Germination Behavior and Geographical Information System-Based Phenotyping of Root Hairs to Evaluate the Effects of Different Sources of Black Soldier Fly (Hermetia illucens) Larval Frass on Herbaceous Crops
Abstract
1. Introduction
- (i)
- to propose a protocol for standardizing germination tests for the evaluation of frass phytotoxicity, regarding both extract preparation and the use of multiple dilutions to highlight stimulation or toxicity on plant above- and below-ground growth;
- (ii)
- to develop a GIS (geographic information system)-based method for the quantification of root hair features. Applications of this method in root research would span from the fast evaluation of frass and other organic materials in germination tests to agronomic research on below-ground plant features.
2. Results
2.1. Chemical and Microbiological Properties of Frass and Frass Extracts
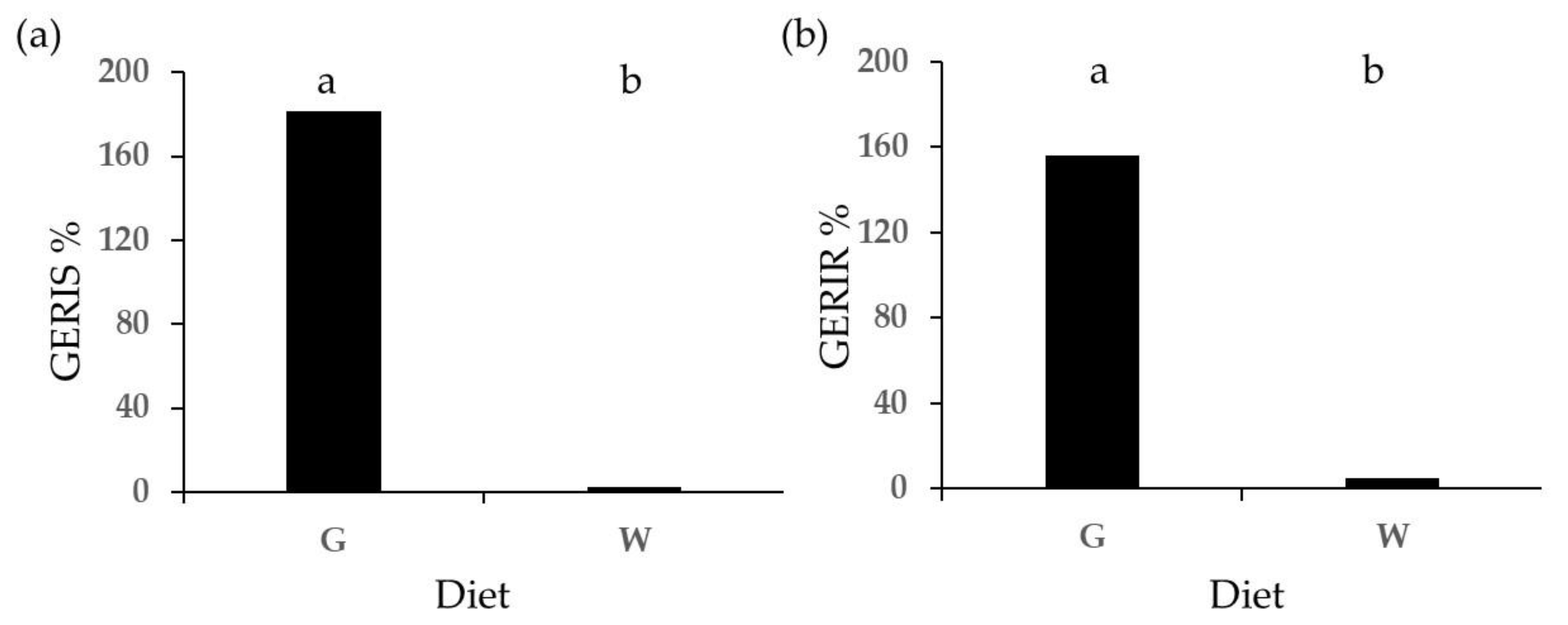
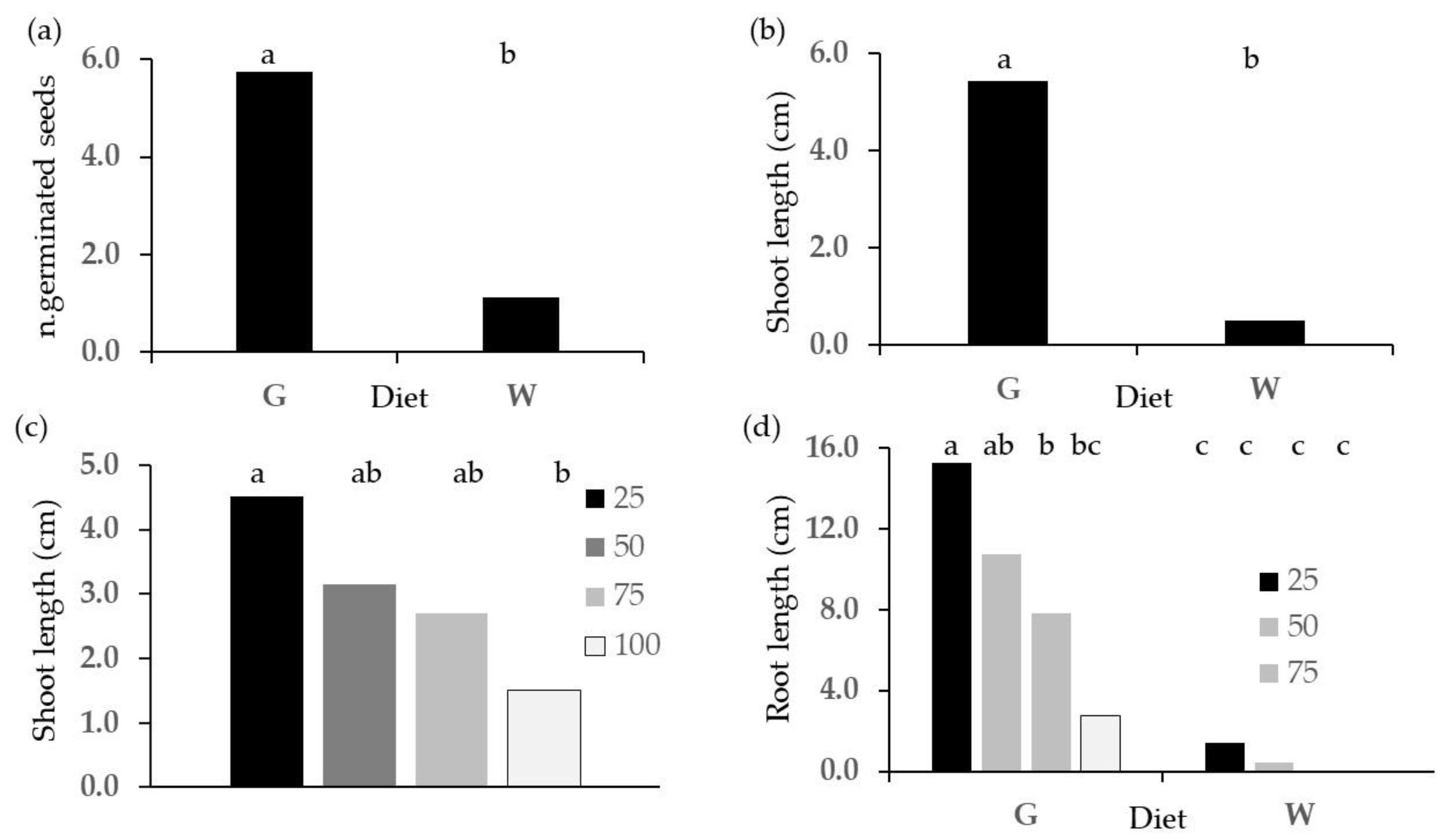
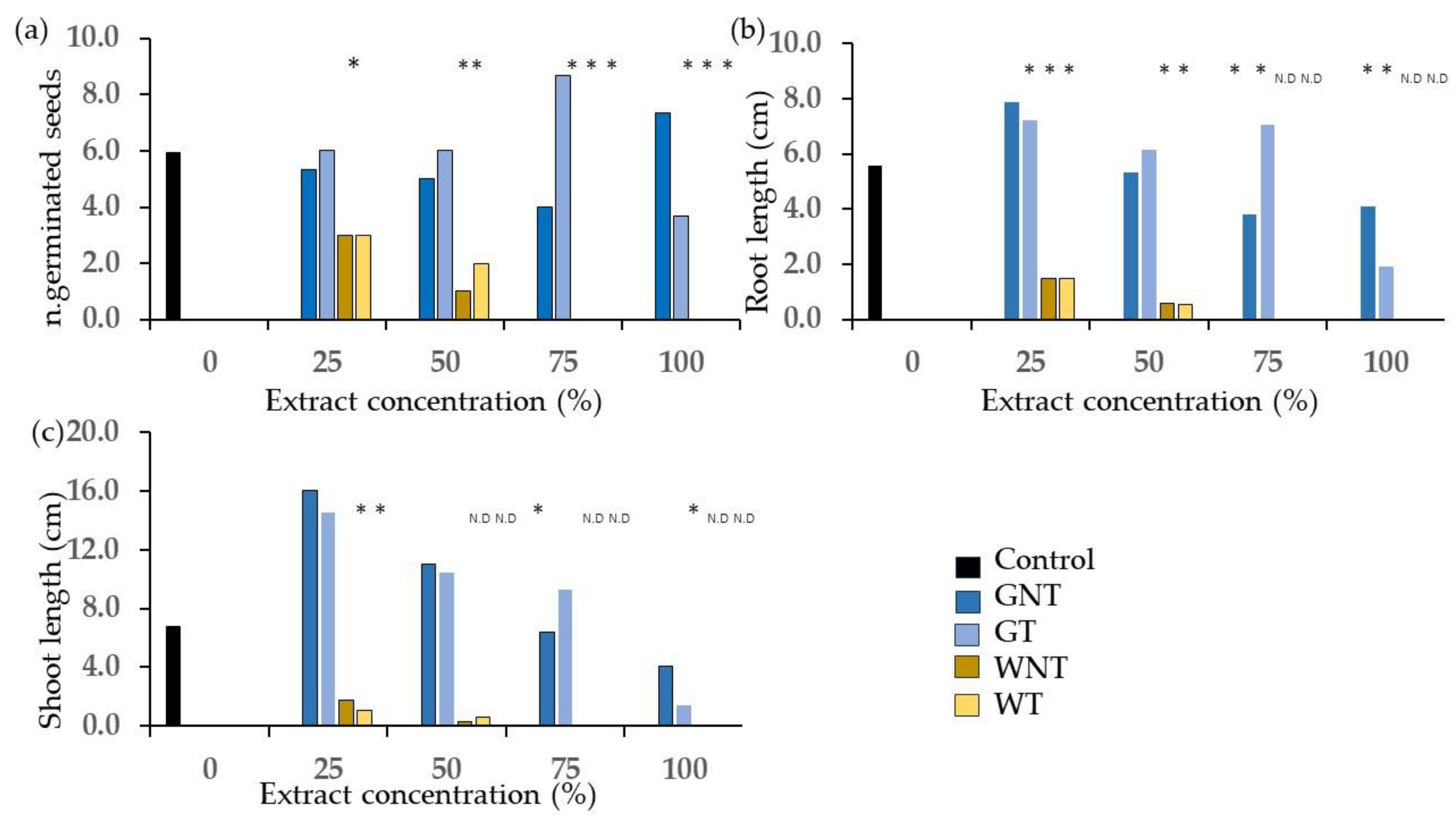
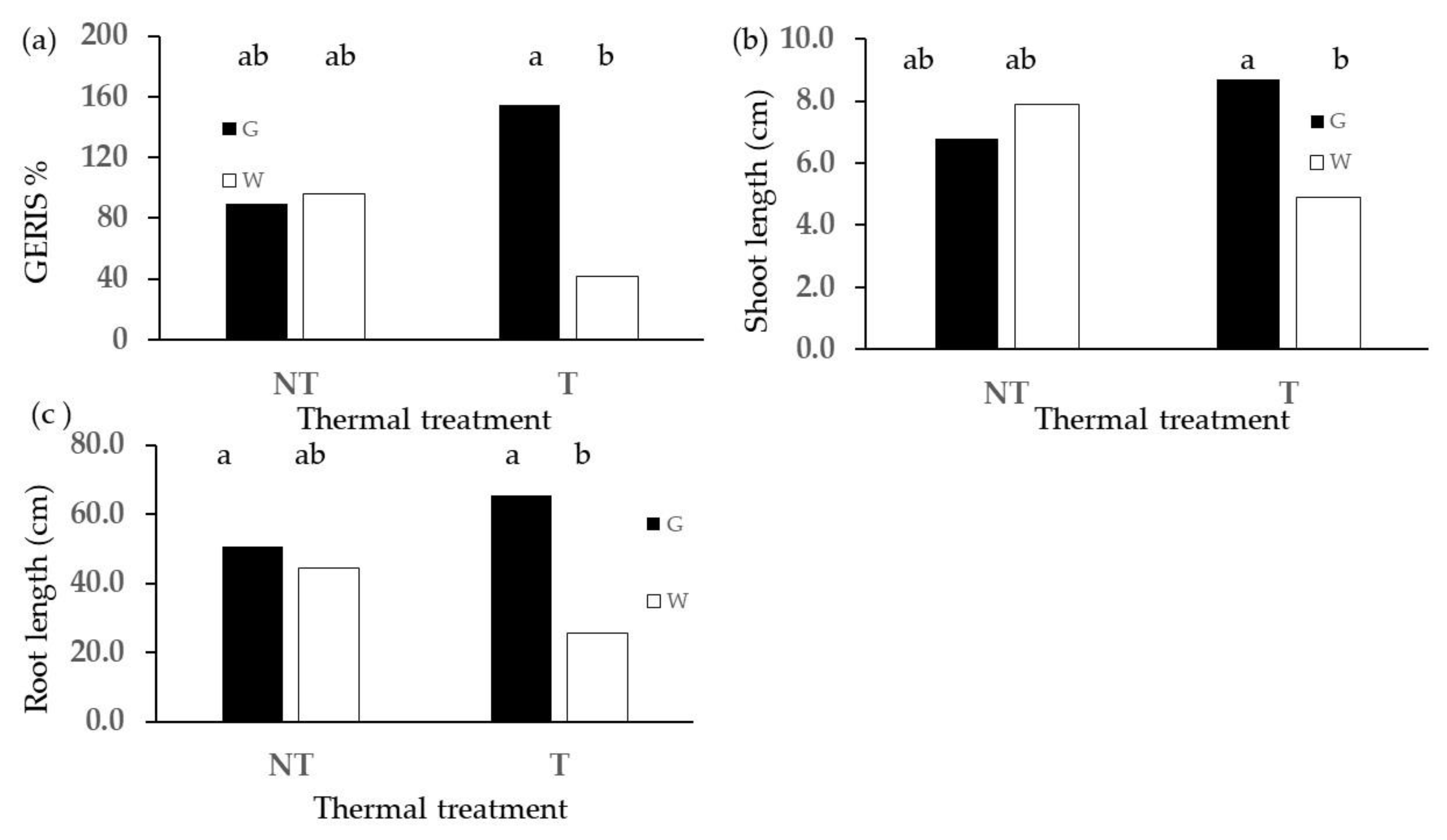
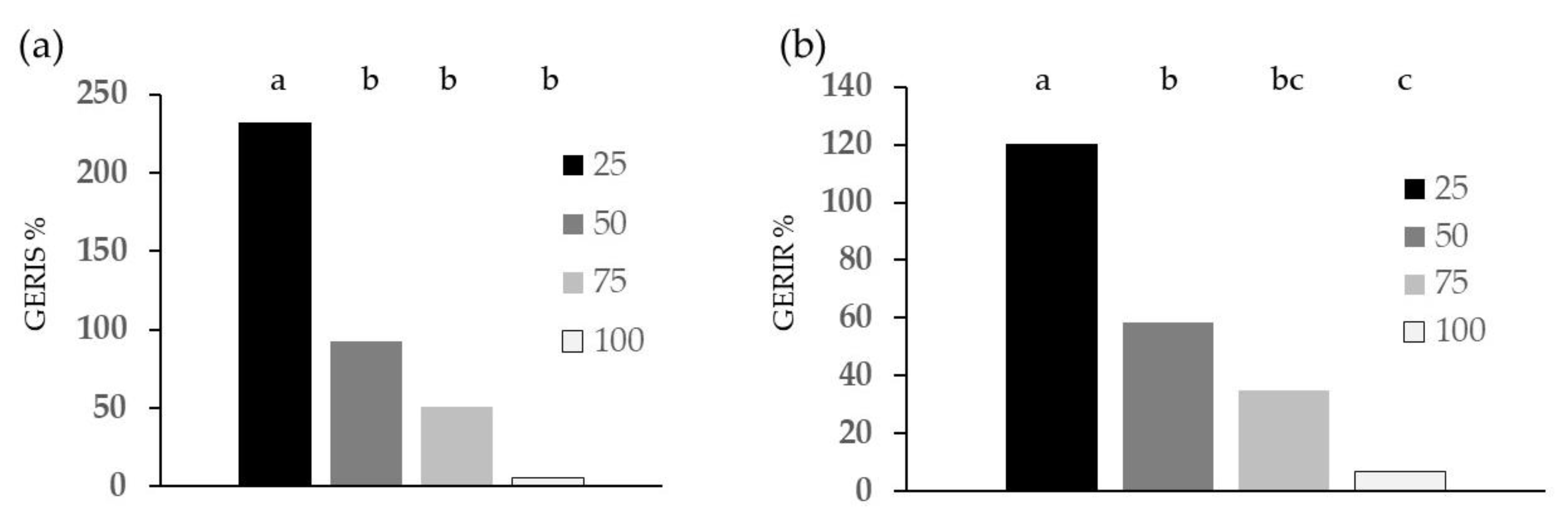
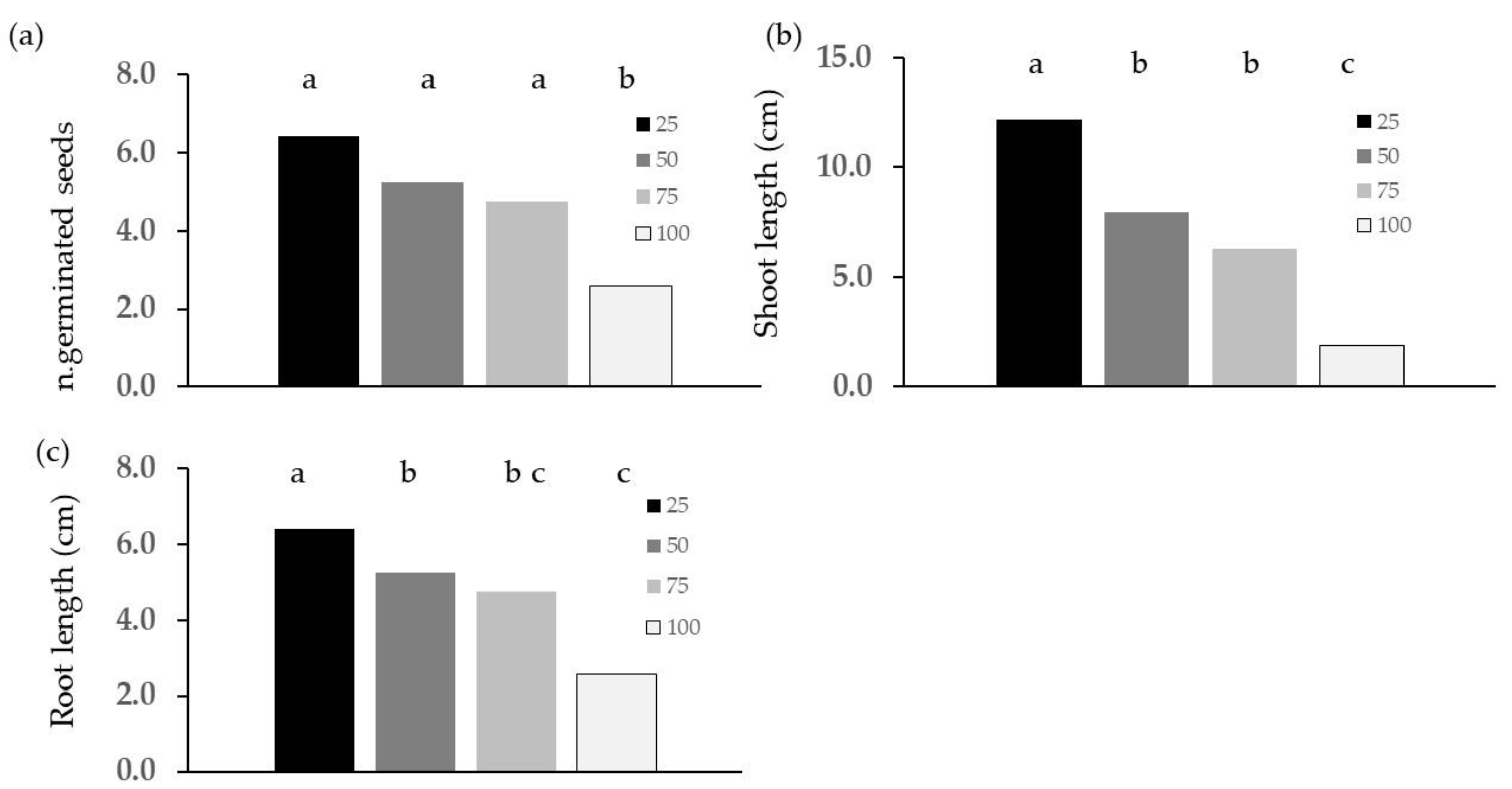
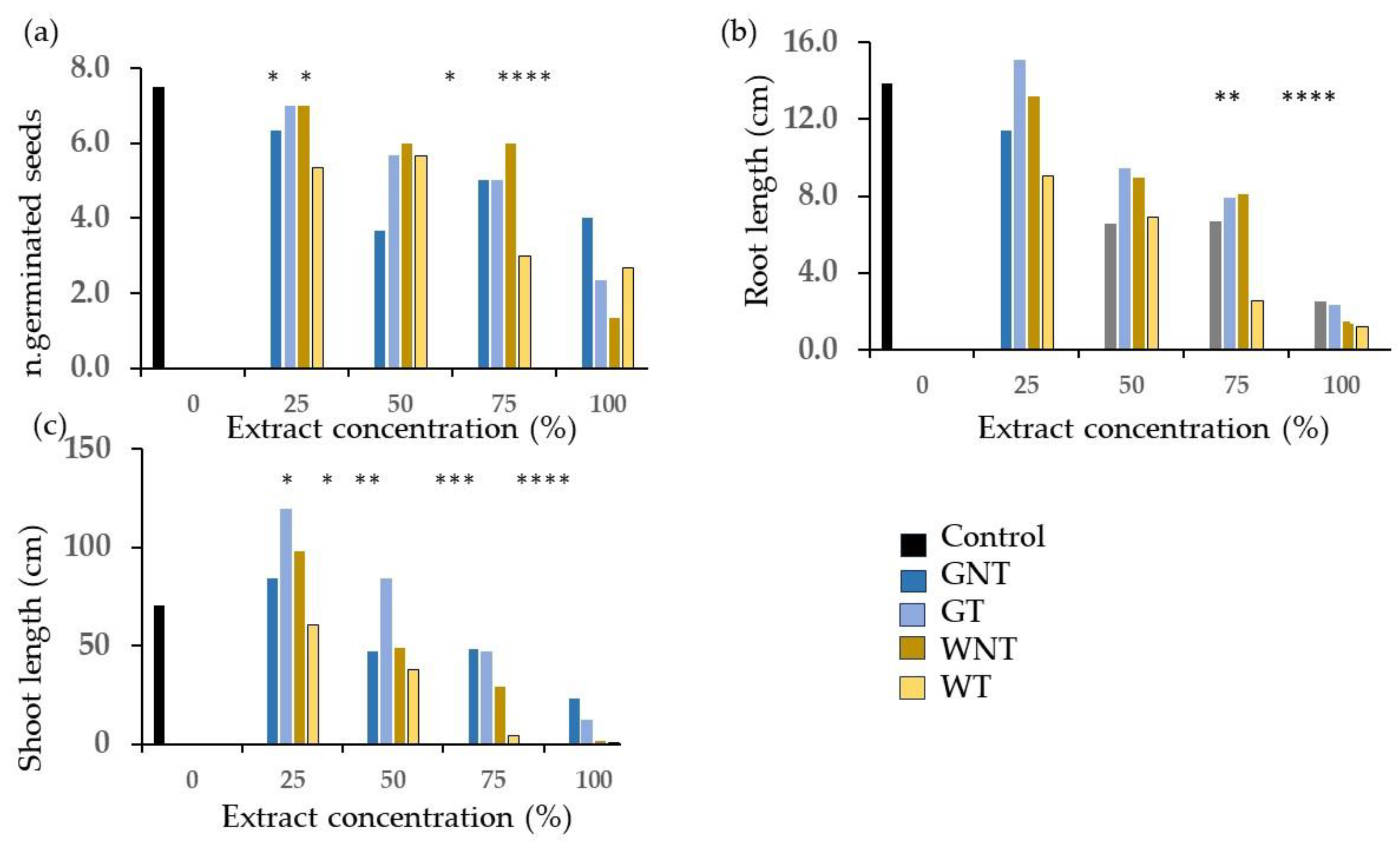
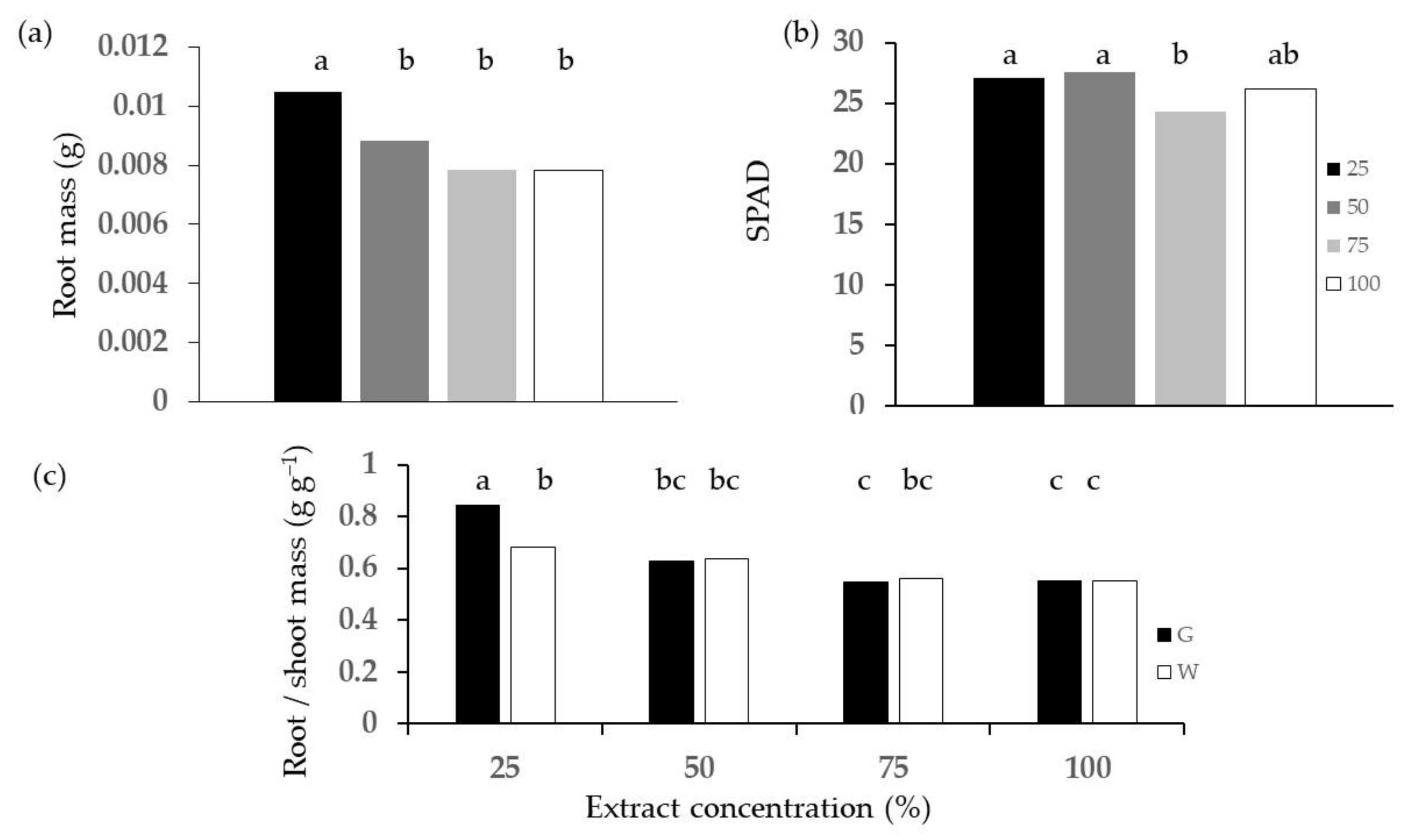
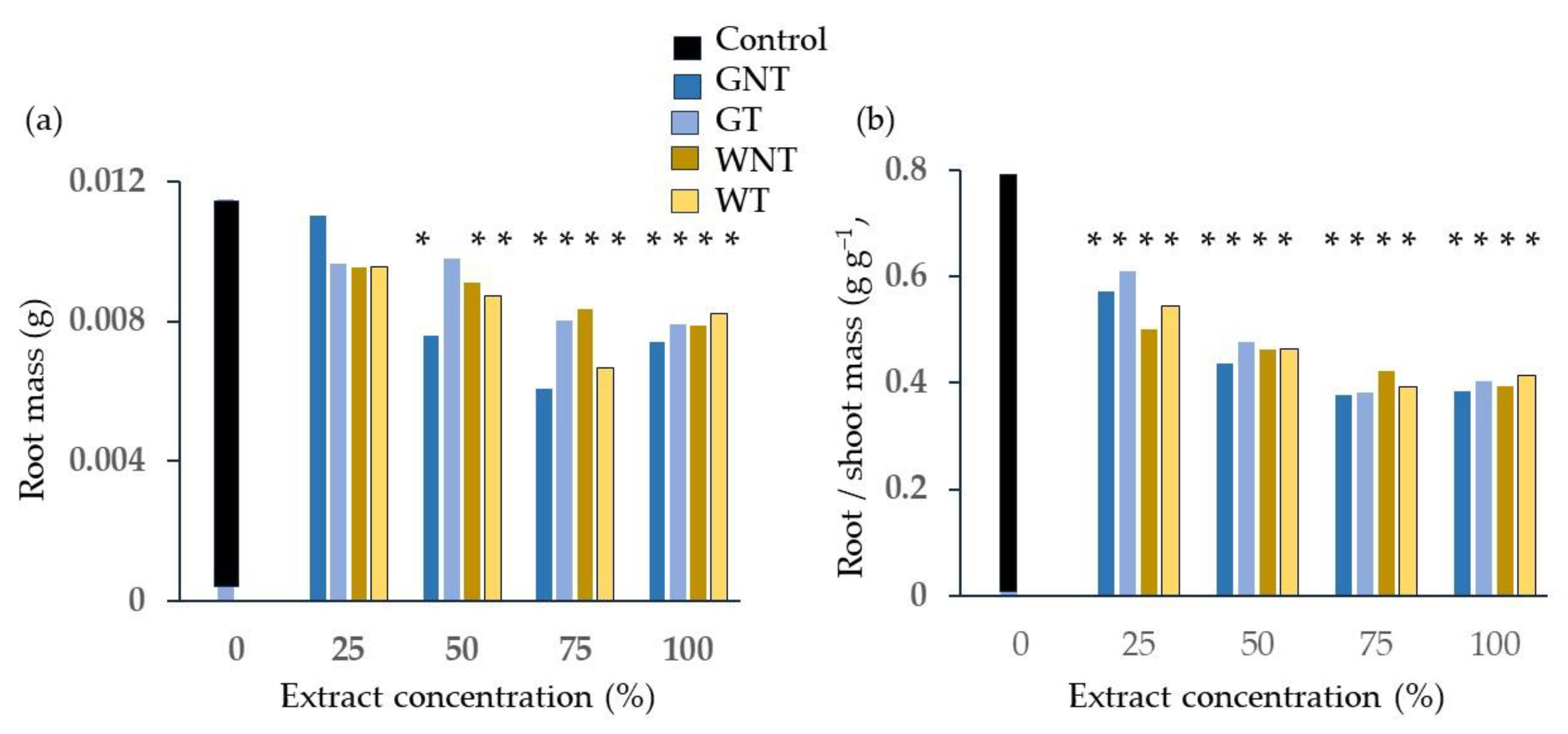
2.2. Phytotoxicity Tests
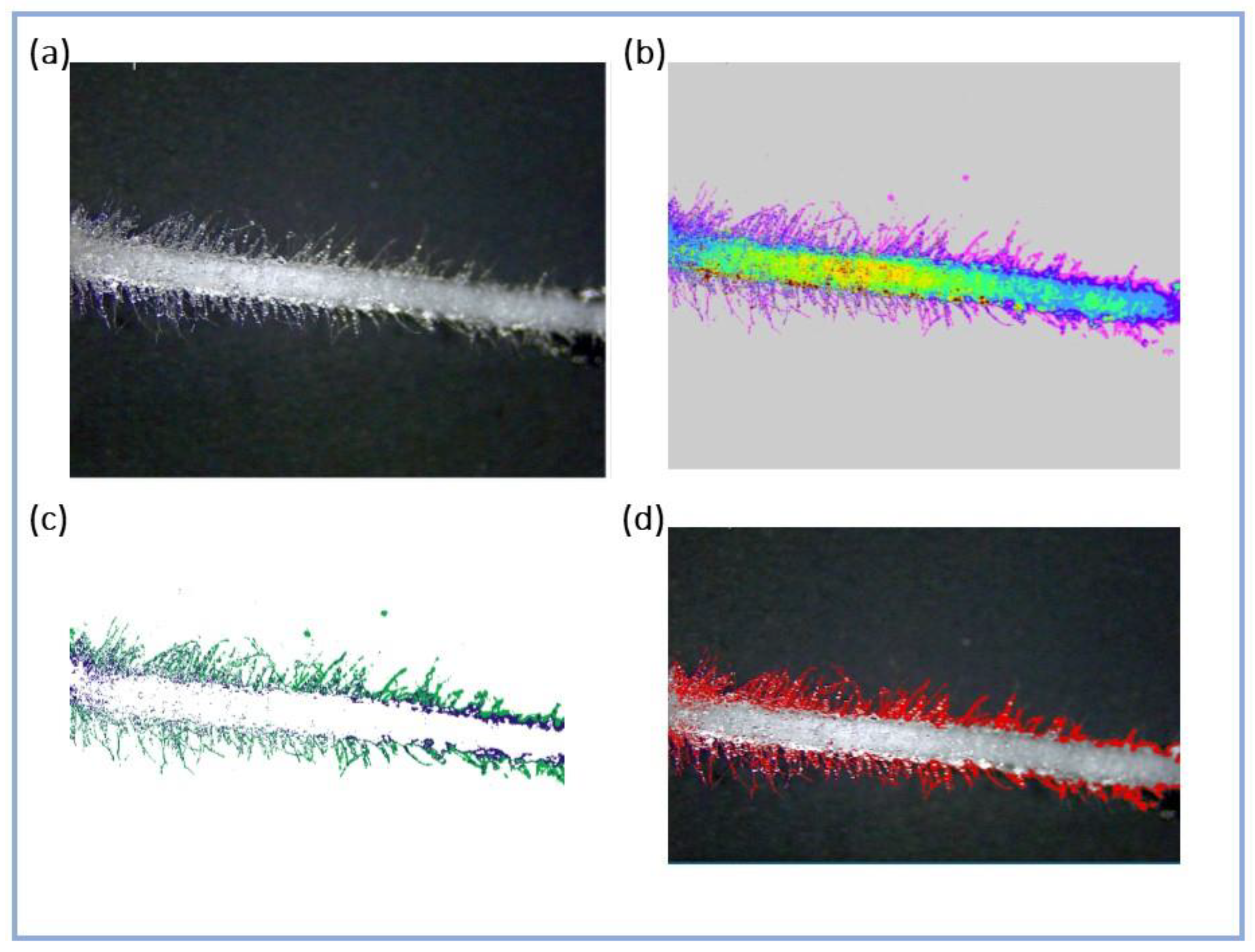
2.3. Root Hairs
3. Discussion
3.1. Chemical Composition
3.2. Phytotoxicity: Germination and Emergence Tests
3.3. Root Hairs
4. Materials and Methods
4.1. Frass Properties and Extract Preparation
4.2. Phytotoxicity Test
4.3. Emergence Tests
- -
- The chlorophyll content was measured on three leaves per plant with a leaf transmittance leaf clip chlorophyll concentration meter (MC-100 Apogee instruments— Inc., Logan, UT, USA) and converted into SPAD (Soil Plant Analysis Development) units [50] as the difference between leaf transmittance at the red light wavelength of 653 nm and leaf transmittance at the near-infrared wavelength of 931 nm.
- -
- Biometric measurements were performed as follows: the above-ground dry mass was determined after oven drying at 70 °C until reaching a constant weight. The soil–root complex was gently extracted from the micro-pots by washing over a 0.5 mm mesh and oven-dried at 70 °C until reaching a constant weight to obtain the root dry mass.
4.4. Root Hairs
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Guidini Lopes, I.; Yong, J.W.H.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef]
- Fowles, T.M.; Nansen, C. Insect-based bioconversion: Value from food waste. In Food Waste Management: Solving the Wicked Problem, 1st ed.; Närvänen, E., Mesiranta, N., Mattila, M., Heikkinen, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 321–346. [Google Scholar]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae mediated valorization of industrial, agriculture and food wastes: Biorefinery concept through bioconversion, processes, procedures, and products. Processes 2020, 8, 857. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; van de Zande, E.M.; Wantulla, M.; van Loon, J.J.A.; de Boer, W.; Dicke, M. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 2022, 7, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Francia, A.D.; et al. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.; Tomberlin, J.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Sharp, R.G. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy 2013, 3, 757–793. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef]
- Hsiao, S.; Kuo, I.; Su, C.; Wang, Y.; Mei, H.; Lee, C. Metabolite characterisation and profiling of Hermetia illucens L. larvae at various growth stages using Sesamum indicum residues as nutrient source. J. Insects Food Feed. 2022, 8, 223–235. [Google Scholar] [CrossRef]
- Alattar, M.A.; Alattar, F.N.; Popa, R. Effects of microaerobic fermentation and black soldier fly larvae food scrap processing residues on the growth of corn plants (Zea mays). Plant Sci. Today 2016, 3, 57–62. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of Black Soldier Fly (Hermetia illucens (L.), Diptera: Stratiomyidae) Larvae Processing Residue in Peat-Based Growing Media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ee, A.W.L.; Tan, J.K.N.; Cheong, J.C.; Chiam, Z.; Arora, S.; Lam, W.N.; Tan, H.T.W. Upcycling food waste using black soldier fly larvae: Effects of further composting on frass quality, fertilising effect and its global warming potential. J. Clean. Prod. 2021, 288, 125664. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, M.K.; Chen, H.; Duan, Y.; Awasthi, S.K.; Zhang, Z. Performance of black soldier fly larvae (Diptera: Stratiomyidae) for manure composting and production of cleaner compost. J. Environ. Manag. 2019, 251, 109593. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Gonnella, M.; Montesano, F.F.; Gai, F.; Parente, A. Valutazione preliminare dell’utilizzo di frass di Hermetia illucens L. e Tenebrio molitor L. come fertilizzante organico nella coltivazione di pomodoro regina e cima di rapa. Giornate scientifiche SOI 21-23 giugno 2023 Torino. Acta Italus Hortus—Riassunti dei lavori XIV 28, 136.
- Clarkson, D.T. Root structure and sites of ion uptake. In Plant Roots: The Hidden Half; Eshel, A., Beeckaman, T., Eds.; Taylor & Francis: Abingdon, UK, 1991; pp. 417–453. [Google Scholar]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Biol. 2001, 52, 329–339. [Google Scholar]
- Rongsawat, T.; Peltier, J.J.; Boyer, J.C.; Véry, A.A.; Sentenac, H. Looking for Root Hairs to Overcome Poor Soils. Trends Plant Sci. 2021, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Hallett, P.D.; Feeney, D.S.; Brown, L.K.; Naveed, M.; Koebernick, N.; Ruiz, S.; Bengough, A.S.; Roose, T.; George, T.S. Impact of root hairs on microscale soil physical properties in the field. Plant Soil 2022, 476, 491–509. [Google Scholar] [CrossRef]
- Marin, M.; Feeney, D.D.; Brown, L.K.; Naveed, S.; Ruiz, N.; Koebernick, A.G.; Bengough, P.D.; Hallett, T.; Roose, J.; Puértolas, I.; et al. Significance of root hairs for plant performance under contrasting field conditions and water deficit. Ann. Bot. 2021, 128, 1–16. [Google Scholar] [CrossRef]
- Vincent, C.; Ebert, R.; Hermans, C. Root hair quantification is an accessible approach to phenotyping important functional traits. J. Exp. Bot. 2022, 73, 3304–3307. [Google Scholar] [CrossRef]
- Mari, C.; Kirchgessner, N.; Marschall, D.; Walter, A.; Hund, A. Rhizoslides: Paper-based growth system for non-destructive, high throughput phenotyping of root development by means of image analysis. Plant Methods 2014, 10, 13. [Google Scholar] [CrossRef]
- Borianne, P.; Subsol, G.; Fallavier, F.; Dardou, A.; Audebert, A. GT-Root S: An integrated software for automated root system measurement from high-throughput phenotyping platform images. Comput. Electron. Agric. 2018, 150, 328–342. [Google Scholar] [CrossRef]
- Narukawa, M.; Kanbara, K.; Tominaga, Y.; Aitani, Y.; Fukuda, K.; Kodama, T.; Murayama, N.; Nara, Y.; Arai, T.; Konno, M.; et al. Chlorogenic acid facilitates root hair formation in lettuce seedlings. Plant Cell Physiol. 2009, 50, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, X.; Wang, F. Adaptive minirhizotron for pepper roots observation and its installation based on root system architecture traits. Plant Methods 2019, 15, 29. [Google Scholar] [CrossRef]
- Guichard, M.; Allain, J.-M.; Bianchi, M.W.; Frachisse, J.-M. Root Hair Sizer: An algorithm for high throughput recovery of different root hair and root developmental parameters. Plant Methods 2019, 15, 104. [Google Scholar] [CrossRef]
- Burrough, P.A.; McDonnell, R.A.; Lloyd, C.D. Principles of Geographical Information Systems; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. In Agricultural Drainage; Skaggs, R.W., van Schilfgaarde, J., Eds.; Agron. Monograph No. 38; ASA: Madison, WI, USA, 1999; pp. 55–108. [Google Scholar]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant Salt Tolerance. In Agricultural Salinity Assessment and Management, 2nd ed.; Wallender, W.W., Tanji, K.K., Eds.; ASCE Manuals and Reports on Engineering Practice No. 71; American Society of Civil Engineers (ASCE): Reston, VA, USA, 2012; pp. 405–459. [Google Scholar]
- Liu, Y.; Liu, J.; Cheng, H.; Luo, Y.; Oh, K.; Meng, X.; Zhang, H.; Liu, N.; Chang, M. Seedling Establishment Test for the Comprehensive Evaluation of Compost Phytotoxicity. Sustainability 2022, 14, 11920. [Google Scholar] [CrossRef]
- Balestrini, R.; Chitarra, W.; Fotopoulos, V.; Ruocco, M. Potential role of beneficial soil microorganisms in plant tolerance to abiotic stress factors. In Soil Biological Communities and Ecosystem Resilience; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 191–207. [Google Scholar]
- Mącik, M.; Gryta, A.; Frac, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar]
- Wynants, E.; Frooninckx, L.; Crauwels, S.; Verreth, C.; De Smet, J.; Sandrock, C.; Wohlfahrt, J.; Van Schelt, J.; Depraetere, S.; Lievens, B.; et al. Assessing the microbiota of black soldier fly larvae (Hermetia illucens) reared on organic waste streams on four different locations at laboratory and large scale. Microb. Ecol. 2019, 77, 913–930. [Google Scholar] [CrossRef]
- Gold, M.; Von Allmen, F.; Zurbrügg, C.; Zhang, J.; Mathys, A. Identification of bacteria in two food waste black soldier fly larvae rearing residues. Front. Microbiol. 2020, 11, 582867. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; Bertoldi, M. Evaluating toxicity of immature compost maturity. Biocycle 1981, 22, 54–57. [Google Scholar]
- Liu, T.; Awasthi, M.K.; Awasthi, S.K.; Duan, Y.; Zhang, Z. Effects of black soldier fly larvae (Diptera: Stratiomyidae) on food waste and sewage sludge composting. J. Environ. Manag. 2020, 256, 109967. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami-Rad, S.; Zeinalzade, N.; Atazadeh, E.; Akhani, H.; Poschenrieder, C. Differential functional traits underlying the contrasting salt tolerance in Lepidium species. Plant Soil 2020, 448, 315–334. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Mei Wang, R.M.; Visser, R.M.; Mei Wang, K.; Van Schaik, H.-K.; Van Duijn, B.; De Boer, A.H. Effects of dormancy-breaking chemicals on ABA levels in barley grain embryos. Seed Sci. Res. 1998, 8, 128–137. [Google Scholar]
- Pill, W.G.; Fret, J.J.; Morneau, D.C. Germination and seedling emergence of primed tomato and asparagus seeds under adverse conditions. HortScience 1991, 26, 1160–1162. [Google Scholar] [CrossRef]
- Ikeya, S.; Aoyanagi, T.; Ishizuka, I.; Takeuchi, A.; Kozaki, A. Nitrate Promotes Germination Under Inhibition by NaCl or High Concentration of Glucose. Plants 2020, 9, 707. [Google Scholar] [CrossRef]
- Verma, S.K.; Chen, Q.; White, J.F. Evaluation of colonization and mutualistic endophytic symbiosis of Escherichia coli with tomato and Bermuda grass seedlings. PeerJ. 2022, 10, e13879. [Google Scholar] [CrossRef]
- De Pessemier, J.; Moturu, T.R.; Nacry, P.; Ebert, R.; De Gernier, H.; Tillard, P.; Swarup, K.; Wells, D.M.; Haseloff, J.; Murray, S.C.; et al. Root system size and root hair length are key phenes for nitrate acquisition and biomass production across natural variation in Arabidopsis. J. Exp. Bot. 2022, 73, 3569–3583. [Google Scholar] [CrossRef]
- Kjeldahl, J. A New Method for the Determination of Nitrogen in Organic Matter. Z. Für Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- European Committee for Standardization. Fertilizers—Determination of Nitric and Ammoniacal Nitrogen according to Ulsch (EN 15558:2009); European Committee for Standardization: Bruxelles, Belgium, 2009; Available online: https://standards.cencenelec.eu/dyn/www/f?p=CEN:110:0::::FSP_PROJECT,FSP_ORG_ID:30751,6241&cs=1FEA0076FFB732841821DDF850B0DDE3F (accessed on 1 December 2023).
- Springer, U.; Klee, J. Pru¨fung der leistungsfa¨higkeit von einigen wichtigen verfahren zur bestimmung des kohlenstoffs mittels chromschwefelsaure sowie vorschlag einer neuen schnellmethode. J. Plant Nutr. Soil Sci 1954, 64, 1–26. [Google Scholar]
- ISO 6579-1: 2017; Microbiology of the Food Chain–Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella–part 1: Horizontal Method for the Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/56712.html (accessed on 1 December 2023).
- ISO 16649-2: 2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-Chloro-3-Indolyl Beta-D-Glucuronide. International Organization for Standardization: Geneva, Switzerland, 2001. Available online: https://www.iso.org/standard/29824.html (accessed on 1 December 2023).
- Uddling, J.; Gelang-Alfredsson, J.; Piikki, K.; Pleijel, H. Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth. Res. 2007, 91, 37–46. [Google Scholar] [CrossRef]
- Jenks, G.F. The Data Model Concept in Statistical Mapping. Int. Yearb. Cartogr. 1967, 7, 186–190. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 December 2023).


| Water Content | Dry Matter | Total N | Organic Matter | Organic Carbon | |
| % | % | % | % | % | |
| GNT | 19.53 ∓ 0.058 b | 80.47 ± 0.058 c | 1.97 ± 0.015 a | 88.10 ± 0.548 b | 51.10 ± 0.318 b |
| GT | 36.00 ± 0.000 a | 64.00 ± 0.000 d | 1.28 ± 0.050 d | 88.83 ± 0.277 b | 51.52 ± 0.161 b |
| WNT | 13.40 ± 0.000 d | 86.60 ± 0.000 a | 1.86 ± 0.015 b | 93.53 ± 0.200 a | 54.25 ± 0.116 a |
| WT | 15.10 ± 0.000 c | 84.90 ± 0.000 b | 1.60 ± 0.017 c | 93.72 ± 0.142 a | 54.36 ± 0.082 a |
| N-NH4+ | N-NO3- | pH | Conductivity | Protein | |
| mg/Kg | mg/Kg | mS cm−1 | % | ||
| GNT | 4741.0 ± 3.61 c | 3.82 ± 0.010 a | 7.20 ± 0.000 c | 4.63 ± 0.039 b | 11.15 ± 0.241 a |
| GT | 4770.1 ± 0.51 c | 3.02 ± 0.007 b | 7.04 ± 0.000 d | 4.56 ± 0.040 b | 7.73 ± 0.040 c |
| WNT | 5980.3 ± 1.53 b | 2.47 ± 0.013 c | 7.32 ± 0.000 b | 5.09 ± 0.000 a | 11.60 ± 0.273 a |
| WT | 6270.0 ± 4.00 a | 2.49 ± 0.020 c | 7.64 ± 0.000 a | 4.60 ± 0.035 b | 10.13 ± 0.260 b |
| Fibre | Carbohydrates | Lipids | Ash | Ca | |
| % | % | % | % | mg/Kg | |
| GNT | 14.25 ± 0.006 a | 2.27 ± 0.261 b | 1.46 ± 0.021 b | 9.58 ± 0.108 a | 4412.0 ± 1.00 a |
| GT | 11.94 ± 0.025 b | 2.68 ± 0.345 b | 1.16 ± 0.015 c | 7.15 ± 0.126 b | 2064.7 ± 4.51 b |
| WNT | <0.01 | 23.43 ± 0.310 a | 1.61 ± 0.024 a | 5.60 ± 0.160 c | 1317.4 ± 0.47 c |
| WT | <0.01 | 22.82 ± 0.360 a | 1.56 ± 0.030 a | 5.33 ± 0.183 c | 1044.7 ± 0.46 d |
| K | Mg | Na | P | Cd | |
| mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | |
| GNT | 19,276.0 ± 201.85 a | 7152.2 ± 162.45 a | 974.1 ± 39.17 b | 9592.5 ± 31.07 a | 0.136 ± 0.115 b |
| GT | 19,378.0 ± 187.72 a | 6833.3 ± 152.81 a | 987.8 ± 46.08 b | 9670.3 ± 40.25 a | 0.120 ± 0.053 b |
| WNT | 11,698.1 ± 193.17 b | 3765.5 ± 136.17 b | 2022.3 ± 42.39 a | 5288.0 ± 39.00 b | 0.124 ± 0.056 b |
| WT | 11,333.0 ± 168.49 b | 3679.8 ± 112.50 b | 1944.4 ± 49.17 a | 5039.4 ± 40.41 b | 0.535 ± 0.030 a |
| Ni | Pb | Zn | As | Escherichia coli | |
| mg/Kg | mg/Kg | mg/Kg | mg/Kg | UFC/g | |
| GNT | 49.533 ± 4.11 a | 0.901 ± 0.010 a | 93.167 ± 2.20 a | 0.228 ± 0.037 a | >150,000 |
| GT | 42.033 ± 2.58 a | 0.714 ± 0.014 b | 89.267 ± 2.11 b | 0.243 ± 0.021 a | 2567 ± 208.17 |
| WNT | 27.200 ± 2.06 b | 1.671 ± 0.003 c | 55.167 ± 3.05 c | 0.133 ± 0.022 b | <10 |
| WT | 25.200 ± 1.01 b | 0.819 ± 0.003 d | 50.600 ± 2.91 c | 0.128 ± 0.014 b | <10 |
| Water Content | Dry Matter | Total N | Organic Matter | Organic Carbon | |
| % | % | % | % | % | |
| GNT | 97.00 ± 0.351 a | 3.00 ± 0.351 a | 0.0061 ± 0.0003 b | 98.98 ± 0.402 a | 57.41 ± 0.233 a |
| GT | 97.10 ± 0.252 a | 2.90 ± 0.252 a | 0.0039 ± 0.0003 c | 99.07 ± 0.499 a | 57.46 ± 0.290 a |
| WNT | 97.50 ± 0.153 a | 2.50 ± 0.152 a | 0.0088 ± 0.0002 a | 99.34 ± 0.204 a | 57.62 ± 0.118 a |
| WT | 97.40 ± 0.200 a | 2.60 ± 0.200 a | 0.0062 ± 0.0004 b | 99.40 ± 0.199 a | 57.66 ± 0.115 a |
| N-NH4+ | N-NO3- | pH | Conductivity | Protein | |
| mg/Kg | mg/Kg | mS cm−1 | % | ||
| GNT | 1597.7 ± 1.528 c | 0.76 ± 0.005 a | 6.32 ± 0.000 b | 12.22 ± 0.040 c | 0.038 ± 0.001 a |
| GT | 1228.3 ± 3.055 d | 0.60 ± 0.004 b | 6.26 ± 0.000 b | 11.63 ± 0.030 d | 0.024 ± 0.000 c |
| WNT | 2287.7 ± 1.527 a | 0.46 ± 0.002 c | 6.28 ± 0.000 b | 13.78 ± 0.142 a | 0.055 ± 0.001 b |
| WT | 1865.3 ± 5.686 b | 0.46 ± 0.001 c | 6.74 ± 0.000 a | 13.11 ± 0.070 b | 0.039 ± 0.001 a |
| Fibre | Carbohydrates | Lipids | Ash | Ca | |
| % | % | % | % | mg/Kg | |
| GNT | 0.25 ± 0.058 a | 0.42 ± 0.061 b | 0.24 ± 0.031 b | 0.03 ± 0.006 a | 425.6 ± 2.551 a |
| GT | 0.13 ± 0.058 b | 0.51 ± 0.051 b | 0.21 ± 0.028 b | 0.02 ± 0.006 a | 446.6 ± 2.847 a |
| WNT | <0.01 | 4.38 ± 0.020 a | 0.34 ± 0.021 a | 0.02 ± 0.006 a | 42.8 ± 1.762 b |
| WT | <0.01 | 4.26 ± 0.025 a | 0.32 ± 0.035 a | 0.02 ± 0.06 a | 44.6 ± 1.583 b |
| K | Mg | Na | P | Cd | |
| mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | |
| GNT | 3362.3 ± 99.65 a | 631.2 ± 2.493 a | 261.3 ± 2.528 b | 1124.9 ± 15.70 a | 0.044 ± 0.002 a |
| GT | 3284.4 ± 106.93 a | 627.3 ± 2.115 a | 262.9 ± 1.265 b | 1096.3 ± 13.32 b | 0.042 ± 0.002 a |
| WNT | 2112.8 ± 80.02 b | 115.6 ± 2.265 b | 381.7 ± 3.155 a | 559.9 ± 12.31 c | 0.037 ± 0.003 b |
| WT | 2085.6 ± 75.17 b | 112.3 ± 2.351 b | 378.4 ± 2.451 a | 556.2 ± 8.06 c | 0.034 ± 0.002 b |
| Ni | Pb | Zn | As | Escherichia coli | |
| mg/Kg | mg/Kg | mg/Kg | mg/Kg | UFC/g | |
| GNT | 3.533 ± 0.156 a | 0.155 ± 0.003 d | 7.400 ± 0.030 a | 0.060 ± 0.002 a | >150,000 |
| GT | 3.167 ± 0.328 a | 0.186 ± 0.002 c | 7.100 ± 0.000 b | 0.057 ± 0.004 a | 14,000 ± 1000.00 |
| WNT | 0.800 ± 0.101 b | 0.320 ± 0.002 a | 5.200 ± 0.000 c | 0.059 ± 0.006 a | 30 ± 4.041 |
| WT | 0.600 ± 0.163 b | 0.296 ± 0.003 b | 5.067 ± 0.015 d | 0.056 ± 0.005 a | 28 ± 5.00 |
| Water Content | Dry Matter | Total N | Organic Matter | Organic Carbon | |
| % | % | % | % | % | |
| GNT | 98.57 ± 0.153 a | 1.43 ± 0.153 a | 0.0018 ± 0.0002 b | 99.02 ± 0.330 a | 57.44 ± 0.192 a |
| GT | 98.40 ± 0.118 a | 1.60 ± 0.118 a | 0.0016 ± 0.0001 b | 99.35 ± 0.225 a | 57.63 ± 0.131 a |
| WNT | 98.43 ± 0.058 a | 1.57 ± 0.058 a | 0.0029 ± 0.0001 a | 99.65 ± 0.330 a | 57.80 ± 0.191 a |
| WT | 98.43 ± 0.058 a | 1.57 ± 0.058 a | 0.0027 ± 0.0001 a | 99.64 ± 0.274 a | 57.79 ± 0.159 a |
| N-NH4+ | N-NO3- | pH | Conductivity | Protein | |
| mg/Kg | mg/Kg | mS cm−1 | % | ||
| GNT | 581.0 ± 8.78 b | 0.39 ± 0.004 a | 6.74 ± 0.015 a | 8.88 ± 0.041 c | 0.011 ± 0.004 b |
| GT | 563.7 ± 9.06 c | 0.39 ± 0.005 a | 6.73 ± 0.025 a | 9.14 ± 0.040 b | 0.010 ± 0.003 b |
| WNT | 820.3 ± 10.81 a | 0.32 ± 0.002 b | 5.19 ± 0.053 b | 9.76 ± 0.039 a | 0.018 ± 0.004 a |
| WT | 696.7 ± 9.31 b | 0.22 ± 0.015 c | 5.31 ± 0.061 b | 9.22 ± 0.042 b | 0.017 ± 0.004 a |
| Fibre | Carbohydrates | Lipids | Ash | Ca | |
| % | % | % | % | mg/Kg | |
| GNT | 0.07 ± 0.015 b | 0.43 ± 0.043 b | 0.37 ± 0.008 a | 0.01 ± 0.001 a | 218.3 ± 4.09 a |
| GT | 0.11 ± 0.014 a | 0.50 ± 0.032 b | 0.39 ± 0.012 a | 0.01 ± 0.001 a | 121.9 ± 2.32 b |
| WNT | 0.03 ± 0.011 c | 2.48 ± 0.046 a | 0.13 ± 0.015 b | 0.01 ± 0.001 a | 44.8 ± 1.51 c |
| WT | 0.06 ± 0.013 b | 2.39 ± 0.041 a | 0.10 ± 0.057 b | 0.01 ± 0.001 a | 12.3 ± 0.61 d |
| K | Mg | Na | P | Cd | |
| mg/Kg | mg/Kg | mg/Kg | mg/Kg | mg/Kg | |
| GNT | 2460.5 ± 108.8 a | 216.8 ± 0.400 a | 144.1 ± 1.70 c | 670.7 ± 3.51 a | 0.034 ± 0.002 c |
| GT | 2243.2 ± 110.1 a | 138.3 ± 1.405 b | 147.6 ± 1.60 c | 586.1 ± 0.808 b | 0.059 ± 0.003 a |
| WNT | 1253.0 ± 89.5 b | 96.4 ± 1.200 c | 224.5 ± 1.42 a | 361.0 ± 2.69 c | 0.055 ± 0.004 a |
| WT | 1152.8 ± 103.8 b | 81.4 ± 0.529 d | 207.6 ± 2.43 b | 314.5 ± 5.00 d | 0.041 ± 0.002 b |
| Ni | Pb | Zn | As | ||
| mg/Kg | mg/Kg | mg/Kg | mg/Kg | ||
| GNT | 1.723 ± 0.031 a | 0.164 ± 0.04 a | 3.585 ± 0.004 c | 0.217 ± 0.018 b | |
| GT | 0.695 ± 0.007 b | 0.224 ± 0.03 a | 5.901 ± 0.013 a | 0.253 ± 0.019 b | |
| WNT | 0.235 ± 0.003 c | 0.081 ± 0.03 b | 4.023 ± 0.007 b | 0.214 ± 0.022 b | |
| WT | 0.168 ± 0.006 d | 0.051 ± 0.04 b | 2.420 ± 0.016 d | 0.335 ± 0.022 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labella, R.; Bochicchio, R.; Addesso, R.; Labella, D.; Franco, A.; Falabella, P.; Amato, M. Germination Behavior and Geographical Information System-Based Phenotyping of Root Hairs to Evaluate the Effects of Different Sources of Black Soldier Fly (Hermetia illucens) Larval Frass on Herbaceous Crops. Plants 2024, 13, 230. https://doi.org/10.3390/plants13020230
Labella R, Bochicchio R, Addesso R, Labella D, Franco A, Falabella P, Amato M. Germination Behavior and Geographical Information System-Based Phenotyping of Root Hairs to Evaluate the Effects of Different Sources of Black Soldier Fly (Hermetia illucens) Larval Frass on Herbaceous Crops. Plants. 2024; 13(2):230. https://doi.org/10.3390/plants13020230
Chicago/Turabian StyleLabella, Rosanna, Rocco Bochicchio, Rosangela Addesso, Donato Labella, Antonio Franco, Patrizia Falabella, and Mariana Amato. 2024. "Germination Behavior and Geographical Information System-Based Phenotyping of Root Hairs to Evaluate the Effects of Different Sources of Black Soldier Fly (Hermetia illucens) Larval Frass on Herbaceous Crops" Plants 13, no. 2: 230. https://doi.org/10.3390/plants13020230
APA StyleLabella, R., Bochicchio, R., Addesso, R., Labella, D., Franco, A., Falabella, P., & Amato, M. (2024). Germination Behavior and Geographical Information System-Based Phenotyping of Root Hairs to Evaluate the Effects of Different Sources of Black Soldier Fly (Hermetia illucens) Larval Frass on Herbaceous Crops. Plants, 13(2), 230. https://doi.org/10.3390/plants13020230









