Chemical Compositions, Pharmacological Properties and Medicinal Effects of Genus Passiflora L.: A Review
Abstract
1. Introduction
2. Taxonomy
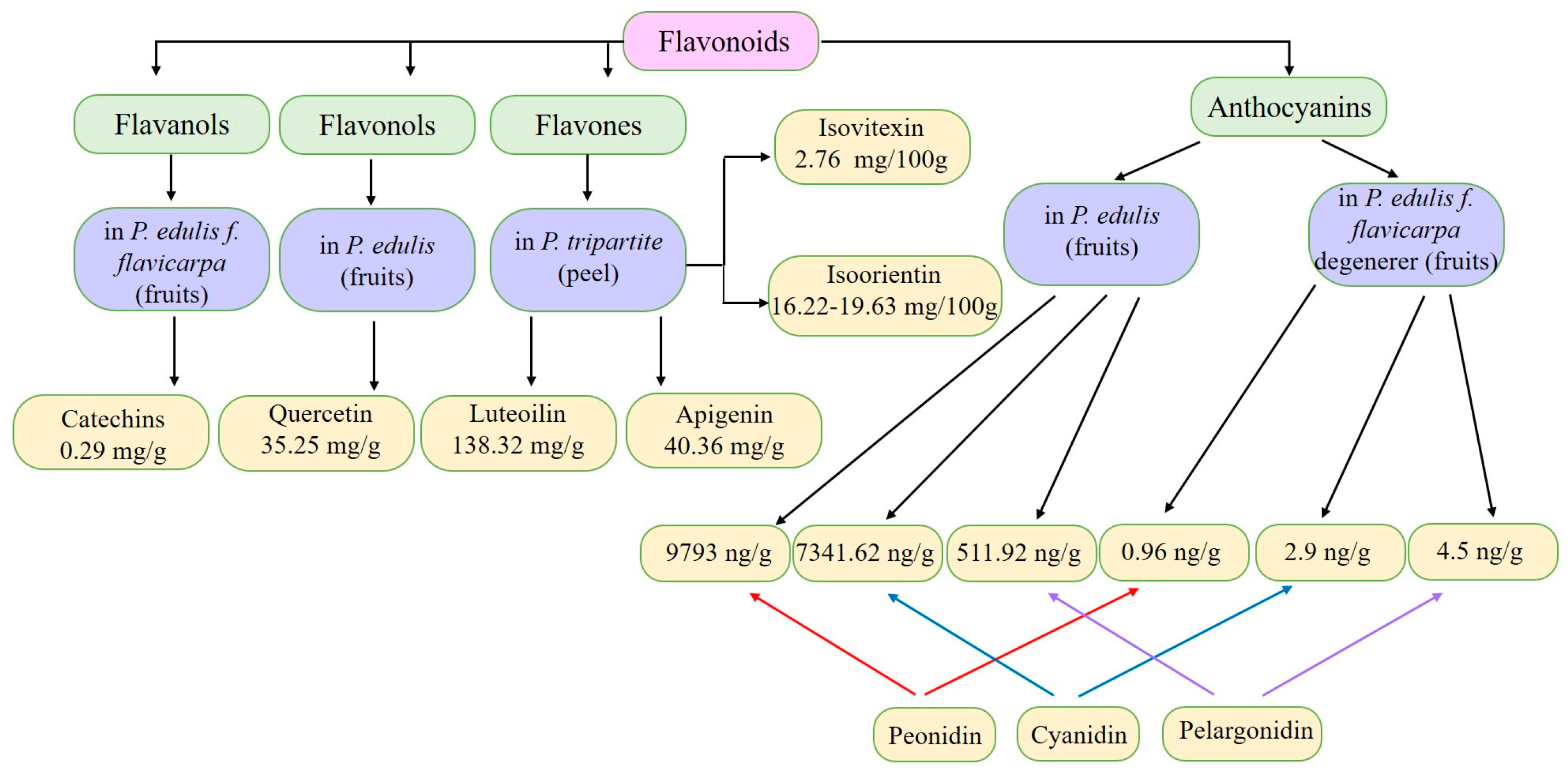
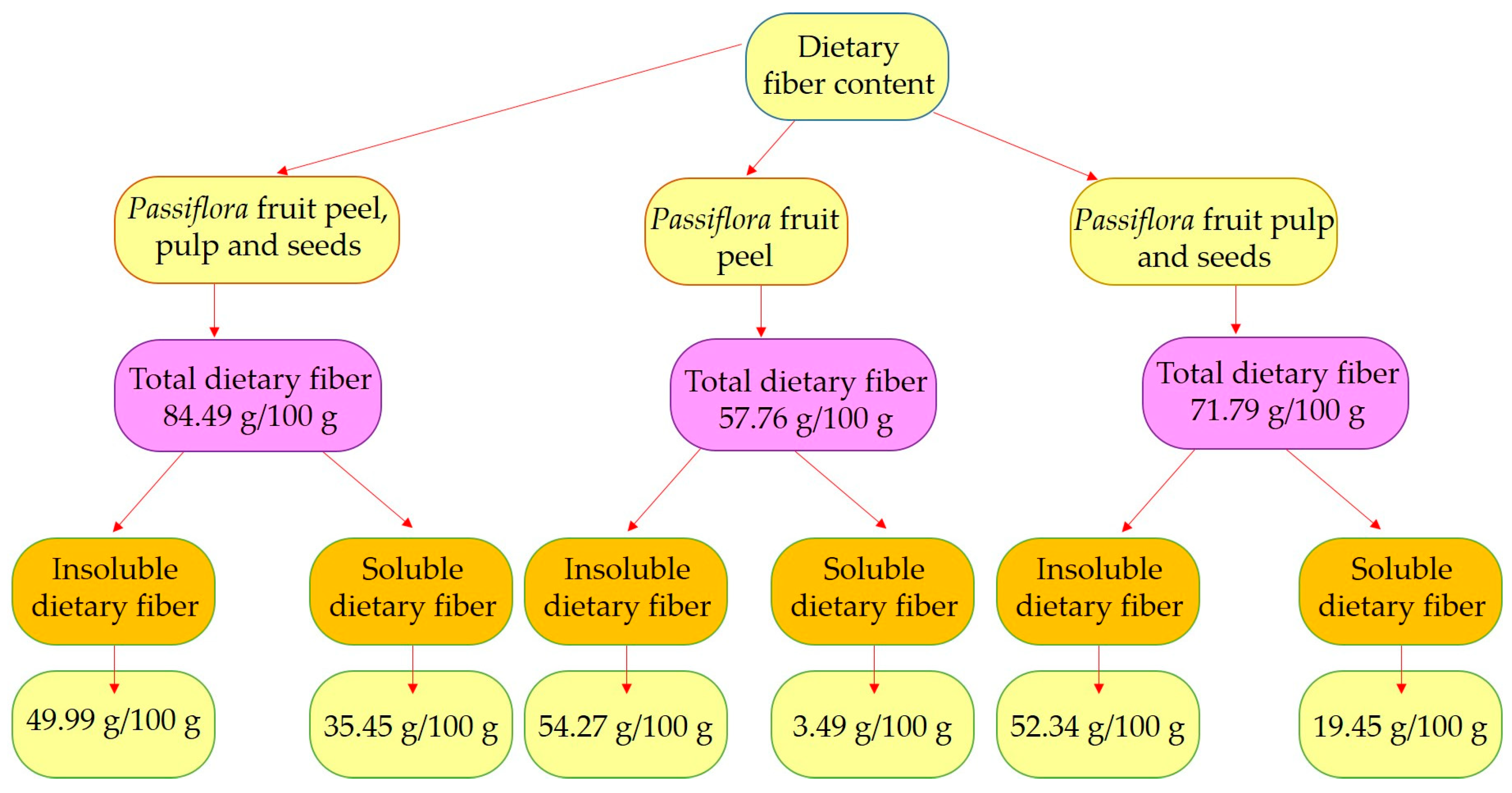
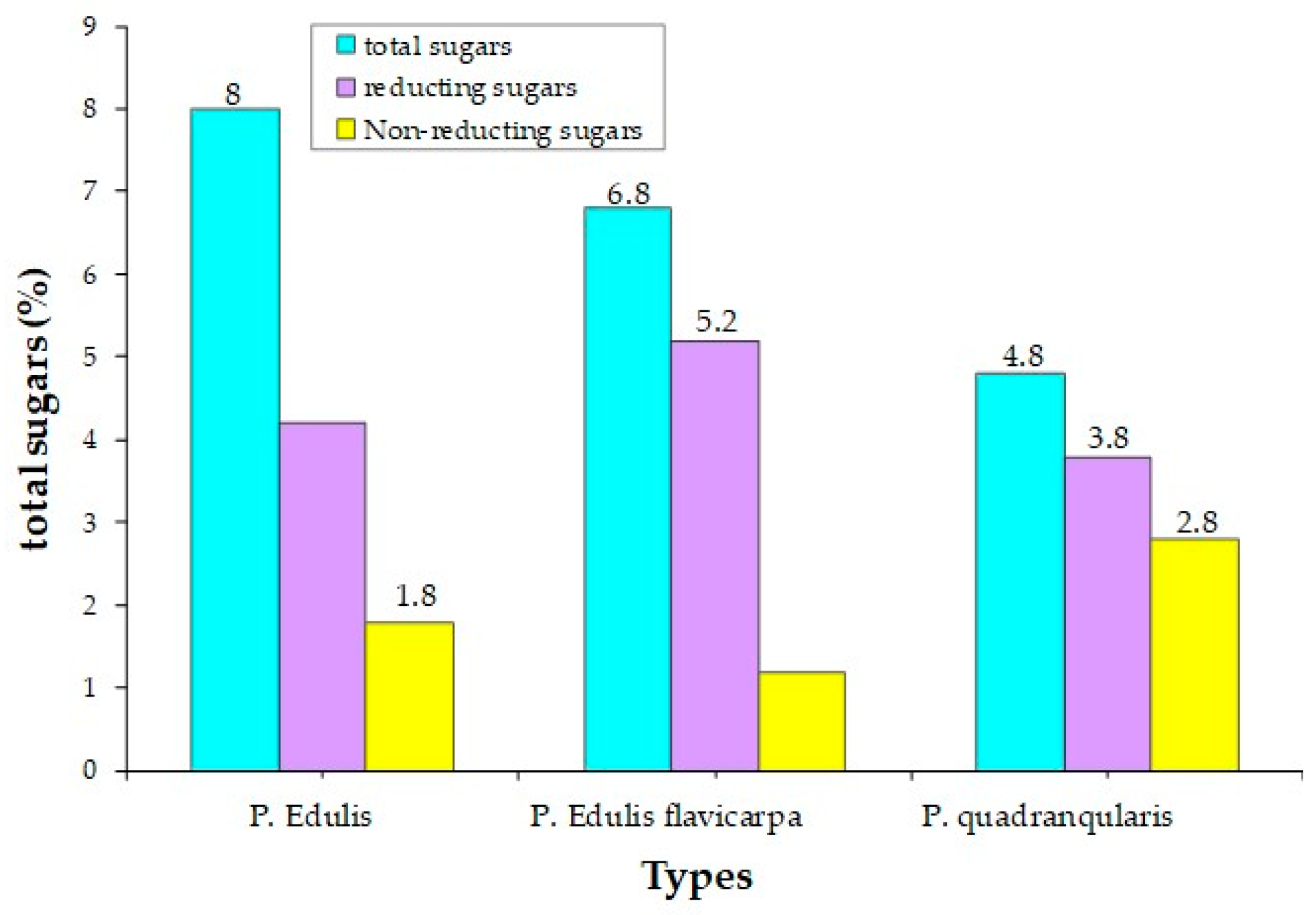
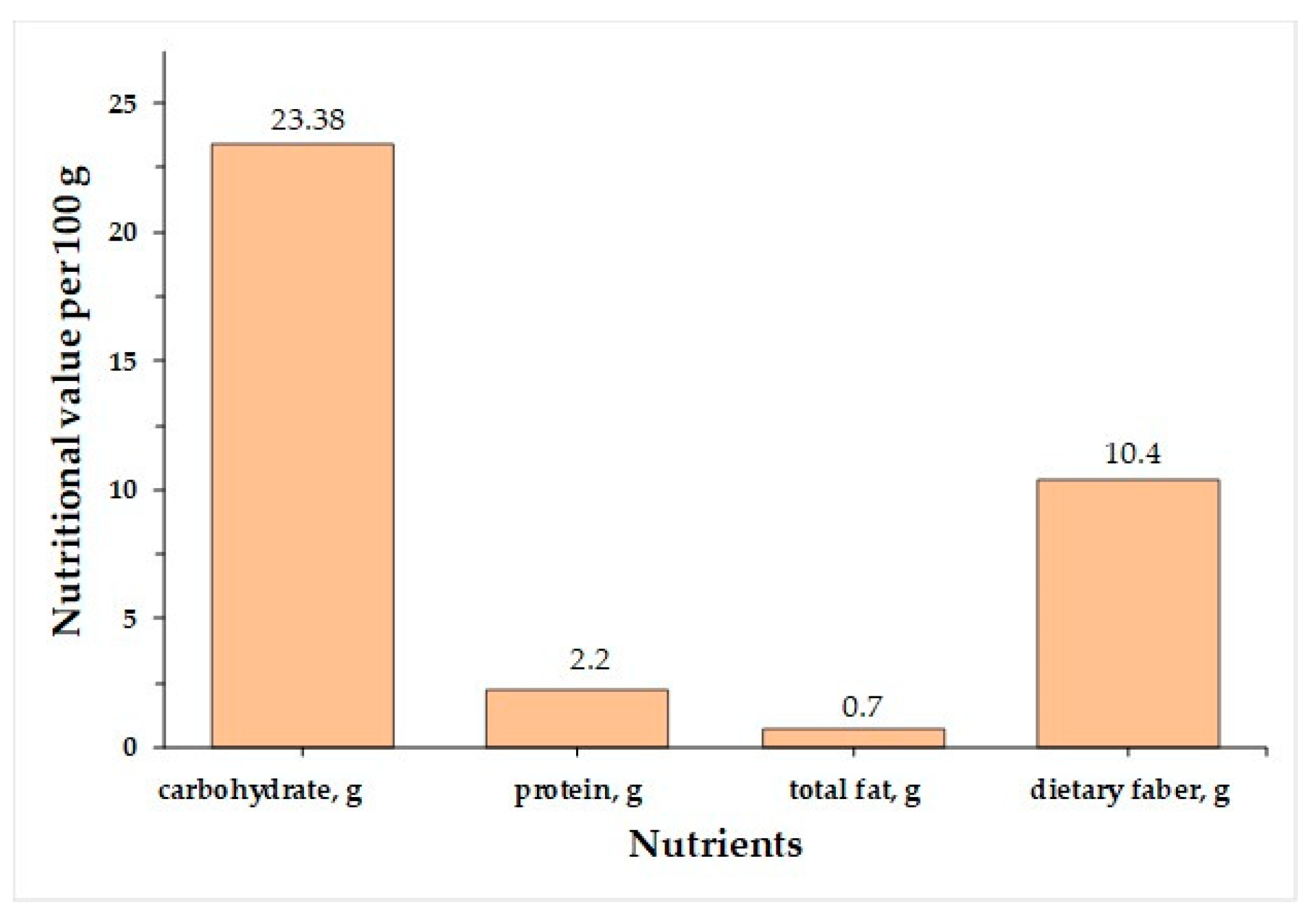
3. Chemical Composition
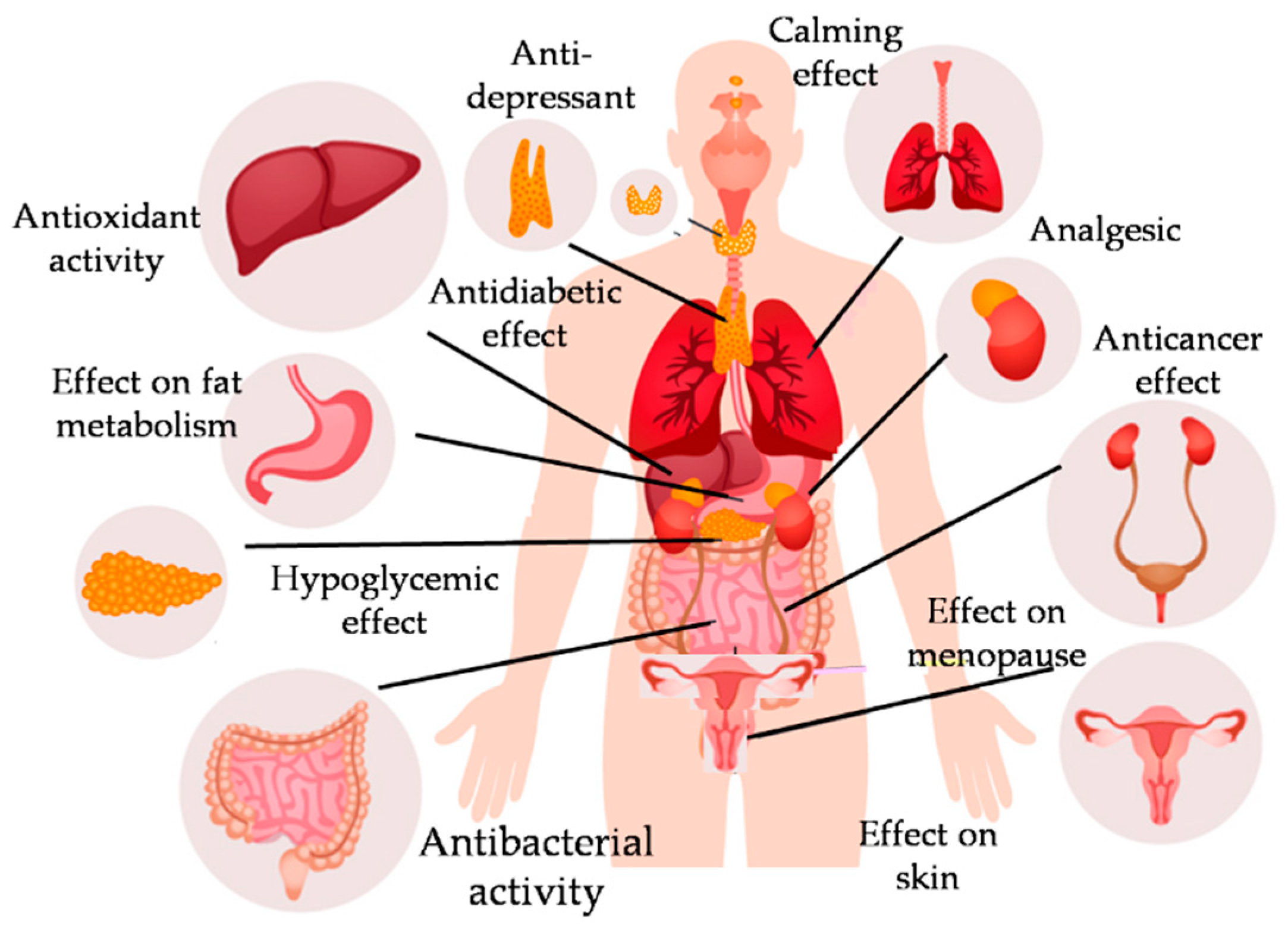
4. Medical Effects and Activities in Pharmacy
4.1. Sedative and Anxiolytic Activities
4.2. Anti-Depressant Activities
4.3. Antidiabetic Activities
4.4. Hepatoprotective Activities
4.5. Antibacterial and Antifungal Activity
4.6. Anti-Inflammatory and Antipyretic Activities
4.7. Antitumor Activity
4.8. Cardiovascular Effects
4.9. Application in Cosmetics
5. Adverse Reactions and Clinical Side Effects
5.1. Pharmacology, Pharmacokinetics, Safety, and Toxicity
5.2. Synergic Effects
6. Patented Products Containing Passiflora, Different from the Widely Available Nutritional Supplements Containing Passiflora Extracts
- Aromatic capsules with chitosan and P. edulis peel extract were designed to reduce lipid and carbohydrate absorption, making them suitable for use in overweight individuals [131].
- A dermatological complex against wrinkles and skin aging was developed, which includes extracts from Papaver, Metha, and Myrtus, as well as seeds of P. edulis, P. incarnata, and P. laurifolia L. The product was tested on 18 patients with dry skin and resulted in a 41% increase in skin moisture compared to the use of the same dermatological complex without the plant extracts [132].
- An antidepressant product containing L-glycine, L-methylfolate, magnesium L-threonate, and P. incarnata extract was patented. This product was shown to increase the swimming and climbing times of mice in antidepressant models compared to those of controls [133].
- A product designed to improve the condition of migraine patients was patented, involving a liquid extract of P. incarnata obtained in water–ethanol mixtures with an ethanol concentration of 30–70%. After three weeks of intake, most patients reported a reduction in the frequency, strength, and duration of migraine attacks [134]. According to Lorente et al., the general condition of patients improved by over 89% [135].
- Pharmaceutical formulations with antiulcer activity were developed, utilizing gelatin nanoparticles with P. alata dry leaf and stem extract (2–5%) in addition to gelatin (95–98%) [135].
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knight, R.J.; Winters, H.F. Pollination and fruit set of yellow passion fruit in southern Florida. Fla. State Hort. Soc. Proc. 1962, 75, 412–418. [Google Scholar]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Hamdi, A.; Jaramillo-Carmona, S.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Guillén-Bejarano, R. Asparagus. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press, Faculty of Sciences and Health, Technological University: Dublin, Ireland, 2020; pp. 581–585. [Google Scholar]
- Thokchom, R.; Mandal, G. Production Preference and Importance of Passion Fruit (Passiflora Edulis): A Review. J. Agric. Eng. Food Technol. 2017, 4, 27–30. [Google Scholar]
- Kishore, K.K.; Pathak, A.; Yadav, D.S.; Bujarbaruah, K.M.; Shukla, R. Passion Fruit; Tech Bull: Maharashtra, India, 2006; pp. 2–4. [Google Scholar]
- Pereira, M.G.; Maciel, M.G.; Haminiuk, G.M.; Hamerski, C.W.I.; Bach, F.; Hamerski, F.; de Paula Scheer, A.; Corazza, M.L. Effect of extraction process on composition, antioxidant and antibacterial activity of oil from Yellow Passion Fruit (Passiflora edulis Var. Flavicarpa) Seeds. Waste Biomass Valorizati 2019, 10, 2611–2625. [Google Scholar] [CrossRef]
- Holanda, D.K.R.; Wurlitzer, N.J.; Dionisio, A.P.; Campos, A.R.; Moreira, R.A.; de Sousa, P.H.M.; de Brito, E.S.; Ribeiro, P.R.V.; Iunes, M.F.; Costa, A.M. Garlic passion fruit (Passiflora tenuifila Killip): Assessment of eventual acute toxicity, anxiolytic, sedative, and anticonvulsant effects using in vivo assays. Food Res. Int. 2020, 128, 108813. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, H.-S.; Lee, H.-H.; Kim, T.-H. Role Identification of PassifloraIncarnata Linnaeus: A mini review. J. Menopausal Med. 2017, 23, 156–160. [Google Scholar] [CrossRef]
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef]
- Ichimura, T.; Yamanaka, A.; Ichiba, T.; Toyokawa, T.; Kamada, Y.; Tamamura, T.; Maruyama, S. Antihypertensive effect of an extract of Passiflora edulis rind in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 718–721. [Google Scholar] [CrossRef]
- Panelli, M.F.; Pierine, D.T.; de Souza, S.L.; Ferron, A.J.T.; Garcia, J.L.; Santos, K.C.D.; Corrêa, C.R. Bark of Passiflora edulis treatment stimulates antioxidant capacity and reduces dyslipidemia and body fat in db/db mice. Antioxidants 2018, 7, 120. [Google Scholar] [CrossRef]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial and antibiotic-modifying activities of three food plants (Xanthosoma mafaffa Lam., Moringa oleifera (L.) Schott and Passiflora edulis Sims) against multidrug-resistant (MDR) Gram-negative bacteria. BMC Complement. Altern. Med. 2016, 16, 9. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhou, T.; Wang, F.; Zhou, Y.; Li, Y.; Zhang, J.J.; Zheng, J.; Xu, D.P.; Li, H.B. The Effects of Syzygiumsamarangense, Passiflora edulis and Solanum muricatum on Alcohol-Induced Liver Injury. Int. J. Mol. Sci. 2016, 17, 1616. [Google Scholar] [CrossRef]
- Lima, N.L.M.; Cunha, E.C.T.; Nascimento, A.R.A. Passiflora species used in folk medicine: Pharmacological, chemical and biological aspects. Rev. Bras. Farmacogn. 2015, 25, 670–678. [Google Scholar] [CrossRef][Green Version]
- Yuan, T.Z.; Kao, C.L.; Li, W.J.; Li, H.T.; Chen, C.Y. Chemical constituents of leaves of Passiflora edulis. Chem. Nat. Compd. 2017, 53, 1165–1166. [Google Scholar] [CrossRef]
- Hu, Y.; Jiao, L.; Jiang, M.H.; Yin, S.; Dong, P.; Zhao, Z.M.; Yang, D.P.; Ho, P.T.; Wang, D.M. A new C-glycosyl flavone and a new neolignan glycoside from Passiflora edulis Sims peel. Nat. Prod. Res. 2018, 32, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.S.; Albuquerque, M.M.; Ferreira, A.C.V.; Pinto, G.G. Passiflora species used in food industry: A review of the principal components and its functional properties. Food Res. Int. 2015, 73, 143–149. [Google Scholar] [CrossRef]
- Kaikade, A.R.; Gurunani, S.G.; Pandel, T.W.; Sherekar, S.A.; Kaikade, P.R.; Mehare, S.R.; Gunjarkar, S.B.; Parate, M.W.; Jaiswal, S.V.; Dhawale, Y.V.; et al. Phyto-Pharmacognostic review on Passiflora Species. J. Med. Plants Stud. 2023, 11, 35–50. [Google Scholar]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef]
- Wen, L.J.; Mao, H.J.; Zhang, Y.C.; Li, Y.J. Compositions and antioxidant activity of Passifloraedulis rind. Food Sci. 2008, 29, 54–58. [Google Scholar]
- Konta, E.M.; Almeida, M.R.; Amaral, C.L.; Darin, J.D.C.; de Rosso, V.V.; Mercadante, A.Z.; Antunes, L.M.G. Evaluation of the Antihypertensive Properties of Yellow Passion Fruit Pulp (Passiflora edulis Sims f. flavicarpa Deg.) in Spontaneously Hypertensive Rats. Phytother. Res. 2014, 28, 28–32. [Google Scholar] [CrossRef]
- Hettiarachchi, H.A.C.O.; Chiranthika, N.N.G.; Janarny, G.; Gunathilake, K.D.P.P. Passion Fruit (Passiflora edulis). Handb. Phytonutrients Indig. Fruits Veg. 2022, 16, 238–250. [Google Scholar] [CrossRef]
- Santosa, T.B.; de Araujo, F.P.; Neto, A.F.; de Freitas, S.T.; Araújo, J.S.; Vilar, S.B.O.; Araújo, A.J.B.; Lima, M.S. Phytochemical Compounds and Antioxidant Activity of the Pulp of Two Brazilian Passion Fruit Species: Passiflora cincinnata Mast. and Passiflora edulis Sims. Int. J. Fruit Sci. 2021, 21, 255–269. [Google Scholar] [CrossRef]
- Teles, R.B.A.; Diniz, T.C.; Pinto, T.C.C.; Júnior, R.G.O.; Silva, M.G.; de Lavor, É.M.; Fernandes, A.W.C.; de Oliveira, A.P.; Ribeiro, F.P.R.A.; da Silva, A.A.M.; et al. Flavonoids as Therapeutic Agents in Alzheimer’s and Parkinson’s Diseases: A Systematic Review of Preclinical Evidences. Oxidative Med. Cell. Longev. 2018, 21, 7043213. [Google Scholar] [CrossRef]
- Dhawan, K.; Kumar, R.; Kumar, S.; Sharma, A. Correct Identification of Passiflora incarnata Linn., a Promising Herbal Anxiolytic and Sedative. J. Med. Food 2004, 4, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The antityrosinase and antioxidant activities of flavonoids dominated by the number and location of phenolic hydroxyl groups. Chin. Med. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Carmona-Hernández, J.C.; Taborda-Ocampo, G.; Valdez, J.C.; Bolling, B.W.; González-Correa, C.H. Polyphenol extracts from three Colombian passifloras (passion fruits) prevent inflammation-induced barrier dysfunction of caco-2 cells. Molecules 2019, 24, 4614. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ali, M.M.; He, Y.; Ma, S.; Rizwan, H.M.; Yang, Q.; Li, B.; Lin, Z.; Chen, F. Flavonoids Accumulation in Fruit Peel and Expression Profiling of Related Genes in Purple (Passiflora edulis f. edulis) and Yellow (Passiflora edulis f. flavicarpa) Passion Fruits. Plants 2021, 10, 2240. [Google Scholar] [CrossRef]
- Kumazawa, T.; Kimura, T.; Matsuba, S.; Sato, S.; Onodera, J.I. Synthesis of 8-C-glucosylflavones. Carbohydr. Res. 2001, 334, 183–193. [Google Scholar] [CrossRef]
- Choo, C.Y.; Sulong, N.Y.; Mana, F.; Wong, T.W. Vitexin and isovitexin from the Leaves of Ficus deltoidea with in vivo a-glucosidase inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef]
- Rotta, E.M.; Rodrigues, C.A.; Jardim, I.C.S.F.; Maldaner, L.; Visentainer, J.V. Determination of Phenolic Compounds and Antioxidant Activity in Passion Fruit Pulp (Passiflora Spp.) Using a Modified QuEChERS Method and UHPLC-MS/MS. LWT 2019, 100, 397–403. [Google Scholar] [CrossRef]
- Zeraik, M.L.; Yariwake, J.H. Quantification of Isoorientin and Total Flavonoids in Passiflora edulis Fruit Pulp by HPLC-UV/DAD. Microchem. J. 2010, 96, 86–91. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Garrido-Mesa, J.; Guerra-Hernández, E.; de Campos Braga, P.A.; Reyes, F.G.R.; Maróstica, M.R.; et al. Intestinal Anti-Inflammatory Effects of Passiflora edulis Peel in the Dextran Sodium Sulphate Model of Mouse Colitis. J. Funct. Foods 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Giambanelli, E.; Gómez-Caravaca, A.M.; Ruiz-Torralba, A.; Guerra-Hernández, E.J.; Figueroa-Hurtado, J.G.; García-Villanova, B.; Verardo, V. New advances in the determination of free and bound phenolic compounds of Banana Passion Fruit Pulp (Passiflora tripartita, var. Mollissima (Kunth) LH Bailey) and their in vitro antioxidant and hypoglycemic capacities. Antioxidants 2020, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; dos Santos Lima, B.; de Souza Araújo, A.A.; Narain, N.; Thangaraj, P. UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, α-amylase and α-glucosidase key enzymes inhibition properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef]
- do Carmo Santos, J.T.; Petry, F.C.; de Castro Tobaruela, E.; Mercadante, A.Z.; Gloria, M.B.A.; Costa, A.M.; Lajolo, F.M.; Hassimotto, N.M.A. Brazilian native passion fruit (Passiflora tenuifila Killip) is a rich source of proanthocyanidins, carotenoids, and dietary fiber. Food Res. Int. 2021, 147, 110521. [Google Scholar] [CrossRef]
- Viera, W.; Shinohara, T.; Samaniego, I.; Sanada, A.; Terada, N.; Ron, L.; Koshio, K. Phytochemical composition and antioxidant activity of Passiflora spp. germplasm grown in Ecuador. Plants 2022, 11, 328. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Di Simone, S.C.; Sinan, K.I.; Ciferri, M.C.; Angeles Flores, G.; Zengin, G.; Orlando, G. Pharmacological properties and chemical profiles of Passiflora foetida L. extracts: Novel insights for pharmaceuticals and nutraceuticals. Processes 2020, 8, 1034. [Google Scholar] [CrossRef]
- Liu, S.; Yang, F.; Li, J.; Zhang, C.; Ji, H.; Hong, P. Physical and chemical analysis of Passiflora seeds and seed oil from China. Int. J. Food Sci. Nutr. 2008, 59, 706–715. [Google Scholar] [CrossRef]
- della Cuna, F.S.R.; Giovannini, A.; Braglia, L.; Sottani, C.; Grignani, E.; Preda, S. Chemical Composition of the Essential Oils From Leaves and Flowers of Passiflora sexocellata and Passiflora trifasciata. Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Rizwana, H.; Al Otibi, F.; Al-Malki, N. Chemical composition, FTIR studies and antibacterial activity of Passiflora edulis f. edulis (Fruit). J. Pure Appl. Microbiol. 2019, 13, 2489–2498. [Google Scholar] [CrossRef]
- Nunes, M.; Oliveira, L.F.S.; Fernandes, J.C.; Giacometti, R.L.C.; de Moura, E.G.P. Chemical composition and in vitro antioxidant activity of Passiflora alata and Passiflora cincinnata leaves. Ind. Crops Prod. 2018, 124, 97–105. [Google Scholar] [CrossRef]
- Silva, S.C.M.; Barros, E.M.S.S.; de Souza, A.C.A.X.; Amorim, A.G.F.; Guedes, M.G.S.; Santos, A.C.F. Phytochemical characterization of the leaves and roots of Passiflora foetida L. Nat. Prod. Res. 2019, 33, 2107–2112. [Google Scholar]
- dos Santos, D.F.; dos Santos, F.A.H.; da Silva, T.R.; de Carvalho Melo Cavalcante, E.A.; de Souza, A.C.A.; Santos, A.C.F. Phytochemical analysis and antioxidant activity of the leaves of Passiflora nitida Kunth (Passifloraceae). Nat. Prod. Res. 2021, 35, 417–422. [Google Scholar]
- Ramos, L.; Pesamosca, E.; Salvador, M.; Hickmann, S.; de Oliveira, A. Antioxidant Potential and Physicochemical Characterization of Yellow, Purple and Orange Passion Fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar]
- Galvão-Novaes, K.; Da Silva-Romão, I.L.; Gomes-Santos, B.; Palhares-Ribeiro, J.; Almeida-Bezerra, M.; Galvão-Paranhos da Silva, E. Screening of Passiflora L. Mineral Content Using Principal Component Analysis and Kohonen Self-Organizing Maps. Food Chem. 2017, 233, 507–513. [Google Scholar] [CrossRef]
- Kumssa, D.B.; Joy, E.J.M.; Broadley, M.R. Global Trends (1961–2017) in Dietary Potassium (K) Intake. Nutrients 2021, 13, 1369. [Google Scholar] [CrossRef]
- Carvajal, L.; Turbay, S.; Álvarez, L.; Rodríguez, A.; Alvarez, M.; Bonilla, K.; Restrepo, S.; Parra, M. Functional and Nutritional Properties of Six Species of Passiflora (Passifloraceae) from the Department of Huila. Colomb. Caldasia 2014, 36, 1–15. [Google Scholar] [CrossRef]
- Bueno, R.C.; de Souza, A.C.A.X.; Amorim, A.G.F.; Guedes, M.G.S.; Santos, A.C.F. Phytochemical characterization and antioxidant activity of the fruit of Passiflora cincinnata Mast. Nat. Prod. Res. 2016, 30, 943–947. [Google Scholar]
- Martins, C.C.V.; Bueno, R.C.; de Souza, A.C.A.X.; Amorim, A.G.F.; Guedes, M.G.S.; Santos, A.C.F. Phytochemical characterization and antioxidant activity of the fruit of Passiflora tenuifila Killip. Nat. Prod. Res. 2017, 31, 240–244. [Google Scholar]
- Ferreira, S.F.; Moreira, J.C.F.; Ribeiro, M.P.; Pinto, V.E.F.; Aquino, S.M. Phenolic compounds, carotenoids, and antioxidant activity in three Passiflora species. Food Sci. Technol. (Camp.) 2017, 37, 271–276. [Google Scholar]
- Aguiar, C.A.; Neves, G.F.; Morais-Braga, R.M.; Vieira Filho, R.F.F.; Moreira, G.L.R.; Vieira, I.C. Antioxidant and antibacterial activity of Passiflora fruit extracts. Braz. J. Biol. 2019, 79, 686–692. [Google Scholar]
- Soares, R.J.; Vasconcelos, E.R.; Carvalho, L.S.S.; Coelho, M.E.S.R.; Aquino, S.M. Phenolic compounds and antioxidant activity in Passiflora edulis Sims fruits at different maturity stages. Food Chem. 2018, 263, 125–130. [Google Scholar] [CrossRef]
- Lal, A. Iron in Health and Disease: An Update. P. edulis var. flavicarpa Degenerer contains twice as much iron (5.50 mg/100 g pulp) as Passiflora edulis Sims. Indian J. Pediatr. 2020, 87, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Tadashi, E.; Grassi, H.; Briancon, R.; Mosca, J. Physical Characterization and Quantity of Nutrients in Sweet Passion Fruit. Rev. Bras. Frutic. 2001, 23, 690–694. [Google Scholar]
- Nedjimi, B. Can Trace Element Supplementations (Cu, Se, and Zn) Enhance Human Immunity against COVID-19 and Its New Variants. J. Basic Appl. Sci. 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.S.M.; Monnerat, P.H.; Vieira, I.J.C.; Carvalho, A.J.C. Flavonoids and mineral composition of leaves in yellow passion fruit plants as a function of leaves positions in the branch. Cienc. Rural 2007, 37, 1634–1639. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Sganzerla, M.; Jacques, A.C.; Barcia, M.T.; Zambiazi, R.C. Carotenoids, tocopherols and ascorbic acid content in yellow passion fruit (Passiflora edulis) grown under different cultivation systems, LWT. Food Sci. Technol. 2015, 64, 259–263. [Google Scholar] [CrossRef]
- USDA Food Composition Databases. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 7 January 2024).
- Wang, C.; Xu, F.Q.; Shang, J.H.; Xiao, H.; Fan, W.W.; Dong, F.W.; Hu, J.M.; Zhou, J. Cycloar-tane triterpenoid saponins from water soluble of Passiflora edulis Sims and their antide-pressant-like effects. J. Ethnopharmacol. 2013, 148, 812–817. [Google Scholar] [CrossRef]
- Vieira, L.Q.; Aguiar, J.A.M.; Santana, A.L.A.; de Souza, A.C.A.X.; Amorim, A.G.F.; Guedes, M.G.S.; Santos, A.C.F. Amino acid composition and nutritional properties of the pulp of three yellow passion fruit (Passiflora edulis Sims) varieties. Food Chem. 2019, 276, 584–589. [Google Scholar]
- Souza, J.R.; Sousa, L.F.; Araújo, F.F.A.; Aragão, F.A.S.; Amorim, A.G.F.; Guedes, M.G.S.; Santos, A.C.F. Free amino acids and peptides in the pulp of three Passiflora species. Food Sci. Technol. (Camp.) 2018, 38, 261–267. [Google Scholar]
- Li, S.P.; Wang, Y.W.; Qi, S.L.; Zhang, Y.P.; Deng, G.; Ding, W.Z.; Ma, C.; Lin, Q.-Y.; Guan, H.-D.; Liu, W.; et al. Analogous b-carboline alkaloids harmaline and harmine ameliorate scopolamine-induced cognition dysfunction by attenuating acetylcholinesterase activity, oxidative stress, and inflammation in mice. Front. Pharmacol. 2018, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Yin, C.M.; Huang, Y.C.; Geng, F.; Yang, F.; Huang, C.D. Progress in extraction, separation and purification, structural characteristics and bioactivities of polysaccharides of Passiflora edulis peel. Sci. Technol. Food Ind. 2018, 39, 335–351. [Google Scholar] [CrossRef]
- Sema, A.; Maiti, C.S. Status & Prospects of Passion Fruit Industry in Northeast India; Central Institute of Horticulture: Medziphema, Nagaland, India, 2006; pp. 70–73. [Google Scholar]
- Domínguez-Rodríguez, G.; García, M.C.; Plaza, M.; Marina, M.L. Revalorization of Passiflora species peels as a sustainable source of antioxidant phenolic compounds. Sci. Total Environ. 2019, 696, 134030. [Google Scholar] [CrossRef]
- Yepes, A.; Ochoa-Bautista, D.; Murillo-Arango, W.; Quintero-Saumeth, J.; Bravo, K.; Osorio, E. Purple passion fruit seeds (Passiflora edulis f. Edulis Sims) as a promising source of skin anti-aging agents: Enzymatic, antioxidant and multi-level computational studies. Arab. J. Chem. 2021, 14, 102905. [Google Scholar] [CrossRef]
- Jusuf, N.K.; Putra, I.B.; Dewi, N.K. Antibacterial Activity of Passion Fruit Purple Variant (Passiflora edulis Sims var. edulis) Seeds Extract Against Propionibacterium acnes. Clin. Cosmet. Investig. Dermatol. 2020, 13, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nerdy, N.; Ritarwan, K. Hepatoprotective activity and nephroprotective activity of, peel extract from three varieties of the passion fruit (Passiflora sp.) in the albino rat. J. Med. Sci. 2019, 7, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Soković, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef]
- Hu, M.; Du, J.; Du, L.; Luo, Q.; Xiong, J. Anti-fatigue activity of purified anthocyanins prepared from purple passion fruit (P. edulis Sim) epicarp in mice. J. Funct. Foods 2020, 65, 103725. [Google Scholar] [CrossRef]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Bloomer, R.J.; MacDonnchadh, J.J.; Gunnels, T.A.; Schriefer, J.H.M. The influence of a passion flower extract on free testosterone in healthy men: A two part investigation involving younger and older men. Integr. Mol. Med. 2016, 10, 15761. [Google Scholar] [CrossRef]
- Al-kuraishy, H.; Alwindy, S.; Al-Gareeb, A. Beneficial Neuro-Pharmacological Effect of Passionflower (Passiflora Incarnate L). Online J. Neurol. Brain Disord. 2020, 3, 285–289. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Naghavi, H.R.; Vazirian, M.; Shayeganpour, A.; Rashidi, H.; Khani, M. Passionflower in the treatment of generalized anxiety: A pilot double-blind randomized controlled trial with oxazepam. J. Clin. Pharm. Ther. 2001, 26, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Azimaraghi, O.; Yousefshahi, F.; Khatavi, F.; Zamani, M.M.; Movafegh, A. Both Oral Passiflora Incarnata and Oxazepam Can Reduce Pre-Operative Anxiety in Ambulatory Surgery Patients: A Double-Blind, Placebo-Controlled Study. Asian J. Pharm. Clin. Res. 2017, 10, 331–334. [Google Scholar] [CrossRef][Green Version]
- Aslanargun, P.; Cuvas, O.; Dikmen, B.; Aslan, E.; Yuksel, M.U. Passiflora Incarnata inneaus as an anxiolytic before spinal anesthesia. J. Anesth. 2012, 26, 39–44. [Google Scholar] [CrossRef]
- Kaviani, N.; Tavakoli, M.; Tabanmehr, M.; Havaei, R. The efficacy of Passiflora Incarnata Linnaeus in reducing dental anxiety in patients undergoing periodontal treatment. J. Dent. (Shiraz) 2013, 14, 68–72. [Google Scholar]
- Ngan, A.; Conduit, R. A double-blind, placebo-controlled investigation of the effects of Passiflora Incarnata (passionflower) herbal tea on subjective sleep quality. Phytother. Res. 2011, 25, 1153–1159. [Google Scholar] [CrossRef]
- Appel, K.; Rose, T.; Fiebich, B.; Kammler, T.; Hoffmann, C.; Weiss, G. Modulation of the γ-aminobutyric acid (GABA) system by Passiflora incarnata L. Phytother. Res. 2011, 25, 838–843. [Google Scholar] [CrossRef]
- Ayres, A.S.F.S.J.; De Araújo, L.L.S.; Soares, T.C.; Costa, G.M.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Schenkel, E.P.; Soares-Rachetti, V.P.; Zucolotto, S.M.; et al. Comparative central effects of the aqueous leaf extract of two populations of Passiflora edulis. Braz. J. Pharm. 2015, 25, 499–505. [Google Scholar] [CrossRef]
- Alves, J.S.F.; Silva, A.M.D.S.; da Silva, R.M.; Tiago, P.R.F.; de Carvalho, T.G.; de Araújo Júnior, R.F.; de Azevedo, E.P.; Lopes, N.P.; Ferreira, L.S.; Gavioli, E.C.; et al. In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics 2020, 12, 383. [Google Scholar] [CrossRef]
- Ayres, A.S.F.S.J.; Santos, W.B.; Junqueira-Ayres, D.D.; Costa, G.M.; Ramos, F.A.; Castellanos, L.; Alves, J.S.F.; Asth, L.; de Medeiros, I.U.; Zucolotto, S.M.; et al. Monoaminergic neurotransmission is mediating the antidepressant-like effects of Passiflora edulis Sims fo. Edulis. Neurosci. Lett. 2017, 660, 79–85. [Google Scholar] [CrossRef]
- Kandandapani, S.; Balaraman, A.K.; Ahamed, H.N. Extracts of passion fruit peel and seed of Passiflora edulis (Passifloraceae) attenuate oxidative stress in diabetic rats. Chin. J. Nat. Med. 2015, 13, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.H.; Ning, D.S.; Fu, Y.X.; Li, D.P.; Zou, Z.Q.; Xie, Y.C.; Yu, L.L.; Li, L.C. Preparative Isolation of Piceatannol Derivatives from Passion Fruit (Passiflora edulis) Seeds by High-Speed Countercurrent Chromatography Combined with High-Performance Liquid Chromatography and Screening for α-Glucosidase Inhibitory Activities. J. Agric. Food Chem. 2020, 68, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I.; et al. The Effect of Piceatannol from Passion Fruit (Passiflora edulis) Seeds on Metabolic Health in Humans. Nutrients 2017, 9, 1142. [Google Scholar] [CrossRef] [PubMed]
- Marquesa, S.S.F.; Libonati, R.M.F.; Sabaa-Srur, A.; Oliveira, U.; Luo, R.; Shejwalkar, P.; Hara, K.; Dobbs, T.; Smith, R.E. Evaluation of the effects of passion fruit peel flour (Passiflora edulis fo. flavicarpa) on metabolic changes in HIV patients with lipodystrophy syndrome secondary to antiretroviral therapy. Rev. Brasil. Farmacogn. 2016, 26, 420–426. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.K.; Yadav, R.K.S. Hepatoprotective activity of Passiflora edulis Sims. leaves against CCl4-induced hepatotoxicity in rats. Asian Pac. J. Trop. Dis. 2014, 4, S939–S944. [Google Scholar]
- Munhoz, A.C.; Leal, C.Q.; Duarte, M.C.T.; Marques, M.C.A.; Facundo, V.A. Protective effect of Passiflora edulis peel flour in non-alcoholic fatty liver disease. Food Res. Int. 2018, 108, 40–47. [Google Scholar] [CrossRef]
- Goss, M.J.; Nunes, M.L.O.; Machado, I.D.; Merlin, L.; Macedo, N.B.; Silva, A.M.O.; Bresolin, T.M.B.; Santin, J.R. Peel flour of Passiflora edulis Var. Flavicarpa supplementation prevents the insulin resistance and hepatic steatosis induced by low-fructose-diet in young rats. Biomed. Pharmacother. 2018, 102, 848–854. [Google Scholar] [CrossRef]
- Mohanasundari, C.; Natarajan, D.; Srinivasan, K.; Umamaheswari, S.; Ramachandran, A. Antibacterial properties of Passiflora foetida L.–A common exotic medicinal plant. Afr. J. Biotechnol. 2007, 6, 2650–2653. [Google Scholar] [CrossRef]
- Ribeiro, S.F.; Taveira, G.B.; Carvalho, A.O.; Dias, G.B.; Da Cunha, M.; Santa-Catarina, C.; Rodrigues, R.; Gomes, V.M. Antifungal and other biological activities of two 2S albumin-homologous proteins against Pathogenic Fungi. Protein J. 2012, 31, 59–67. [Google Scholar] [CrossRef]
- Jagessar, R.C.; Hafeez, A.; Chichester, M.; Crepaul, Y. Antimicrobial activity of the ethanolic and aqueous extract of passion fruit (Passiflora edulis Sims), in the absence and presence of Zn (OAc)2.-H2O. World J. Pharm. Pharmaceut. Sci. 2017, 6, 230–246. [Google Scholar] [CrossRef]
- Saravanan, S.; Arunachalam, K.; Parimelazhagan, T. Antioxidant, analgesic, anti-inflammatory and antipyretic effects of polyphenols from Passiflora subpeltata leaves a promising species of Passiflora. Ind. Crops Prod. 2014, 54, 272–280. [Google Scholar] [CrossRef]
- Sasikala, V.; Saravanan, S.; Parimelazhagan, T. Analgesic and anti–inflammatory activities of Passiflora foetida L. Asian Pac. J. Trop. Med. 2011, 4, 600–603. [Google Scholar] [CrossRef]
- Koriem, K.M.M. Importance of Herbapassiflorae in medicinal applications: Review on experimental and clinical pharmacology. Biointerface Res. Appl. Chem. 2021, 11, 12886–12900. [Google Scholar]
- Montanher, A.B.; Zucolotto, S.M.; Schenkel, E.P.; Frode, T.S. Evidence of anti-inflammatory effects of Passiflora edulis in an inflammation model. J. Ethnopharmacol. 2007, 109, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, C.B.B.; da Silva, J.K.; Colomeu, T.C.; Batista, A.G.; Meletti, M.; Paschoal, J.J.R.; Bogusz, S.; Braga, P.A.G.; Reyes, F.; Augusto, F.; et al. Intake of Passiflora edulis leaf extract improves antioxidant and anti-inflammatory status in rats with 2,4,6-trinitrobenzenesulphonic acid induced colitis. J. Funct. Foods 2015, 17, 575–586. [Google Scholar] [CrossRef]
- Saravanan, S.; Iniyavan, M.; Bruno, S.L.; Adriano, A.S.A.; Narain, N.; Mairim, R.S.; Parimelazhagan, T. HPLC–DAD–MS identification of polyphenols from Passiflora leschenaultii and determination of their antioxidant, analgesic, anti-inflammatory and antipyretic properties. Arab. J. Chem. 2016, 12, 760–771. [Google Scholar] [CrossRef]
- Amaral, R.G.; Gomes, S.V.F.; Luciano, M.C.D.S.; Pessoa, C.D.Ó.; Andrade, L.N.; Severino, P.; Brandão, G.C.; Bomfim, L.M.; Soares, M.B.P.; Bezerra, D.P.; et al. Cytotoxic potential of 14 Passiflora species against cancer cells. J. Med. Plants Res. 2019, 13, 157–166. [Google Scholar]
- Aguillón, J.; Arango, S.S.; Uribe, D.F.; Loango, N. Citotoxyc and apoptotic activity of extracts from leaves and juice of Passiflora edulis. J. Liver Res. Disord. Ter. 2018, 4, 67–71. [Google Scholar] [CrossRef]
- Mota, N.S.R.S.; Kviecinski, M.R.; Zeferino, R.C.; de Oliveira, D.A.; Bretanha, L.C.; Ferreira, S.R.S.; Micke, G.A.; Filho, D.W.; Pedrosa, R.C.; Ourique, F. In vivo antitumor activity of by-products of Passiflora edulis f. flavicarpa Deg. Rich in medium and long chain fatty acids evaluated through oxidative stress markers, cell cycle arrest and apoptosis induction. Food Chem. Toxicol. 2018, 118, 557–565. [Google Scholar] [CrossRef]
- Dantas, L.N.S.; de Oliveira, D.M.; de Oliveira, A.S.; Cunha, V.M.N.; Carvalho, F.A.A.; Costa, J.P.; de Sousa, D.P. Hypotensive and vasorelaxant effects of ethanolic extract from Passiflora edulis Sims leaves. J. Ethnopharmacol. 2017, 204, 127–132. [Google Scholar]
- Leslie, G.B. A pharmacometric evaluation of nine Bio-Strath herbal remedies. Medica 1978, 8, 3–19. [Google Scholar]
- Lewis, B.J.; Herrlinger, K.A.; Craig, T.A.; Mehring-Franklin, C.E.; DeFreitas, Z.; Hinojosa-Laborde, C. Antihypertensive effect of passion fruit peel extract and its major bioactive components following acute supplementation in spontaneously hypertensive rats. J. Nutr. Biochem. 2013, 24, 1359–1366. [Google Scholar] [CrossRef]
- Zibadia, S.; Faridc, R.; Moriguchid, S.; Lue, Y.R.; Fooe, L.Y.; Tehranic, P.M. Oral administration of purple passion fruit peel extract attenuates blood pressure in female spontaneously hypertensive rats and humans. Nutr. Res. 2007, 27, 408–416. [Google Scholar] [CrossRef]
- -Kinoshita, Y.; Kawakami, S.; Yanae, K.; Sano, S.; Uchida, H.; Inagaki, H.; Ito, T. Effect of long-term piceatannol treatment on eNOS levels in cultured endothelial cells. Bioc hem. Biophys. Res. Commun. 2013, 430, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Duarte, I.d.A.E.; Milenkovic, D.; Borges, T.K.d.S.; Rosa, A.J.d.M.; Morand, C.; Oliveira, L.d.L.d.; Costa, A.M. Acute Effects of the Consumption of Passiflora setacea Juice on Metabolic Risk Factors and Gene Expression Profile in Humans. Nutrients 2020, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Lourith, N.; Kanlayavattanakul, M. Antioxidant activities and phenolics of Passiflora edulis seed recovered from juice production residue. J. Oleo Sci. 2013, 62, 235–240. [Google Scholar] [CrossRef]
- Leao, K.M.M.; Sampaio, K.L.; Pagani, A.A.C.; da Silva, M.A.A.P. Odor potency, aroma profile and volatiles composition of cold pressed oil from industrial passion fruit residues. Ind. Crops Prod. 2014, 58, 280–286. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Krambeck, K.; Santos, D.; Otero-Espinar, F.; Sousa Lobo, J.M.; Amaral, M.H. Lipid nanocarriers containing Passiflora edulis seeds oil intended for skin application. Colloids Surf. B Biointerfaces 2020, 193, 111057. [Google Scholar] [CrossRef]
- Smith, G.W.; Chalmers, T.M.; Nuki, G. Vasculitis Associated with Herbal Preparation Containing Passijiora Extract. Rheumatology 1993, 32, 87–88. [Google Scholar] [CrossRef]
- Giavina-Bianchi, P.F., Jr.; Castro, F.F.; Machado, M.L.; Duarte, A.J. Occupational respiratory allergicdisease induced by Passiflora alata and Rhamnus purshiana. Ann. Allergy Asthma Immunol. 1997, 79, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Gibbert, J.; Kreimendahl, F.; Lebert, J.; Rychlik, R.; Trompetter, I. Improvement of Stress Resistance and Quality of Life of Adults with Nervous Restlessness after Treatment with a Passion Flower Dry Extract. Complement. Med. Res. 2017, 24, 83–89. [Google Scholar] [CrossRef]
- Gow, P.J.; Connelly, N.J.; Crowley, P.; Angus, P.W.; Hill, R.L. Fatal fulminant hepatic failure induced by a natural therapy containing kava. Med. J. Aust. 2003, 178, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, K.; Kumar, S.; Sharma, A. Reversal of cannabinoids (delta 9-THC) by the benzoflavone moiety from methanol extract of Passiflora incarnata Linn in mice: A possible therapy for cannabinoid addiction. J. Pharm. Pharmacol. 2002, 54, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, K.; Kumar, S.; Sharma, A. Nicotine reversal effects of the benzoflavone moiety from Passiflora incarnata Linn in mice. Addict. Biol. 2002, 7, 435–441. [Google Scholar] [CrossRef]
- WHO. Monographs on Selected Medicinal Plants, Volume 3; World Health Organization Press: Geneva, Switzerland, 2007; pp. 257–267. ISBN 9789241547024.
- Cicero, A.F.G.; Colletti, A. Handbook of Nutraceuticals for Chemical Use; Springer: Cham, Switzerland, 2018; pp. 1–23. [Google Scholar]
- Machado, H.; Nagem, T.J.; Peters, V.M.; Fonseca, C.S.; Oliveira, T.T. Flavonoides e seupotencialterapeutico. Bol. Cent. Biol. Reprod. 2008, 27, 33–39. [Google Scholar]
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, O.E.; Falcão-Silva, V.S.; Junior-Siqueira, J.P. In vitro interference of Momordica charantia in the resistance to aminoglycosides. Pharm. Biol. 2008, 47, 1056–1059. [Google Scholar] [CrossRef]
- Capasso, A.; Sorrentino, L. Pharmacological studies on the sedative and hypnotic effect of Kava kava and Passiflora extracts combination. Phytomedicine 2005, 12, 39–45. [Google Scholar] [CrossRef]
- Madabushi, R.; Frank, B.; Drewelow, B.; Derendorf, H.; Butterweck, V. Hyperforin in St. John’s wort drug interactions. Eur. J. Clin. Pharmacol. 2006, 62, 225–233. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Knörle, R.; Appel, K.; Kammler, T.; Weiss, G. Pharmacological studies in an herbal drug combination of St. John’s Wort (Hypericum perforatum) and passion flower (Passiflora incarnata): In vitro and in vivo evidence of synergy between Hypericum and Passiflora in antidepressant pharmacological models. Fitoterapia 2011, 82, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, L.D.; Ramamoorthy, S. Regulation of monoamine transporters: Influence of psychostimulants and therapeutic antidepressants. AAPS J. 2005, 7, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Hansen, K.B.; Boyle, N.J.; Han, K.; Muske, G.; Huang, X.; Egebjerg, J.; Sánchez, C. An allosteric binding site at the human serotonin transporter mediates the inhibition of escitalopram by R-citalopram: Kinetic binding studies with the ALI/VFL-SI/TT mutant. Neurosci. Lett. 2009, 462, 207–212. [Google Scholar] [CrossRef]
- Yamauchi, R.H. Cápsulas com Aromatizante para Mémoire da Absorpción de Lipídes e Carbohidrados. BR Patent BRPI0800705-A2, 21 June 2011. [Google Scholar]
- Golz-Berner, K.; Zastrow, L.; Doucet, O. Productocosméticoantiarrugas. US Patent US20070134189, 14 June 2007. [Google Scholar]
- Yusubov, N. A Novel Combination Containing Passiflora, Glycine, Metylfolate and Magnesium Threonate and Production Method Thereof. TR Patent WO2020162859, 20 August 2020. [Google Scholar]
- Pereira Leal, A.E.B.; de Lavor, É.M.; de Menezes Barbosa, J.; de Moura Fontes Araújo, M.T.; Dos Santos Cerqueira Alves, C.; de Oliveira Júnior, R.G.; de Lima, Á.A.N.; da Silva Almeida, J.R.G. Pharmacological Activities of the Genus Passiflora (Passifloraceae): A Patent Review. Curr. Top. Med. Chem. 2022, 22, 2315–2328. [Google Scholar] [CrossRef] [PubMed]
- Lorente, R.R.; Fernandez, O.S.L.; Sánches, G.M.; Jure, M.M. Pharmaceutical Composition Based on Plant Extracts for the Treatment and/or Prevention of Migraines. CU Patent WO2007048356A1, 1 May 2007. [Google Scholar]
- Bacchi, E.M.; Chacra, N.A.B.; Salazar, P.M.N. Nanopartícula. BR Patent BR102012021728-A2, 1 July 2014. [Google Scholar]
- Dantas, L.P.; de Oliveira-Ribeiro, A.; de Almeida-Souza, L.M.; Groppo, F.C. Effects of Passiflora incarnata and midazolam for control of anxiety in patients undergoing dental extraction. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, 95–101. [Google Scholar] [CrossRef]
- Rokhtabnak, F.; Ghodraty, M.R.; Kholdebarin, A.; Khatibi, A.; Alizadeh, S.S.S.; Koleini, Z.S.; Zamani, M.M.; Pournajafian, A. Comparing the Effect of Preoperative Administration of Melatonin and Passiflora incarnata on Postoperative Cognitive Disorders in Adult Patients Undergoing Elective Surgery. Anesth. Pain Med. 2016, 7, 41238. [Google Scholar] [CrossRef] [PubMed]
- Movafegh, A.; Alizadeh, R.; Hajimohamadi, F.; Esfehani, F.; Nejatfar, M. Preoperative oral Passiflora incarnata reduces anxiety in ambulatory surgery patients: A double-blind, placebo-controlled study. Anesth. Analg. 2008, 106, 1728–1732. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Mohammadi, M.R.; Momeni, F. Passiflora incarnata in the treatment of attention deficit hyperactivity disorder in children and adolescents. Therapy 2005, 2, 609–614. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Kashani, L.; Mobaseri, M.; Hosseini, S.H.; Nikzad, S.; Khani, M. Passionflower in the treatment of opiates withdrawal: A double-blind randomized controlled trial. J. Clin. Pharm. Ther. 2001, 26, 369–373. [Google Scholar] [CrossRef]

| Species | Habitat/Distribution | Characteristics of Fruits | References |
|---|---|---|---|
| Passiflora edulis Sims (purple passion fruit) | South America and Mexico Adapted for cultivation in China | The fruit is round, purple, 4–7 cm in diameter, and 4–9 cm long, weighing about 35–45 g | [4] |
| Passiflora edulis Sims f. flavicarpa O. Deg. (yellow passion fruit) | A mutation of the purple variety or natural hybrid. Commonly found in Northeast or South India [5] | The fruit is round, golden yellow, 4–7 cm in diameter, and 6–12 cm long, weighing about 60 g | [4,5,6] |
| Passiflora quadrangularis L. (giant granadilla) | The tropical regions of America and Asia | The fruit is greenish-yellow, 15–20 cm long, and weighs about 600 g | [4] |
| ‘Kaveri’ Hybrid passion fruit (purple and yellow) | A hybrid, developed in India by crossing purple and yellow passion fruit | The fruit is purple, weighing about 85–110 g | [4] |
| Chemical Compound | Species | Part of the Plant | References |
|---|---|---|---|
| Citric acid | Passiflora mollissima L.H. Bailey | Fruit pulp | [35] |
| Syringic acid | Passiflora mollissima L.H. Bailey | Fruit pulp | [35] |
| Caffeic acid | Passiflora subpeltata Ortega | Leaves | [36] |
| Caffeoylquinic acid | Passiflora tenuifila Killip | Fruits | [37] |
| Malic acid | Passiflora edulis Sims, Passiflora edulis f. flavicarpa O. Deg, Passiflora alata Curtis | Fruits | [38] |
| Apigenin-C-hexoside-C-pentoside | Passiflora foetida L. | Aerial parts (as mixed) | [39] |
| Vitexin (Apigenin-8-C-glucoside) | Passiflora foetida L. | Aerial parts (as mixed) | [39] |
| Luteolin-C-hexoside-C-pentoside | Passiflora foetida L. | Aerial parts (as mixed) | [39] |
| Luteolin | Passiflora edulis Sims, Passiflora edulis f. flavicarpa O. Deg | Fruit peel fruit | [29] |
| Quercetin | Passiflora edulis Sims, Passiflora edulis f. flavicarpa O. Deg | Fruit peel fruit | [29] |
| Naringenin | Passiflora mollissima L.H. Bailey | Fruit pulp | [35] |
| Trihydroxy(iso)flavanol-(epi)catechin | Passiflora mollissima L.H. Bailey | Fruit pulp | [35] |
| Tyrosine | Passiflora edulis Sims (cultivar ‘Tainung No. 1’) | Seed | [40] |
| Valine | Passiflora edulis Sims (cultivar ‘Tainung No. 1’) | Seed | [40] |
| Proline | Passiflora edulis Sims (cultivar ‘Tainung No. 1’) | Seed | [40] |
| Glycine | Passiflora edulis Sims (cultivar ‘Tainung No. 1’) | Seed | [40] |
| α-Humulene | Passiflora sexocellata Schltdl., Passiflora trifasciata Lem. | Leaves, flowers | [41] |
| Pentadecanoic acid | Passiflora sexocellata Schltdl. Passiflora trifasciata Lem. | Leaves, flowers | [41] |
| Methyl salicylate | Passiflora trifasciata Lem. | Leaves, flowers | [41] |
| Benzyl alcohol | Passiflora sexocellata Schltdl., Passiflora trifasciata Lem. | Leaves, flowers | [41] |
| Cyclooctasiloxane, Hexadecamethyl | Passiflora edulis Sims | Fruit pulp | [42] |
| Cyclononasiloxane, Octadecamethyl | Passiflora edulis Sims | Fruit pulp | [42] |
| Type of Vitamin, Units | Juice from P. edulis f. flavicarpa | Juice from P. edulis |
|---|---|---|
| Vitamin B6, mg | 0.06 | 0.05 |
| Vitamin A RAE, μg | 47 | 36 |
| Vitamin A, IU | 943 | 717 |
| Vitamin E (alpha tocopherol), mg | 0.01 | 0.01 |
| Vitamin K, μg | 0.4 | 0.4 |
| Chemical Formula | Effect | Reference |
|---|---|---|
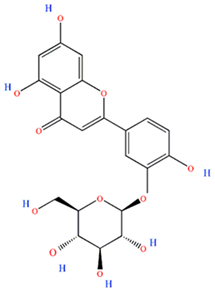 Luteolin-3-glucoside | Antitumor | [21] |
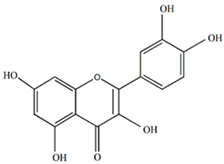 Quercetin | Antiallergic, antitumor, antiviral, and neuroprotective | [21,43,44,45] |
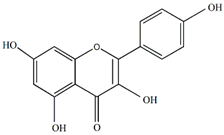 Kaempferol | Antiallergic, antitumor, antiviral, and neuroprotective | [21,43,44,45] |
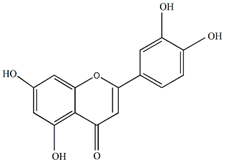 Luteolin | Antiallergic, antitumor, antiviral, and neuroprotective | [43,44,45] |
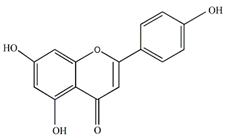 Apigenin | Antiallergic, antitumor, antiviral, and neuroprotective | [21,43,44,45] |
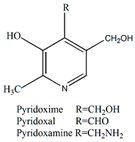 Vitamin B6 | Antiviral, immunological, and neuroprotective | [59] |
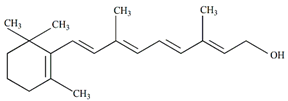 Vitamin A | Antiviral, immunological, and neuroprotective | [59] |
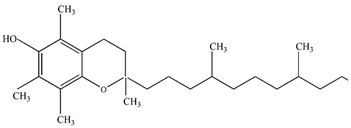 Vitamin E | Antiviral, immunological, and neuroprotective | [59] |
 Vitamin K | Antiviral, immunological | [59] |
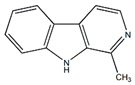 Harmane | Anti-inflammatory, antitumor | [59] |
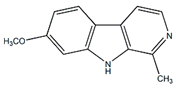 Harmine | Anti-inflammatory, antitumor | [4] |
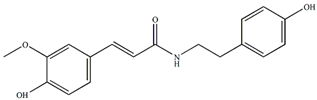 N-trans-feruloyltyramine | Anti-inflammatory, antitumor | [4] |
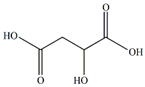 Malic acids | Antioxidant | [4] |
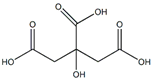 Citric acids | Antioxidant | [4] |
| Passiflora Species | Ailment | Dose | Ref. |
|---|---|---|---|
| P. incarnata | Dental anxiety | 20 drops/day | [80] |
| 260 mg | [136] | ||
| P. incarnata | Preoperative anxiety | 1000 mg | [137] |
| 500 mg | [138] | ||
| 700 mg/5 mL | [79] | ||
| P. incarnata | Anxiety disorder | 45 drops/day | [77] |
| P. incarnata | Sleep disorder | infusion (2 g in 250 mL) | [81] |
| P. incarnata | Attention-deficit hyperactivity disorder (ADHD) | 0.04 mg/kg/day (twice daily) | [139] |
| P. incarnata | Opiate detoxification | 60 drops/day + 0.8 mg of clonidine | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, K.; Velikova, M.; Gentscheva, G.; Gerasimova, A.; Slavov, P.; Harbaliev, N.; Makedonski, L.; Buhalova, D.; Petkova, N.; Gavrilova, A. Chemical Compositions, Pharmacological Properties and Medicinal Effects of Genus Passiflora L.: A Review. Plants 2024, 13, 228. https://doi.org/10.3390/plants13020228
Nikolova K, Velikova M, Gentscheva G, Gerasimova A, Slavov P, Harbaliev N, Makedonski L, Buhalova D, Petkova N, Gavrilova A. Chemical Compositions, Pharmacological Properties and Medicinal Effects of Genus Passiflora L.: A Review. Plants. 2024; 13(2):228. https://doi.org/10.3390/plants13020228
Chicago/Turabian StyleNikolova, Krastena, Margarita Velikova, Galia Gentscheva, Anelia Gerasimova, Pavlo Slavov, Nikolay Harbaliev, Lubomir Makedonski, Dragomira Buhalova, Nadezhda Petkova, and Anna Gavrilova. 2024. "Chemical Compositions, Pharmacological Properties and Medicinal Effects of Genus Passiflora L.: A Review" Plants 13, no. 2: 228. https://doi.org/10.3390/plants13020228
APA StyleNikolova, K., Velikova, M., Gentscheva, G., Gerasimova, A., Slavov, P., Harbaliev, N., Makedonski, L., Buhalova, D., Petkova, N., & Gavrilova, A. (2024). Chemical Compositions, Pharmacological Properties and Medicinal Effects of Genus Passiflora L.: A Review. Plants, 13(2), 228. https://doi.org/10.3390/plants13020228