Genetic Dissection of Panicle Morphology Traits in Super High-Yield Hybrid Rice Chaoyou 1000
Abstract
1. Introduction
2. Result
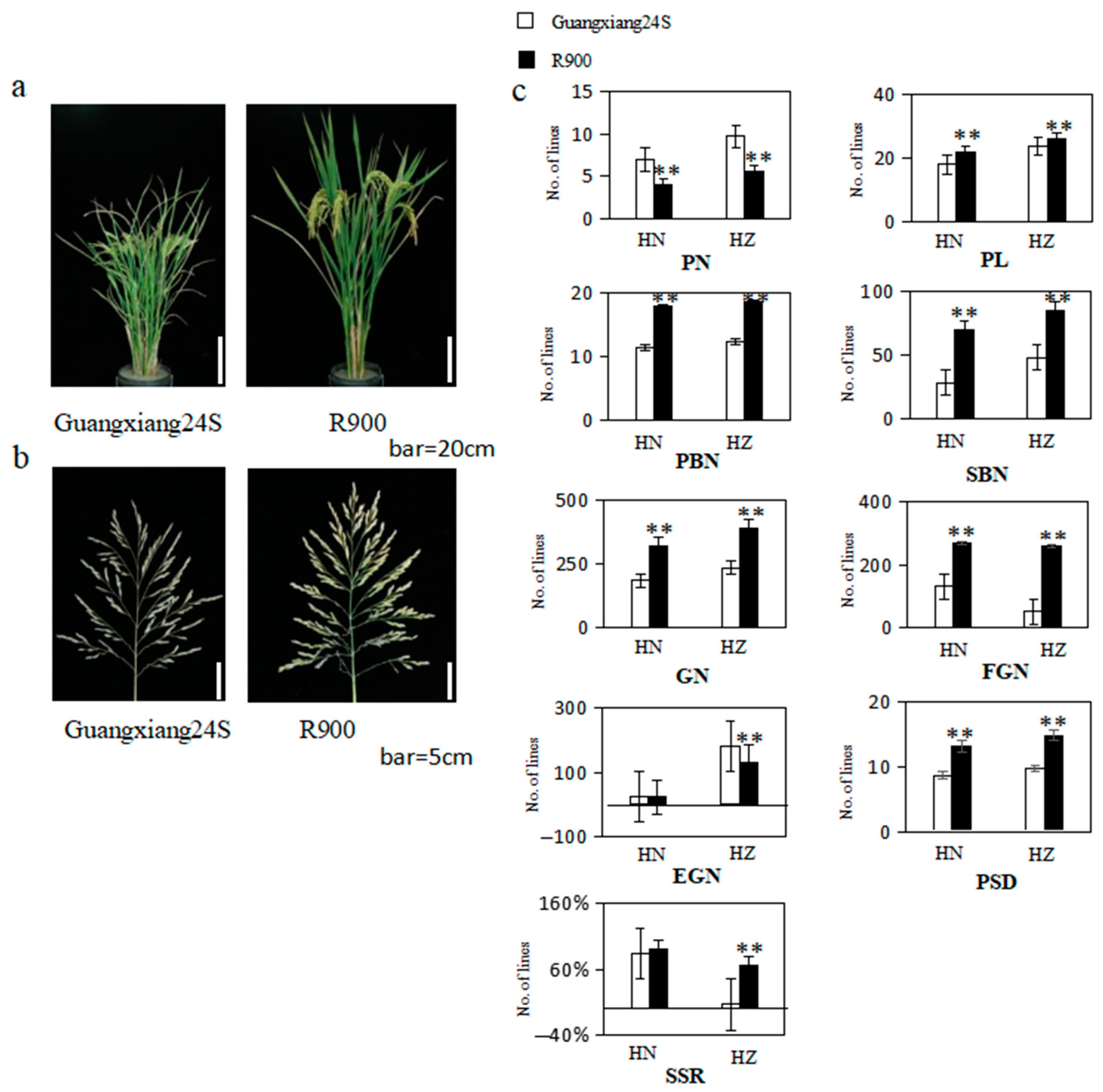
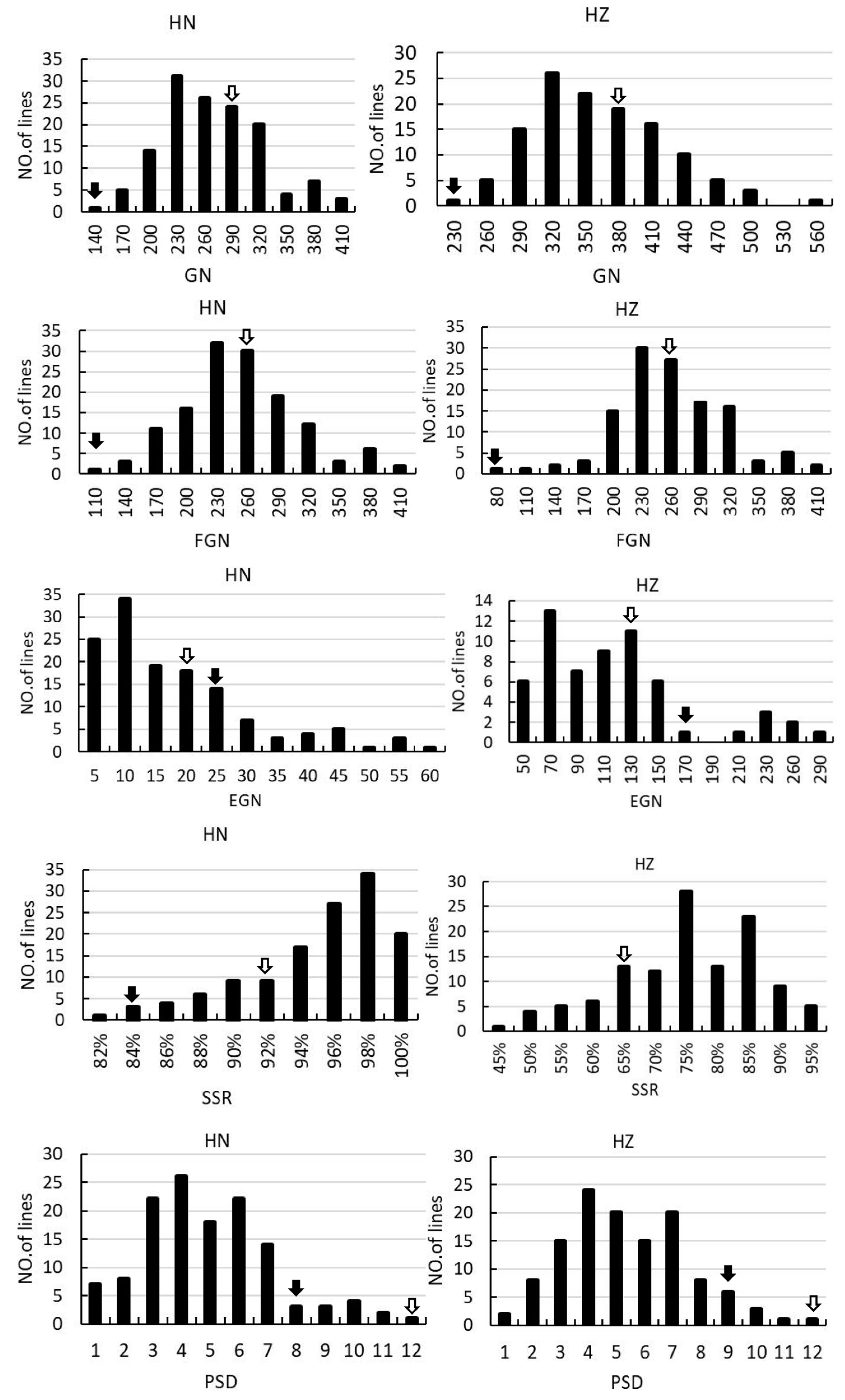
2.1. Phenotypic Analysis of Parents and Populations
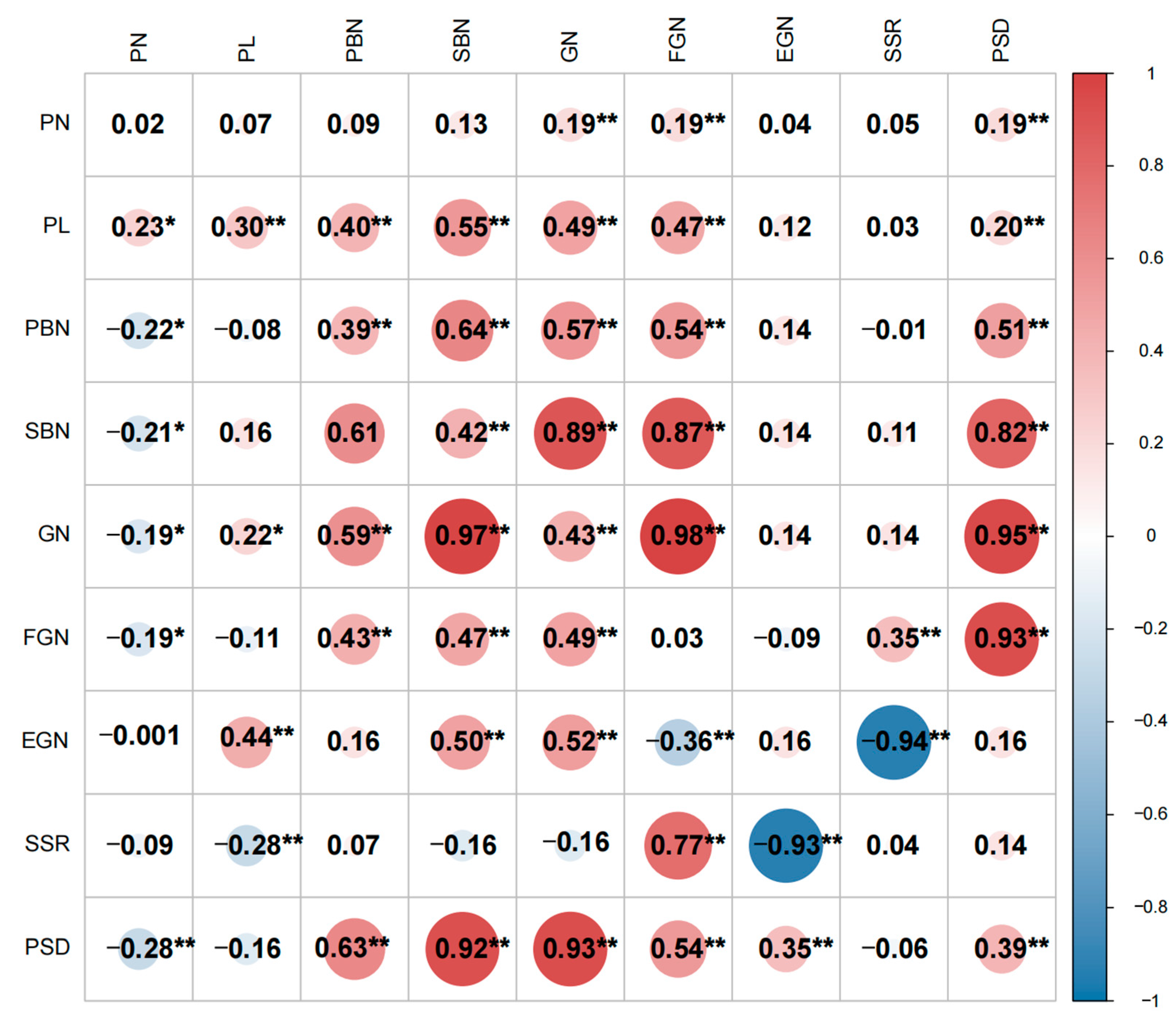
2.2. Correlation Analysis of Panicle-Related Traits
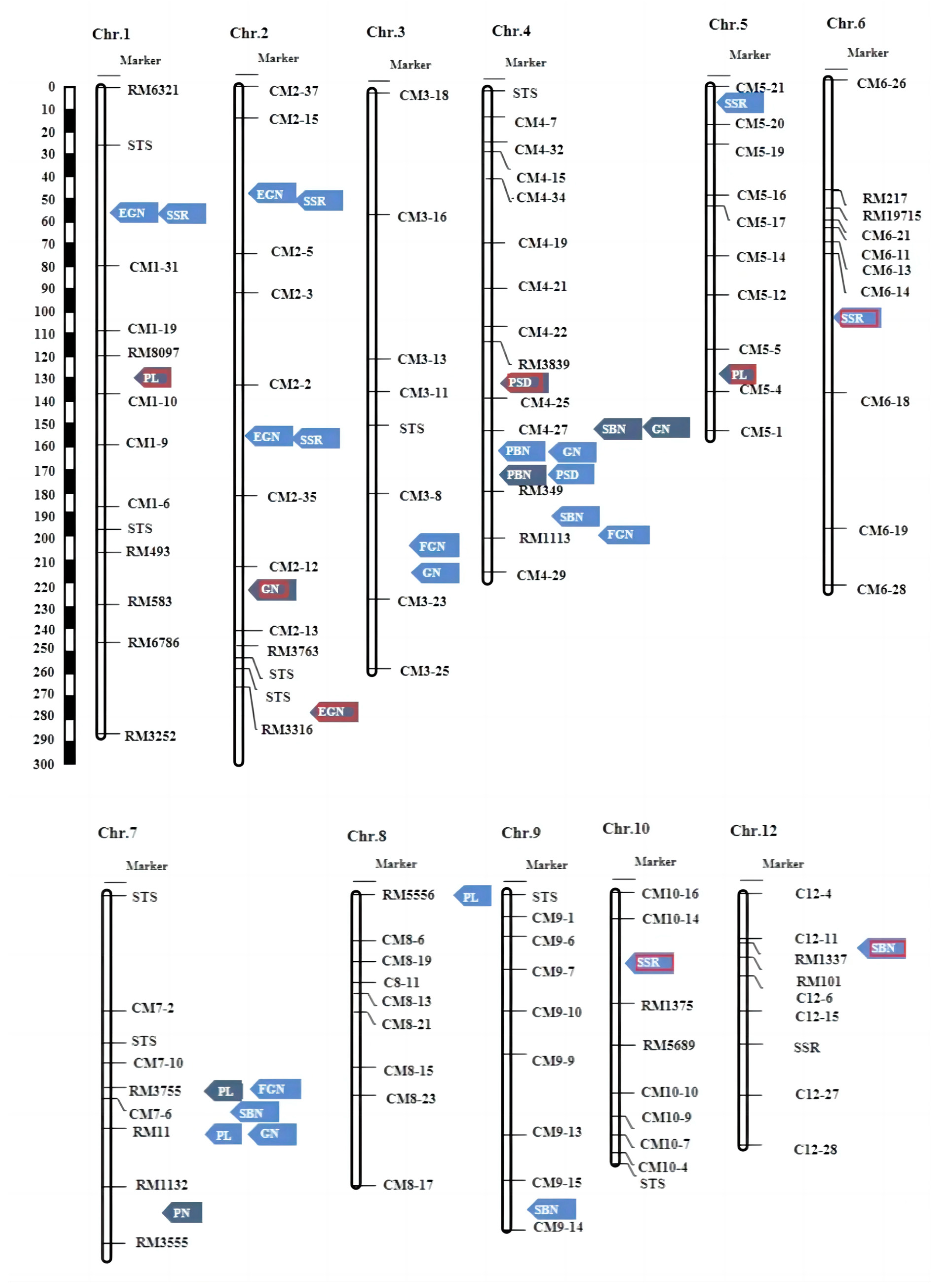
2.3. Construction of Genetic Map
2.4. Main-QTL Analysis
2.5. Digenic Epistatic QTL Pairs (E-QTL) Underlying Panicle Morphology Traits
2.6. Q × E Interaction Analysis in F2 and F2:3 Populations
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Field Experiment
4.3. Traits Investigation and Data Analysis
4.4. DNA Extraction and Marker Analysis
4.5. Genetic Map Construction and QTL Detection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and thesustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends areinsufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar]
- Molina, J.; Sikora, M.; Garud, N.; Flowers, J.M.; Rubinstein, S.; Reynolds, A.; Huang, P.; Jackson, S.; Schaal, B.A.; Bustamante, C.D.; et al. Molecular evidence for a single evolutionary origin of domesticated rice. Proc. Natl. Acad. Sci. USA 2011, 108, 8351–8356. [Google Scholar] [PubMed]
- Cheng, S.; Hu, P. Development strategy of rice science and technology in china. Chin. J. Rice Sci. 2008, 22, 223–226. [Google Scholar]
- Zhang, Q. Strategies for developing Green Super Rice. Mol. Plant Breed. 2005, 3, 601–602. [Google Scholar] [CrossRef]
- Zhang, Q. Strategies for developing Green Super Rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar] [CrossRef]
- Leng, Y.J.; Xue, D.W.; Huang, L.C.; Cheng, L.; Ren, D.Y.; Yang, Y.L.; Zhang, G.H.; Hu, J.; Zhu, L.; Guo, L.B.; et al. Mapping QTL with Main Effect, Digenic Epistatic and QTL × Environment Interactions of Panicle Related Traits in Rice (Oryza sativa). Int. J. Agiculture Biol. 2017, 16, 1814–1820. [Google Scholar]
- Liu, J.; Yao, X.Y.; Liu, D.; Yu, L.; Li, H.; Wang, Q.; Wang, J.; Li, M. Identification of QTL for Panicle Traits under Multiple Environments in Rice (Oryza sativa L.). China J. Rice Sci. 2019, 33, 32–42. [Google Scholar]
- Nasim, R.; Jafari, M.T. Agronomic characteristics and grain yield of 30 Iranian rice genotypes as affected by genotype x environment interaction. Ecol. Genet. Genom. 2023, 26, 100153. [Google Scholar]
- Zhong, Q.; Jia, Q.; Yin, W.; Wang, Y.; Rao, Y.; Mao, Y. Advances in cloning functional genes for rice yield traits and molecular design breeding in China. Front. Plant Sci. 2023, 14, 1206165. [Google Scholar]
- Xu, Y.; Ma, K.; Zhao, Y.; Wang, X.; Zhou, K.; Yu, G.; Li, C.; Li, P.; Yang, Z.; Xu, C.; et al. Genomic selection: A breakthrough technology in rice breeding. Crop J. 2021, 9, 669–677. [Google Scholar] [CrossRef]
- Ashikari, M.H.; Lin, S.; Yamamoto, T.; Takashi, T.; Nishimura, A.; Angeles, E.R.; Qian, Q.; Kitano, H.; Matsuoka, M. Cytokinin oxidase regulates rice grain production. Science 2005, 309, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Piao, R.; Jiang, W.; Ham, T.H.; Choi, M.S.; Qiao, Y.; Chu, S.H.; Park, J.H.; Woo, M.O.; Jin, Z.; An, G.; et al. Map-based cloning of the ERECT PANICLE 3 gene in rice. Theor. Appl. Genet. 2009, 119, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wu, B.; Xing, Y. Yield-related QTLs and their applications in rice genetic improvement. J. Integr. Plant Biol. 2012, 54, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, L.; Liu, T.; Xu, C.; Xing, Y. Four rice QTL controlling number of spikelets per panicle expressed the characteristics of single Mendelian gene in near isogenic backgrounds. Theor. Appl. Genet. 2009, 118, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Singh, A.; Jain, N.; Anand, S.; Gacche, R.; Singh, A.; Gaikwad, K.; Sharma, T.; Mohapatra, T.; Singh, N. Identification of candidate genes for grain number in rice (Oryza sativa L.). Funct. Integr. Genom. 2010, 10, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.J.; Hao, W.; Jin, J.; Gao, J.P.; Lin, H.X. Fine mapping of Spr3, a locus for spreading panicle from African cultivated rice (Oryza glaberrima Steud.). Mol. Plant 2008, 1, 830–838. [Google Scholar] [CrossRef]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef]
- Veyrieras, J.B.; Goffinet, B.; Charcosset, A. Meta QTL: A package of new computational methods for the meta-analysis of QTL mapping experiments. BMC Bioinform. 2007, 8, 49. [Google Scholar]
- Zhu, J.; Zhou, Y.; Liu, Y.; Wang, Z.; Tang, Z.; Yi, C.; Tang, S.; Gu, M.; Liang, G. Fine mapping of a major QTL controlling panicle number in rice. Mol. Breed. 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Wada, G.; Cruz, P.C.S. Varietal difference in nitrogen response of rice plants with special reference to growth duration. Jpn. J. Crop Sci. 1989, 58, 732–739. [Google Scholar] [CrossRef]
- Hu, H.; Lv, W.; Li, Q.; Ou, X.; Xu, J.; Li, Z.; Xing, D.; Yang, L.; Xu, J.; Qiu, X.; et al. Characterization of main effects, epistatic effects and genetic background effects on QTL for yield related traits by two sets of reciprocal introgression lines in rice (Oryza sativa). Int. J. Agric. Biol. 2018, 20, 2125–2132. [Google Scholar]
- Tao, Y.; Zhu, J.; Xu, J.; Wang, L.; Gu, H.; Zhou, R.; Yang, Z.; Zhou, Y.; Liang, G. Exploitation of heterosis loci for yield and yield components in rice using chromosome segment substitution lines. Sci. Rep. 2016, 6, 36802. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Zhang, J.; Zhang, Q.; Tao, Y.; Feng, B.; Zheng, T.; Xu, J.; Li, Z. Genetic background effects on QTL and QTL× environment interaction for yield and its component traits as revealed by reciprocal introgression lines in rice. Crop J. 2014, 2, 345–357. [Google Scholar] [CrossRef]
- Liu, T.; Yu, T.; Xing, Y. Identification and validation of a yield-enhancing QTL cluster in rice (Oryza sativa L.). Euphytica 2013, 192, 145–153. [Google Scholar] [CrossRef]
- Singh, V.K.; Ellur, R.K.; Singh, A.K.; Nagarajan, M.; Singh, B.D.; Singh, N.K. Effect of qGN4.1 qtl for grain number per panicle in genetic backgrounds of twelve different mega varieties of rice. Rice 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Rao, Y.; Huang, L.; Leng, Y.; Hu, J.; Lu, M.; Zhang, G.; Zhu, L.; Gao, Z.; Dong, G.; et al. Fine Mapping Identifies a New QTL for Brown Rice Rate in Rice (Oryza Sativa L.). Rice 2016, 9, 4. [Google Scholar] [CrossRef]
- Khush, G.S. Increasing the genetic yield potential of rice: Prospects and approaches. Int. Rice Comm. Newsl. 1995, 43, 57–71. [Google Scholar]
- Mc Couch, S.R. Report on QTL nomenclature. Rice Genet. Newsl. 1997, 14, 11–13. [Google Scholar]

| Chr. | Physical Distance (Mb) | Mean Physical Distance (Mb) | Genetic Distance (cM) | Mean Genetic Distance (cM) | The Number of Marker |
|---|---|---|---|---|---|
| 1 | 43.20 | 3.32 | 288.52 | 22.19 | 13 |
| 2 | 36.00 | 3.00 | 293.95 | 24.50 | 12 |
| 3 | 37.85 | 4.73 | 260.65 | 32.58 | 8 |
| 4 | 34.1 | 2.44 | 212.8 | 15.20 | 14 |
| 5 | 29.79 | 2.98 | 151.17 | 15.12 | 10 |
| 6 | 30.96 | 3.10 | 229.03 | 22.90 | 10 |
| 7 | 28.87 | 3.21 | 160.66 | 17.85 | 9 |
| 8 | 28.56 | 3.17 | 129.42 | 14.38 | 9 |
| 9 | 23.61 | 2.62 | 149.86 | 16.65 | 9 |
| 10 | 24.39 | 2.71 | 125.43 | 13.94 | 9 |
| 11 | 30.3 | 2.75 | 146.68 | 13.33 | 11 |
| 12 | 25.37 | 2.82 | 114.32 | 12.70 | 9 |
| Total | 373 | 3.07 | 2262.48 | 18.45 | 123 |
| Trait Name | Environment | QTL | Chr. | Marker Interval | LOD | R2 (%) |
|---|---|---|---|---|---|---|
| PN | HZ | qPN-7F | 7 | RM1132-RM3555 | 2.83 | 10.60 |
| PBN | HN | qPBN-4L | 4 | CM4-27-RM349 | 12.08 | 46.09 |
| PBN | HZ | qPBN-4F | 4 | CM4-27-RM349 | 4.49 | 17.52 |
| SBN | HN | qSBN-4.1L | 4 | RM349-RM1113 | 2.59 | 9.26 |
| SBN | HZ | qSBN-4.2F | 4 | CM4-25-CM4-27 | 2.64 | 9.71 |
| SBN | HN | qSBN-7L | 7 | CM7-6-RM11 | 4.25 | 11.80 |
| SBN | HN | qSBN-9L | 9 | CM9-15-CM9-14 | 4.57 | 16.75 |
| SBN | HN | qSBN-12L | 12 | RM1337-RM101 | 2.78 | 7.26 |
| PL | HZ | qPL-1F | 1 | RM8097-CM1-10 | 2.56 | 11.97 |
| PL | HZ | qPL-5F | 5 | CM5-5-CM5-4 | 2.65 | 10.28 |
| PL | HN | qPL-7L | 7 | CM7-6-RM11 | 6.79 | 21.33 |
| PL | HZ | qPL-7F | 7 | CM7-6-RM11 | 2.65 | 8.67 |
| PL | HN | qPL-8L | 8 | RM5556-CM8-6 | 2.69 | 7.21 |
| GN | HZ | qGN-2F | 2 | CM2-12-CM2-13 | 2.80 | 15.79 |
| GN | HN | qGN-3L | 3 | CM3-8-CM3-23 | 3.28 | 26.64 |
| GN | HN | qGN-4.1L | 4 | CM4-27-RM349 | 2.73 | 15.91 |
| GN | HZ | qGN-4.2F | 4 | CM4-25-CM4-27 | 2.80 | 10.75 |
| GN | HN | qGN-7L | 7 | CM7-6-RM11 | 2.54 | 9.22 |
| FGN | HN | qFGN-3L | 3 | CM3-8-CM3-23 | 2.63 | 20.36 |
| FGN | HN | qFGN-4L | 4 | RM349-RM1113 | 2.74 | 12.02 |
| FGN | HN | qFGN-7L | 7 | RM3755-CM7-6 | 2.65 | 9.65 |
| EGN | HN | qEGN-1L | 1 | STS-CM1-31 | 4.65 | 28.89 |
| EGN | HN | qEGN-2L | 2 | CM2-15-CM2-5 | 4.85 | 28.24 |
| EGN | HN | qEGN-2.2L | 2 | CM2-2-CM2-35 | 6.29 | 29.98 |
| EGN | HZ | qEGN-2.3F | 2 | RM3316-CM2-6 | 3.03 | 9.25 |
| SSR | HN | qSSR-1L | 1 | STS-CM1-31 | 3.23 | 24.54 |
| SSR | HN | qSSR-2.1L | 2 | CM2-5-CM2-15 | 5.30 | 27.55 |
| SSR | HN | qSSR-2.2L | 2 | CM2-2-CM2-35 | 2.84 | 22.01 |
| SSR | HN | qSSR-5L | 5 | RM3616-CM5-17 | 2.89 | 4.96 |
| SSR | HN | qSSR-6L | 6 | CM6-14-CM6-18 | 4.12 | 30.86 |
| SSR | HN | qSSR-10L | 10 | CM10-14-RM1375 | 3.02 | 26.66 |
| PSD | HN | qPSD-4.1L | 4 | CM4-27-RM349 | 2.73 | 13.82 |
| PSD | HZ | qPSD-4.2F | 4 | RM3839-CM4-25 | 3.98 | 15.28 |
| Population | Trait | Region1 | Region2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chr. | Marker Interval | M-QTL | Chr. | Marker Interval | M-QTL | LOD | AA | R2 | ||
| F2 | PN | 2 | CM2-15-CM2-5 | 8 | CM8-15-CM8-23 | 5.90 | −0.77 | 20.16 | ||
| PN | 3 | CM3-13-CM3-11 | 8 | CM8-15-CM8-23 | 7.16 | 0.16 | 23.24 | |||
| PL | 8 | RM5556-CM8-6 | 12 | CM12-15-SSR | 5.51 | 0.43 | 10.47 | |||
| PBN | 4 | RM349-RM1113 | 11 | CM11-21-CM11-41 | 6.80 | −0.99 | 8.60 | |||
| PBN | 2 | CM2-2-CM2-35 | 4 | RM349-RM1113 | 6.51 | 0.86 | 8.97 | |||
| PBN | 4 | CM4-27-RM349 | 8 | CM8-13-CM8-21 | 5.73 | 0.32 | 9.35 | |||
| SBN | 4 | RM3839-CM4-25 | 9 | CM9-13-CM9-15 | qSBN-9L | 5.65 | 0.53 | 14.89 | ||
| GN | 4 | CM4-27-RM349 | 8 | CM8-13-CM8-21 | 6.87 | −6.83 | 17.09 | |||
| GN | 2 | CM2-2-CM2-35 | 7 | RM11-RM1132 | 6.29 | 1.78 | 20.90 | |||
| FGN | 2 | CM2-5-CM2-3 | 4 | RM349-RM1113 | qFGN-4L | 6.13 | 37.80 | 13.52 | ||
| FGN | 2 | CM2-5-CM2-3 | 11 | CM11-29-CM11-21 | 5.87 | 2.02 | 19.40 | |||
| EGN | 2 | CM2-15-CM2-5 | qEGN-2L | 2 | CM2-15-CM2-5 | qEGN-2L | 11.68 | −9.71 | 22.18 | |
| EGN | 1 | STS-CM1-31 | qEGN-1L | 2 | CM2-3-CM2-1 | 12.88 | 14.70 | 18.29 | ||
| EGN | 1 | STS-CM1-31 | qEGN-1L | 3 | CM3-13-CM3-11 | 13.80 | −2.45 | 21.78 | ||
| EGN | 2 | CM2-15-CM2-5 | qEGN-2L | 3 | CM3-23-CM3-35 | 11.98 | −9.40 | 22.85 | ||
| EGN | 2 | CM2-15-CM2-5 | qEGN-2L | 4 | CM4-25-CM4-27 | 9.74 | −8.90 | 17.66 | ||
| EGN | 1 | STS-CM1-31 | qEGN-1L | 5 | CM5-4-CM5-1 | 11.37 | -8.82 | 21.78 | ||
| EGN | 2 | CM2-15-CM2-5 | qEGN-2L | 5 | CM5-4-CM5-1 | 10.15 | 6.75 | 16.95 | ||
| EGN | 2 | CM2-15-CM2-5 | qEGN-2L | 6 | CM6-18-CM5-20 | 12.55 | −4.77 | 21.59 | ||
| EGN | 2 | CM2-15-CM2-5 | qEGN-2L | 7 | CM6-28-STS | 12.12 | −7.90 | 21.75 | ||
| SSR | 1 | CM6-28-STS | 2 | CM2-15-CM2-5 | qSSR-2.1L | 8.68 | 0.03 | 18.39 | ||
| SSR | 2 | CM2-15-CM2-5 | qSSR-2.1L | 5 | RM3616-CM5-17 | 8.49 | −0.05 | 17.83 | ||
| SSR | 2 | CM2-15-CM2-5 | qSSR-2.1L | 6 | CM6-14-CM6-18 | qSSR-6L | 9.52 | −0.03 | 18.77 | |
| SSR | 6 | CM6-14-CM6-18 | qSSR-6L | 6 | CM6-14-CM6-18 | qSSR-6L | 10.56 | 0.03 | 18.84 | |
| SSR | 1 | STS-CM1-31 | qSSR-1L | 7 | STS-CM7-2 | 9.57 | −0.09 | 19.60 | ||
| SSR | 1 | STS-CM1-31 | qSSR-1L | 10 | CM10-14-RM1375 | 8.54 | -0.02 | 17.91 | ||
| SSR | 2 | CM2-15-CM2-5 | qSSR-2.1L | 10 | CM10-14-RM1375 | 9.98 | −0.04 | 18.62 | ||
| SSR | 2 | CM2-15-CM2-5 | qSSR-2.1L | 11 | STS-CM11-5 | 7.37 | −0.01 | 18.44 | ||
| PSD | 2 | CM2-3-CM2-1 | 4 | CM4-27-RM349 | qPSD-4.1L | 5.08 | 2.01 | 11.82 | ||
| PSD | 3 | CM3-18-CM3-13 | 4 | CM4-27-RM349 | qPSD-4.1L | 5.72 | 2.49 | 14.00 | ||
| PSD | 4 | CM4-27-RM349 | qPSD-4.1L | 7 | RM11-RM1132 | 5.04 | 0.12 | 11.19 | ||
| PSD | 4 | CM4-27-RM349 | qPSD-4.1L | 8 | CM8-13-CM8-21 | 7.51 | −0.44 | 11.53 | ||
| F2:3 | PN | 2 | CM2-15-CM2-5 | 11 | CM11-5-CM11-37 | 5.90 | −0.25 | 30.52 | ||
| PN | 4 | CM4-19-CM4-21 | 12 | SSR-CM12-27 | 5.75 | −1.06 | 13.17 | |||
| PN | 4 | RM349-RM1113 | 7 | RM11-RM1132 | qPN-7F | 7.68 | -2.18 | 23.22 | ||
| PN | 7 | RM11-RM1132 | qPN-7F | 9 | CM9-7-CM9-10 | 6.54 | −0.93 | 24.74 | ||
| PN | 10 | CM10-14-RM1375 | 10 | RM5689-CM10-10 | 6.09 | 0.44 | 19.83 | |||
| PL | 9 | CM8-17-STS | 10 | CM10-9-CM10-7 | 5.72 | −1.31 | 8.76 | |||
| PL | 4 | CM4-25-CM4-27 | 7 | CM6-28-STS | 5.67 | −0.90 | 15.36 | |||
| PBN | 1 | STS-CM1-31 | 8 | RM5556-CM8-6 | 5.58 | −0.43 | 23.29 | |||
| PBN | 4 | CM4-19-CM4-21 | 7 | CM6-28-STS | 5.91 | 0.82 | 16.80 | |||
| PBN | 6 | CM6-14-CM6-18 | 8 | CM8-11-CM8-13 | 5.70 | 1.33 | 21.43 | |||
| SBN | 4 | CM4-27-RM349 | 8 | CM8-15-CM8-23 | 6.73 | −3.22 | 20.34 | |||
| SBN | 4 | CM4-15-CM4-34 | 9 | CM9-13-CM9-15 | 5.82 | 13.56 | 15.50 | |||
| GN | 2 | CM2-15-CM2-5 | 9 | CM9-9-CM9-7 | 5.96 | 1.26 | 28.02 | |||
| GN | 4 | RM3839-CM4-25 | qGFN-4.2F | 8 | CM8-15-CM8-23 | 5.56 | −0.82 | 21.04 | ||
| SSR | 2 | CM2-15-CM2-5 | 2 | CM2-5-CM2-3 | 5.90 | 0.12 | 21.43 | |||
| SSR | 2 | CM2-15-CM2-5 | 5 | CM5-19-RM3616 | 5.36 | 0.06 | 16.30 | |||
| SSR | 4 | CM4-25-CM4-27 | 5 | CM5-19-RM3616 | 5.30 | 0.07 | 10.67 | |||
| SSR | 2 | CM2-15-CM2-5 | 6 | CM6-14-CM6-18 | 7.59 | 0.13 | 30.75 | |||
| PSD | 2 | CM2-15-CM2-5 | 3 | CM3-8-CM3-23 | 5.12 | −0.63 | 25.82 | |||
| PSD | 1 | STS-CM1-31 | 4 | CM4-25-CM4-27 | qPSD-4.2F | 5.60 | −0.03 | 22.70 | ||
| PSD | 4 | CM4-25-CM4-27 | qPSD-4.2F | 9 | CM9-13-CM9-15 | 6.45 | −0.63 | 16.18 | ||
| QTLs | Traits | Chr. | Marker Interval | R2 (%) | R2byA (%) | R2byE (%) | AbyE01 | AbyE02 |
|---|---|---|---|---|---|---|---|---|
| qPN-7F | PN | 7 | RM1132-RM3555 | 5.88 | 1.63 | 4.25 | 0.23 | −0.23 |
| qPL-5F | PL | 5 | CM5-5-CM5-4 | 5.45 | 1.24 | 4.21 | 0.15 | −0.15 |
| qPL-7F | PL | 7 | CM7-6-RM11 | 12.24 | 12.04 | 0.20 | −0.10 | 0.10 |
| qPL-8L | PL | 8 | RM5556-CM8-6 | 3.21 | 2.52 | 0.69 | −0.17 | 0.17 |
| qPBN-4F | PBN | 4 | CM4-27-RM349 | 22.16 | 20.91 | 1.25 | −0.20 | 0.20 |
| qSBN-4.2F | SBN | 4 | CM4-25-CM4-27 | 6.25 | 5.46 | 0.79 | 0.62 | −0.62 |
| qSBN-7L | SBN | 7 | CM7-6-RM11 | 6.01 | 5.27 | 0.74 | −1.33 | 1.33 |
| qSBN-9L | SBN | 9 | CM9-15-CM9-14 | 4.03 | 2.23 | 1.80 | −2.11 | 2.11 |
| qSBN-12L | SBN | 12 | RM1337-RM101 | 5.20 | 4.90 | 0.30 | −0.42 | 0.42 |
| qGN-4L | GN | 4 | CM4-27-RM349 | 7.47 | 6.98 | 0.49 | −0.05 | 0.05 |
| qGN-7L | GN | 7 | CM7-6-RM11 | 5.56 | 5.41 | 0.15 | −2.09 | 2.09 |
| qFGN-7L | FGN | 7 | RM3755-CM7-6 | 3.41 | 1.45 | 1.95 | −10.03 | 10.03 |
| qSSR-5L | SSR | 5 | RM3616-CM5-17 | 2.25 | 1.14 | 1.12 | 0.00 | 0.00 |
| qPSD-4.2F | PSD | 4 | M45-M46 | 7.89 | 7.32 | 0.57 | 0.09 | −0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Wang, L.; Fan, G.; Long, Y.; Lu, X.; Wang, R.; Liu, H.; Qiu, X.; Zeng, D.; Li, Z. Genetic Dissection of Panicle Morphology Traits in Super High-Yield Hybrid Rice Chaoyou 1000. Plants 2024, 13, 179. https://doi.org/10.3390/plants13020179
Jiang J, Wang L, Fan G, Long Y, Lu X, Wang R, Liu H, Qiu X, Zeng D, Li Z. Genetic Dissection of Panicle Morphology Traits in Super High-Yield Hybrid Rice Chaoyou 1000. Plants. 2024; 13(2):179. https://doi.org/10.3390/plants13020179
Chicago/Turabian StyleJiang, Jing, Li Wang, Gucheng Fan, Yu Long, Xueli Lu, Run Wang, Haiyang Liu, Xianjin Qiu, Dali Zeng, and Zhixin Li. 2024. "Genetic Dissection of Panicle Morphology Traits in Super High-Yield Hybrid Rice Chaoyou 1000" Plants 13, no. 2: 179. https://doi.org/10.3390/plants13020179
APA StyleJiang, J., Wang, L., Fan, G., Long, Y., Lu, X., Wang, R., Liu, H., Qiu, X., Zeng, D., & Li, Z. (2024). Genetic Dissection of Panicle Morphology Traits in Super High-Yield Hybrid Rice Chaoyou 1000. Plants, 13(2), 179. https://doi.org/10.3390/plants13020179







