Sesquiterpene Lactones Isolated from Centaurea cineraria L. subsp. cineraria Inhibit the Radicle Growth of Broomrape Weeds
Abstract
1. Introduction
2. Results
2.1. Plant Extraction
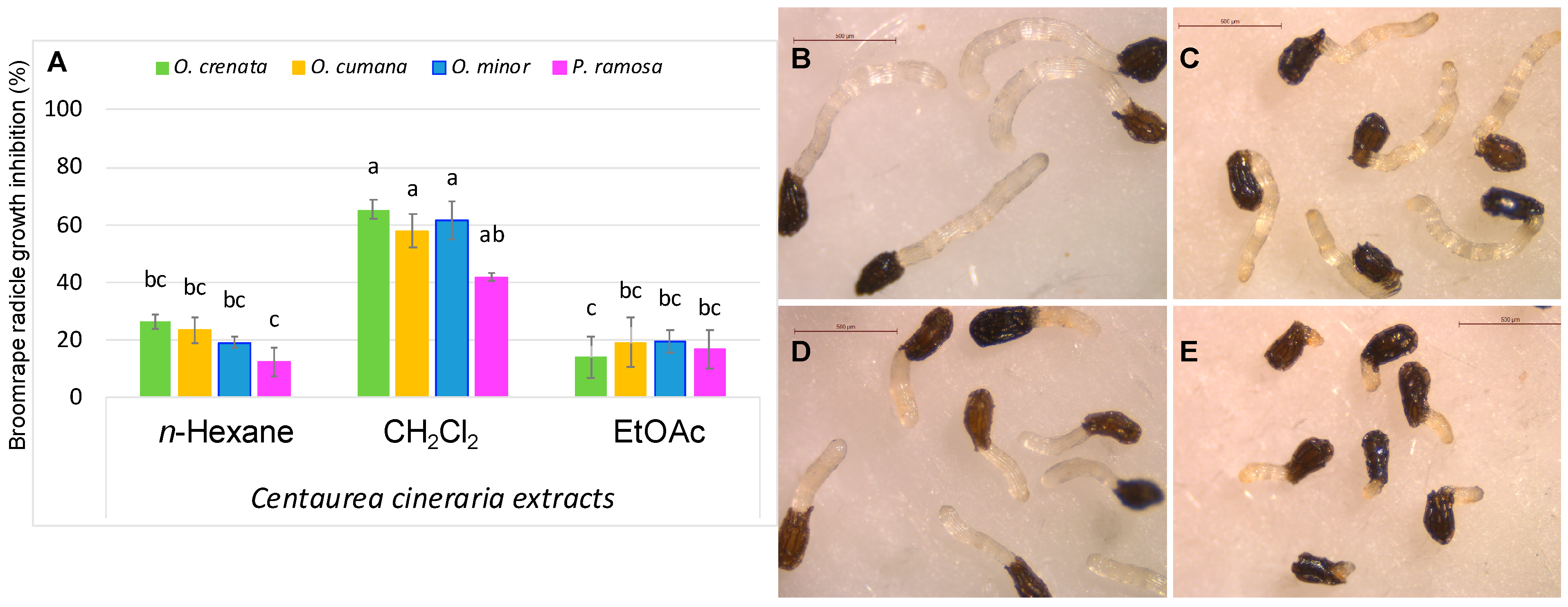
2.2. Bio-Activity-Guided Purification of Secondary Metabolites
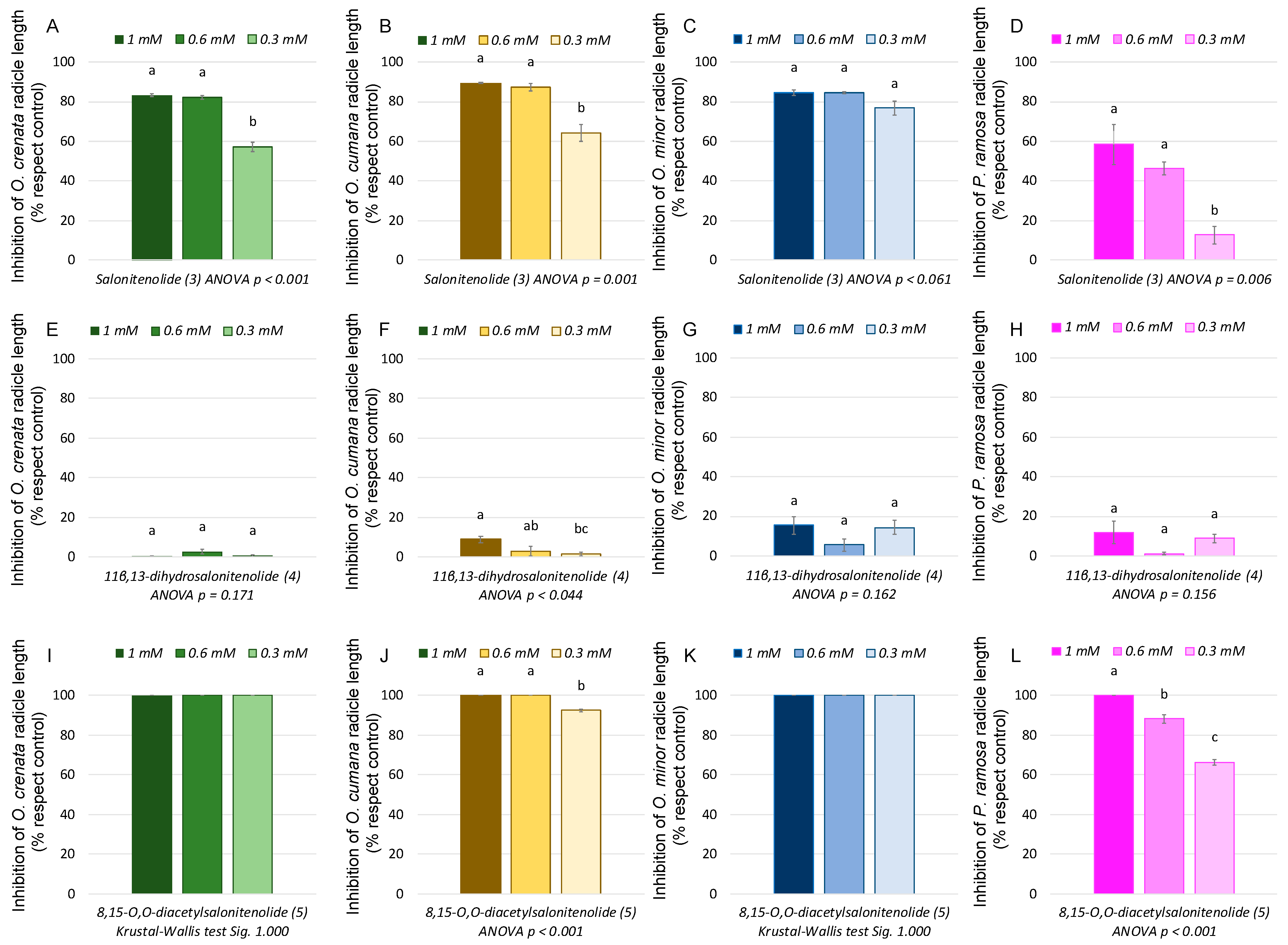
2.3. Bioassays against Broomrapes
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Purification and Identification Compounds 1–4
4.4. Synthesis of 8,15-O,O′-Diacetylsalonitenolide (5)
4.5. Growth Inhibition Assays on Broomrape Species
4.6. Statistical Analysis and Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest. Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of infection by parasitic weeds: A review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef]
- Parker, C. Observations on the current status of Orobanche and Striga problems worldwide. Pest Manag. Sci. 2009, 65, 453–459. [Google Scholar] [CrossRef]
- Parker, C. The parasitic weeds of the Orobanchaceae. In Parasitic Orobanchaceae; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 313–344. [Google Scholar]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The effect of Orobanche crenata infection severity in faba bean, field pea and grass pea productivity. Front. Plant Sci. 2016, 7, 1049. [Google Scholar] [CrossRef]
- Eizenberg, H.; Goldwasser, Y. Control of Egyptian broomrape in processing tomato: A summary of 20 years of research and successful implementation. Plant Dis. 2018, 102, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Cvejić, S.; Radanović, A.; Dedić, B.; Jocković, M.; Jocić, S.; Miladinović, D. Genetic and genomic tools in sunflower breeding for broomrape resistance. Genes 2020, 11, 152. [Google Scholar] [CrossRef]
- Soto-Cruz, F.J.; Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Igartuburu, J.M.; Macías, F.A. Allelopathic activity of strigolactones on the germination of parasitic plants and arbuscular mycorrhizal fungi growth. Agronomy 2021, 11, 2174. [Google Scholar] [CrossRef]
- Peralta, A.C.; Soriano, G.; Zorrilla, J.G.; Masi, M.; Cimmino, A.; Fernández-Aparicio, M. Characterization of Conyza bonariensis allelochemicals against broomrape weeds. Molecules 2022, 27, 7421. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Cimmino, A.; Soriano, G.; Masi, M.; Vilariño, S.; Evidente, A. Assessment of weed root extracts for allelopathic activity against Orobanche and Phelipanche species. Phytopathol. Mediterr. 2021, 60, 455–466. [Google Scholar] [CrossRef]
- Rial, C.; Tomé, S.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Phytochemical study of safflower roots (Carthamus tinctorius) on the induction of parasitic plant germination and weed control. J. Chem. Ecol. 2020, 46, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhang, M.; Zhang, M.; Ma, Y. Assessing the performance of maize (Zea mays L.) as trap crops for the management of sunflower broomrape (Orobanche cumana Wallr.). Agronomy 2020, 10, 100. [Google Scholar] [CrossRef]
- Lejeune, K.D.; Seastedt, T.R. Centaurea species: The forb that won the west. Conserv. Biol. 2001, 15, 1568–1574. [Google Scholar] [CrossRef]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative assessment of the phytochemical composition and biological activity of extracts of flowering plants of Centaurea cyanus L., Centaurea jacea L. and Centaurea scabiosa L. Plants 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Hilpold, A.; Schönswetter, P.; Susanna, A.; Garcia-Jacas, N.; Vilatersana, R. Evolution of the central Mediterranean Centaurea cineraria group (Asteraceae): Evidence for relatively recent, allopatric diversification following transoceanic seed dispersal. Taxon 2011, 60, 528–538. [Google Scholar] [CrossRef]
- Valletta, A.; Santamaria, A.R.; Fabrini, G.; Tocci, N.; Filho, V.C.; Wagner, T.; Brasili, E.; Pasqua, G. Strategies for ex situ conservation of Centaurea cineraria subsp. circae (Asteraceae), an endemic plant from Lazio (Italy). Plant Biosyst. 2016, 150, 323–332. [Google Scholar] [CrossRef]
- Del Guacchio, E.; Iamonico, D.; Cennamo, P.; Caputo, P. Nomenclatural and taxonomic notes on some Centaurea taxa (Asteraceae) from southern Italy. Turk. J. Bot. 2020, 44, 441–454. [Google Scholar] [CrossRef]
- Hashim, S.S.; Mahmood, Z.F.; Abdulateef, S.F.; Dheeb, B.I. Evaluation cytotoxicity effects of Centaurea cineraria extracts against some of cancer cell lines. Biomed. Pharmacol. J. 2023, 16, 223–225. [Google Scholar] [CrossRef]
- Salachna, P.; Pietrak, A.; Łopusiewicz, Ł. Antioxidant potential of flower extracts from Centaurea spp. depends on their content of phenolics, flavonoids and free amino acids. Molecules 2021, 26, 7465. [Google Scholar] [CrossRef]
- Fletcher, R.A.; Renney, A.J. A growth inhibitor found in Centaurea spp. Can. J. Plant Sci. 1963, 43, 475–481. [Google Scholar] [CrossRef]
- Raupp, F.M.; Spring, O. New sesquiterpene lactones from sunflower root exudate as germination stimulants for Orobanche cumana. J. Agric. Food Chem. 2013, 61, 10481–10487. [Google Scholar] [CrossRef] [PubMed]
- Sokovic, M.; Ciric, A.; Glamoclija, J.; Skaltsa, H. Biological activities of sesquiterpene lactones isolated from the genus Centaurea L. (Asteraceae). Curr. Pharm. Des. 2017, 23, 2767–2786. [Google Scholar] [CrossRef] [PubMed]
- Kebbi, S.; Ciavatta, M.L.; Mahmoud, A.M.; Carbone, M.; Ligresti, A.; Seghiri, R.; Gavagnin, M. Sesquiterpene lactones with the 12,8-guaianolide skeleton from Algerian Centaurea omphalotricha. Biomolecules 2021, 11, 1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Li, Z.M.; Ma, N.; Wang, B.L.; Jiang, L.; Pang, S.S.; Lee, Y.T.; Guddat, L.W.; Duggleby, R.G. Structure-activity relationships for a new family of sulfonylurea herbicides. J. Comput. Aided Mol. Des. 2005, 19, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.B.; DesRochers, N.; Hoogstra, S.; Garnham, C.P.; Sumarah, M.W. Structure activity relationship for fumonisin phytotoxicity. Chem. Res. Toxicol. 2021, 34, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Herz, W. Sesquiterpene lactones and flavones from Centaurea cineraria subsp. umbrosa. Phytochemistry 1988, 27, 1873–1875. [Google Scholar] [CrossRef]
- Marco, J.A.; Sanz, J.F.; Sancenon, F.; Susanna, A.; Rustaiyan, A.; Saberi, M. Sesquiterpene lactones and lignans from Centaurea species. Phytochemistry 1992, 31, 3527–3530. [Google Scholar] [CrossRef]
- Kurita, M.; Tanigawa, M.; Narita, S.; Usuki, T. Synthetic study of cnicin: Synthesis of the side chain and its esterification. Tetrahedron Lett. 2016, 57, 5899–5901. [Google Scholar] [CrossRef]
- Fernández, I.; Pedro, J.; Polo, E. Sesquiterpene lactones from Centaurea alba and C. conifera. Phytochemistry 1995, 38, 655–657. [Google Scholar] [CrossRef]
- Djeddi, S.; Karioti, A.; Sokovic, M.; Koukoulitsa, C.; Skaltsa, H. A novel sesquiterpene lactone from Centaurea pullata: Structure elucidation, antimicrobial activity, and prediction of pharmacokinetic properties. Bioorg. Med. Chem. 2008, 16, 3725–3731. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Gonzalez, G.F.; dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with high toxicity. Crit. Rev. Plant Sci. 2016, 35, 18–37. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Locken, L.J. Phytotoxic properties of cnicin, a sesquiterpene lactone from Centaurea maculosa (spotted knapweed). J. Chem. Ecol. 1987, 13, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Tiwana, G.; Fua, J.; Lu, L.; Cheesman, M.J.; Cock, I.E. A review of the traditional uses, medicinal properties and phytochemistry of Centaurea benedicta L. Pharmacogn. J. 2021, 13, 798–812. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Facile synthesis of anhydrojudaicin and 11,13-dehydroanhydrojudaicin, two eudesmanolide-skeleton lactones with potential allelopathic activity. Phytochem. Lett. 2019, 31, 229–236. [Google Scholar] [CrossRef]
- Tice, C.M. Selecting the right compounds for screening: Does Lipinski’s rule of 5 for 652 pharmaceuticals apply to agrochemicals? Pest Manag. Sci. 2001, 57, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Spring, O.; Schmauder, K.; Lackus, N.D.; Schreiner, J.; Meier, C.; Wellhausen, J.; Smith, L.V.; Frey, M. Spatial and developmental synthesis of endogenous sesquiterpene lactones supports function in growth regulation of sunflower. Planta 2020, 252, 2. [Google Scholar] [CrossRef] [PubMed]
- Takei, S.; Uchiyama, Y.; Bürger, M.; Suzuki, T.; Okabe, S.; Chory, J.; Seto, Y. A Divergent clade KAI2 protein in the root parasitic plant Orobanche minor is a highly sensitive strigolactone receptor and is involved in the perception of sesquiterpene lactones. Plant Cell Physiol. 2023, 64, 996–1007. [Google Scholar] [CrossRef]
- Andolfi, A.; Zermane, N.; Cimmino, A.; Avolio, F.; Boari, A.; Vurro, M.; Evidente, A. Inuloxins A–D, phytotoxic bi-and tri-cyclic sesquiterpene lactones produced by Inula viscosa: Potential for broomrapes and field dodder management. Phytochemistry 2013, 86, 112–120. [Google Scholar] [CrossRef]
- Molinaro, F.; Monterumici, C.M.; Ferrero, A.; Tabasso, S.; Negre, M. Bioherbicidal activity of a germacranolide sesquiterpene dilactone from Ambrosia artemisiifolia L. J. Environ. Sci. Health B 2016, 51, 847–852. [Google Scholar] [CrossRef]
- Ulloa, J.L.; Spina, R.; Casasco, A.; Petray, P.B.; Martino, V.; Sosa, M.A.; Frank, F.M.; Muschietti, L.V. Germacranolide-type sesquiterpene lactones from Smallanthus sonchifolius with promising activity against Leishmania mexicana and Trypanosoma cruzi. Parasit. Vectors 2017, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Strategies for the synthesis of canonical, non-canonical and analogues of strigolactones, and evaluation of their parasitic weed germination activity. Phytochem. Rev. 2022, 21, 1627–1659. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.; Raccuglia, R.A.; Bruno, M. Rearrangement of germacranolides. Synthesis and absolute configuration of elemane and heliangolane derivatives from cnicin. Eur. J. Org. Chem. 2003, 2003, 2690–2694. [Google Scholar] [CrossRef]
- Monsalve, L.N.; Rosselli, S.; Bruno, M.; Baldessari, A. Lipase-catalysed preparation of acyl derivatives of the germacranolide cnicin. J. Mol. Catal. B Enzym. 2009, 57, 40–47. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia, 4th ed.; Edagricole: Milan, Italy, 2019; ISBN 978-88-506-5245-7. [Google Scholar]
- Fernández-Aparicio, M.; Moral, A.; Kharrat, M.; Rubiales, D. Resistance against broomrapes (Orobanche and Phelipanche spp.) in faba bean (Vicia faba) based in low induction of broomrape seed germination. Euphytica 2012, 186, 897–905. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Vraka, C.; Nics, L.; Wagner, K.H.; Hacker, M.; Wadsak, W.; Mitterhauser, M. LogP, a yesterday’s value? Nucl. Med. Biol. 2017, 50, 1–10. [Google Scholar] [CrossRef]
- PerkinElmer. Available online: http://perkinelmer.com (accessed on 20 December 2023).





| Isocnicin (1) | Cnicin (2) | Salonitenolide (3) | 11β,13-Dihydrosalonitenolide (4) | 8,15-O,O′-Diacetylsalonitenolide (5) | |
|---|---|---|---|---|---|
| Clog P | 0.37 | 0.63 | 0.61 | 0.64 | 2.45 |
| Rotable bonds | 8 | 6 | 1 | 1 | 5 |
| H-bond acceptors | 7 | 7 | 4 | 4 | 6 |
| H-bond donors | 3 | 3 | 2 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorrilla, J.G.; Innangi, M.; Cala Peralta, A.; Soriano, G.; Russo, M.T.; Masi, M.; Fernández-Aparicio, M.; Cimmino, A. Sesquiterpene Lactones Isolated from Centaurea cineraria L. subsp. cineraria Inhibit the Radicle Growth of Broomrape Weeds. Plants 2024, 13, 178. https://doi.org/10.3390/plants13020178
Zorrilla JG, Innangi M, Cala Peralta A, Soriano G, Russo MT, Masi M, Fernández-Aparicio M, Cimmino A. Sesquiterpene Lactones Isolated from Centaurea cineraria L. subsp. cineraria Inhibit the Radicle Growth of Broomrape Weeds. Plants. 2024; 13(2):178. https://doi.org/10.3390/plants13020178
Chicago/Turabian StyleZorrilla, Jesús G., Michele Innangi, Antonio Cala Peralta, Gabriele Soriano, Maria Teresa Russo, Marco Masi, Mónica Fernández-Aparicio, and Alessio Cimmino. 2024. "Sesquiterpene Lactones Isolated from Centaurea cineraria L. subsp. cineraria Inhibit the Radicle Growth of Broomrape Weeds" Plants 13, no. 2: 178. https://doi.org/10.3390/plants13020178
APA StyleZorrilla, J. G., Innangi, M., Cala Peralta, A., Soriano, G., Russo, M. T., Masi, M., Fernández-Aparicio, M., & Cimmino, A. (2024). Sesquiterpene Lactones Isolated from Centaurea cineraria L. subsp. cineraria Inhibit the Radicle Growth of Broomrape Weeds. Plants, 13(2), 178. https://doi.org/10.3390/plants13020178











