An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry
Abstract
1. Introduction
2. Results and Discussion
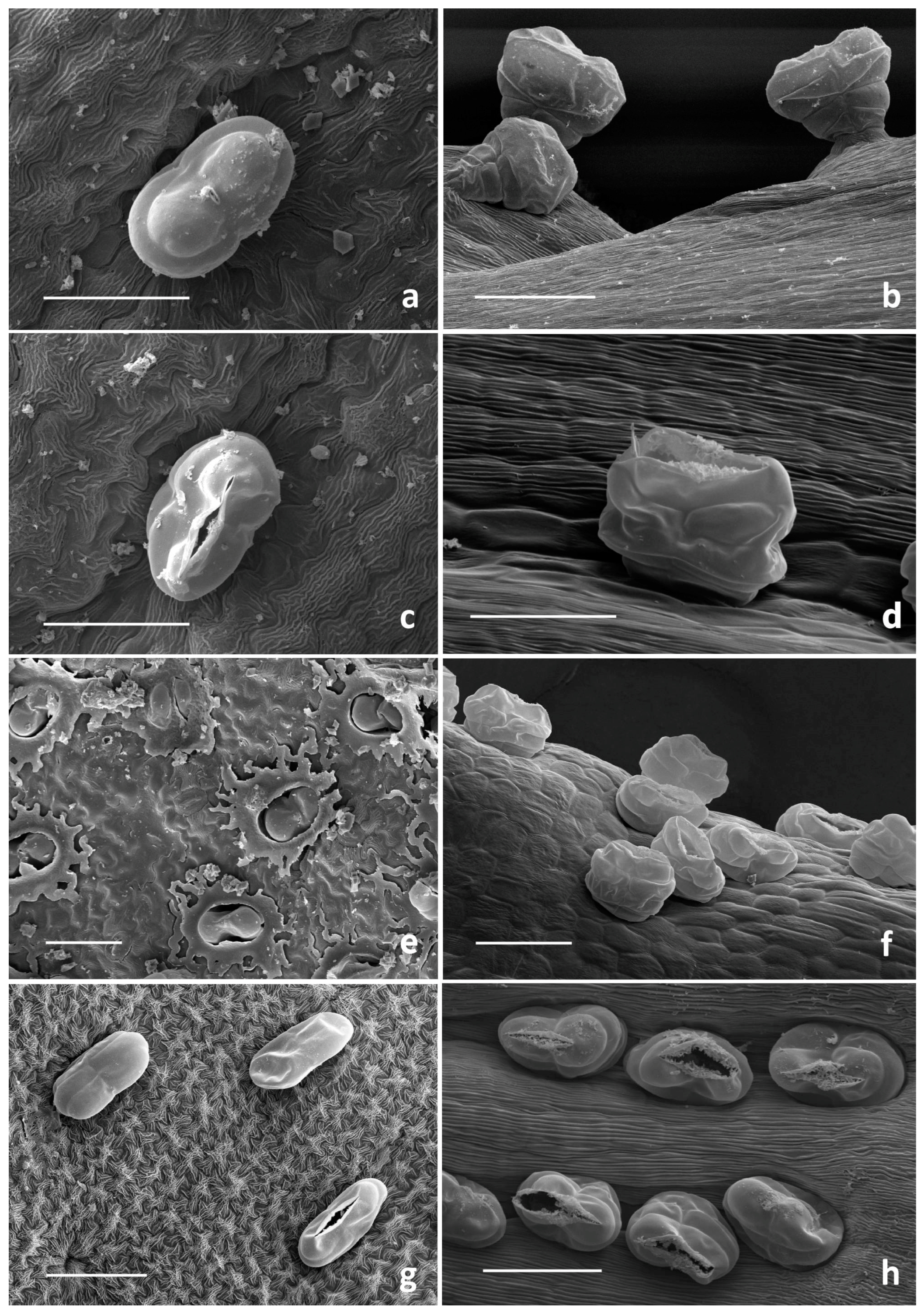
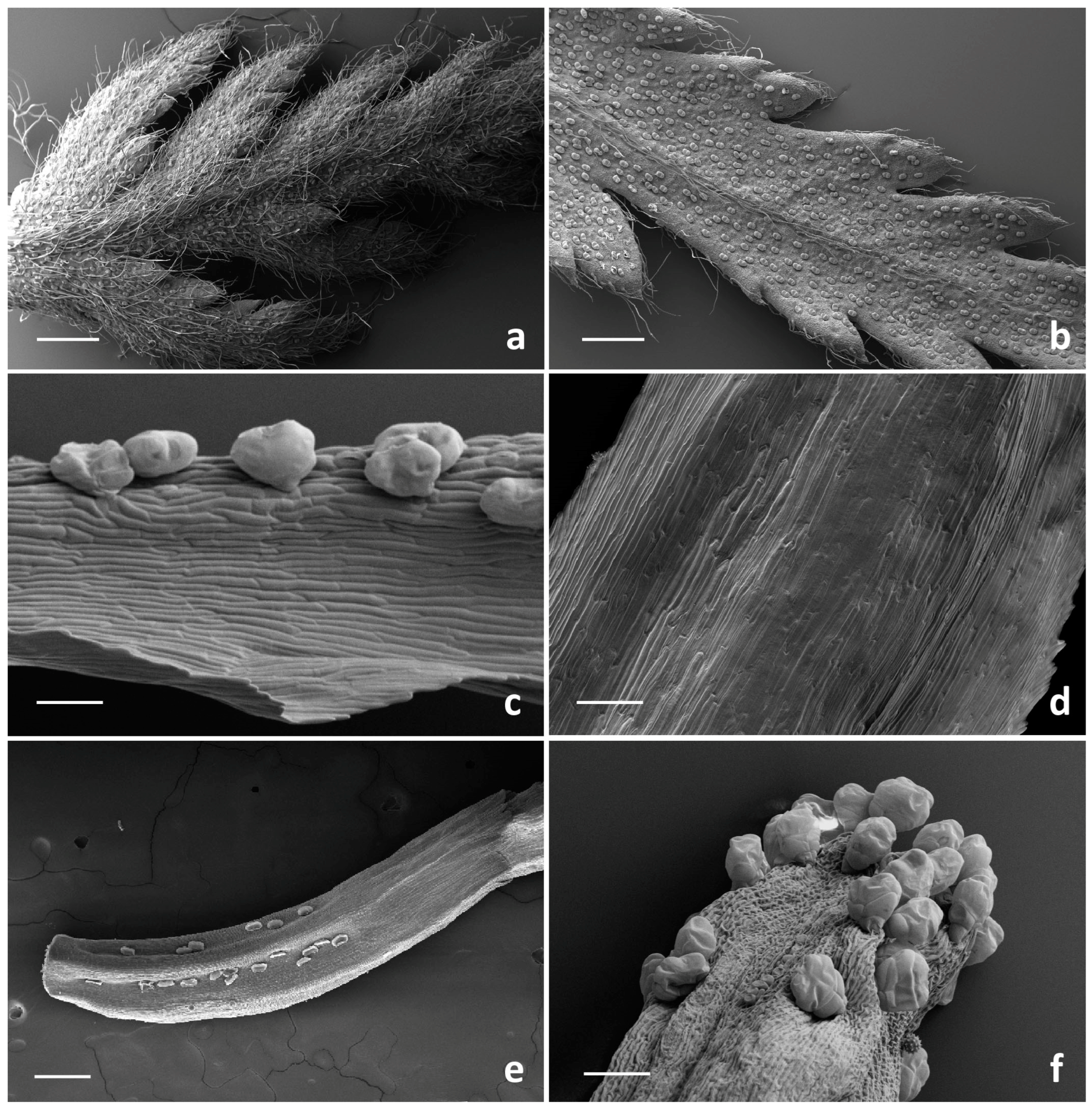
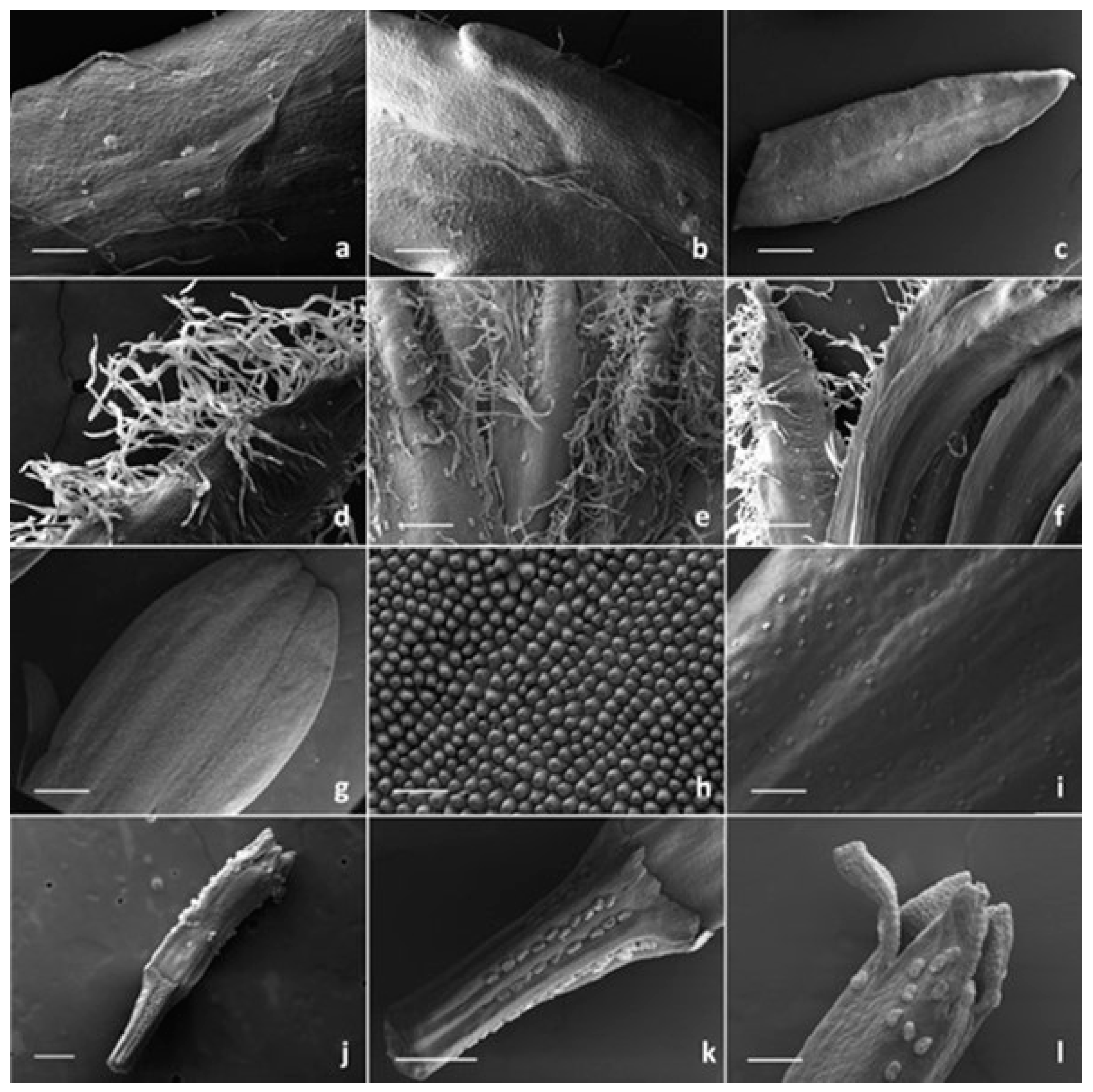
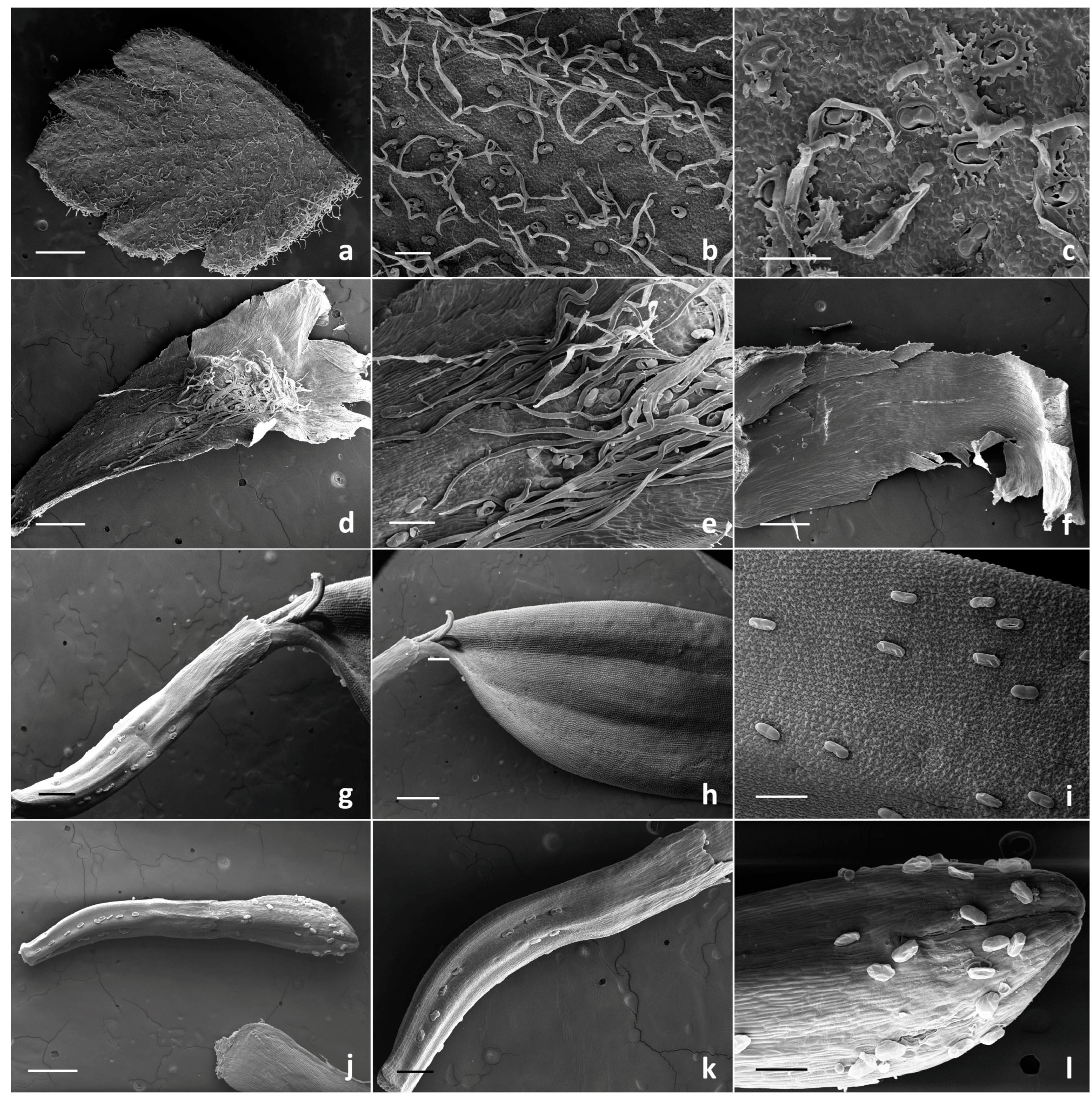
2.1. Micromorphological Investigation
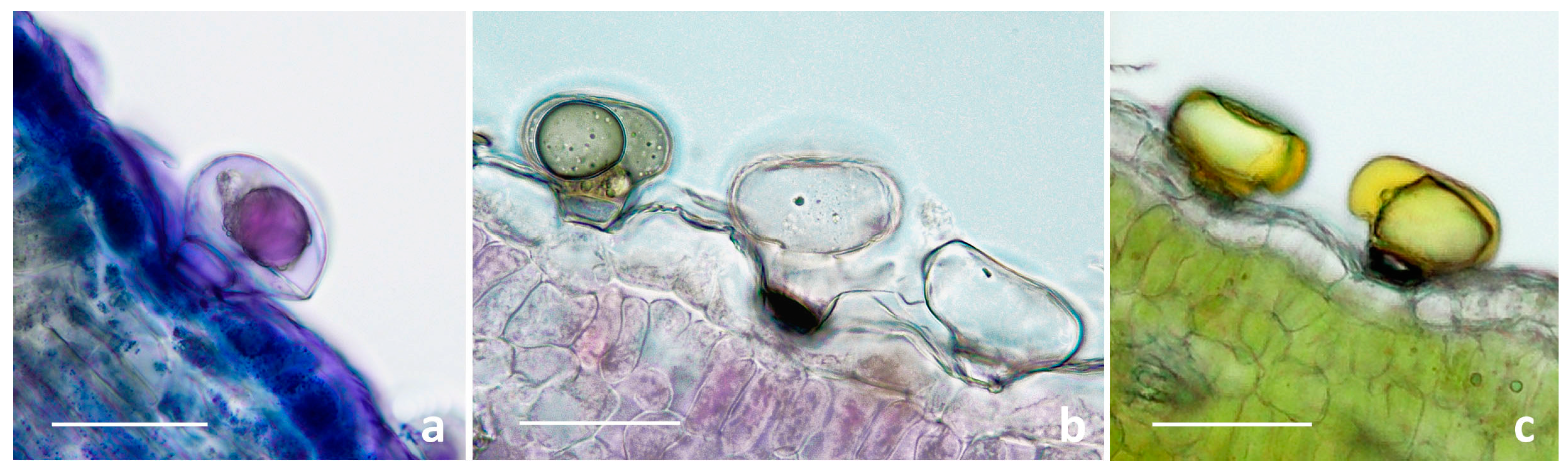
2.1.1. Trichome Morphotypes and Distribution Pattern
2.1.2. Histochemistry of the Biseriate Glands
2.2. Phytochemical Investigation
2.3. Scientific Dissemination
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Micromorphological Investigation
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Light Microscopy and Fluorescence Microscopy
3.4. Phytochemical Investigation
3.4.1. Preparation of Essential Oils (EOs)
3.4.2. GC-MS Analysis of Essential Oils
4. Conclusions
- (i).
- The combination of digital light microscopy and scanning electron microscopy enabled us to deepen the knowledge about the non-glandular and glandular indumenta on the vegetative and reproductive organs; specifically, our investigation proved a great affinity regarding the trichome morphotypes, their distribution pattern and density across the studied taxa, along with a similar histochemical profiles of the secretory products (mainly terpenes and flavonoids) of the glandular hairs; specifically, the overall information on Tanacetum corymbosum represented an element of novelty in the panorama of the current literature.
- (ii).
- The phytochemical characterization explained the variability of the EO profiles, when compared with the literature data. We addressed our work on the investigation of species cultivated at the same study site and collected in the same period with the special intent to make an internal comparison, limiting every single variable. The study revealed great complexity in the profile of the EOs obtained from the flowering aerial parts in the case of T. vulgare, compared to T. parthenium and T. corymbosum: the latter appeared to represent an established source of compounds such as camphor, farnesol, or α-santalone.
- (iii).
- The phytochemical diversity, particularly in relation to the major compounds present, may reflect the diversified use of these three species in relation to the interest versus a peculiar biological activity.
- (iv).
- Although the composition of the volatile organic fraction was not investigated, based on the literature data, we assumed that the main EO components exert ecological roles related to a dominant defense action in all the studied species. Specifically, they include repulsive agents against plant pathogens such as microbes, beetles, and mites.
- (v).
- The scientific results were channeled into the creation of an original interpretative apparatus for the target species at the Ghirardi Botanic Garden, giving rise to a direct exchange of information with the public, immediately following the research, thus emphasizing the concept of Open Science policy.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Funk, V.A.; Susanna, A.; Stuessy, T.F.; Bayer, R.J. Systematics, Evolution, and Biogeography of Compositae; IAPT: Vienna, Austria, 2009. [Google Scholar]
- Sonboli, A.; Stroka, K.; Kazempour Osaloo, S.; Oberprieler, C. Molecular phylogeny and taxonomy of Tanacetum L. (Compositae, Anthemideae) inferred from nrDNA ITS and cpDNA trnH–psbA sequence variation. Plant Syst. Evol. 2012, 298, 431–444. [Google Scholar] [CrossRef]
- Oberprieler, C.; Töpfer, A.; Dorfner, M.; Stock, M.; Vogt, R. An updated subtribal classification of Compositae tribe Anthemideae based on extended phylogenetic reconstructions. Willdenowia 2022, 52, 117–149. [Google Scholar] [CrossRef]
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot. Rev. 1982, 48, 121–594. [Google Scholar] [CrossRef]
- Da Costa, F.B.; Terfloth, L.; Gasteiger, J. Sesquiterpene lactone-based classification of three Asteraceae tribes: A study based on self-organizing neural networks applied to chemosystematics. Phytochemistry 2005, 66, 345–353. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Brant, A.J.; Alvarenga, S.A.; Emerenciano, V.P. Neural networks in chemosystematic studies of Asteraceae: A classification based on a dichotomic approach. Chem. Biodiv. 2005, 2, 633–644. [Google Scholar] [CrossRef]
- Shulha, O.; Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae revisited: An update (2008–2017). Phytochemistry 2019, 163, 149–177. [Google Scholar] [CrossRef]
- Harborne, J.B.; Heywood, V.H.; Saleh, N.A.M. Chemosystematics of the Compositae: Flavonoid patterns in the Chrysanthemum complex of the tribe Anthemideae. Phytochemistry 1970, 9, 2011–2017. [Google Scholar] [CrossRef]
- Abad, M.J.; Bermejo, P.; Villar, A. An approach to the genus Tanacetum L. (Compositae): Phytochemical and pharmacological review. Phytother. Res. 1995, 9, 79–92. [Google Scholar] [CrossRef]
- Hordiei, K.; Gontova, T.; Gaponenko, V. Prospects of studying of volatile oils of Tanacetum parthenium (L.) Schultz Bip. For issues in chemosystematics of Tanacetum genus. EUREKA Health Sci. 2020, 6, 102–107. [Google Scholar] [CrossRef]
- Gören, N.; Arda, N.; Caliskan, Z. Chemical characterization and biological activities of the genus Tanacetum (Compositae). Stud. Nat. Prod. Chem. 2002, 27, 547–658. [Google Scholar]
- Özbilgin, S.; Akkol, E.K.; Ergene Öz, B.; Ilhan, M.; Saltan, G.; Bahadir Acıkara, Ö.; Tekin, M.; Keleş, H.; Süntar, I. In vivo activity assessment of some Tanacetum species used as traditional wound healer along with identification of the phytochemical profile by a new validated HPLC method. Iran. J. Basic Med. Sci. 2018, 21, 145–152. [Google Scholar]
- Baczek, K.B.; Kosakowska, O.; Przybyl, J.L.; Pioro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Weglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Kumar, V.; Tyagi, D. Chemical composition and biological activities of essential oils of genus Tanacetum—A review. J. Pharmacogn. Phytochem. 2013, 2, 159–163. [Google Scholar]
- Bașer, K.H.C.; Demirci, B.; Tabanca, N.; Özek, T.; Gören, N. Composition of the essential oils of Tanacetum armenium (DC.) Schultz Bip., T. balsamita L., T. chiliophyllum (Fisch & Mey) Schultz Bip. var. chiliophyllum and T. haradjani (Rech. Fil.) Grierson and the enantiomeric distribution of camphor and carvone. Flav. Fragr. J. 2001, 16, 195–200. [Google Scholar]
- Sanz, J.F.; Marco, J.A. NMR studies of Tatridin A and some related sesquiterpene lactones from Tanacetum vulgare. J. Nat. Prod. 1991, 54, 591–596. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, Y. Chemical composition and antibacterial activity of essential oils of Tanacetum longifolium. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 836–841. [Google Scholar] [CrossRef]
- Todorova, M.N.; Evstatieva, L.N. Comparative study of Tanacetum species growing in Bulgaria. Z. Naturforsch. C J. Biosci. 2001, 56, 506–512. [Google Scholar] [CrossRef]
- Long, C.; Sauleau, P.; David, B.; Lavaud, C.; Cassabois, V.; Ausseil, F.; Massiot, G. Bioactive flavonoids of Tanacetum parthenium revisited. Phytochemistry 2003, 64, 567–569. [Google Scholar] [CrossRef]
- Mantle, D.; Eddeb, F.; Pickering, A.T. Comparison of relative antioxidant activities of British medicinal plant species in vitro. J. Ethnopharmacol. 2000, 72, 47–51. [Google Scholar] [CrossRef]
- Coté, H.; Boucher, M.A.; Pichette, A.; Legault, J. Anti-inflammatory, antioxidant, antibiotic and cytotoxic activities of Tanacetum vulgare L. essential oil and its constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef]
- Ivănescu, B.; Tuchiluș, C.; Cociorvă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.-M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Schinella, G.R.; Giner, R.M.; Del Carmen Recio, M.; Mordujovich de Buschiazzo, P.; Rios, J.L.; Manez, S. Anti-inflammatory effects of South American Tanacetum vulgare. J. Pharm. Pharmacol. 1998, 50, 1069–1074. [Google Scholar] [CrossRef]
- Bagci, E.; Kursat, M.; Kosak, A.; Gur, S. Composition and antimicrobial activity of the essential oils of Tanacetum balsamita L. subsp. balsamita and T. chiliophyllum (Fisch. et Mey.) Schultz Bip. var. chiliophyllum (Asteraceae) from Turkey. J. Essent. Oil-Bear. Plants 2008, 11, 476–484. [Google Scholar] [CrossRef]
- Masoudi, S.; Abbassi, J. Antibacterial activity and comparison of the volatile oils of Tanacetum tenuisectum (Boiss.) Podl. obtained by three different methods of extraction. Iran. J. Pharm. Res. 2017, 16, 188–196. [Google Scholar]
- Susurluk, H.; Çalișkan, Z.; Gürkan, O.; Kirmizigül, S.; Gören, N. Antifeedant activity of some Tanacetum species and bioassay guided isolation of the secondary metabolites of Tanacetum cadmeum ssp. cadmeum (Compositae). Ind. Crops Prod. 2007, 26, 220–228. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Milano, Italy, 2017; Volume 2. [Google Scholar]
- Douglas, E.; Soltis, P.S.; Soltis, J.J.; Doyle, J. Molecular Systematics of Plants 2; Kluwe Academic Publisher: Hingham, MA, USA, 1998; Volume 2. [Google Scholar]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Maggi, F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.). Ind. Crops Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Simmons, C.B.; Raj, S.K.; Saxena, P.K. Morphocytological characterization of feverfew, Tanacetum parthenium (L.) Schultz Bip. J. Herbs Spices Med. Plants 2002, 9, 29–45. [Google Scholar] [CrossRef]
- Majdi, M.; Liu, Q.; Karimzadeh, G.; Malboobi, M.A.; Beekwilder, J.; Cankar, K.; de Vos, R.; Todorović, S.; Simonović, A.; Bouwmeester, H. Biosynthesis and localization of parthenolide in glandular trichomes of feverfew (Tanacetum parthenium L. Schulz. Phytochemistry 2011, 72, 1739–1750. [Google Scholar] [CrossRef]
- Dere, S.; Akcin, T.A. Anatomical and micromorphological properties of some Tanacetum L. (Asteraceae) taxa from Turkey and their systematic implications. Acta Bot. Croat. 2017, 76, 138–145. [Google Scholar] [CrossRef]
- Devrnja, N.; Krstić-Milošević, D.; Janošević, D.; Tešević, V.; Vinterhalter, B.; Savić, J.; Ćalić, D. In vitro cultivation of tansy (Tanacetum vulgare L.): A tool for the production of potent pharmaceutical agents. Protoplasma 2021, 258, 587–599. [Google Scholar] [CrossRef]
- Sharma, G.; Ali, M.I.; Moin, S. The genus Tanacetum: A comprehensive review. Int. J. Pharm. Qual. Assur. 2022, 13, 208–213. [Google Scholar]
- Keskitalo, M.; Pehu, E.; Simon, J.E. Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem. Syst. Ecol. 2001, 29, 267–285. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)-A wild-growing aromatic medicinal plant with a variable essential oil composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Aćimović, M.; Puvača, N. Tanacetum vulgare L.—A Systematic Review. J. Agron. Technol. Eng. Manag. 2020, 3, 416–422. [Google Scholar]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn. Rev. 2011, 5, 103. [Google Scholar] [CrossRef]
- Shahhoseini, R.; Azizi, M.; Asili, J.; Moshtaghi, N.; Samiei, L. Comprehensive assessment of phytochemical potential of Tanacetum parthenium (L.): Phenolic compounds, antioxidant activity, essential oil and parthenolide. J. Essent. Oil-Bear. Plants 2019, 22, 614–629. [Google Scholar] [CrossRef]
- Mohsenzadeh, F.; Chehregani, A.; Amiri, H. Chemical composition, antibacterial activity and cytotoxicity of essential oils of Tanacetum parthenium in different developmental stages. Pharm. Biol. 2011, 49, 920–926. [Google Scholar] [CrossRef]
- Polatoglu, K.; Demirci, F.; Demirci, B.; Gören, N.; Baser, K.H.C. Antibacterial activity and the variation of Tanacetum parthenium (L.) Schultz Bip. essential oils from Turkey. J. Oleo Sci. 2010, 59, 177–184. [Google Scholar] [CrossRef]
- Pavela, R.; Sajfrtová, M.; Sovová, H.; Bárnet, M.; Karban, J. The insecticidal activity of Tanacetum parthenium (L.) Schultz Bip. extracts obtained by supercritical fluid extraction and hydrodistillation. Ind. Crops Prod. 2010, 31, 449–454. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lupaşcu, L.; Aricu, A.; Dragalin, I.; Ciocarlan, N.; Zinicovscaia, I.; Slănină, V.; Yushin, N. Chemical composition of the essential oil and antimicrobial properties of crude extract from Tanacetum corymbosum (L.) Shi. Bip. Chem. J. Moldova 2021, 16, 83–90. [Google Scholar] [CrossRef]
- Bottoni, M.; Baron, G.; Gado, F.; Milani, F.; Santagostini, L.; Colombo, L.; Colombo, P.S.; Caporali, E.; Spada, A.; Biagi, M.; et al. Achillea moschata Wulfen: From ethnobotany to phytochemistry, morphology, and biological activity. Molecules 2022, 27, 8318. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Garbari, F.; Pagni, A.M. Glandular hairs of the ovary: A helpful character for Asteroideae (Asteraceae) taxonomy? Ann. Bot. Fenn. 2007, 44, 1–7. [Google Scholar]
- Adedeji, O. Leaf epidermal studies of the species of Emilia Cass. (Senecioneae, Asteraceae) in Nigeria. Bot. Lith. 2004, 10, 121–133. [Google Scholar]
- Raal, A.; Orav, A.; Gretchushnikova, T. Essential oil content and composition in Tanacetum vulgare L. herbs growing wild in Estonia. J. Essent. Oil-Bear. Plants 2014, 17, 670–675. [Google Scholar] [CrossRef]
- Rohloff, J.; Mordal, R.; Dragland, S. Chemotypical variation of tansy (Tanacetum vulgare L.) from 40 different locations in Norway. J. Agric. Food Chem. 2004, 52, 1742–1748. [Google Scholar] [CrossRef]
- Judzentiene, A.; Mockute, D. The inflorescence and leaf oils of Tanacetum vulgare L. var. vulgare growing wild in Lithuania. Biochem. Syst. Ecol. 2005, 33, 487–498. [Google Scholar] [CrossRef]
- Mockute, D.; Judzentiene, A. The myrtenol chemotype of essential oil of Tanacetum vulgare L. vulgare (tansy) growing wild in the Vilnius region. Chemija 2005, 14, 103–107. [Google Scholar]
- Formisano, C.; Senatore, F.; Bruno, M.; Rosselli, S.; Bellone, G.; Spadaro, V. Essential oil composition of Tanacetum vulgare subsp. siculum (Guss.) Raimondo et Spadaro (Asteraceae) from Sicily. Nat. Prod. Commun. 2009, 4, 1934578X0900400425. [Google Scholar] [CrossRef]
- Lechkova, B.; Karcheva-Bahchevanska, D.; Ivanov, K.; Todorova, V.; Benbassat, N.; Penkova, N.; Atanassova, P.; Peychev, L.; Hrischev, P.; Peychev, Z.; et al. A Study of the chemical composition, acute and subacute toxicity of Bulgarian Tanacetum parthenium essential oil. Molecules 2023, 28, 4906. [Google Scholar] [CrossRef]
- Liu, P.; Deng, B.; Long, C.A.; Min, X. Effect of farnesol on morphogenesis in the fungal pathogen Penicillium expansum. Ann. Micr. 2009, 59, 33–38. [Google Scholar] [CrossRef]
- Thomas, O.O. Chemical taxonomy and chemosystematics of the genus Tanacetum. Part 2. Phytochemistry of the leaf and flower oil of Tanacetum corymbosum. Fitoterapia 1989, 60, 225–228. [Google Scholar]
- Chauvin, G.; Vannier, G. Insecticidal properties of paradichlorobenzene and camphor—Effects on behaviour, transpiration and heat-resistance—A preliminary study on Tineola bisselliella Hum (Lepidoptera, Tineidae). Acta Oecol. Int. J. Ecol. 1994, 15, 23–29. [Google Scholar]
- Hada, T.; Shiraishi, A.; Furuse, S.; Inoue, Y.; Hamashima, H.; Matsumoto, Y.; Masuda, K.; Shiojima, K.; Shimada, J. Inhibitory effects of terpenes on the growth of Staphylococcus aureus. Nat. Med. 2003, 57, 64–67. [Google Scholar]
- Jung, Y.Y.; Hwang, S.T.; Sethi, G.; Fan, L.; Arfuso, F.; Ahn, K.S. Potential Anti-Inflammatory and Anti-Cancer Properties of Farnesol. Molecules 2018, 23, 2827. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon—A review. Molecules 2013, 18, 5434. [Google Scholar] [CrossRef]
- Kong, W.B.; Huo, H.R.; Gu, Y.; Cao, Y.Q.; Wang, J.L.; Liang, J.Y.; Niu, S.Q. Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Obeng-Ofori, D.; Reichmuth, C.H.; Bekele, A.J.; Hassanali, A. Toxicity and protectant potential of camphor a major component of essential oil of Ocimum kilimandscharicum against four stored product beetles. Int. J. Pest Manag. 1998, 44, 203–209. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, D.; Sundaresan, S.; Iyadurai, A.; Subramanian, K.S.; Janavi, G.J.; Paliyath, G.; Subramanian, J. Hexanal vapor induced resistance against major postharvest pathogens of banana (Musa acuminata L.). Plant Pathol J. 2020, 36, 133–147. [Google Scholar] [CrossRef]
- Sholberg, P.; Randall, P. Hexanal vapor for postharvest decay control and aroma production in stored pome fruit. Phytopathology 2005, 95, S96. [Google Scholar]
- Cheema, A.; Padmanabhan, P.; Subramanian, J.; Blom, T.; Paliyath, G. Improving quality of greenhouse tomato (Solanum lycopersicum L.) by pre- and postharvest applications of hexanal-containing formulations. Postharvest Biol. Technol. 2014, 95, 13–19. [Google Scholar] [CrossRef]
- Yumbya, P.; Ambuko, J.; Hutchinson, M.; Owino, W.; Juma, J.; Machuka, E.; Mutuku, J.M. Transcriptome analysis to elucidate hexanal’s mode of action in preserving the postharvest shelf life and quality of banana fruits (Musa acuminata). J. Agric. Food Res. 2021, 3, 100114. [Google Scholar]
- Francke, W.; Schulz, S. Pheromones of Terrestrial Invertebrates—Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier Pubs.: Amsterdam, The Netherlands, 2010; pp. 153–223. [Google Scholar]
- U.S. Environmental Protection Agency, Office of Pesticide Programs, Biopesticides and Pollution Prevention Division. Farnesol and Nerolidol; Biopesticides Registration Action Document; U.S. Environmental Protection Agency, Office of Pesticide Programs, Biopesticides and Pollution Prevention Division: Washington, DC, USA, 2009.
- Vukovic, N.; Milosevic, T.; Sukdolak, S.; Solujic, S. Antimicrobial Activities of Essential Oil and Methanol Extract of Teucrium montanum. Evid. Based Complement. Alternat. Med. 2007, 4, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]


| Trichomes Type | Species | Leaf | Involucral Bract | Ligulate Floret | Tubular Floret | ||||
|---|---|---|---|---|---|---|---|---|---|
| adax. | abax. | adax. | abax. | ovary | corolla | ovary | corolla | ||
| Flagellar non-glandular | T. vulgare | ++ | +++ | − | − | − | − | ||
| T. parthenium | ± | + | − | ++ | − | − | − | − | |
| T. corymbosum | + | ++ | − | ++ | − | − | − | − | |
| Ten-celled biseriateglandular | T. parthenium | + | + | − | ++ | ++ | ++ | +++ | ++ |
| T. vulgare | ++ | +++ | − | ++ | ++ | ++ | |||
| T. corymbosum | ++ | +++ | − | ++ | ++ | ++ | ++ | ++ | |
| Stains | Target Compounds | T. vulgare | T. parthenium | T. corymbosum |
|---|---|---|---|---|
| Fluoral Yellow-088 | Total lipids | ++ | + | + |
| Nile Red | Neutral lipids | + | + | + |
| Nadi reagent | Terpenoids | ++ | ++ | ++ |
| PAS reagent | Total polysaccharides | − | + | − |
| Ruthenium Red | Acid polysaccharides | − | ± | − |
| Alcian Blue | Muco-polysaccharides | − | − | ± |
| FeCl3 | Polyphenols | ++ | ++ | ++ |
| AlCl3 | Flavonoids | + | + | + |
| Naturstoff reagent A | Flavonols | + | + | + |
| Relative Abundance (%) | |||||||
|---|---|---|---|---|---|---|---|
| No. | LRIa | LRIb | Type | Compound | Tv | Tp | Tc |
| 1 | 769 | 776 | NH | hexanal | 0.59 | 0.55 | 11.00 |
| 2 | 821 | 821 | NH | 2-hexyn-1-ol | - | 0.21 | - |
| 3 | 843 | 835 | NH | 2-hexenal | 0.25 | 0.41 | 4.57 |
| 4 | 863 | 854 | MH | 1-hexanol | - | 0.27 | - |
| 5 | 869 | 868 | NH | 1,6-dimethylcyclohexene | 0.26 | - | - |
| 6 | 897 | 902 | MH | santolina triene | 2.48 | - | - |
| 7 | 922 | 926 | NH | 2,5,5-trimethyl-1,3,6-heptatriene | 0.82 | - | - |
| 8 | 926 | 925 | MH | 3-thujene | 0.36 | - | - |
| 9 | 937 | 933 | MH | α-pinene | 1.11 | - | - |
| 10 | 955 | 946 | MH | camphene | 1.95 | - | - |
| 11 | 981 | 973 | MH | β-pinene | 0.82 | - | - |
| 12 | 991 | 1017 | MO | myroxide | 1.57 | - | - |
| 13 | 1004 | 1000 | MH | 2,5,5-trimethyl-3,6-heptadien-2-ol | 0.43 | - | 0.47 |
| 14 | 1037 | 1022 | MO | 1,8-cineole | 1.35 | 0.13 | 1.49 |
| 15 | 1037 | 1025 | NH | p-cymene | 3.37 | - | - |
| 16 | 1062 | 1072 | NH | hotrienol | 2.51 | - | - |
| 17 | 1067 | 1060 | MH | γ-terpinene | 0.29 | 0.31 | - |
| 18 | 1075 | 1070 | MH | cis-sabinene hydrate | 2.38 | 0.53 | - |
| 19 | 1076 | 1072 | MO | artemisia alcohol | - | - | 0.66 |
| 20 | 1079 | 1079 | MH | terpinolene | 0.18 | - | - |
| 21 | 1086 | 1086 | MO | linalool | 1.53 | - | - |
| 22 | 1091 | 1074 | MH | p-cymenene | - | - | 1.54 |
| 23 | 1103 | 1083 | NH | nonanal | 0.97 | 0.15 | - |
| 24 | 1106 | 1108 | NH | 2,2,6-trimethyl-3-keto-6-vinyltetrahydropyran | - | 0.61 | - |
| 25 | 1107 | 1167 | NH | 2-nonen-1-ol | 0.65 | 0.72 | - |
| 26 | 1115 | 1207 | MO | carveol | 2.05 | - | - |
| 27 | 1128 | 1105 | MO | fenchol | 0.46 | 0.27 | - |
| 28 | 1132 | 1126 | MO | p-menth-2-en-1-ol | 1.79 | 0.33 | - |
| 29 | 1156 | 1146 | MO | camphor | 1.80 | 56.83 | 49.36 |
| 30 | 1170 | 1181 | MO | myrtenol | 0.97 | - | - |
| 31 | 1146 | 1164 | MO | α-pinocarvone | - | 0.22 | - |
| 32 | 1150 | 1180 | MO | isopinocarveol | - | 0.93 | 0.30 |
| 33 | 1182 | 1167 | MO | endo-borneol | 2.23 | 1.11 | - |
| 34 | 1189 | 1182 | MO | terpinen-4-ol | 0.66 | 0.78 | - |
| 35 | 1205 | 1171 | MO | myrtenal | 1.92 | - | - |
| 36 | 1215 | 1192 | MO | (Z)-piperitol | 0.18 | - | - |
| 37 | 1233 | 1237 | MO | cis-geraniol | - | 0.90 | - |
| 38 | 1238 | 1305 | MO | myrtenyl acetate | 1.81 | - | - |
| 39 | 1246 | 1260 | MO | lyratyl acetate | 0.30 | - | - |
| 40 | 1265 | 1261 | MO | 6-isopropyl-3-methyl-7-oxabicyclo [4.1.0]-heptan-2-one | 1.05 | - | 1.20 |
| 41 | 1274 | 1250 | MO | geranyl vinyl ether | 0.32 | - | - |
| 42 | 1281 | 1272 | MO | p-mentha-1,8-dien-3-one | 0.12 | - | - |
| 43 | 1288 | 1285 | MO | bornyl acetate | 1.28 | - | - |
| 44 | 1289 | 1287 | NH | safrole | - | 0.25 | - |
| 45 | 1307 | 1331 | NH | silphiperfol-5-ene | - | - | 0.74 |
| 46 | 1319 | 1350 | MO | α-terpinyl acetate | 0.27 | - | - |
| 47 | 1353 | 1398 | MO | β-elemene | 1.06 | - | - |
| 48 | 1363 | 1399 | SH | cyperene | 3.22 | - | - |
| 49 | 1378 | 1376 | SH | α-copaene | 0.22 | - | - |
| 50 | 1392 | 1398 | SH | ciclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl) | 2.13 | - | - |
| 51 | 1426 | 1419 | SH | caryophyllene | 1.99 | - | - |
| 52 | 1462 | 1496 | SH | cis-α-bisabolene | 0.53 | - | - |
| 53 | 1470 | 1510 | SO | trans-verbenyl isovalerate | - | - | 0.84 |
| 54 | 1482 | 1483 | SH | α-curcumene | 0.95 | - | - |
| 55 | 1496 | 1499 | SH | eremophilia-1(10),11-diene | 1.62 | - | - |
| 56 | 1502 | 1405 | SH | longifolene | 0.55 | - | - |
| 57 | 1516 | 1433 | SH | γ-elemene | 0.61 | - | 0.79 |
| 58 | 1523 | 1524 | SH | δ-cadinene | 0.63 | - | - |
| 59 | 1539 | 1532 | SO | cubenene | 0.11 | - | - |
| 60 | 1546 | 1576 | SO | spathulenol | 0.45 | - | - |
| 61 | 1549 | 1542 | SH | α-calacorene | 4.92 | 0.27 | - |
| 62 | 1551 | 1581 | SO | caryophyllene oxide | - | - | - |
| 63 | 1570 | 1572 | SO | 8-acetoxycarvo-tanacetone | 2.08 | - | - |
| 64 | 1579 | 1586 | SO | ledol | 2.05 | - | - |
| 65 | 1595 | 1632 | SO | longiverbenone | 2.00 | 0.44 | - |
| 66 | 1622 | 1606 | SO | humulene epoxide | 0.29 | - | - |
| 67 | 1642 | 1637 | SO | caryophylladienol | 5.92 | 2.19 | - |
| 68 | 1654 | 1638 | SO | isospathulenol | 0.86 | - | - |
| 69 | 1665 | 1672 | SO | aromadendrene oxide (I) | 1.30 | - | - |
| 70 | 1672 | 1681 | SO | α-santalol | 0.79 | 0.98 | 0.50 |
| 71 | 1688 | 1694 | SO | β-santalol | 1.36 | - | - |
| 72 | 1707 | 1693 | SO | germacra-3,7(11),9-trien-6-one | 0.44 | - | - |
| 73 | 1716 | 1678 | SO | aromadendrene oxide (II) | 1.43 | - | - |
| 74 | 1757 | 1713 | SO | farnesol | 5.92 | 28.83 | - |
| 75 | 1783 | 1778 | SO | costol | 1.15 | 0.58 | 0.80 |
| 76 | 1788 | 1701 | SO | shyobunol | - | - | 1.92 |
| 77 | 1790 | 1724 | SO | thujopsenal | - | - | 2.23 |
| 78 | 1795 | 1695 | SO | 7-isopropyl-4,10-dimethylenecyclodec-5-enol | 1.39 | - | - |
| 79 | 1822 | 1763 | SO | cis-lanceol | 0.36 | 0.43 | - |
| 80 | 1838 | 1777 | SO | 15-hydroxy-α-muurolene | 0.34 | - | - |
| 81 | 1853 | 1844 | SO | hexahydrofarnesyl acetone | 1.89 | - | - |
| 82 | 1869 | 1867 | SO | α-santalone | - | - | 21.58 |
| Oil Yield (%) | 0.35 | 0.09 | 0.05 | ||||
| Total Identified | 89.67 | 100.00 | 100.00 | ||||
| Non-terpenic derivatives (NH) | 9.42 | 3.18 | 16.32 | ||||
| Monoterpene hydrocarbons (MH) | 10.02 | 1.32 | 2.01 | ||||
| Oxygenated monoterpenes (MO) | 22.70 | 61.51 | 53.01 | ||||
| Sesquiterpene hydrocarbons (SH) | 17.38 | 0.27 | 0.79 | ||||
| Oxygenated sesquiterpenes (SO) | 30.15 | 33.72 | 27.88 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, C.; Bottoni, M.; Milani, F.; Spada, A.; Falsini, S.; Papini, A.; Santagostini, L.; Fico, G. An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry. Plants 2024, 13, 155. https://doi.org/10.3390/plants13020155
Giuliani C, Bottoni M, Milani F, Spada A, Falsini S, Papini A, Santagostini L, Fico G. An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry. Plants. 2024; 13(2):155. https://doi.org/10.3390/plants13020155
Chicago/Turabian StyleGiuliani, Claudia, Martina Bottoni, Fabrizia Milani, Alberto Spada, Sara Falsini, Alessio Papini, Laura Santagostini, and Gelsomina Fico. 2024. "An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry" Plants 13, no. 2: 155. https://doi.org/10.3390/plants13020155
APA StyleGiuliani, C., Bottoni, M., Milani, F., Spada, A., Falsini, S., Papini, A., Santagostini, L., & Fico, G. (2024). An Integrative Approach to Selected Species of Tanacetum L. (Asteraceae): Insights into Morphology and Phytochemistry. Plants, 13(2), 155. https://doi.org/10.3390/plants13020155







