Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Extraction Based on Total Phenolic Compounds and Antioxidant Activity
2.1.1. Influence of the Extraction Parameters on TPCs and TEAC
2.1.2. Optimization and Validation of Extraction Parameters
2.2. Chemical Characterization of Araticum Peel Extract Obtained under Optimized Conditions
2.2.1. Determination of TPCs, CTs, and Antioxidant Activity
2.2.2. Identification and Quantification of Phenolic Compounds by HPLC-DAD
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Ultrasound-Assisted Extraction
3.4. Experimental Design
3.5. Determination of Total Phenolic Compounds (TPCs)
3.6. Determination of Condensed Tannins (CTs) Content
3.7. Antioxidant Activity
3.7.1. Ferric Reducing Antioxidant Power (FRAP) Assay
3.7.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.7.3. Peroxyl Radical (ROO•) Scavenging Activity
3.7.4. Hydroxyl Radical (•OH) Scavenging Activity
3.7.5. Hypochlorous Acid (HOCl) Scavenging Activity
3.7.6. Superoxide Radical (•O2−) Scavenging Activity
3.8. HPLC-DAD Analysis of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prado, L.G.; Arruda, H.S.; Peixoto Araujo, N.M.; de Oliveira Braga, L.E.; Banzato, T.P.; Pereira, G.A.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Eberlin, M.N.; de Carvalho, J.E.; et al. Antioxidant, Antiproliferative and Healing Properties of Araticum (Annona crassiflora Mart.) Peel and Seed. Food Res. Int. 2020, 133, 109168. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Araújo, M.V.L.; Marostica Junior, M.R. Underexploited Brazilian Cerrado Fruits as Sources of Phenolic Compounds for Diseases Management: A Review. Food Chem. Mol. Sci. 2022, 5, 100148. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Angolini, C.F.F.; Eberlin, M.N.; Pastore, G.M.; Marostica Junior, M.R. UHPLC-ESI-QTOF-MS/MS Profiling of Phytochemicals from Araticum Fruit (Annona crassiflora Mart.) and Its Antioxidant Activity. Foods 2023, 12, 3456. [Google Scholar] [CrossRef]
- Ramos, A.L.C.C.; Silva, M.R.; Mendonça, H.d.O.P.; Mazzinghy, A.C.d.C.; Silva, V.D.M.; Botelho, B.G.; Augusti, R.; Ferreira, R.M.d.S.B.; de Sousa, I.M.N.; Batista-Santos, P.; et al. Use of Pulp, Peel, and Seed of Annona crassiflora Mart. in Elaborating Extracts for Fingerprint Analysis Using Paper Spray Mass Spectrometry. Food Res. Int. 2022, 160, 111687. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of Free, Esterified, Glycosylated and Insoluble-Bound Phenolics Composition in the Edible Part of Araticum Fruit (Annona crassiflora Mart.) and Its by-Products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pastore, G.M. Araticum (Annona crassiflora Mart.) as a Source of Nutrients and Bioactive Compounds for Food and Non-Food Purposes: A Comprehensive Review. Food Res. Int. 2019, 123, 450–480. [Google Scholar] [CrossRef]
- Almeida, R.F.; Moreno, I.F.; Machado, A.P.O.; Meireles, M.A.A.; da Silva, L.K.F.; Batista, E.A.C. Araticum (Annona crassiflora Mart.): A Critical Review for the Food Industry. Food Res. Int. 2024, 184, 114241. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Vilela, D.D.; Peixoto, L.G.; Martins, M.M.; Teixeira, R.R.; Miranda, N.C.; da Silva, N.M.; de Sousa, R.M.F.; de Oliveira, A.; et al. Peel of Araticum Fruit (Annona crassiflora Mart.) as a Source of Antioxidant Compounds with α-Amylase, α-Glucosidase and Glycation Inhibitory Activities. Bioorg. Chem. 2016, 69, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.L.C.C.; Minighin, E.C.; Soares, I.I.C.; Ferreira, R.M.d.S.B.; de Sousa, I.M.N.; Augusti, R.; Labanca, R.A.; de Araújo, R.L.B.; Melo, J.O.F. Evaluation of the Total Phenolic Content, Antioxidative Capacity, and Chemical Fingerprint of Annona crassiflora Mart. Bioaccessible Molecules. Food Res. Int. 2023, 165, 112514. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Angolini, C.F.F.; Eberlin, M.N.; Meireles, M.A.A.; Pastore, G.M. Effects of High-Intensity Ultrasound Process Parameters on the Phenolic Compounds Recovery from Araticum Peel. Ultrason. Sonochem. 2019, 50, 82–95. [Google Scholar] [CrossRef]
- Barbosa, M.F.; Justino, A.B.; Martins, M.M.; Belaz, K.R.A.; Ferreira, F.B.; de Oliveira, R.J.; Danuello, A.; Espindola, F.S.; Pivatto, M. Cholinesterase Inhibitors Assessment of Aporphine Alkaloids from Annona crassiflora and Molecular Docking Studies. Bioorg. Chem. 2022, 120, 105593. [Google Scholar] [CrossRef]
- Justino, A.B.; Florentino, R.M.; França, A.; Filho, A.C.M.L.; Franco, R.R.; Saraiva, A.L.; Fonseca, M.C.; Leite, M.F.; Espindola, F.S. Alkaloid and Acetogenin-Rich Fraction from Annona crassiflora Fruit Peel Inhibits Proliferation and Migration of Human Liver Cancer HepG2 Cells. PLoS ONE 2021, 16, e0250394. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.N.; Justino, A.B.; Martins, M.M.; Peixoto, L.G.; Vilela, D.D.; Santos, P.S.; Teixeira, T.L.; da Silva, C.V.; Goulart, L.R.; Pivatto, M.; et al. Stephalagine, an Alkaloid with Pancreatic Lipase Inhibitory Activity Isolated from the Fruit Peel of Annona crassiflora Mart. Ind. Crops Prod. 2017, 97, 324–329. [Google Scholar] [CrossRef]
- Ramos, L.P.A.; Justino, A.B.; Tavernelli, N.; Saraiva, A.L.; Franco, R.R.; de Souza, A.V.; Silva, H.C.G.; de Moura, F.B.R.; Botelho, F.V.; Espindola, F.S. Antioxidant Compounds from Annona crassiflora Fruit Peel Reduce Lipid Levels and Oxidative Damage and Maintain the Glutathione Defense in Hepatic Tissue of Triton WR-1339-Induced Hyperlipidemic Mice. Biomed. Pharmacother. 2021, 142, 112049. [Google Scholar] [CrossRef] [PubMed]
- Justino, A.B.; Pereira, M.N.; Peixoto, L.G.; Vilela, D.D.; Caixeta, D.C.; de Souza, A.V.; Teixeira, R.R.; Silva, H.C.G.; de Moura, F.B.R.; Moraes, I.B.; et al. Hepatoprotective Properties of a Polyphenol-Enriched Fraction from Annona Crassiflora Mart. Fruit Peel against Diabetes-Induced Oxidative and Nitrosative Stress. J. Agric. Food Chem. 2017, 65, 4428–4438. [Google Scholar] [CrossRef]
- Justino, A.B.; Costa, M.S.; Saraiva, A.L.; Silva, P.H.; Vieira, T.N.; Dias, P.; Linhares, C.R.B.; Dechichi, P.; Avila, V.d.M.R.; Espindola, F.S.; et al. Protective Effects of a Polyphenol-Enriched Fraction of the Fruit Peel of Annona crassiflora Mart. on Acute and Persistent Inflammatory Pain. Inflammopharmacology 2020, 28, 759–771. [Google Scholar] [CrossRef]
- da Silva, J.J.; Cerdeira, C.D.; Chavasco, J.M.; Cintra, A.B.P.; da Silva, C.B.P.; de Mendonça, A.N.; Ishikawa, T.; Boriollo, M.F.G.; Chavasco, J.K. In vitro Screening Antibacterial Activity of Bidens pilosa Linné and Annona crassiflora Mart. against Oxacillin Resistant Staphylococcus aureus (Orsa) from the Aerial Environment at the Dental Clinic. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 333–340. [Google Scholar] [CrossRef]
- Arruda, H.S.; Borsoi, F.T.; Andrade, A.C.; Pastore, G.M.; Marostica Junior, M.R. Scientific Advances in the Last Decade on the Recovery, Characterization, and Functionality of Bioactive Compounds from the Araticum Fruit (Annona crassiflora Mart.). Plants 2023, 12, 1536. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J.; Li, J.; Li, P.; Zhao, M.; Xia, G. Optimization of Ultrasonic-Assisted Extraction of Polyphenol from Areca Nut (Areca catechu L.) Seeds Using Response Surface Methodology and Its Effects on Osteogenic Activity. Ultrason. Sonochem. 2023, 98, 106511. [Google Scholar] [CrossRef]
- Hefied, F.; B Ahmed, Z.; Yousfi, M. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activities From Pistacia atlantica Desf. Galls Using Response Surface Methodology. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100449. [Google Scholar] [CrossRef]
- Chaabani, E.; Abert Vian, M.; Bettaieb Rebey, I.; Bourgou, S.; Zar Kalai, F.; Chemat, F.; Ksouri, R. Ethanol–Water Binary Solvent Affects Phenolic Composition and Antioxidant Ability of Pistacia lentiscus L. Fruit Extracts: A Theoretical versus Experimental Solubility Study. J. Food Meas. Charact. 2023, 17, 4705–4714. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Optimization of Extraction Parameters of Total Phenolics from Annona crassiflora Mart. (Araticum) Fruits Using Response Surface Methodology. Food Anal. Methods 2017, 10, 100–110. [Google Scholar] [CrossRef]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Namdar, B.; Akbarmivehie, M.; Mirsaeedghazi, H.; Azarikia, F. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds from Pomegranate and Orange Peels and Their Antioxidant Activity in a Functional Drink. Food Biosci. 2022, 49, 101918. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Kauffmann, A.C.; Castro, V.S. Phenolic Compounds in Bacterial Inactivation: A Perspective from Brazil. Antibiotics 2023, 12, 645. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response Surface Optimization of Ultrasound-Assisted Flavonoids Extraction from the Flower of Citrus aurantium L. Var. Amara Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [CrossRef]
- Bae, I.K.; Ham, H.M.; Jeong, M.H.; Kim, D.H.; Kim, H.J. Simultaneous Determination of 15 Phenolic Compounds and Caffeine in Teas and Mate Using RP-HPLC/UV Detection: Method Development and Optimization of Extraction Process. Food Chem. 2015, 172, 469–475. [Google Scholar] [CrossRef]
- Özbek, H.N.; Halahlih, F.; Göğüş, F.; Koçak Yanık, D.; Azaizeh, H. Pistachio (Pistacia vera L.) Hull as a Potential Source of Phenolic Compounds: Evaluation of Ethanol–Water Binary Solvent Extraction on Antioxidant Activity and Phenolic Content of Pistachio Hull Extracts. Waste Biomass Valorization 2020, 11, 2101–2110. [Google Scholar] [CrossRef]
- Kaur, S.; Ubeyitogullari, A. Extraction of Phenolic Compounds from Rice Husk via Ethanol-Water-Modified Supercritical Carbon Dioxide. Heliyon 2023, 9, e14196. [Google Scholar] [CrossRef] [PubMed]
- Drevelegka, I.; Goula, A.M. Recovery of Grape Pomace Phenolic Compounds through Optimized Extraction and Adsorption Processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Barba, F.J.; Alcántara, C.; Abdelkebir, R.; Bäuerl, C.; Pérez-Martínez, G.; Lorenzo, J.M.; Collado, M.C.; García-Pérez, J.V. Ultrasonically-Assisted and Conventional Extraction from Erodium glaucophyllum Roots Using Ethanol:Water Mixtures: Phenolic Characterization, Antioxidant, and Anti-Inflammatory Activities. Molecules 2020, 25, 1759. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- El Adnany, E.M.; Elhadiri, N.; Mourjane, A.; Ouhammou, M.; Hidar, N.; Jaouad, A.; Bitar, K.; Mahrouz, M. Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology. Separations 2023, 10, 326. [Google Scholar] [CrossRef]
- Simić, V.M.; Rajković, K.M.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.; Lazić, M.L.; Karabegović, I.T. Optimization of Microwave-Assisted Extraction of Total Polyphenolic Compounds from Chokeberries by Response Surface Methodology and Artificial Neural Network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Le, X.T.; Lan Vi, V.L.; Toan, T.Q.; Bach, L.G.; Truc, T.T.; Hai Ha, P.T. Extraction Process of Polyphenols from Soybean (Glycine max L.) Sprouts: Optimization and Evaluation of Antioxidant Activity. Processes 2019, 7, 489. [Google Scholar] [CrossRef]
- Haas, I.C.d.S.; Toaldo, I.M.; Burin, V.M.; Bordignon-Luiz, M.T. Extraction Optimization for Polyphenolic Profiling and Bioactive Enrichment of Extractives of Non-Pomace Residue from Grape Processing. Ind. Crops Prod. 2018, 112, 593–601. [Google Scholar] [CrossRef]
- Khan, U.; Hayat, F.; Khanum, F.; Shao, Y.; Iqbal, S.; Munir, S.; Abdin, M.; Li, L.; Ahmad, R.M.; Qiu, J.; et al. Optimizing Extraction Conditions and Isolation of Bound Phenolic Compounds from Corn Silk (Stigma maydis) and Their Antioxidant Effects. J. Food Sci. 2023, 88, 3341–3356. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and Antioxidant Assays of Polyphenols: A Review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.V.S.B.; Dash, K.K. Ultrasound-Assisted Extraction of Phytocompounds from Dragon Fruit Peel: Optimization, Kinetics and Thermodynamic Studies. Ultrason. Sonochem. 2020, 68, 105180. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)Phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef]
- Begum, S.N.; Hossain, M.; Adnan, M.; Rahaman, C.H.; Reza, A. Optimization and Characterization of Phenolic Extraction Conditions and Antioxidant Activity Evaluation of Adenanthera pavonina L. Bark. Plants 2023, 12, 3902. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Ramos, A.M.; Pertuzatti, P.B.; Barcia, M.T.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Flour of Banana (Musa AAA) Peel as a Source of Antioxidant Phenolic Compounds. Food Res. Int. 2014, 55, 397–403. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant Activity/Capacity Measurement. 3. Reactive Oxygen and Nitrogen Species (ROS/RNS) Scavenging Assays, Oxidative Stress Biomarkers, and Chromatographic/Chemometric Assays. J. Agric. Food Chem. 2016, 64, 1046–1070. [Google Scholar] [CrossRef]
- Lin, S.; Ye, C.; Lin, Z.; Huang, L.; Li, D. Recent Progress of Near-Infrared Fluorescent Probes in the Determination of Reactive Oxygen Species for Disease Diagnosis. Talanta 2024, 268, 125264. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; Dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.F.D.S.; Rosalen, P.L.; de Alencar, S.M. Winery By-Products: Extraction Optimization, Phenolic Composition and Cytotoxic Evaluation to Act as a New Source of Scavenging of Reactive Oxygen Species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Berto, A.; Ribeiro, A.B.; de Souza, N.E.; Fernandes, E.; Chisté, R.C. Bioactive Compounds and Scavenging Capacity of Pulp, Peel and Seed Extracts of the Amazonian Fruit Quararibea cordata against ROS and RNS. Food Res. Int. 2015, 77, 236–243. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Berindan Neagoe, I. The Relationships Between Biological Activities and Structure of Flavan-3-ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Bobrowska-Korczak, B. Active Compounds in Fruits and Inflammation in the Body. Nutrients 2022, 14, 2496. [Google Scholar] [CrossRef]
- Zhou, Y.; Suo, W.; Zhang, X.; Yang, Y.; Zhao, W.; Li, H.; Ni, Q. Targeting Epigenetics in Diabetic Cardiomyopathy: Therapeutic Potential of Flavonoids. Biomed. Pharmacother. 2023, 157, 114025. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Silva, J.D.R.; Arruda, H.S.; Andrade, A.C.; Berilli, P.; Borsoi, F.T.; Monroy, Y.M.; Rodrigues, M.V.N.; Sampaio, K.A.; Pastore, G.M.; Marostica Junior, M.R. Eugenia calycina and Eugenia stigmatosa as Promising Sources of Antioxidant Phenolic Compounds. Plants 2024, 13, 2039. [Google Scholar] [CrossRef]
- Aboulghazi, A.; Bakour, M.; Fadil, M.; Lyoussi, B. Simultaneous Optimization of Extraction Yield, Phenolic Compounds and Antioxidant Activity of Moroccan Propolis Extracts: Improvement of Ultrasound-Assisted Technique Using Response Surface Methodology. Processes 2022, 10, 297. [Google Scholar] [CrossRef]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef]
- Roesler, R.; Malta, L.G.; Carrasco, L.C.; Holanda, R.B.; Sousa, C.A.S.; Pastore, G.M. Atividade Antioxidante de Frutas do Cerrado. Food Sci. Technol. 2007, 27, 53–60. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.S.M.C.; Quirino, D.J.G.; Favaro-Trindade, C.S.; Sartori, A.G.d.O.; Massarioli, A.P.; Lazarini, J.G.; Silva, A.P.d.S.; de Alencar, S.M. Effects of Simulated Gastrointestinal Digestion/Epithelial Transport on Phenolics and Bioactivities of Particles of Brewer’s Spent Yeasts Loaded with Brazilian Red Propolis. Food Res. Int. 2023, 173, 113345. [Google Scholar] [CrossRef] [PubMed]
- Mariutti, L.R.B.; Rodrigues, E.; Chisté, R.C.; Fernandes, E.; Mercadante, A.Z. The Amazonian Fruit Byrsonima crassifolia Effectively Scavenges Reactive Oxygen and Nitrogen Species and Protects Human Erythrocytes against Oxidative Damage. Food Res. Int. 2014, 64, 618–625. [Google Scholar] [CrossRef]
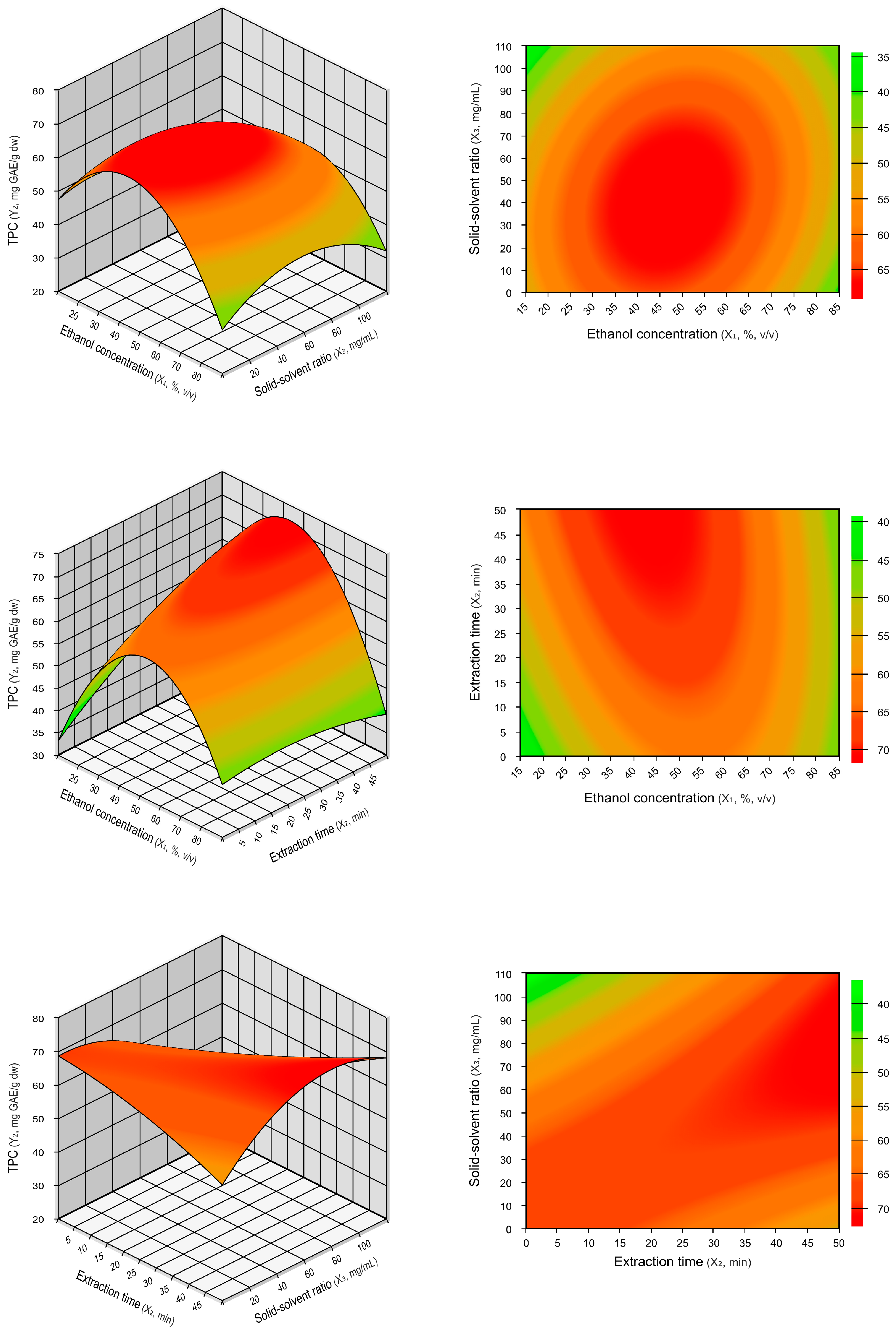
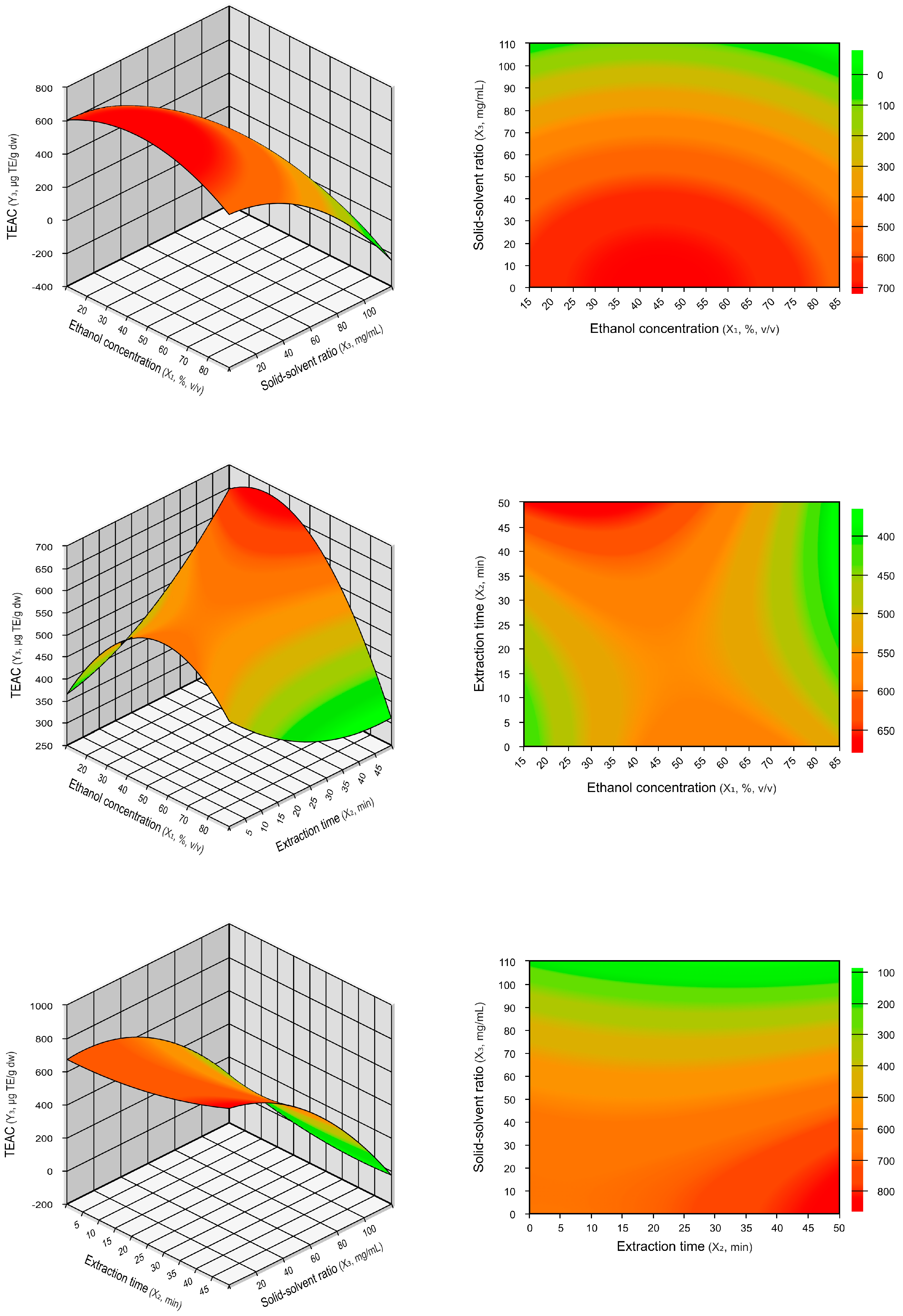
| Run | Coded Values | Actual Values | Response Variables | |||||
|---|---|---|---|---|---|---|---|---|
| EC | ET | SSR | EC (%, v/v) | ET (min) | SSR (mg/mL) | TPCs (mg GAE/g dw) | TEAC (µmol TE/g dw) | |
| x1 | x2 | x3 | X1 | X2 | X3 | R1 | R2 | |
| 1 | −1.00 | −1.00 | −1.00 | 32.14 | 13.10 | 28.21 | 62.71 ± 0.88 | 654.56 ± 21.37 |
| 2 | 1.00 | −1.00 | −1.00 | 67.86 | 13.10 | 28.21 | 69.91 ± 0.99 | 724.31 ± 21.06 |
| 3 | −1.00 | 1.00 | −1.00 | 32.14 | 36.90 | 28.21 | 74.48 ± 3.25 | 832.43 ± 6.04 |
| 4 | 1.00 | 1.00 | −1.00 | 67.86 | 36.90 | 28.21 | 59.66 ± 2.73 | 661.53 ± 18.04 |
| 5 | −1.00 | −1.00 | 1.00 | 32.14 | 13.10 | 81.79 | 56.61 ± 0.88 | 354.82 ± 12.36 |
| 6 | 1.00 | −1.00 | 1.00 | 67.86 | 13.10 | 81.79 | 51.68 ± 1.65 | 270.04 ± 9.34 |
| 7 | −1.00 | 1.00 | 1.00 | 32.14 | 36.90 | 81.79 | 62.66 ± 0.62 | 323.33 ± 14.31 |
| 8 | 1.00 | 1.00 | 1.00 | 67.86 | 36.90 | 81.79 | 69.21 ± 3.10 | 285.06 ± 5.10 |
| 9 | −1.68 | 0.00 | 0.00 | 19.97 | 25.00 | 55.00 | 53.83 ± 0.47 | 486.02 ± 14.10 |
| 10 | 1.68 | 0.00 | 0.00 | 80.03 | 25.00 | 55.00 | 50.34 ± 1.82 | 426.59 ± 8.39 |
| 11 | 0.00 | −1.68 | 0.00 | 50.00 | 4.98 | 55.00 | 62.04 ± 1.76 | 555.54 ± 7.19 |
| 12 | 0.00 | 1.68 | 0.00 | 50.00 | 45.02 | 55.00 | 65.92 ± 3.45 | 597.68 ± 11.35 |
| 13 | 0.00 | 0.00 | −1.68 | 50.00 | 25.00 | 9.95 | 59.94 ± 2.24 | 570.53 ± 13.67 |
| 14 | 0.00 | 0.00 | 1.68 | 50.00 | 25.00 | 100.05 | 59.39 ± 1.82 | 316.99 ± 5.36 |
| 15 | 0.00 | 0.00 | 0.00 | 50.00 | 25.00 | 55.00 | 68.62 ± 0.76 | 520.96 ± 17.33 |
| 16 | 0.00 | 0.00 | 0.00 | 50.00 | 25.00 | 55.00 | 69.14 ± 1.53 | 583.27 ± 12.15 |
| 17 | 0.00 | 0.00 | 0.00 | 50.00 | 25.00 | 55.00 | 69.02 ± 0.75 | 576.79 ± 14.51 |
| Model Components | TPCs | TEAC | ||||||
|---|---|---|---|---|---|---|---|---|
| RC | SE | t-Value | p-Value | RC | SE | t-Value | p-Value | |
| Mean | 68.58 | 3.65 | 18.81 | <0.0001 * | 558.72 | 57.89 | 9.65 | <0.0001 * |
| x1 | −0.87 | 1.71 | −0.51 | 0.6274 | −23.74 | 27.19 | −0.87 | 0.4116 |
| x2 | 2.32 | 1.71 | 1.35 | 0.2184 | 12.41 | 27.19 | 0.46 | 0.6619 |
| x3 | −2.02 | 1.71 | −1.18 | 0.2777 | −151.28 | 27.19 | −5.56 | 0.0008 * |
| x12 | −4.75 | 1.88 | −2.52 | 0.0397 * | −31.19 | 29.92 | −1.04 | 0.3318 |
| x22 | −0.55 | 1.88 | −0.29 | 0.7803 | 11.34 | 29.92 | 0.38 | 0.7160 |
| x32 | −2.07 | 1.88 | −1.10 | 0.3080 | −35.63 | 29.92 | −1.19 | 0.2726 |
| x1x2 | −1.32 | 2.24 | −0.59 | 0.5745 | −24.27 | 35.52 | −0.68 | 0.5165 |
| x1x3 | 1.16 | 2.24 | 0.52 | 0.6216 | −2.74 | 35.52 | −0.08 | 0.9407 |
| x2x3 | 2.76 | 2.24 | 1.23 | 0.2576 | −16.45 | 35.52 | −0.46 | 0.6574 |
| Source | TPCs | TEAC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SS | DF | MS | F-Value | p-Value | SS | DF | MS | F-Value | p-Value | |
| Model | 497.79 | 9.00 | 55.31 | 1.38 | 0.3428 | 358,061.73 | 9.00 | 39,784.64 | 3.94 | 0.0421 * |
| Residual | 280.37 | 7.00 | 40.05 | 70,660.66 | 7.00 | 10,094.38 | ||||
| Lack of fit | 280.22 | 5.00 | 56.04 | 756.00 | 0.0013 * | 68,313.49 | 5.00 | 13,662.70 | 11.64 | 0.0810 |
| Pure error | 0.15 | 2.00 | 0.07 | 2347.17 | 2.00 | 1173.59 | ||||
| Total | 778.16 | 16.00 | 428,722.39 | 16.00 | ||||||
| R2 | 0.6397 | 0.8352 | ||||||||
| Parameters | TPCs (mg GAE/g dw) | TEAC (µmol TE/g dw) | |||||
|---|---|---|---|---|---|---|---|
| Optimum Conditions | PV | EV | RSE (%) | PV | EV | RSE (%) | |
| EC (%, v/v) | 50 | 68.47 a | 70.16 ± 2.34 a | 2.41 | 677.04 a | 667.22 ± 42.24 a | −1.47 |
| ET (min) | 5 | ||||||
| SSR (mg/mL) | 10 | ||||||
| Analysis | Parameters | Araticum Peel |
|---|---|---|
| Phytochemicals | TPCs (mg GAE/g dw) | 70.16 ± 2.34 |
| CTs (mg CE/g dw) | 76.49 ± 1.61 | |
| Synthetic free radical | TEAC (µmol TE/g dw) | 667.22 ± 42.24 |
| FRAP (µmol TE/g dw) | 730.57 ± 26.82 | |
| ROS | ROO• (µmol TE/g dw) | 1596.05 ± 40.43 |
| •OH (IC50 µg/mL dw) | 0.26 ± 0.01 | |
| HOCl (IC50 µg/mL dw) | 40.40 ± 0.89 | |
| •O2− (IC50 µg/mL dw) | 89.23 ± 9.84 |
| Class | Compound | Content (µg/g dw) |
|---|---|---|
| Phenolic acids | 4-Hydroxybenzoic acid | n.d. |
| Benzoic acid | n.d. | |
| Caffeic acid | n.d. | |
| Chlorogenic acid (5-caffeoylquinic acid) | 29.04 ± 0.30 | |
| Ferulic acid | 13.86 ± 0.37 | |
| Gallic acid | n.d. | |
| Gentisic acid (2,5-dihydroxybenzoic acid) | n.d. | |
| p-Coumaric acid | 5.52 ± 0.25 | |
| Protocatechuic acid (3,4-dihydroxybenzoic acid) | n.d. | |
| Sinapic acid | n.d. | |
| Syringic acid | n.d. | |
| Trans-cinnamic acid | n.d. | |
| Vanillic acid | 61.15 ± 1.13 | |
| α-Resorcylic acid (3,5-dihydroxybenzoic acid) | 28.16 ± 0.51 | |
| Total phenolic acids | 137.73 ± 2.18 | |
| Flavonoids | Apigenin | n.d. |
| Apigetrin (apigenin-7-O-glucoside) | n.d. | |
| Astragalin (kaempferol-3-O-glucoside) | 22.45 ± 0.48 | |
| Catechin | 100.96 ± 1.46 | |
| Epicatechin | 2526.12 ± 23.22 | |
| Hesperetin | n.d. | |
| Hyperoside (quercetin-3-O-galactoside) | 45.67 ± 1.42 | |
| Kaempferol | n.d. | |
| Luteolin | n.d. | |
| Myricetin | n.d. | |
| Naringenin | n.d. | |
| Procyanidin A2 | n.d. | |
| Procyanidin B1 | n.d. | |
| Procyanidin B2 | 3248.77 ± 33.52 | |
| Quercetin | n.d. | |
| Quercetrin (quercetin-3-O-rhamnoside) | 19.94 ± 1.35 | |
| Rutin (quercetin-3-O-rutinoside) | 79.10 ± 1.27 | |
| Vitexin (apigenin-8-C-glucoside) | n.d. | |
| Vitexin-2″-O-rhamnoside | n.d. | |
| Total flavonoids | 6043.01 ± 52.30 | |
| Total phenolic compounds | 6180.74 ± 52.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, A.C.; Borsoi, F.T.; Saliba, A.S.M.C.; de Alencar, S.M.; Pastore, G.M.; Arruda, H.S. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology. Plants 2024, 13, 2560. https://doi.org/10.3390/plants13182560
Andrade AC, Borsoi FT, Saliba ASMC, de Alencar SM, Pastore GM, Arruda HS. Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology. Plants. 2024; 13(18):2560. https://doi.org/10.3390/plants13182560
Chicago/Turabian StyleAndrade, Amanda Cristina, Felipe Tecchio Borsoi, Ana Sofia Martelli Chaib Saliba, Severino Matias de Alencar, Glaucia Maria Pastore, and Henrique Silvano Arruda. 2024. "Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology" Plants 13, no. 18: 2560. https://doi.org/10.3390/plants13182560
APA StyleAndrade, A. C., Borsoi, F. T., Saliba, A. S. M. C., de Alencar, S. M., Pastore, G. M., & Arruda, H. S. (2024). Optimization of Ultrasonic-Assisted Extraction of Phenolic Compounds and Antioxidant Activity from Araticum Peel Using Response Surface Methodology. Plants, 13(18), 2560. https://doi.org/10.3390/plants13182560









