebadera, E.; Barros, L.; Pires, T.C.; Molina, A.K.; da Silveira, T.F.F.; Vidal-Diez de Ulzurrun, G.; Tadío, J.; Cámara, M.; et al. Natural Food Colorant Obtained from Wild Berberis vulgaris L. by Ultrasound-Assisted Extraction: Optimization and Characterization. Foods 2025, 14, 183. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.E.; Thomas-Valdés, S.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Zampini, I.C.; Torres, S.; Alberto, M.R.; Ramos, L.L.; Schmeda-Hirschmann, G.; Isla, M.I. Chemical and functional characterization of skin, pulp and seed powder from the Argentine native fruit mistol (Ziziphus mistol): Effects of phenolic fractions on key enzymes involved in metabolic syndrome and oxidative stress. J. Funct. Foods 2017, 37, 531–540. [Google Scholar] [CrossRef]
- Desalegn, B.B. Effect of soaking and germination on proximate composition, mineral bioavailability and functional properties of chickpea flour. J. Food Public Health 2015, 5, 108–113. [Google Scholar]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Boeri, P.; Piñuel, L.; Dalzotto, D.; Monasterio, R.; Fontana, A.; Sharry, S.; Barrios, D.A.; Carrillo, W. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J. Food Biochem. 2020, 44, e13254. [Google Scholar] [CrossRef]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef]
- Andola, H.C.; Rawal, R.S.; Bhatt, I.D. Comparative studies on the nutritive and anti-nutritive properties of fruits in selected Berberis species of West Himalaya, India. Food Res. Int. 2011, 44, 2352–2356. [Google Scholar] [CrossRef]
- Ji, S.H.; Kang, J.H.; Jo, G.S.; Lee, S.K.; Kim, H.R.; Choi, Y.M.; Lee, Y.S. Comparison of ash and mineral contents in local agricultural products. Korean J. Food Nutr. 2016, 29, 1015–1022. [Google Scholar] [CrossRef]
- Dhungel, S.; Joshi, G.P.; Pant, D.R. Antioxidant and antibacterial activities of fruit extracts of Berberis species from Nepal. BOTOR 2016, 10, 6–11. [Google Scholar] [CrossRef]
- Khromykh, N.O.; Lykholat, Y.V.; Kovalenko, I.M.; Kabar, A.M.; Didur, O.O.; Nedzvetska, M.I. Variability of the antioxidant properties of Berberis fruits depending on the plant species and conditions of habitat. Regul. Mech. Biosyst. 2018, 9, 56–61. [Google Scholar] [CrossRef]
- Krasteva, D.; Ivanov, Y.; Chengolova, Z.; Godjevargova, T. Antimicrobial potential, antioxidant activity, and phenolic content of grape seed extracts from four grape varieties. Microorganisms 2023, 11, 395. [Google Scholar] [CrossRef]
- Wang, S.Y.; Camp, M.J.; Ehlenfeldt, M.K. Antioxidant capacity and α-glucosidase inhibitory activity in peel and flesh of blueberry (Vaccinium spp.) cultivars. Food Chem. 2012, 132, 1759–1768. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.-Y.; Zhao, C.-N.; Feng, X.-L.; Xu, X.-Y.; Cao, S.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, H.-B. Comparison of antioxidant activities of different grape varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef]
- de Andrade, R.B.; Machado, B.A.S.; Barreto, G.d.A.; Nascimento, R.Q.; Corrêa, L.C.; Leal, I.L.; Tavares, P.P.L.G.; Ferreira, E.d.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Antimicrobial Bioactive Compounds. Biology 2021, 10, 1262. [Google Scholar] [CrossRef]
- Nath, H.; Samtiya, M.; Dhewa, T. Beneficial attributes and adverse effects of major plant-based foods anti-nutrients on health: A review. Hum. Nutr. Metab. 2022, 28, 200147. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. J. Food Sci. 2008, 73, C241–C248. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Wrolstad, R.E. Extraction of anthocyanins and polyphenolics from blueberry processing waste. J. Food Sci. 2004, 69, 564–573. [Google Scholar] [CrossRef]
- Chander, V.; Aswal, J.S.; Dobhal, R.; Uniyal, D.P. A review on Pharmacological potential of Berberine; an active component of Himalayan Berberis aristata. J. Phytopharmacol. 2017, 6, 53–58. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, R.; Li, Q.; Liu, Y.; Li, Y.; Chen, H.; Ai, G.; Xie, J.; Zeng, H.; Chen, J.; et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed. Pharmacother. 2021, 137, 111312. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.J.; et al. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Li, J.; Liu, J.; Xu, F. Nutritional composition and antioxidant properties of the fruit of Berberis heteropoda Schrenk. PLoS ONE 2022, 17, e0262622. [Google Scholar] [CrossRef]
- Jin, H.J.; Lee, J.H.; Kim, D.H.; Kim, K.T.; Lee, G.W.; Choi, S.J.; Chang, P.S.; Paik, H.D. Antioxidative and nitric oxide scavenging activity of branched-chain amino acids. Food Sci. Biotechnol. 2015, 24, 1555–1558. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Nuncio-Jáuregui, N.; Calín-Sánchez, A.; Carbonell-Barrachina, A.; Hernández, F. Changes in quality parameters, proline, antioxidant activity and color of pomegranate (Punica granatum L.) as affected by fruit position within tree, cultivar and ripening stage. Sci. Hortic. 2014, 165, 181–189. [Google Scholar] [CrossRef]
- Mariangel, E.; Reyes-Diaz, M.; Lobos, W.; Bensch, E.; Schalchli, H.; Ibarra, P. The antioxidant properties of calafate (Berberis microphylla) fruits from four different locations in southern Chile. Cienc. Investig Agrar. 2013, 40, 161–170. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020, 87, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 588. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Murshed, A.; Zaky, M.Y.; Khan, I.; Rahman, F.U.; Abdellattif, M.H.; Liu, G.; Lu, J. Berberis lycium: A Miracle Medicinal Plant with Multifaceted Health Benefits. J. Food Qual. 2024, 2024, 3152456. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Barbary, M.A.; El-Ghorab, D.M.; Bohlin, L.; Borg-Karlson, A.K.; Göransson, U.; Verpoorte, R. Recent insights into the biosynthesis and biological activities of natural xanthones. Curr. Med. Chem. 2010, 17, 854–901. [Google Scholar] [CrossRef]
- Sukpondma, Y.; Rukachaisirikul, V.; Phongpaichit, S. Antibacterial caged-tetraprenylated xanthones from the fruits of Garcinia hanburyi. Chem. Pharm. Bull. 2005, 53, 850–852. [Google Scholar] [CrossRef]
- Jin, Y.M.; Kim, J.Y.; Lee, S.M.; Tan, X.F.; Park, K.H. α-Glucosidase inhibitory caged xanthones from the resin of Garcinia hanburyi. J. Appl. Biol. Chem. 2019, 62, 81–86. [Google Scholar] [CrossRef]
- Mortazaeinezahad, F.; Safavi, K. Investigation of epicatechin in barberry fruits. In Proceedings of the International Conference on Life Science and Technology (ICLST 2011), Mumbai, India, 7–9 January 2011. [Google Scholar]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef]
- Liu, T.; Cao, L.; Zhang, T.; Fu, H. Molecular docking studies, anti-Alzheimer’s disease, antidiabetic, and anti-acute myeloid leukemia potentials of narcissoside. Arch. Physiol. Biochem. 2023, 129, 405–415. [Google Scholar] [CrossRef]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. [Google Scholar] [CrossRef]
- Feng, J.; Xu, B.; Ma, D.; Hao, Z.; Jia, Y.; Wang, C.; Wang, L. Metabolite identification in fresh wheat grains of different colors and the influence of heat processing on metabolites via targeted and non-targeted metabolomics. Food Res. Int. 2022, 160, 111728. [Google Scholar] [CrossRef] [PubMed]
- Bernjak, B.; Kristl, J. A review of tannins in berries. Sci. Agric. 2020, 17, 27–36. [Google Scholar] [CrossRef]
- Tie, F.; Wang, J.; Liang, Y.; Zhu, S.; Wang, Z.; Li, G.; Wang, H. Proanthocyanidins ameliorated deficits of lipid metabolism in type 2 diabetes mellitus via inhibiting adipogenesis and improving mitochondrial function. Int. J. Mol. Sci. 2020, 21, 2029. [Google Scholar] [CrossRef] [PubMed]
- Gidik, B. Antioxidant, antimicrobial activities and fatty acid compositions of wild Berberis spp. by different techniques combined with chemometrics (PCA and HCA). Molecules 2021, 26, 7448. [Google Scholar] [CrossRef]
- Balta, I.; Stef, L.; Pet, I.; Iancu, T.; Stef, D.; Corcionivoschi, N. Essential fatty acids as biomedicines in cardiac health. Biomedicines 2021, 9, 1466. [Google Scholar] [CrossRef]
- Özgen, M.; Saraçoğlu, O.; Geçer, E.N. Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits. Hortic. Environ. Biotechnol. 2012, 53, 447–451. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Leal, M.; Mercado, M.I.; Moreno, M.A.; Chamas, J.J.M.; Zampini, I.C.; Ponessa, G.I.; Simirgiotis, M.J.; Isla, M.I. Gochnatia glutinosa (D. Don) D. Don ex Hook. & Arn.: A plant with medicinal value against inflammatory disorders and infections. Heliyon 2023, 9, e15276. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial effects of phenolic compounds on gut microbiota and metabolic syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.D.A.A.; Rossetto, R.; Brugnari, T.; Ribeiro, V.R.; Haminiuk, C.W.I. Biological potential and technological applications of red fruits: An overview. Food Chem. Adv. 2022, 1, 100014. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Moura, M.H.C.; de Araujo, R.L.; de Lima Santiago, G.; de Moraes Barros, H.R.; Genovese, M.I. Polyphenols from Brazilian native Myrtaceae fruits and their potential health benefits against obesity and its associated complications. Curr. Opin. Food Sci. 2018, 19, 42–49. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Moein, S.; Moein, M.; Javid, H. Inhibition of α-Amylase and α-Glucosidase of Anthocyanin Isolated from Berberis integerrima Bunge Fruits: A Model of Antidiabetic Compounds. Evid. Based Complement. Altern. Med. 2022, 2022, 6529590. [Google Scholar] [CrossRef] [PubMed]
- Moazezi, Z.; Qujeq, D. Berberis fruit extract and biochemical parameters in patients with type II diabetes. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e13490. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Szot, I. The Possibility of Using Fruit-Bearing Plants of Temperate Climate in the Treatment and Prevention of Diabetes. Life 2023, 13, 1795. [Google Scholar] [CrossRef]
- He, X.; Chen, L.; Pu, Y.; Wang, H.; Cao, J.; Jiang, W. Fruit and vegetable polyphenols as natural bioactive inhibitors of pancreatic lipase and cholesterol esterase: Inhibition mechanisms, polyphenol influences, application challenges. Food Biosci. 2023, 55, 103054. [Google Scholar] [CrossRef]
- Sosnowska, D.; Podsędek, A.; Kucharska, A.Z. Proanthocyanidins as the main pancreatic lipase inhibitors in chokeberry fruits. Food Funct. 2022, 13, 5616–5625. [Google Scholar] [CrossRef]
- Orqueda, M.E.; Torres, S.; Zampini, I.C.; Cattaneo, F.; Di Pardo, A.F.; Valle, E.M.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Isla, M.I. Integral use of Argentinean Solanum betaceum red fruits as functional food ingredient to prevent metabolic syndrome: Effect of in vitro simulated gastroduodenal digestion. Heliyon 2020, 6, e03387. [Google Scholar] [CrossRef]
- Correa Uriburu, F.M.; Zampini, I.C.; Maldonado, L.M.; Gómez Mattson, M.; Salvatori, D.; Isla, M.I. Chemical Characterization and Biological Activities of a Beverage of Zuccagnia punctata, an Endemic Plant of Argentina with Blueberry Juice and Lemon Honey. Plants 2024, 13, 3143. [Google Scholar] [CrossRef]
- Usenik, V.; Štampar, F.; Veberic, R. Anthocyanins and fruit colour in plums (Prunus domestica L.) during ripening. Food Chem. 2009, 114, 529–534. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Somogyi, M. A new reagent for the determination of sugars. J. Biol. Chem. 1945, 160, 61–68. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- AOCS. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1989. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nico, C.; Payne, M.J.; Reed, J. Multilaboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decoloration assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Optimized enzymatic colorimetric assay for determination of hydrogen peroxide (H2O2) scavenging activity of plant extracts. Methods X 2015, 2, 283–291. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Ordoñez, R.M.; Zampini, I.C.; Sayago, J.E.; Isla, M.I. Nutritional and antioxidant properties and toxicity of Geoffroea decorticans, an Argentinean fruit and products derived from them (flour, arrope, decoction and hydroalcoholic beverage). Food Res. Int. 2013, 54, 160–168. [Google Scholar] [CrossRef]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, A.S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols rich fraction from Geoffroea decorticans fruits flour affects key enzymes involved in metabolic syndrome, oxidative stress and inflammatory process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.M.; Mathiasson, L.; Martensson, L.; Bergatröm, S. Artemia salina as test organism for assessment of acute toxicity of leachate water from landfills. Environ. Monit. Assess. 2005, 102, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res./Environ. Mutagen. Relat. Subjects 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2015. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar/ (accessed on 12 December 2024).



| Plant Species | Fruit Parts | Chromatic Parameters | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| Berberis burruyacuensis | Peel | 36.14 ± 0.13 B | 4.75 ± 0.19 A | 1.86 ± 0.15 B |
| Pulp | 34.58 ± 0.45 B | 4.26 ± 0.15 B | 0.98 ± 0.09 B | |
| Seed | 42.07 ± 0.40 B | 8.00 ± 0.11 B | 9.58 ± 0.01 B | |
| Berberis mikuna | Peel | 34.08 ± 0.01 A | 4.15 ± 0.04 A | 0.72 ± 0.02 A |
| Pulp | 33.23 ± 0.12 A | 2.70 ± 0.07 A | 0.07 ± 0.01 A | |
| Seed | 39.91 ± 0.06 A | 4.69 ± 0.01 A | 7.42 ± 0.28 A | |
| Plant Species | Fruit Parts | Moisture # (%) | Total Soluble Sugars (g GE/100 g Powder) | Reducing Sugars (g GE/100 g Powder) | Total Protein (%) | Fat (%) | Total Fibers (g/100 g Powder) | Ashes (%) |
|---|---|---|---|---|---|---|---|---|
| Berberis burruyacuensis | Peel | 62 ± 3 C | 25.38 ± 2.43 B | 23.75 ± 2.02 B,C | 6.2 ± 0.3 A | 2.0 ± 0.1 C | 54.04 ± 0.51 D | 0.38 ± 0.03 A,B |
| Pulp | 82 ± 2 D | 24.68 ± 1.27 B | 26.31 ± 0.05 C | 6.9 ± 0.3 A | 1.8 ± 0.1 C | 25.84 ± 0.48 B | 0.41 ± 0.04 A,B | |
| Seed | 47 ± 2 B | 26.99 ± 1.11 B | 12.07 ± 0.70 A | 14.9 ± 0.8 C | 2.4 ± 0.1 D | 63.86 ± 2.03 E | 0.96 ± 0.08 C | |
| Berberis mikuna | Peel | 57 ± 2 C | 23.68 ± 1.40 B | 16.03 ± 2.66 A,B | 6.1 ± 0.3 A | 1.3 ± 0.1 B | 35.40 ± 0.53 C | 0.50 ± 0.05 B |
| Pulp | 82 ± 2 D | 37.55 ± 1.37 C | 36.80 ± 3.96 D | 6.3 ± 0.2 A | 0.9 ± 0.04 A | 12.55 ± 0.85 A | 0.26 ± 0.02 A | |
| Seed | 38 ± 2 A | 16.72 ± 0.95 A | 8.90 ± 0.33 A | 12.8 ± 0.7 B | 1.5 ± 0.1 B | 54.96 ± 2.14 D | 1.33 ± 0.11 D |
| Plant Species | Fruit Parts | Total Phenolic Compounds (g GAE/100 g Powder) | Total Flavonoids (g QE/100 g Powder) | Total Anthocyanins (mg C3GE/100 g Powder) | Condensed Tannins (mg PB2/100 g Powder) |
|---|---|---|---|---|---|
| Berberis burruyacuensis | Peel | 0.52 ± 0.01 A | 1.35 ± 0.02 A | 54.60 ± 0.97 B | 0.03 ± 0.00 A |
| Pulp | 0.78 ± 0.00 B | 2.10 ± 0.01 A | 137.78 ± 3.18 C | 0.04 ± 0.00 A | |
| Seed | 7.94 ± 0.07 D | 43.31 ± 0.41 C | 20.56 ± 0.65 A | 6.09 ± 0.06 C | |
| Berberis mikuna | Peel | 0.62 ± 0.01 A,B | 1.16 ± 0.01 A | 195.55 ± 7.75 D | 0.06 ± 0.00 A |
| Pulp | 0.76 ± 0.01 B | 1.25 ± 0.02 A | 283.49 ± 6.55 E | 0.05 ± 0.00 A | |
| Seed | 6.16 ± 0.19 C | 29.30 ± 0.93 B | 7.82 ± 0.79 A | 3.64 ± 0.11 B |
| Peak | Tentative Identification | Retention Time (min) | [M + H]+ | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (ppm) | Metabolite Type | MS Ions (ppm) | B. burruyacuensis Samples | B. mikuna Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Na formiate (internal standard) | 0.37 | NaC2H2O4 | 113.9829 | 113.9842 | 2.3 | Standard | - | a,b,c | a,b,c |
| 2 | L-Histidine | 0.6 | C6H9N3O2 | 156.0766 | 156.0733 | −21.14 | Amino acid | 83.0599 | a,b,c | a,b,c |
| 3 | L-Glutamic acid | 0.8 | C5H9NO4 | 148.0609 | 148.0535 | −49.8 | Amino acid | 84.0456 | a,c | c |
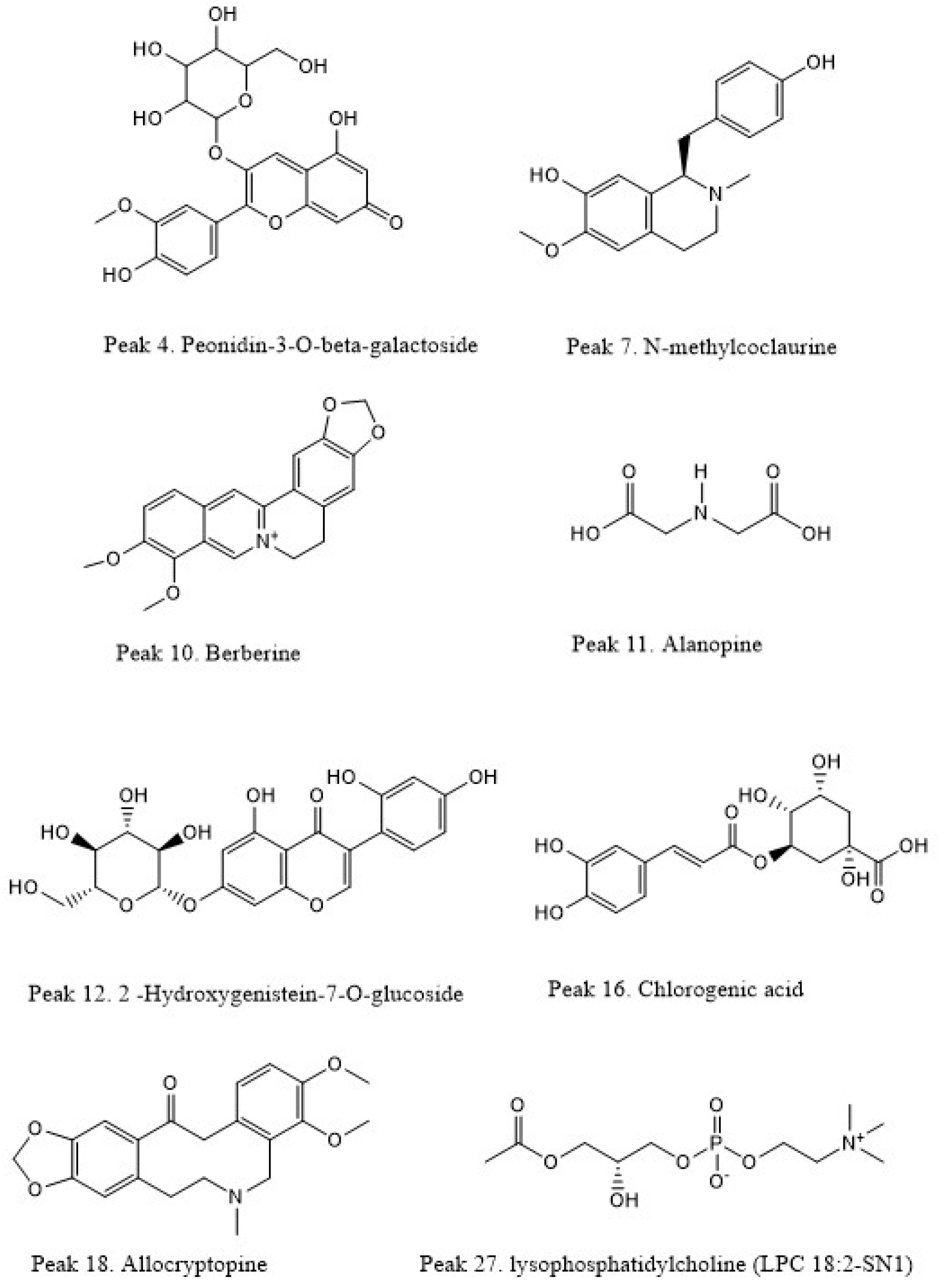
| 4 | Peonidin-3-O-beta-galactoside | 0.9 | C22H22O11 | 463.1211 | 463.1138 | −15.7 | Anthocyanin | 298.0452, 283.0243, 212.0443 | b | a,b |
| 5 | Caffeic acid | 2.1 | C9H9O4 | 181.0641 | 181.0622 | −21.14 | Phenolic acid | 83.0599 | b,c | c |
| 6 | Trigonelline | 2.25 | C7H7NO2 | 138.0551 | 138.0578 | 19.5 | Alkaloid (piridin) | 110.0601, 94.0655 | - | c |
| 7 | N-methylcoclaurine | 5.57 | C18H21NO3 | 200.1590 | 200.1532 | −28.9 | Alkaloid | 269.1181, 107.0491 | - | c |
| 8 | Ascorbic acid | 9.07 | C6H8O6 | 177.0543 | 177.0566 | 5.2 | Vitamin | 115.0547 | b,c | a,b,c |
| 9 | Reticuline | 8.28 | C19H23NO4 | 330.1698 | 330.1672 | −7.8 | Alkaloid | 192.1023, 177.082 | - | c |
| 10 | Berberine | 8.28 | C20H18NO4 | 336.1446 | 336.1482 | 4.7 | Alkaloid | 320.1128, 292.1154 | b | b,c |
| 11 | Alanopine2-(1-carboxyethylamino) | 9.10 | C6H11NO4 | 162.0764 | 162.0791 | 16.6 | Alkaloid | 116.0692 | - | c |
| 12 | 2-Hydroxygenistein-7-O-glucoside | 9.30 | C21H20O11 | 449.1068 | 449.0996 | −16.0 | Isoflavone | 287.0524 | - | c |
| 13 | Tryptophan | 9.92 | C11H12N2O | 205.0971 | 205.0898 | −35.5 | Amino acid | 118.0642, 115.0532 | - | a,b,c |
| 14 | Quercetin 4-O-glucoside | 10.17 | C21H20O12 | 465.1041 | 465.0969 | −15.4 | Flavonoid | 303.0523, 229.0586 | c | b,c |
| 15 | Rutin | 10.38 | C27H30O16 | 611.1635 | 611.1662 | 4.4 | Flavonoid | 303.0498, 127.0379 | a,c | a,b,c |
| 16 | Chlorogenic acid | 10.69 | C16H18O9 | 355.1014 | 355.0941 | −20.5 | Phenolic ester | 163.0389 | - | a,c |
| 17 | Tetrahydropalmatine | 11.20 | C21H25NO4 | 356.1789 | 356.1716 | −20.4 | Alkaloid | 176.0778, 148.0768 | - | c |
| 18 | Allocryptopine | 11.25 | C21H23NO5 | 370.1650 | 370.1677 | 7.29 | Alkaloid | 188.0707, 149.0596 | c | c |
| 19 | Procyanidin B1 | 11.40 | C30H26O12 | 579.1488 | 579.1535 | 8.11 | Tannin | 407.0802, 290.0697 | a,b,c | a,b,c |
| 20 | Epicatechin | 12.11 | C15H14O6 | 291.0853 | 291.0880 | 9.27 | Tannin | 147.0472, 123.0462 | c | c |
| 21 | 5,7-Dihydroxy-4-methylcoumarin | 12.81 | C10H8O4 | 215.0162 | 215.0184 | 10.23 | Coumarin | 147.0442 | a,b | - |
| 22 | Tetrahydrocolumbamine | 12.69 | C20H23NO4 | 341.1695 | 341.1891 | 57.2 | Alkaloid | 303.3251, 294.1150 | a,b,c | - |
| 23 | Isorhamnetin-3-O-rutinoside | 12.90 | C28H32O16 | 625.1750 | 625.1677 | −11.6 | Flavonoid | 271.0239, 243.0265 | a,b,c | a,c |
| 24 | Oxyberberine | 13.38 | C20H17NO5 | 352.1176 | 352.1165 | −3.12 | Alkaloid | 374.0992 (M + Na), 344.0573, | b,c | a,b,c |
| 25 | Alpinetin | 13.52 | C16H14O4 | 271.0960 | 271.0887 | Flavonoid | 167.0365, 152.0123 | a,b | a,c | |
| 26 | lysophosphatidylcholine (LPC 18:3-SN1) | 13.66 | C26H48NO7P | 518.3227 | 518.3254 | 5.20 | Lipid | 494.3489, 475.3257, 466.3167, 459.3174 | b,c | b,c |
| 27 | lysophosphatidylcholine (LPC 18:2-SN1) | 13.77 | C26H50NO7P | 520.3380 | 520.3357 | −4.4 | Lipid | 494.3489, 475.3257, 466.3167, 459.3174 | a,b,c | a,b,c |
| 28 | Linolenic acid | 14.01 | C18H30O2 | 279.2315 | 279.2242 | −26.14 | Lipid | 95.0875, 67.0538 | a,b | a |
| 29 | Columbamine | 14.15 | C20H20NO4 | 338.3653 | 338.3623 | −8.8 | Alkaloid | 322.1085, 303.3255, 294.1153 | a,b,c | b,c |
| 30 | Erucic acid | 14.52 | C22H42O2 | 339.3106 | 339.3258 | 24.6 | Fatty acid | 255.2514 | b | - |
| 31 | Linoleic acid | 14.86 | C18H32O2 | 281.2470 | 281.2487 | Fatty acid | 255.2432 | b | b | |
| 32 | Moreollic Acid | 15.08 | C34H41O9 | 593.3158 | 593.2945 | −29 | Xanthone | b,c | c | |
| 33 | Mellein | 13.27 | C10H10O3 | 179.0701 | 179.07356 | 19.15 | Coumarin | a | a |
| Plant Species | Fruit Parts | ABTS•+ | H2O2 |
|---|---|---|---|
| SC50 (μg GAE/mL) | |||
| Berberis burruyacuensis | Peel | 1.80 ± 0.14 E | 41.5 ± 0.7 C |
| Pulp | 1.13 ± 0.01 C,D | 31.5 ± 0.7 B | |
| Seed | 1.00 ± 0.03 B,C | 24.8 ± 0.3 A | |
| Berberis mikuna | Peel | 1.34 ± 0.00 D | * 22.4 ± 0.4 |
| Pulp | 1.63 ± 0.04 E | 52.9 ± 0.5 D | |
| Seed | 0.71 ± 0.01 A | 32.4 ± 0.3 B | |
| Plant Species | Fruit Parts | α-Glucosidase | α-Amylase | Lipase |
|---|---|---|---|---|
| IC50 (µg GAE/mL) | ||||
| Berberis burruyacuensis | Peel | 26.3 ± 4.8 B | 15.9 ± 1.1 C | 0.85 ± 0.01 B |
| Pulp | 42.1 ± 0.6 C | 23.0 ± 1.2 D | 1.16 ± 0.09 C | |
| Seed | 7.5 ± 1.4 A | 3.5 ± 0.8 A | 0.26 ± 0.01 A | |
| Berberis mikuna | Peel | 51.6 ± 0.1 D | 7.4 ± 0.3 B | 1.04 ± 0.09 B,C |
| Pulp | 42.3 ± 0.8 C | 33.6 ± 1.0 E | 1.01 ± 0.04 B,C | |
| Seed | 2.1 ± 0.8 A | 6.5 ± 0.2 B | 0.37 ± 0.14 A | |
| Positive controls (µg/mL) | ||||
| Acarbose | 25.0 ± 1.0 | 1.2 ± 0.1 | - | |
| Orlistat | - | - | 0.08 ± 0.01 | |
| Sample | Powder Extract | Treatment (μg GAE/Plate) | MR TA98 | MR TA100 |
|---|---|---|---|---|
| Berberis burrayucuensis | Peel | 500 | 1.0 | 0.8 |
| 250 | 0.9 | 0.6 | ||
| 125 | 0.7 | 0.7 | ||
| Pulp | 500 | 0.8 | 0.8 | |
| 250 | 0.8 | 0.7 | ||
| 125 | 0.6 | 0.8 | ||
| Seed | 500 | 1.0 | 0.6 | |
| 250 | 0.9 | 0.6 | ||
| 125 | 0.7 | 0.6 | ||
| Berberis Mikuna | Peel | 500 | 0.8 | 0.6 |
| 250 | 1.2 | 0.6 | ||
| 125 | 0.9 | 0.7 | ||
| Pulp | 500 | 0.8 | 0.8 | |
| 250 | 0.8 | 0.8 | ||
| 125 | 0.7 | 0.8 | ||
| Seed | 500 | 0.6 | 0.9 | |
| 250 | 0.9 | 0.7 | ||
| 125 | 0.9 | 0.8 | ||
| Positive control | 40.37 | 4.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, E.A.; Orqueda, M.E.; Leal, M.; Isla, M.I.; Simirgiotis, M.; Zampini, I.C.; Dantur, O.R.; Moreno, M.A. Nutritional, Phytochemical, and Biological Characterization of Peel, Pulp, and Seed Powder from the Fruits of Berberis mikuna and Berberis burruyacuensis: Potential as a Functional Ingredient. Plants 2025, 14, 1418. https://doi.org/10.3390/plants14101418
Matteucci EA, Orqueda ME, Leal M, Isla MI, Simirgiotis M, Zampini IC, Dantur OR, Moreno MA. Nutritional, Phytochemical, and Biological Characterization of Peel, Pulp, and Seed Powder from the Fruits of Berberis mikuna and Berberis burruyacuensis: Potential as a Functional Ingredient. Plants. 2025; 14(10):1418. https://doi.org/10.3390/plants14101418
Chicago/Turabian StyleMatteucci, Enzo Agustín, María Eugenia Orqueda, Mariana Leal, María Inés Isla, Mario Simirgiotis, Iris Catiana Zampini, Oscar R. Dantur, and María Alejandra Moreno. 2025. "Nutritional, Phytochemical, and Biological Characterization of Peel, Pulp, and Seed Powder from the Fruits of Berberis mikuna and Berberis burruyacuensis: Potential as a Functional Ingredient" Plants 14, no. 10: 1418. https://doi.org/10.3390/plants14101418
APA StyleMatteucci, E. A., Orqueda, M. E., Leal, M., Isla, M. I., Simirgiotis, M., Zampini, I. C., Dantur, O. R., & Moreno, M. A. (2025). Nutritional, Phytochemical, and Biological Characterization of Peel, Pulp, and Seed Powder from the Fruits of Berberis mikuna and Berberis burruyacuensis: Potential as a Functional Ingredient. Plants, 14(10), 1418. https://doi.org/10.3390/plants14101418










