Towards Pathogen-Free Coconut Germplasm Exchange
Abstract
1. Introduction
2. The Disease-Causing Agents of Concern
2.1. Phytoplasma
| Agent | Disease | Localization | Symptoms | References |
|---|---|---|---|---|
| Phytoplasma | Coconut lethal yellowing disease (LY) | Husk, shell, endosperm embryo, plumule, phloem cells and haustorial tissue from diseased coconut palms | Untimely shedding of fruit, discoloration in younger leaves, and darkening of the inflorescence, finally leading to the death of the coconut palm. | [22,38,39] |
| Coconut root wilt disease (CRWD) | Leaves, inflorescence, and roots | Flaccid bending of leaflets, accompanied by leaf yellowing, necrosis, compromised stomatal regulation, and damage to the root system. | [24] | |
| Weligama coconut leaf wilt disease (WCLW) | Sieve tubes, leaflets | The leaves exhibit flaccidity and marginal necrosis, accompanied by intense yellowing of the fronds. With disease advancement, the crown diminishes in size, and the trunk starts to taper. In addition, the female flower production declines, as does the palm’s productivity. Moderately affected palms show necrosis in the root tips, while severely affected palms lack young roots. Some of the infected palms also exhibit leaf rot disease. However, no studies have reported the death of a coconut palm caused by this disease. | [23,40,41,42] | |
| Cape St. Paul wilt disease (CSPW) | Fronds, inflorescence, and leaves | Premature nut drops, yellowing of fronds, and necrosis of immature inflorescences, followed by progressive yellowing of the crown from the older leaves upwards, and death of palms. | [43] | |
| Bogia coconut syndrome (BCS) | Fruit husk, shell, endosperm, embryo, trunk phloem | Premature dropping of fruit of all ages. The outer fronds of the crown will droop and develop a pale yellow colour. Fronds then turn brown and hang down the stem, like a skirt. A dry rot develops in the newly expanding spear, progressing downwards to the growing point. Complete necrosis—death of the palm. | [22] | |
| Virus | Coconut foliar decay virus disease (CFDV) | Leaflet, frond, phloem, tissue adjacent to and within necrotic zones | Yellowing in certain leaflets below the unfolding spear leaf. Then, a broader yellowing occurs in both the affected fronds and neighboring organs. Synchronously, these fronds undergo a lateral necrosis near the petiole’s base, resulting in their collapse. | [44] |
| Viroid | Coconut cadang-cadang viroid disease (CCCVd) | Vascular tissues, nucleolus of mesophyll cells, embryos. | The fruit assumes a rounded shape, displaying distinctive scarifications at its equator, while the initial non-necrotic, translucent, bright yellow leaf spots emerge. Inflorescences undergo necrosis, halting fruit production, slowing down new frond development, and causing larger and more frequent leaf spots. Later, fronds start to appear chlorotic when observed from a distance. Finally, preceding death, leaf spots merge, and the entire crown exhibits a distinct yellow or bronze hue, significantly reduced in size with a diminished number of fronds. | [45,46,47] |
| Coconut tinangaja viroid disease (CTiVd) | Unreported | A decrease in frond quantity, along with diminished size and quantity of fruit, leads to the shriveling and deformation of the fruit. This results in the cessation of fruit production, failure to generate inflorescences, tapering of the distal end of the trunk, persistent stipules, stippling of leaflets characterized by fine chlorotic spots, and the development of brittleness in both leaflets and fronds. | [48] |
2.2. Viruses
2.3. Viroids
3. Tissues Used for Coconut Germplasm Exchange
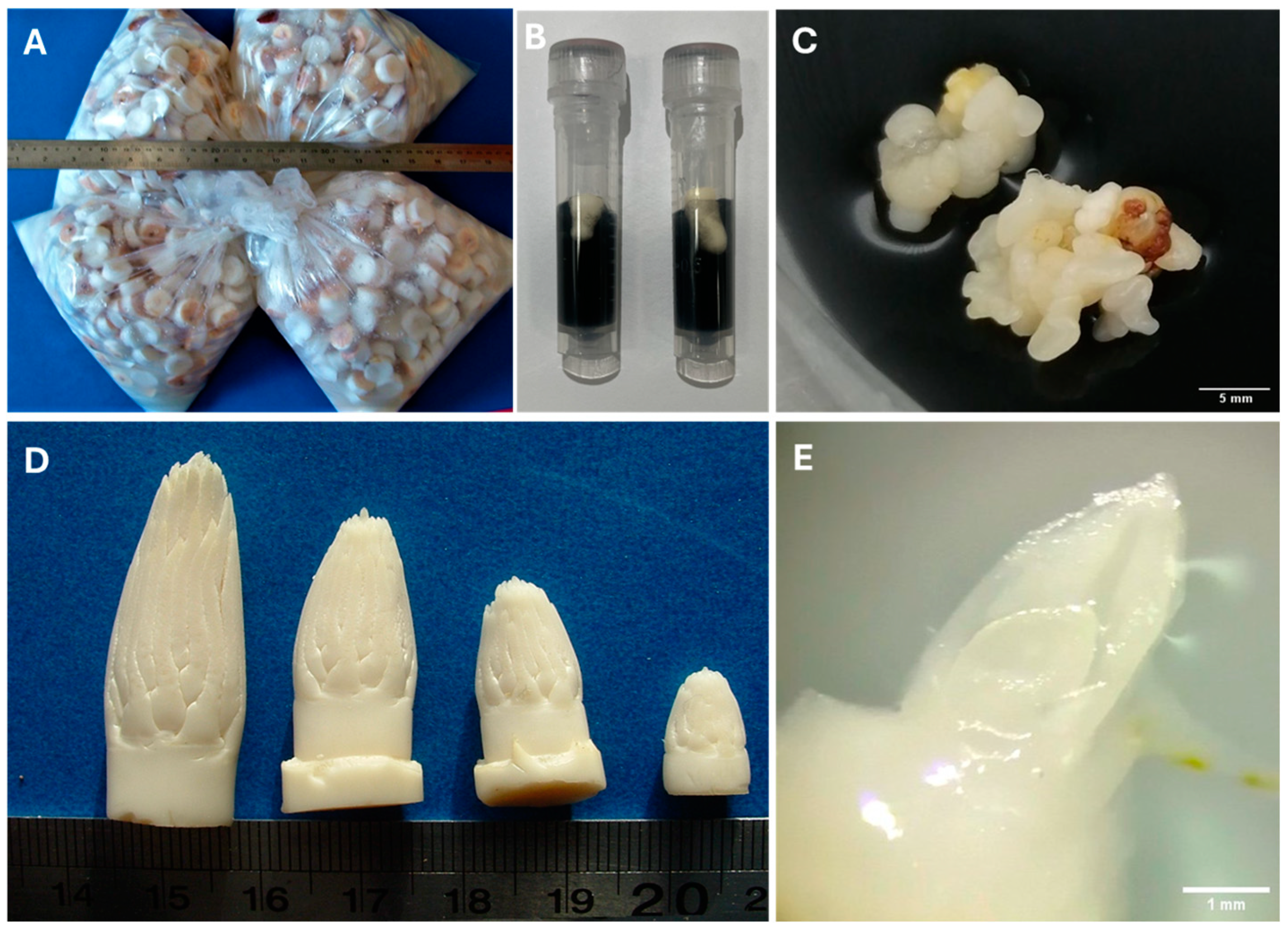
3.1. Zygotic Embryos
3.2. Apical Meristems
3.3. Somatic Embryos/Embryogenic Callus
4. Methods for the Detection of Lethal Pathogens
4.1. Detection Using Artificial Transmission Test Systems
4.2. Visual Detection Methods
4.3. Immunological Detection Methods
4.4. Molecular Detection Methods
4.4.1. Molecular Hybridization Assays
4.4.2. Polymerase Chain Reaction (PCR)-Based Methods
- (a)
- Conventional PCR: The molecular detection of phytoplasmas present in symptomatic tissue is routinely undertaken by the PCR using phytoplasma-specific universal or phytoplasma group-specific primers designed based on the highly conserved 16S ribosomal RNA (rRNA) gene sequences, the ribosomal protein, and elongation factor genes [101]. Phytoplasmas can be easily detected using PCR with the highest sensitivity in immature rather than mature tissues [101], as evidenced by the detection of LY phytoplasma in coconut leaves [102] and the Coconut yellow decline (CYD) phytoplasma in spear leaves, inflorescences, or trunk tissues of affected symptomatic and asymptomatic coconut palm varieties [103]. A high population of viroid-like molecules has been identified using PCR coconut palms infected with the Coconut tapering disease in Sri Lanka [104]. Similarly, a sense-specific single-primer PCR assay has been employed to identify CFDV DNA from coconut palms in Vanuatu [105].
- (b)
- Reverse transcriptase-PCR (RT-PCR): As most plant viruses have RNA genomes (and even DNA viruses produce RNA transcripts), it is most effective to detect virus infections by analyzing RNA sequences from infected plant samples [106]. The RT-PCR technique has been employed for detecting low concentrations of viruses and viroids in infected plants, with the only requirement being that of obtaining good quality RNA [106]. The application of the RT-PCR technique has successfully helped to detect the CCCVd viroid variant in oil palm leaf tissues [99], the Areca palm necrotic ringspot virus (ANRSV) in areca palm (Areca catechu Linn. [86]), and African oil palm ringspot virus (AOPRV) in infected oil palm leaves [107].
- (c)
- Nested PCR: Nested PCR methods, where four primers are used in two consecutive rounds of DNA amplification, have greatly enhanced specificity and sensitivity, and therefore aid in the detection of a low titer of pathogens [108,109]. Most of the nested PCR protocols developed have been focusing on phytoplasma detection and are used to increase the sensitivity of the available protocol [98]. Nesting of universal group primers with group-specific primers has helped in the diagnosis of phytoplasma infection in well-defined taxonomic groups [110]. For example, the WCLW-causing phytoplasma has been successfully detected using an optimized nested PCR in symptomatic coconut plants in Sri Lanka [111]. Similarly, CRWD- and CSPW-causing phytoplasma have also been detected in coconut palms using nested PCR [15,24,112].
- (d)
- Quantitative PCR (qPCR): In recent years, qPCR has proven to be an indispensable tool for the molecular diagnosis of pathogens. It is about 10 times more sensitive than standard PCR and does not require gel electrophoresis for target confirmation. The qPCR can simultaneously detect and quantify the pathogen, and this method is less affected by cross-contamination and is less work-intensive as compared to RT-PCR and nested PCR. For example, phytoplasma belonging to the 16SrIVgroup causing CRWD have been easily and effectively detected by qPCR [113], especially when compared to conventional and nested PCR [114]. The qPCR approach using the TaqMan probe gives higher sensitivity than nested-PCR when detecting the 16SrIV subgroups of the LY phytoplasma in the coconut [83,115,116]. The CCCVd variants in oil palm have been detected by using the qPCR technique [117]. This technique could be used in the coconut as well.
- (e)
- Digital PCR: Digital PCR (dPCR) is a breakthrough next-generation PCR technology, and it works by partitioning the sample into thousands of separate reaction compartments, conducting single, parallel PCR reactions [118]. It is used to detect phytoplasma and viruses, with several advantages over qPCR diagnostic assays, including higher sensitivity, precision, accuracy in detecting extremely rare target sequences, absolute quantification without standard curve and reference samples, greater tolerance to PCR inhibitors, and suitability for the preparation of in-house reference materials [119]. Reverse transcription (RT) dPCR has been used to detect viruses and viroids in apple and citrus plants [120,121], and this technique can be used for the coconut.
4.4.3. Isothermal Amplification Techniques
4.4.4. Next-Generation Sequencing
4.5. Limitations of Current Detection Methods
5. Methods for the Elimination of Lethal Pathogens
5.1. Chemotherapy
5.2. Thermotherapy and Cryotherapy
5.3. Limitations of Elimination Methods
6. Research Gaps and Research Priorities towards Safe Exchange of Coconut Germplasm
6.1. Lack of Knowledge on Vertical Transmission of Lethal Coconut Pathogens, and Their Detection in Various Tissues
6.2. Lack of Robust Disease Elimination Methods
6.3. Lack of Plant Health and Exchange Regulations among Coconut-Growing Countries
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pham, L.J. Chapter 9—Coconut (Cocos nucifera). In Industrial Oil Crops; McKeon, T.A., Hayes, D.G., Hildebrand, D.F., Weselake, R.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–242. ISBN 978-1-893997-98-1. [Google Scholar]
- Mu, Z.; Li, Z.; Liu, R.; Fan, H. The Development of Coconut (Cocos nucifera L.) Tissue Culture Technology in the Past 40 Years: A Review. Chin. J. Trop. Crop. 2020, 41, 11. [Google Scholar] [CrossRef]
- Vinodhini, C.; Deshmukh, K.V. An Economic Analysis of Coconut Farming in Karur District of Tamil Nadu, India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1566–1573. [Google Scholar] [CrossRef]
- Kalaipandian, S.; Biddle, J.; Adkins, S.W. Global Coconut Trade, Economy and the Value Chain. In Botany, Production and Uses; Adkins, S.W., Biddle, J.M., Bazrafshan, A., Kalaipandian, S., Eds.; CABI: Wallingford, UK, 2024; pp. 193–205. ISBN 978-1-78924-971-2. [Google Scholar]
- Shankar, B.; Roshan, D. Coconut Products Market by Type (Coconut Water, Coconut Oil, Coconut Milk, Dried Coconut Products, and Others), Application (Food, Beverage, Cosmetics, and Others), and Form (Solid and Liquid: Global Opportunity Analysis and Industry Forecast, 2019–2026; Allied Market Research: Maharashtra, India, 2019. [Google Scholar]
- Wang, C.; Hu, S.; Gardner, C.; Lübberstedt, T. Emerging Avenues for Utilization of Exotic Germplasm. Trends Plant Sci. 2017, 22, 624–637. [Google Scholar] [CrossRef]
- Koskela, J.; Vinceti, B.; Dvorak, W.; Bush, D.; Dawson, I.K.; Loo, J.; Kjaer, E.D.; Navarro, C.; Padolina, C.; Bordács, S.; et al. Utilization and Transfer of Forest Genetic Resources: A Global Review. For. Ecol. Manag. 2014, 333, 22–34. [Google Scholar] [CrossRef]
- Kumar, P.L.; Cuervo, M.; Kreuze, J.F.; Muller, G.; Kulkarni, G.; Kumari, S.G.; Massart, S.; Mezzalama, M.; Alakonya, A.; Muchugi, A.; et al. Phytosanitary Interventions for Safe Global Germplasm Exchange and the Prevention of Transboundary Pest Spread: The Role of CGIAR Germplasm Health Units. Plants 2021, 10, 328. [Google Scholar] [CrossRef]
- Samosir, Y.M.S.; Adkins, S. Improving Acclimatization through the Photoautotrophic Culture of Coconut (Cocos nucifera) Seedlings: An In Vitro System for the Efficient Exchange of Germplasm. Vitr. Cell. Dev. Biol.-Plant 2014, 50, 493–501. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Bandupriya, H.D.D.; López-Villalobos, A.; Sisunandar, S.; Foale, M.; Adkins, S.W. Tissue Culture and Associated Biotechnological Interventions for the Improvement of Coconut (Cocos nucifera L.): A Review. Planta 2015, 242, 1059–1076. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Plant Tissue Culture: An Introductory Text; Springer India: Delhi, India, 2013; ISBN 978-81-322-1025-2. [Google Scholar]
- Rao, N. Kameswara Plant Genetic Resources: Advancing Conservation and Use through Biotechnology. Afr. J. Biotechnol. 2003, 3, 136–145. [Google Scholar] [CrossRef]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. Vitr. Cell. Dev. Biol. Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Bertaccini, A.; Lee, I.-M. Phytoplasmas: An Update. In Phytoplasmas: Plant Pathogenic Bacteria—I; Rao, G.P., Bertaccini, A., Fiore, N., Liefting, L.W., Eds.; Springer: Singapore, 2018; pp. 1–29. ISBN 9789811301186. [Google Scholar]
- Nipah, J.O.; Jones, P.; Dickinson, M.J. Detection of Lethal Yellowing Phytoplasma in Embryos from Coconut Palms Infected with Cape St Paul Wilt Disease in Ghana. Plant Pathol. J. 2007, 56, 777–784. [Google Scholar] [CrossRef]
- Yankey, E.N.; Swarbrick, P.J.; Nipah, J.O.; Quaicoe, R.N.; Dickinson, M.J. Detection of the Cape St. Paul Wilt Phytoplasma in Coconut Palms in Ghana through the Combination of Visual Symptoms Assessment and Molecular Diagnosis Using a secA Gene Based Assay. J. Plant Pathol. 2014, 96, 281–285. [Google Scholar] [CrossRef]
- Bertaccini, A. Phytoplasma Research between Past and Future: What Directions? Phyt. Moll. 2015, 5, S1–S4. [Google Scholar] [CrossRef]
- Rosete, Y.A.; Diallo, H.A.; Konan Konan, J.L.; Yankey, N.; Saleh, M.; Pilet, F.; Contaldo, N.; Paltrinieri, S.; Bertaccini, A.; Scott, J. Detection and Differentiation of the Coconut Lethal Yellowing Phytoplasma in Coconut-Growing Villages of Grand-Lahou, Côte d’Ivoire: Detection and Differentiation of the Côte d’Ivoire Coconut Lethal Yellowing Phytoplasma. Ann. Appl. Biol. 2017, 170, 333–347. [Google Scholar] [CrossRef]
- Bila, J.; Mondjana, A.; Samils, B.; Santos, L.; Hogberg, N. Integrated Management of Coconut Lethal Yellowing Phytoplasma Disease in Mozambique: Current Challenges and Future Perspectives. In Sustainable Management of Phytoplasma Diseases in Crops Grown in the Tropical Belt; Olivier, C.Y., Dumonceaux, T.J., Pérez-López, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 12, pp. 233–249. ISBN 978-3-030-29649-0. [Google Scholar]
- Oropeza, C.; Cordova, I.; Puch-Hau, C.; Castillo, R.; Chan, J.L.; Sáenz, L. Detection of Lethal Yellowing Phytoplasma in Coconut Plantlets Obtained through In Vitro Germination of Zygotic Embryos from the Seeds of Infected Palms. Ann. Appl. Biol. 2017, 171, 28–36. [Google Scholar] [CrossRef]
- Obeng-Darko, S.A.; Quaicoe, R.N.; Yankey, E.N.; Twumasi, P. Comparative PCR Analyses for the Detection of the Cape St. Paul Wilt Disease Phytoplasma in Coconut Palms in Ghana. J. Phytopathol. 2018, 166, 477–483. [Google Scholar] [CrossRef]
- Gurr, G. Bogia Coconut Syndrome and Related Phytoplasma Syndromes in Papua New Guinea: Developing Biological Knowledge and a Risk Management Strategy; ACIAR: Canberra, Australia, 2020. [Google Scholar]
- Perera, L.; Meegahakumbura, M.K.; Wijesekara, H.R.T.; Fernando, W.B.S.; Dickinson, J. A Phytoplasma Is Associated with the Weligama Coconut Leaf Wilt Disease in Sri Lanka. Plant Pathol. J. 2012, 94, 205–209. [Google Scholar]
- Manimekalai, R.; Nair, S.; Soumya, V.P. Evidence of 16SrXI Group Phytoplasma DNA in Embryos of Root Wilt Diseased Coconut Palms. Australas. Plant Pathol. 2014, 43, 93–96. [Google Scholar] [CrossRef]
- Lu, H.; Wilson, B.; Zhang, H.; Woruba, S.B.; Feng, B.; Johnson, A.C.; Komolong, B.; Kuniata, L.; Yang, G.; Gurr, G.M. Detection and Identification of Bogia Coconut Syndrome Phytoplasma from Seed-Associated Tissues and Seedlings of Coconut (Cocos nucifera) and Betel Nut (Areca catechu). Sci. Rep. 2024, 14, 11542. [Google Scholar] [CrossRef] [PubMed]
- Oropeza, C.; Cordova, I.; Chumba, A.; Narváez, M.; Sáenz, L.; Ashburner, R.; Harrison, N. Phytoplasma Distribution in Coconut Palms Affected by Lethal Yellowing Disease. Ann. Appl. Biol. 2011, 159, 109–117. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Oshima, K.; Ammar, E.; Kakizawa, S.; Kingdom, H.N.; Namba, S. Phytoplasmas: Bacteria That Manipulate Plants and Insects. Mol. Plant Pathol. 2008, 9, 403–423. [Google Scholar] [CrossRef]
- Mou, D.-F.; Lee, C.-C.; Hahn, P.G.; Soto, N.; Humphries, A.R.; Helmick, E.E.; Bahder, B.W. Effects of Lethal Bronzing Disease, Palm Height, and Temperature on Abundance and Monitoring of Haplaxius crudus. Insects 2020, 11, 748. [Google Scholar] [CrossRef]
- Soufi, Z.; Komor, E. Latent Infection of Asymptomatic Hawaiian Sugarcane Cultivars with 16SrI and 16SrXI Phytoplasmas. J. Gen. Plant Pathol. 2014, 80, 255–263. [Google Scholar] [CrossRef]
- Shin, H.-D. Impossibility of Seed Transmission in Plant Mycoplasmal Diseases. Korean J. Appl. Entomol. 1980, 19, 141–143. [Google Scholar]
- Mink, G.I. Pollen and Seed-Transmitted Viruses and Viroids. Annu. Rev. Phytopathol. 1993, 31, 375–402. [Google Scholar] [CrossRef]
- Johansen, E.; Edwards, M.C.; Hampton, R.O. Seed Transmission of Viruses: Current Perspectives. Annu. Rev. Phytopathol. 1994, 32, 363–386. [Google Scholar] [CrossRef]
- Satta, E.; Paltrinieri, S.; Bertaccini, A. Phytoplasma Transmission by Seed. In Phytoplasmas: Plant Pathogenic Bacteria—II: Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 131–147. ISBN 978-981-13-2832-9. [Google Scholar]
- Nečas, T.; Mašková, V.; Krška, B. The Possibility of ESFY Phytoplasma Transmission: Through Flowers and Seeds. Acta Hortic. 2008, 781, 443–448. [Google Scholar] [CrossRef]
- Çağlar, B.K.; Satar, S.; Bertaccini, A.; Elbeaino, T. Detection and Seed Transmission of Bermudagrass Phytoplasma in Maize in Turkey. J. Phytopathol. 2019, 167, 248–255. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Salari, M.; Zamharir, M.G.; Ghorbani, M. Detection and Identification of a Phytoplasma Associated with Rapeseedfasciation in Iran. Phytopathogenic Mollicutes 2021, 11, 125–130. [Google Scholar] [CrossRef]
- Mateeti, S.T.; Checchi, G.; Messina, N.A.; Feduzi, G.; Bertaccini, A.; Contaldo, N. Presence and Seed Transmission of Phytoplasmas in Tomato Fields in Italy. Phytopathogenic Mollicutes 2022, 12, 1–6. [Google Scholar] [CrossRef]
- Cordova, I.; Jones, P.; Harrison, N.A.; Oropeza, C. In Situ PCR Detection of Phytoplasma DNA in Embryos from Coconut Palms with Lethal Yellowing Disease. Mol. Plant Pathol. 2003, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Eziashi, E.; Omamor, I. Lethal Yellowing Disease of the Coconut Palms (Cocos nucifera L.): An Overview of the Crises. Afr. J. Biotechnol. 2011, 9, 9122–9127. [Google Scholar]
- Wijesekara, T.; Perera, L.; Wickramananda, I.; Herath, I.; Meegahakumbura, M.; Fernando, W.B.S.; De Silva, P.H.P.R. Preliminary Investigation on Weligama Coconut Leaf Wilt Disease: A New Disease in Southern Part of Sri Lanka. In Second Plantation Crop Symposium, Proceedings of the Proceedings of the Second Plantation Crop Symposium, Sri Lanka, January 2008; Nainanayake, N.A.P.D., Everard, J.M.D.T., Eds.; Samayawardhana Printers: Colombo, Sri Lanka, 2008. [Google Scholar]
- Solomon, J.J.; Hegde, V.; Babu, M.; Geetha, L. Phytoplasmal Diseases. In The Coconut Palm (Cocos nucifera L.)—Research and Development Perspectives; Krishnakumar, V., Thampan, P.K., Nair, M.A., Eds.; Springer: Singapore, 2018; pp. 519–556. ISBN 9789811327537. [Google Scholar]
- Wijesekara, H.T.R.; Perera, S.A.C.N.; Bandupriya, D.; Meegahakumbura, M.K.; Perera, L. Detection of Eligama Coconut Leaf Wilt Disease Phytoplasma by Real-Time Polymerase Chain Reaction. CORD 2020, 36, 11–15. [Google Scholar] [CrossRef]
- Pilet, F.; Philippe, R.; Reignard, S.; Descamps, S.; Quaicoe, R.; Nkansa-Poku, J.; Fabre, S.; Dollet, M. Identification of Potential Insect Vectors of the Cape Saint Paul Wilt Disease of Coconut in Ghana by PCR. OCL 2009, 16, 107–110. [Google Scholar] [CrossRef]
- Randles, J.W.; Miller, D.C.; Morin, J.P.; Rohde, W.; Hanold, D. Localisation of Coconut Foliar Decay Virus in Coconut Palm. Ann. Appl. Biol. 1992, 121, 601–617. [Google Scholar] [CrossRef]
- Hanold, D.; Randles, J.W. Coconut Cadang-Cadang Disease and Its Viroid Agent. Plant Dis. 1991, 75, 330. [Google Scholar] [CrossRef]
- Pacumbaba, E.P.; Zelazny, B.; Orense, J.C.; Rillo, E.P. Evidence for Pollen and Seed Transmission of the Coconut Cadang-cadang Viroid in Cocos nucifera. J. Phytopathol. 1994, 142, 37–42. [Google Scholar] [CrossRef]
- Bonfiglioli, R.G.; Webb, D.R.; Symons, R.H. Tissue and Intra-cellular Distribution of Coconut Cadang Cadang Viroid and Citrus Exocortis Viroid Determined by In Situ Hybridization and Confocal Laser Scanning and Transmission Electron Microscopy. Plant J. 1996, 9, 457–465. [Google Scholar] [CrossRef]
- Vadamalai, G.; Thanarajoo, S.S.; Joseph, H.; Kong, L.L.; Randles, J.W. Coconut Cadang-Cadang Viroid and Coconut Tinangaja Viroid. In Viroids and Satellites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 263–273. ISBN 978-0-12-801498-1. [Google Scholar]
- Gergerich, R.C.; Dolja, V.V. Introduction to Plant Viruses, the Invisible Foe. Plant Health Instr. 2006, 06. [Google Scholar] [CrossRef]
- Randles, J.W.; Wefels, E.; Hanold, D.; Miller, D.C.; Morin, J.P.; Rohde, W. Detection and Diagnosis of Coconut Foliar Decay Disease. In Current Advances in Coconut Biotechnology; Oropeza, C., Verdeil, J.L., Ashburner, G.R., Cardeña, R., Santamaría, J.M., Eds.; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrecht, The Netherlands, 1999; Volume 35, pp. 247–258. ISBN 978-90-481-5265-0. [Google Scholar]
- Rodriguez, M.J.B.; Hanold, D.; Morin, J.P.; Labouisse, J.P.; Randles, J.W.; Tsai, J.H.; Harrison, N.A. Coconut and Other Palm Trees. In Virus and Virus-Like Diseases of Major Crops in Developing Countries; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 567–606. ISBN 978-94-010-3729-7. [Google Scholar]
- Pagán, I. Transmission through Seeds: The Unknown Life of Plant Viruses. PLoS Pathog. 2022, 18, e1010707. [Google Scholar] [CrossRef] [PubMed]
- Diener, T.O. Potato Spindle Tuber “Virus”: IV. A Replicating, Low Molecular Weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Yanagisawa, H.; Sano, T. Vertical and Horizontal Transmission of Pospiviroids. Viruses 2018, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.W. Seed, Pollen, and Insect Transmission of Viroids. In Viroids and Satellites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 521–530. ISBN 978-0-12-801498-1. [Google Scholar]
- Chung, B.-N.; Pak, H.-S. Seed Transmission of Chrysanthemum Stunt Viroid in Chrysanthemum. Plant Pathol. J. 2008, 24, 31–35. [Google Scholar] [CrossRef]
- Bhuvitarkorn, S.; Reanwarakorn, K. Pollen and Seed Transmission of Columnea Latent Viroid in Eggplants. Eur. J. Plant Pathol. 2019, 154, 1067–1075. [Google Scholar] [CrossRef]
- Joseph, H.; Vandamalai, G.; Abdullah, S.N.A.; Lau, W.H.; Randles, J.W.; Abu Seman, I. Transmission of Coconut Cadang-Cadang Viroid Oil Palm Variants to Oil Palm Tissue Culture Plantlets. Borneo Akad. 2017, 2, 28–34. [Google Scholar]
- Hadidi, A.; Sun, L.; Randles, J.W. Modes of Viroid Transmission. Cells 2022, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.K. Anita Karun Coconut Embryo Culture: Present Status and Future Thrust. CORD 1999, 15, 34. [Google Scholar] [CrossRef]
- Biddle, J.; Nguyen, Q.; Mu, Z.; Foale, M.; Adkins, S. Germplasm Reestablishment and Seedling Production: Embryo Culture. In Coconut Biotechnology: Towards the Sustainability of the ‘Tree of Life’; Adkins, S., Foale, M., Bourdeix, R., Nguyen, Q., Biddle, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 199–225. ISBN 978-3-030-44988-9. [Google Scholar]
- Okogbenin, E.; Kahya, S.; Fregene, M. Use of Biotechnology Tools in Cassava Breeding. In The Cassava Handbook: A Reference Manual Based on the Asian Regional Cassava Training Course, Held in Thailand; Howeler, R.H., Ed.; Centro Internacional de Agricultura Tropical (CIAT): Bangkok, Thailand, 2012; pp. 119–145. [Google Scholar]
- Snyman, S.J.; Meyer, G.M.; Koch, A.C.; Banasiak, M.; Watt, M.P. Applications of In Vitro Culture Systems for Commercial Sugarcane Production and Improvement. Vitr. Cell. Dev. Biol. Plant 2011, 47, 234–249. [Google Scholar] [CrossRef]
- Muthoni, J.; Shimelis, H.; Melis, R. Long-Term Conservation of Potato Genetic Resources: Methods and Status of Conservation. Aust. J. Crop Sci. 2019, 13, 717–725. [Google Scholar] [CrossRef]
- Grout, B.W.W. Meristem-Tip Culture for Propagation and Virus Elimination. In Plant Cell Culture Protocols; Hall, R.D., Ed.; Humana Press: Totowa, NJ, USA, 1999; pp. 115–125. ISBN 978-1-59259-583-9. [Google Scholar]
- Raychaudhuri, S.P. Plant Viruses in Tissue Culture. In Advances in Virus Research; Smith, K.M., Lauffer, M.A., Eds.; Academic Press: Cambridge, MA, USA, 1967; Volume 12, pp. 175–206. ISBN 0065-3527. [Google Scholar]
- Faccioli, G. Control of Potato Viruses Using Meristem and Stem-Cutting Cultures, Thermotherapy and Chemotherapy. In Virus and Virus-Like Diseases of Potatoes and Production of Seed-Potatoes; Loebenstein, G., Berger, P.H., Brunt, A.A., Lawson, R.H., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 365–390. ISBN 978-94-010-3736-5. [Google Scholar]
- AlMaarri, K.; Massa, R.; AlBiski, F. Evaluation of Some Therapies and Meristem Culture to Eliminate Potato Y Potyvirus from Infected Potato Plants. Plant Biotechnol. J. 2012, 29, 237–243. [Google Scholar] [CrossRef]
- Islam, N.; Islam, T.; Hossain, M.M.; Bhattacharjee, B.; Hossain, M.M.; Islam, S.S. Embryogenic Callus Induction and Efficient Plant Regeneration in Three Varieties of Soybean (Glycine max). Plant Tissue Cult. Biotech. 2017, 27, 41–50. [Google Scholar] [CrossRef]
- Bazrafshan, A. Ex Situ Conservation of Coconut (Cocos nucifera L.) Germplasm Using Cryopresevation. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2022. [Google Scholar]
- Gambino, G.; Bondaz, J.; Gribaudo, I. Detection and Elimination of Viruses in Callus, Somatic Embryos and Regenerated Plantlets of Grapevine. Eur. J. Plant Pathol. 2006, 114, 397–404. [Google Scholar] [CrossRef]
- Kereša, S.; Kurtović, K.; Ban, S.G.; Vončina, D.; Jerčić, I.H.; Bolarić, S.; Lazarević, B.; Godena, S.; Ban, D.; Mihovilović, A.B. Production of Virus-Free Garlic Plants through Somatic Embryogenesis. Agronomy 2021, 11, 876. [Google Scholar] [CrossRef]
- Parmessur, Y.; Aljanabi, S.; Saumtally, S.; Dookun-Saumtally, A. Sugarcane Yellow Leaf Virus and Sugarcane Yellows Phytoplasma: Elimination by Tissue Culture: Yellow Leaf Syndrome in Sugarcane. Plant Pathol. 2002, 51, 561–566. [Google Scholar] [CrossRef]
- Gambino, G.; Navarro, B.; Vallania, R.; Gribaudo, I.; Di Serio, F. Somatic Embryogenesis Efficiently Eliminates Viroid Infections from Grapevines. Eur. J. Plant Pathol. 2011, 130, 511–519. [Google Scholar] [CrossRef]
- Vicient, C.M.; Martínez, F.X. The Potential Uses of Somatic Embryogenesis in Agroforestry Are Not Limited to Synthetic Seed Technology. Rev. Bras. Fisiol. Veg. 1998, 10, 1–12. [Google Scholar]
- Olah, R.; Turcsan, M.; Olah, K.; Farkas, E.; Deak, T.; Jahnke, G.; Sardy, D.A.N. Somatic Embryogenesis: A Tool for Fast and Reliable Virus and Viroid Elimination for Grapevine and Other Plant Species. Horticulturae 2022, 8, 508. [Google Scholar] [CrossRef]
- Kalaipandian, S.; Mu, Z.; Kong, E.Y.Y.; Biddle, J.; Cave, R.; Bazrafshan, A.; Wijayabandara, K.; Beveridge, F.C.; Nguyen, Q.; Adkins, S.W. Cloning Coconut via Somatic Embryogenesis: A Review of the Current Status and Future Prospects. Plants 2021, 10, 2050. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Davis, R. Prospects for In Vitro Culture of Plant-Pathogenic Mycoplasmalike Organisms. Ann. Rev. Phytopathol. 1986, 24, 339–354. [Google Scholar] [CrossRef]
- Cousin, M.-T.; Roux, J.; Millet, N.; Michel, M.-F. Maintenance of MLOs (Mycoplasma-like Organisms) on Populus alba Micropropagation. J. Phytopathol. 1990, 130, 17–23. [Google Scholar] [CrossRef]
- Bertaccini, A.; Davis, R.E.; Lee, I.M. In Vitro Micropropagation for Maintenance of Mycoplasma-like Organisms in Infected Plant Tissues. HortScience 1992, 27, 1041–1043. [Google Scholar] [CrossRef]
- Eden-Green, S.J. Attempts to Transmit Lethal Yellowing Disease of Coconuts in Jamaica by Leafhoppers (Homoptera: Cicadelloidea). Trop. Agric. 1979, 56. Available online: https://journals.sta.uwi.edu/ojs/index.php/ta/article/view/2949 (accessed on 23 June 2024).
- Howard, F.W.; Thomas, D.L. Transmission of Palm Lethal Decline to Veitchia merrillii by a Planthopper Myndus crudus. J. Econ. Entomol. 1980, 73, 715–717. [Google Scholar] [CrossRef]
- Narváez, M.; Nic-Matos, G.; Oropeza, C. In Vitro Transmission of 16SrIV Phytoplasmas to Coconut Plants by Haplaxius crudus in Yucatan, Mexico. 3 Biotech 2022, 12, 5. [Google Scholar] [CrossRef]
- Vies, D.L.; Clark, M.F. Maintenance of Mycoplasma-like Organisms Occurring in Pyrus Species by Micropropagation and Their Elimination by Tetracycline Therapy. Plant Pathol. 1994, 43, 819–823. [Google Scholar] [CrossRef]
- Kausche, G.A.; Pfankuch, E.; Ruska, H. Die Sichtbarmachung von Pflanzlichem Virus Im Übermikroskop. Naturwissenschaften 1939, 27, 292–299. [Google Scholar] [CrossRef]
- Yang, K.; Shen, W.; Li, Y.; Li, Z.; Miao, W.; Wang, A.; Cui, H. Areca Palm Necrotic Ringspot Virus, Classified within a Recently Proposed Genus Arepavirus of the Family Potyviridae, Is Associated with Necrotic Ringspot Disease in Areca Palm. Phytopathology 2019, 109, 887–894. [Google Scholar] [CrossRef]
- Williamson, D.H.; Fennell, D.J. The Use of Fluorescent DNA-Binding Agent for Detecting and Separating Yeast Mitochondrial DNA. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 1975; Volume 12, pp. 335–351. ISBN 978-0-12-564112-8. [Google Scholar]
- Schuiling, M.; Förstel-Neuhaus, A. The Use of the Fluorochroms DAPI in the Diagnose of Lethal Disease of Coconut Palm (Cocos nucifera) in Tanzania / Die Anwendung Des Zur Fluorochroms DAPI Zur Diagnose Der Letalen Erkrankung von Kokospalmen (Cocos nucifera) in Tanzania. J. Plant Dis. Prot. 1992, 99, 614–616. [Google Scholar]
- Alaa, H.; Waleed, K.; Samir, M.; Tarek, M.; Sobeah, H.; Abdul, M. An Intelligent Approach for Detecting Palm Trees Diseases Using Image Processing and Machine Learning. Int. J. Adv. Comput. Sci. Appl. 2020, 11, 434–441. [Google Scholar] [CrossRef]
- Culman, M.; Delalieux, S.; Van Tricht, K. Individual Palm Tree Detection Using Deep Learning on RGBimagery to Support Tree Inventory. Remote Sens. Lett. 2020, 12, 3476. [Google Scholar] [CrossRef]
- Delalieux, S.; Hardy, T.; Ferry, M.; Gomez, S.; Kooistra, L.; Culman, M.; Tits, L. Red Palm Weevil Detection in Date Palm Using Temporal UAV Imagery. Remote Sens. Lett. 2023, 15, 1380. [Google Scholar] [CrossRef]
- Venbrux, M.; Crauwels, S.; Rediers, H. Current and Emerging Trends in Techniques for Plant Pathogen Detection. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- MSasikala, M.; Prakash, V.R.; Sapna, V.P.; Mayilvaganan, M.; Nair, L.S. Nair Refinement of ELISA and Its Use in Early Detection of Coconut Root (Wilt) Disease. CORD 2005, 21, 34. [Google Scholar] [CrossRef]
- Sasikala, M.; Rajeev, G.; Prakash, V.; Amith, S. Modified Protocol of ELISA for Rapid Detection of Coconut Root (Wilt) Disease. J. Plant. Crops 2010, 38, 16–19. [Google Scholar]
- Kanatiwela-de Silva, C.; Damayanthi, M.; de Silva, N.; Wijesekera, R.; Dickinson, M.; Weerakoon, D.; Udagama, P. Immunological Detection of the Weligama Coconut Leaf Wilt Disease Associated Phytoplasma: Development and Validation of a Polyclonal Antibody Based Indirect ELISA. PLoS ONE 2019, 14, e0214983. [Google Scholar] [CrossRef] [PubMed]
- Ishiie, T.; Doi, Y.; Yora, K.; Asuyama, H. Suppressive Effects of Antibiotics of Tetracycline Group on Symptom Development of Mulberry Dwarf Disease. Jpn. J. Phytopathol. 1967, 33, 267–275. [Google Scholar] [CrossRef]
- McCoy, R. Effect of Various Antibiotics on Development of Lethal Yellowing in Coconut Palm. In Proceedings of the Florida State Horticultural Society; 1973; Volume 86, pp. 503–505. Available online: https://journals.flvc.org/fshs/article/download/98893/94879 (accessed on 23 June 2024).
- López, M.M.; Llop, P.; Olmos, A.; Marco-Noales, E.; Cambra, M.; Bertolini, E. Are Molecular Tools Solving the Challenges Posed by Detection of Plant Pathogenic Bacteria and Viruses? Curr. Issues Mol. Biol. 2009, 11, 13–46. [Google Scholar] [CrossRef] [PubMed]
- Roslan, N.D.; Meilina, O.A.; Mohamed-Azni, I.-N.A.; Seman, I.A.; Sundram, S. Comparison of RNA Extraction Methods for RT-PCR Detection of Coconut cadang-cadang viroid Variant in Orange Spotting Oil Palm Leaves. Can. J. Plant Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef]
- Vadamalai, G. An Investigation of Oil Palm Orange Spotting Disorder. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2005. [Google Scholar]
- Nejat, N.; Vadamalai, G. Phytoplasma Detection in Coconut Palm and Other Tropical Crops. Plant Pathol. J. 2010, 9, 112–121. [Google Scholar] [CrossRef][Green Version]
- Myrie, W.; Harrison, N.; Dollet, M. Molecular Detection and Characterization of Phytoplasmas Associated with Lethal Yellowing Disease of Coconut Palms in Jamaica. Bull. Insectology 2007, 60, 159–160. [Google Scholar]
- Nejat, N.; Sijam, K.; Abdullah, S.N.A.; Vadamalai, G.; Dickinson, M. First Report of a 16SrXIV, ‘Candidatus Phytoplasma Cynodontis’ Group Phytoplasma Associated with Coconut Yellow Decline in Malaysia. Plant Pathol. 2009, 58, 389. [Google Scholar] [CrossRef]
- Wijerathna, Y. Application of Biotechnology in Coconut (Cocos nucifera L.): Sri Lanka. Plant Tissue Cult. Biotech. 2015, 25, 103–116. [Google Scholar] [CrossRef]
- Wefels, E.; Morin, J.P.; Randles, J.W. Molecular Evidence for a Persistent-Circulative Association between Coconut Foliar Decay Virus and Its Vector Myndus Taffini. Australas. Plant Pathol. 2015, 44, 283–288. [Google Scholar] [CrossRef]
- Jones, S.; Baizan-Edge, A.; MacFarlane, S.; Torrance, L. Viral Diagnostics in Plants Using Next Generation Sequencing: Computational Analysis in Practice. Front. Plant Sci. 2017, 8, 1770. [Google Scholar] [CrossRef]
- Lozano, I.; Morales, F.J.; Martinez, A.K.; Peña, E.A. Molecular Characterization and Detection of African Oil Palm Ringspot Virus. J. Phytopathol. 2010, 158, 167–172. [Google Scholar] [CrossRef]
- Porter-Jordan, K.; Rosenberg, E.I.; Keiser, J.F.; Gross, J.D.; Ross, A.M.; Nasim, S.; Garrett, C.T. Nested Polymerase Chain Reaction Assay for the Detection of Cytomegalovirus Overcomes False Positives Caused by Contamination with Fragmented DNA. J. Med. Virol. 1990, 30, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Balfe, P.; Peutherer, J.F.; Ludlam, C.A.; Bishop, J.O.; Brown, A.J. Human Immunodeficiency Virus-Infected Individuals Contain Provirus in Small Numbers of Peripheral Mononuclear Cells and at Low Copy Numbers. J. Virol. 1990, 64, 864–872. [Google Scholar] [CrossRef]
- Marzachì, C. Molecular Diagnosis of Phytoplasmas. Phytopathol. Mediterr. 2004, 43, 228–231. [Google Scholar] [CrossRef]
- De Silva, P.R.; Perera, C.N.; Bahder, B.W.; Attanayake, R.N. Nested PCR-Based Rapid Detection of Phytoplasma Leaf Wilt Disease of Coconut in Sri Lanka and Systemic Movement of the Pathogen. Pathogens 2023, 12, 294. [Google Scholar] [CrossRef]
- Nipah, J.; Jones, P.; Hodgetts, J.; Dickinson, M. Detection of Phytoplasma DNA in Embryos from Coconut Palms in Ghana, and Kernels from Maize in Peru. Bull. Insectology 2007, 60, 385–386. [Google Scholar]
- Manimekalai, R.; Nair, S.; Soumya, V.P.; Roshna, O.M.; Thomas, G.V. Real-Time PCR Technique-Based Detection of Coconut Root (Wilt) Phytoplasma. Curr. Sci. 2011, 101, 1209–1213. [Google Scholar]
- Manimekalai, R.; Soumya, V.P.; Sathish Kumar, R.; Selvarajan, R.; Reddy, K.; Thomas, G.V.; Sasikala, M.; Rajeev, G.; Baranwal, V.K. Molecular Detection of 16SrXI Group Phytoplasma Associated with Root (Wilt) Disease of Coconut (Cocos nucifera) in India. Plant Dis. 2010, 94, 636. [Google Scholar] [CrossRef]
- Córdova, I.; Oropeza, C.; Puch-Hau, C.; Harrison, N.; Collí-Rodríguez, A.; Narvaez, M.; Nic-Matos, G.; Reyes, C.; Sáenz, L. A Real-Time PCR Assay for Detection of Coconut Lethal Yellowing Phytoplasmas of Group 16SrIV Subgroups A, D and E Found in the Americas. Plant Pathol. J. 2014, 96, 343–352. [Google Scholar] [CrossRef]
- Córdova, I.; Oropeza, C.; Harrison, N.; Ku-Rodríguez, S.; Puch-Hau, C.; Narváez, M.; Sáenz, L. Simultaneous Detection of Coconut Lethal Yellowing Phytoplasmas (Group 16SrIV) by Real-Time PCR Assays Using 16Sr- and GroEL-Based TaqMan Probes. Plant Pathol. J. 2019, 101, 609–619. [Google Scholar] [CrossRef]
- Roslan, N.D. Comparison of Real-Time PCR, Conventional PCR and RT-LAMP for the Detection of Coconut Cadang-Cadang Viroid Variant in Oil Palm. J. Oil Palm Res. 2022, 35, 121–132. [Google Scholar] [CrossRef]
- Quan, P.-L.; Sauzade, M.; Brouzes, E. dPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef]
- Morcia, C.; Ghizzoni, R.; Delogu, C.; Andreani, L.; Carnevali, P.; Terzi, V. Digital PCR: What Relevance to Plant Studies? Biology 2020, 9, 433. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, Q.; Zhang, Y.; Shen, W.; Li, R.; Cao, M.; Chen, L.; Li, X.; Zhou, C.; et al. Development of a Sensitive and Reliable Reverse Transcription Droplet Digital PCR Assay for the Detection of Citrus Yellow Vein Clearing Virus. Arch. Virol. 2019, 164, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Leichtfried, T.; Reisenzein, H.; Steinkellner, S.; Gottsberger, R.A. Transmission Studies of the Newly Described Apple Chlorotic Fruit Spot Viroid Using a Combined RT-qPCR and Droplet Digital PCR Approach. Arch. Virol. 2020, 165, 2665–2671. [Google Scholar] [CrossRef]
- Wanjala, B.W.; Ateka, E.M.; Miano, D.W.; Fuentes, S.; Perez, A.; Low, J.W.; Kreuze, J.F. Loop-Mediated Isothermal Amplification Assays for on-Site Detection of the Main Sweetpotato Infecting Viruses. J. Virol. Methods 2021, 298, 114301. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, D. Isothermal Nucleic Acid Amplification and Its Uses in Modern Diagnostic Technologies. 3 Biotech 2023, 13, 200. [Google Scholar] [CrossRef]
- Fakruddin, M.; Mannan, K.B.; Chowdhury, A.; Mazumdar, R.; Hossain, M.; Islam, S.; Chowdhury, M. Nucleic Acid Amplification: Alternative Methods of Polymerase Chain Reaction. J. Pharm. Bioallied Sci. 2013, 5, 245. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef]
- Vadamalai, G.; Hanold, D.; Rezaian, M.A.; Randles, J.W. Variants of Coconut Cadang-Cadang Viroid Isolated from an African Oil Palm (Elaies guineensis Jacq.) in Malaysia. Arch. Virol. 2006, 151, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Vadamalai, G.; Perera, A.A.F.L.K.; Hanold, D.; Rezaian, M.A.; Randles, J.W. Detection of Coconut Cadang-cadang Viroid Sequences in Oil and Coconut Palm by Ribonuclease Protection Assay. Ann. Appl. Biol. 2009, 154, 117–125. [Google Scholar] [CrossRef]
- Gronenborn, B.; Randles, J.W.; Knierim, D.; Barrière, Q.; Vetten, H.J.; Warthmann, N.; Cornu, D.; Sileye, T.; Winter, S.; Timchenko, T. Analysis of DNAs Associated with Coconut Foliar Decay Disease Implicates a Unique Single-Stranded DNA Virus Representing a New Taxon. Sci. Rep. 2018, 8, 5698. [Google Scholar] [CrossRef]
- Nair, S.; Manimekalai, R.; Ganga Raj, P.; Hegde, V. Loop Mediated Isothermal Amplification (LAMP) Assay for Detection of Coconut Root Wilt Disease and Arecanut Yellow Leaf Disease Phytoplasma. World J. Microbiol. Biotechnol. 2016, 32, 108. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; Van Der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in Virus Diagnostics: From ELISA to next Generation Sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Bömer, M.; Rathnayake, A.I.; Visendi, P.; Sewe, S.O.; Sicat, J.P.A.; Silva, G.; Kumar, P.L.; Seal, S.E. Tissue Culture and Next-Generation Sequencing: A Combined Approach for Detecting Yam (Dioscorea spp.) Viruses. Physiol. Mol. Plant Pathol. 2019, 105, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Wambua, L.; Wanga, J.O.; Imali, O.; Wambua, P.N.; Agutu, L.; Olds, C.; Jones, C.S.; Masiga, D.; Midega, C.; Khan, Z.; et al. Development of Field-Applicable Tests for Rapid and Sensitive Detection of Candidatus Phytoplasma Oryzae. Mol. Cell. Probes 2017, 35, 44–56. [Google Scholar] [CrossRef]
- Jo, Y.; Lian, S.; Chu, H.; Cho, J.K.; Yoo, S.-H.; Choi, H.; Yoon, J.-Y.; Choi, S.-K.; Lee, B.C.; Cho, W.K. Peach RNA Viromes in Six Different Peach Cultivars. Sci. Rep. 2018, 8, 1844. [Google Scholar] [CrossRef]
- Bertaccini, A. Containment of Phytoplasma-Associated Plant Diseases by Antibiotics and Other Antimicrobial Molecules. Antibiotics 2021, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.-P.; Mahillon, J.; Bragard, C. On the Use of Antibiotics to Control Plant Pathogenic Bacteria: A Genetic and Genomic Perspective. Front. Microbiol. 2023, 14, 1221478. [Google Scholar] [CrossRef]
- McCoy, R.E.; Carroll, V.J.; Poucher, C.P.; Gwin, G.H. Field Control of Coconut Lethal Yellowing with Oxytetracycline Hydrochloride. Phytopathology 1976, 66, 1148–1150. [Google Scholar] [CrossRef]
- Hocher, V.; Verdeil, J.L.; Malaurie, B. Cocos nucifera Coconut. In Biotechnology of Fruit and Nut Crops; Litz, R.E., Ed.; CAB International: Wallingford, UK, 2005; pp. 90–112. ISBN 978-0-85199-066-8. [Google Scholar]
- AMRPC Steering Group Australian One Health Antimicrobial Resistance Colloquium Background Paper 2013. Available online: https://www.safetyandquality.gov.au/publications-and-resources/resource-library/australian-one-health-antimicrobial-resistance-colloquium-background-paper-july-2013 (accessed on 23 June 2024).
- Marković, Z.; Chatelet, P.; Sylvestre, I.; Kontić, J.K.; Engelmann, F. Cryopreservation of Grapevine (Vitis vinifera L.) In Vitro Shoot Tips. Cent. Eur. J. Biol. 2013, 8, 993–1000. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Costa, M.D.; Gardin, J.P.P.; Kretzschmar, A.A.; Pathirana, R. Cryotherapy: A New Technique to Obtain Grapevine Plants Free of Viruses. Rev. Bras. Frutic. 2016, 38, e-833. [Google Scholar] [CrossRef]
- van Slogteren, E. The Influence of Different Temperatures on Development, Growth and Flowering of Hyacinths, Tulips and Daffodils. Gartenbauwissenschaft 1937, 11, 17–34. [Google Scholar]
- Wang, M.-R.; Cui, Z.-H.; Li, J.-W.; Hao, X.-Y.; Zhao, L.; Wang, Q.-C. In Vitro Thermotherapy-Based Methods for Plant Virus Eradication. Plant Methods 2018, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Laimer, M.; Bertaccini, A. Phytoplasma Elimination from Perennial Horticultural Crops. In Phytoplasmas: Plant Pathogenic Bacteria—II: Transmission and Management of Phytoplasma—Associated Diseases; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 185–206. ISBN 978-981-13-2832-9. [Google Scholar]
- Panis, B. Cryopreservation of Musa Germplasm, 2nd ed.; Technical Guidelines No. 9; Engelmann, F., Benson, E., Eds.; Bioversity International: Montpellier, France, 2009; ISBN 978-2-910810-86-3. [Google Scholar]
- Wang, Q.; Valkonen, J.P. Cryotherapy of Shoot Tips: Novel Pathogen Eradication Method. Trends Plant Sci. 2009, 14, 119–122. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Marković, Z.; Bi, W.; Volk, G.M.; Matsumoto, T.; Wang, Q.-C. Grapevine Shoot Tip Cryopreservation and Cryotherapy: Secure Storage of Disease-Free Plants. Plants 2021, 10, 2190. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-A.; Nguyen, P.T.; Le, M.-A.; Bazrafshan, A.; Sisunandar, S.; Kalaipandian, S.; Adkins, S.W.; Nguyen, Q.T. A Practical Framework for the Cryopreservation of Palm Species. Vitr. Cell. Dev. Biol. Plant 2023, 59, 425–445. [Google Scholar] [CrossRef]
- Wilms, H.; Rhee, J.H.; Rivera, R.L.; Longin, K.; Panis, B. Developing Coconut Cryopreservation Protocols and Establishing Cryo-Genebank at RDA; a Collaborative Project between RDA and Bioversity International. Acta Hortic. 2019, 1234, 343–348. [Google Scholar] [CrossRef]
- Wang, M.-R.; Hamborg, Z.; Ma, X.-Y.; Blystad, D.-R.; Wang, Q.-C. Double-Edged Effects of the Cryogenic Technique for Virus Eradication and Preservation in Shallot Shoot Tips. Plant Pathol. 2022, 71, 494–504. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.; Li, J.; Cui, Z.; Volk, G.M.; Wang, Q. Cryobiotechnology: A Double-Edged Sword for Obligate Plant Pathogens. Plant Dis. 2019, 103, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- FAO Plant Protection Outlook in the Asia-Pacific Region—Including an in-Depth View of the Invasive Fall Armyworm; FAO: Bangkok, Thailand, 2021; ISBN 978-92-5-135264-9.
- Randles, J.; Rodriguez, J.; Vadamalai, G.; Hanold, D.; Perera, L. Coconut Cadang-Cadang Viroid (Cadang Cadang Disease). CABI International. 2008. Available online: https://doi.org/10.1079/cabicompendium.13700 (accessed on 23 June 2024).
- Bioversity International. Validation of a Coconut Embryo-Culture Protocol for the International Exchange of Germplasm; Bioversity International: Rome, Italy, 2012. [Google Scholar]
- Frison, E.A.; Putter, C.; Diekmann, M. FAO/IBPGR Technical Guidelines for the Safe Movement of Coconut Germplasm; Bioversity International: Rome, Italy, 1993; Volume 11, ISBN 92-9043-156-3. [Google Scholar]
- MacLeod, A.; Pautasso, M.; Jeger, M.J.; Haines-Young, R. Evolution of the International Regulation of Plant Pests and Challenges for Future Plant Health. Food Secur. 2010, 2, 49–70. [Google Scholar] [CrossRef]
- Germplasm Health Management for COGENT’s Multi-Site International Coconut Genebank; Ikin, R., Ed.; IPGRI-APO: Serdang, Malaysia, 2004; ISBN 92-9043-630-1. [Google Scholar]
- COGENT. Global Conservation Strategy for Cocos nucifera: A Framework for Promoting the Effective Conservation and Use of Coconut Genetic Resources Developed in Consultation with COGENT Members and Partners; COGENT: Washington, DC, USA, 2008. [Google Scholar]
- Cueto, C.A.; Johnson, V.B.; Engelmann, F.; Kembu, A.; Konan, J.L.; Kouassi Kan, M.; Rivera, R.L.; Vidhanaarachchi, V.; Bourdeix, R.F.; Weise, S.F. Technical Guidelines for the Safe Movement and Duplication of Coconut (Cocos nucifera L.) Germplasm Using Embryo Culture Transfer Protocols; COGENT: Washington, DC, USA; Bioversity International: Montpellier, France, 2012; ISBN 978-92-9043-924-0. [Google Scholar]
- Bourdeix, R.; Prades, A. A Global Strategy for the Conservation and Use of Coconut Genetic Resources, 2018–2028: Summary Brochure; Bioversity International: Montpellier, France, 2018; 29p. [Google Scholar]
- Bourdeix, R.; Sourisseau, J.M. Coconut Risk Management and Mitigation Manual for the Pacific Region; Land Resources Division, Pacific Community (SPC): Noumea, New Caledonia, 2021; ISBN 978-982-00-1429-9. [Google Scholar]
- Randles, J.W. Coconut Foliar Decay Disease and Identification of Its Viral Agent. In Report on ACIAR-Funded Research on Viroids and Viruses of Coconut Palm and Other Tropical Monocotyledons 1985–1993 (ACIAR Working Paper No.51 August 1998); Hanold, D., Randles, J.W., Eds.; Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 1998; pp. 177–187. ISBN 1 86320 219 6. [Google Scholar]
- Card, S.D.; Pearson, M.N.; Clover, G.R.G. Plant Pathogens Transmitted by Pollen. Australas. Plant Pathol. 2007, 36, 455–461. [Google Scholar] [CrossRef]

| Method | Type | Targeted Coconut Pathogens | Source |
|---|---|---|---|
| Field transmission | Artificial transmission test systems | Phytoplasma | [43,81] |
| In vitro transmission | Artificial transmission test systems | Phytoplasma | [20,83] |
| Transmission electron microscope (TEM) | Visual | Viroids, virus, phytoplasma | [86] |
| Fluorescent stain 4′,6-diamidino-2-phenylindole (DAPI). | Visual | Phytoplasma | [88] |
| ELISA assay | Immunological | Phytoplasma | [93,94,95] |
| Antibiotic response | Immunological | Phytoplasma | [97] |
| Hybridization assay | Molecular | Viroids, phytoplasma | [100] |
| Polymerase chain reaction (PCR)-based methods | Molecular | Virus, viroids, phytoplasma | [15,24,83,99,102,103,104,105,111,112,113,114,115,116] |
| Isothermal amplification techniques (IAT) | Molecular | Virus, viroids, phytoplasma | [126,127,128,129] |
| Next-generation sequencing (NGS) | Molecular | Not yet studied in coconut | [106,130] |
| Methods | Targeted Pathogens | Targeted Coconut Pathogens | Source |
|---|---|---|---|
| Chemotherapy | Phytoplasma | Phytoplasma | [136,137] |
| Thermotherapy | Virus | Not yet studied in coconut | [142,143] |
| Cryotherapy | Virus | Not yet studied in coconut | [144,145,146,147] |
| Name of Guideline | Source | Year Published |
|---|---|---|
| FAO/IBPGR Technical Guidelines for the Safe Movement of Coconut Germplasm | [154] | 1993 |
| Germplasm Health Management for COGENT’s Multi-Site International Coconut Genebank | [156] | 2004 |
| COGENT Global Conservation Strategy for Cocos Nucifera: A Framework for Promoting the Effective Conservation and Use of Coconut Genetic Resources Developed in Consultation with COGENT Members and Partners | [157] | 2008 |
| Technical guidelines for the safe movement and duplication of coconut (Cocos nucifera L.) germplasm using embryo culture transfer protocols | [158] | 2012 |
| A Global Strategy for the Conservation and Use of Coconut Genetic Resources, 2018–2028: Summary Brochure | [159] | 2018 |
| Coconut risk management and mitigation manual for the Pacific Region | [160] | 2021 |
| Year | Coconut Germplasm Guidelines | Advances/Gaps in the Understanding of Phytoplasma, Virus and Viroid Diseases |
|---|---|---|
| Before 2003 | Coconut fruit (dehusked and surface-sterilized), some zygotic embryos and pollen were the main internationally exchanged materials. The guidelines stated that pollen had to be tested for the presence of phytoplasma, and de-husked fruit and in vitro cultured embryos needed to be tested for viruses, viroids, or insect pests [154]. The embryos should first be cultured in vitro in the country of origin rather than the receiving country. If the country of origin or the recipient lacks the necessary tissue culture facilities, embryos should be sent to a different, third country for their culture [154]. | Phytoplasma diseases were still largely unexplored. Coconut husk, shell, and endosperm tissues were all shown to contain the Coconut cadang-cadang viroid [45]. DNA of the Coconut foliar decay virus was detected on the coconut fruit husk and in zygotic embryos but was not shown to be transferred through zygotic embryo cultures [161]. |
| 2003–2005 | Germplasm should be distributed in an in vitro form. No strict management guidelines were developed for the donor or recipient countries for zygotic embryos and other in vitro transferred materials. Tissues should not be sourced from disease-stricken areas, and pathogen testing should be undertaken. Pest risk analysis approaches were introduced to assist in pest control [156]. Food and Agriculture Organization/The International Board for Plant Genetic Resources (FAO/IBPGR) released technical guidelines for the safe movement of coconut germplasm, which recommended the transfer of isolated zygotic embryos instead of whole or de-husked fruit [137,156]. | Lethal yellowing phytoplasma DNA was detected in embryos extracted from diseased fruit by in situ PCR [38]. |
| 2005–2012 | The International Coconut Genetic Resources Network (COGENT) adopted the FAO/IBPGR guidelines and incorporated them into its global strategy for 2005–2015 and designated the embryo culture as a key technique to be further refined for the international exchange of coconut germplasm [157]. Pollen transfer was identified as having fewer quarantine complications than other forms of tissue transfer [157]. A general recommendation was made for the transfer of in vitro coconut materials, including a full procedure for the transfer of zygotic embryos. The recommendation was that in vitro germination should be undertaken in the donor country and the ex vitro growth take place in the recipient country [153,158] | Studies show that some embryos taken from phytoplasma-diseased coconut palms do not contain phytoplasma DNA [15,112]. Pollen is reported to transmit several lethal palm virus and viroid diseases [162]. |
| 2012–2020 | Guidelines were developed that required International genebanks to duplicate their collections in other geographical locations due to the threat from phytoplasma diseases [159]. | Phytoplasma DNA was detected in the plumules of in vitro germinated coconut embryos coming from infected fruit [20]. Bogia coconut syndrome and Banana wilt-associated phytoplasma DNA were shown to be introduced to coconut palms when caged with phytoplasma-carrying insects [22]. |
| 2021-present | Pollen transfer was still allowed; however, suspicion was raised about embryo culture; current embryo exchange activities became restricted. Further transmission confirmation research is now considered necessary for zygotic embryo transfer [160]. | An in vitro assay was developed to confirm the transmission of Lethal yellowing phytoplasma by planthoppers (Halaxius crudus) onto in vitro growing coconut seedlings, 16SrIV-group phytoplasmas were detected by nested PCR [83]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Nguyen, V.A.; Nulu, N.P.C.; Kalaipandian, S.; Beveridge, F.C.; Biddle, J.; Young, A.; Adkins, S.W. Towards Pathogen-Free Coconut Germplasm Exchange. Plants 2024, 13, 1809. https://doi.org/10.3390/plants13131809
Yang C, Nguyen VA, Nulu NPC, Kalaipandian S, Beveridge FC, Biddle J, Young A, Adkins SW. Towards Pathogen-Free Coconut Germplasm Exchange. Plants. 2024; 13(13):1809. https://doi.org/10.3390/plants13131809
Chicago/Turabian StyleYang, Chongxi, Van Anh Nguyen, Naga Prafulla Chandrika Nulu, Sundaravelpandian Kalaipandian, Fernanda Caro Beveridge, Julianne Biddle, Anthony Young, and Steve W. Adkins. 2024. "Towards Pathogen-Free Coconut Germplasm Exchange" Plants 13, no. 13: 1809. https://doi.org/10.3390/plants13131809
APA StyleYang, C., Nguyen, V. A., Nulu, N. P. C., Kalaipandian, S., Beveridge, F. C., Biddle, J., Young, A., & Adkins, S. W. (2024). Towards Pathogen-Free Coconut Germplasm Exchange. Plants, 13(13), 1809. https://doi.org/10.3390/plants13131809








