Biomonitoring with the Use of the Herbal Plant Taraxacum officinale as a Source of Information on Environmental Contamination
Abstract
1. Introduction
2. Results and Discussion
2.1. Statistical Analysis
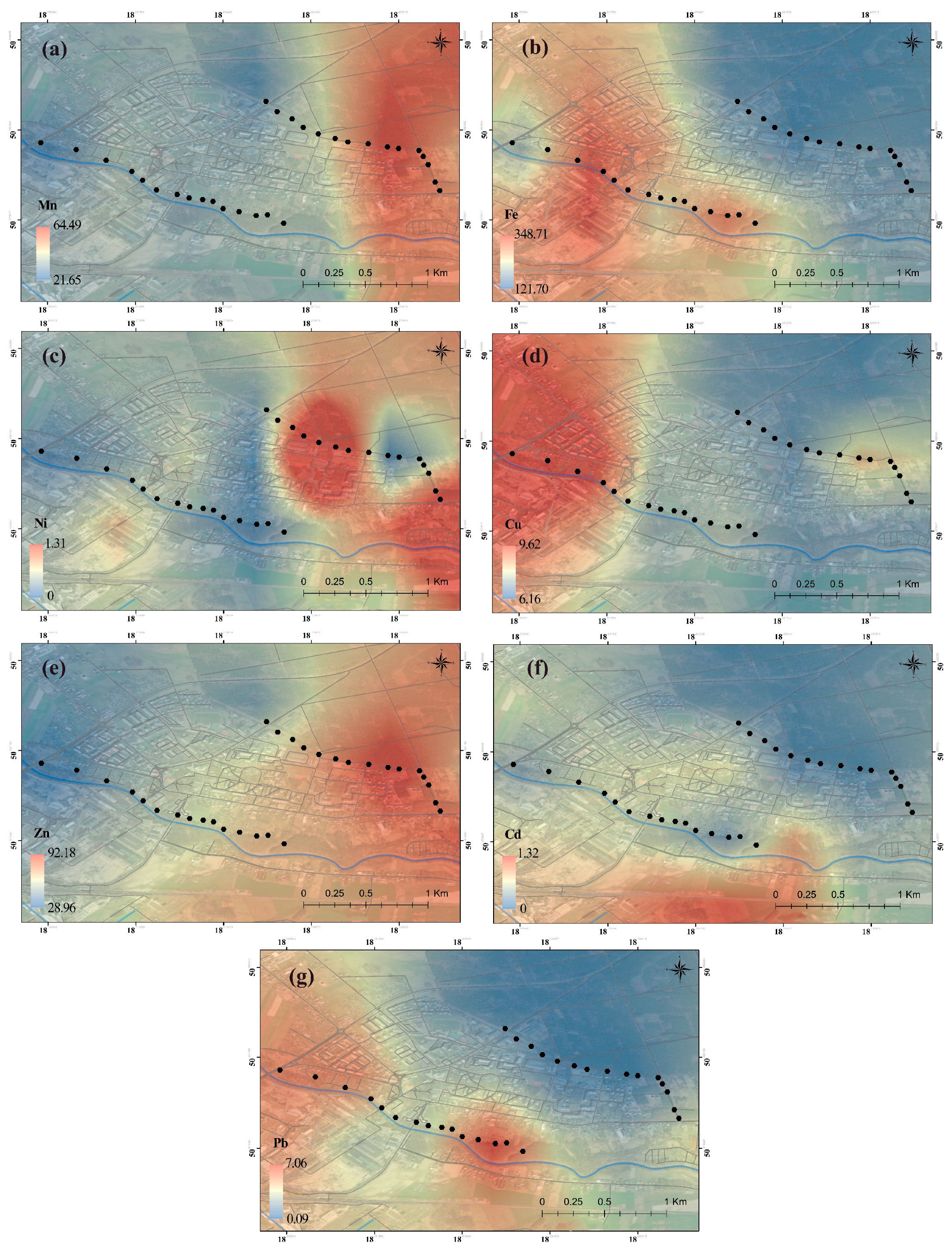
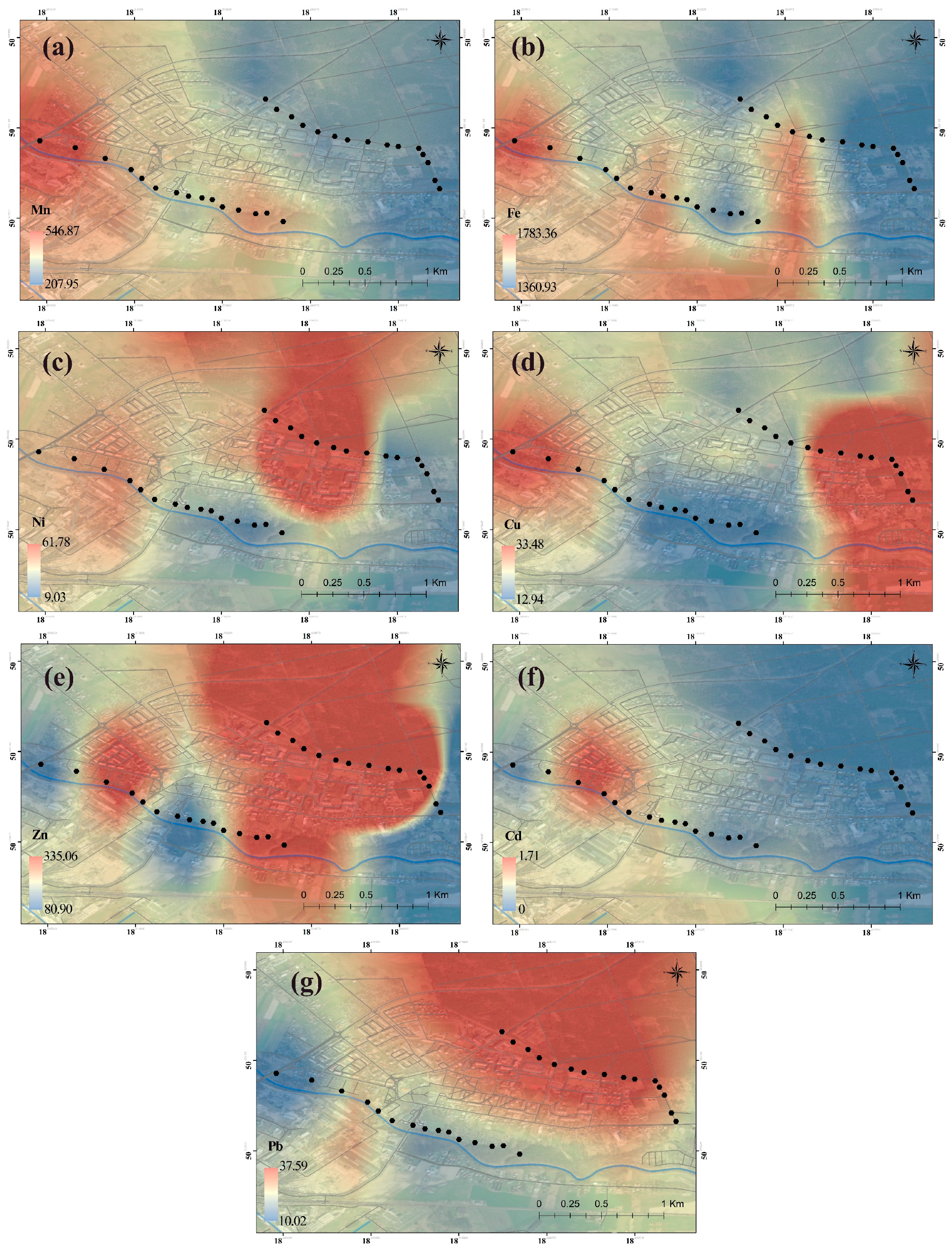
2.2. Spatial Distribution
3. Materials and Methods
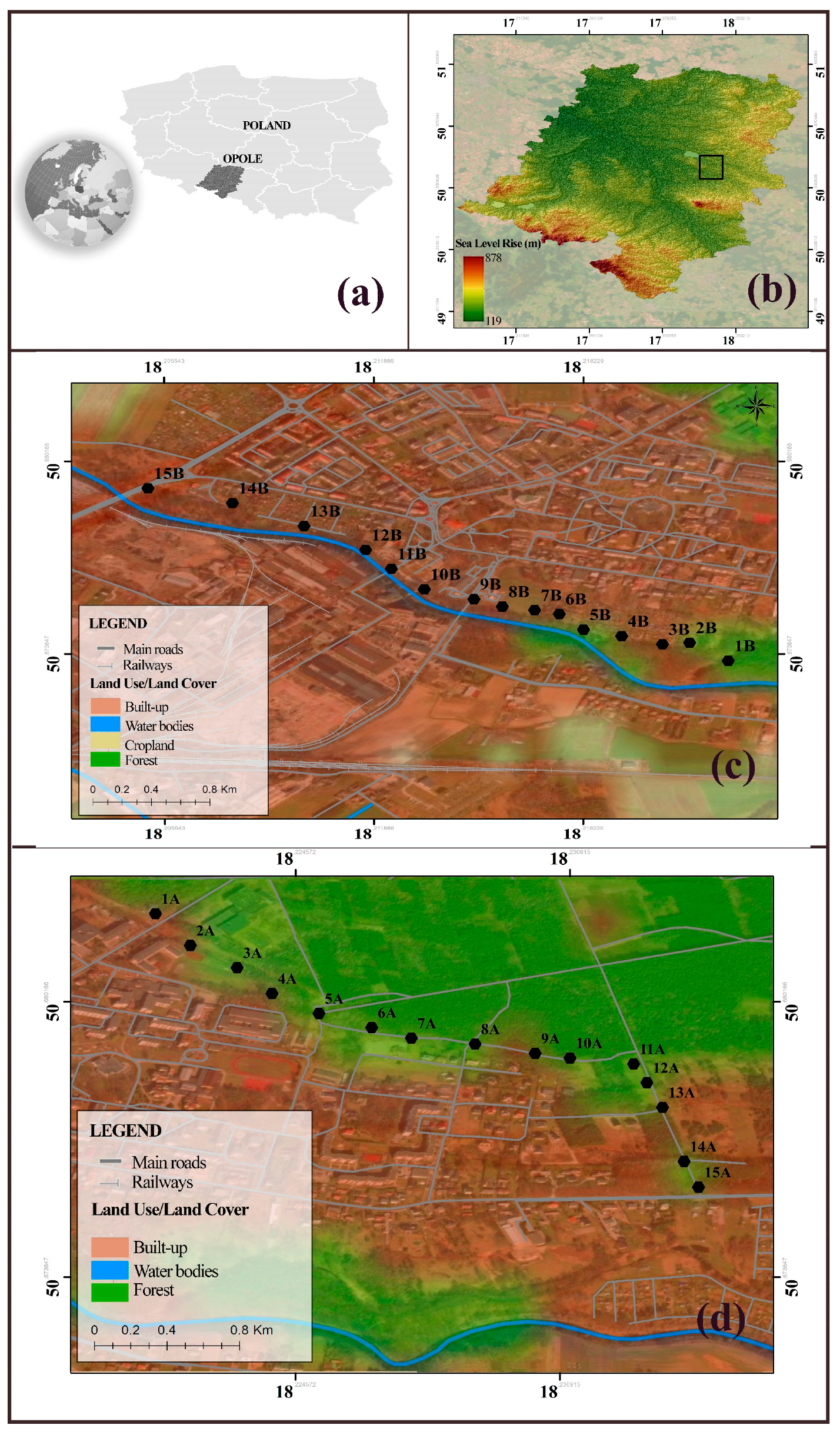
3.1. Study Site
3.2. Passive Biomonitoring and Analytical Procedures
3.3. Quality Control
3.4. Data Processing and Statistics
4. Executive Summary and Conclusions
- (1)
- The concentrations of metals were almost six times higher in soil samples than in dandelion, which is due to the slow process of phytoremediation of soils from metals as well as pollution caused by human anthropogenic activities. The highest concentrations in soil were noted for Fe at 1482 mg/kg d.m. and 1670 mg/kg d.m. in areas A and B, respectively. Nevertheless, in accordance with the Regulation of the European Parliament and of the Council (EC) of 2008, the maximum permissible concentrations of the determined analytes in soil were not exceeded.
- (2)
- In the above-ground parts (leaves, stems) of dandelion, the concentration of Pb and Cd in individual samples exceeded the permitted values given for herbs in the 2023 Commission Regulation.
- (3)
- The dandelion itself and the soil showed the highest concentrations of Mn (52.4 mg/kg d.m.), Fe (635 mg/kg d.m.), and Zn (143 mg/kg d.m.), especially in the contaminated area (B), which is the result of pollutant emissions from the smelter (dust from electric arc furnaces in steel melting, extraction installations in production halls transmitting pollutants into the air from molding sand, or waste from molding and core sand dumped on the heap and blown by the wind from the landfill), but also of high anthropopressure caused by human activity—for example, heating processes or road transport (car exhaust fumes).
- (4)
- The BCF values for Taraxacum officinale showed that Cu and Zn accumulated to a medium degree at both sites A and B, with values from 0.452 to 0.785 [-]. The implication is that dandelion takes up these metals to a moderate to medium degree from the soil.
- (5)
- The study confirmed that the analyzed plant can be used as a bioindicator in passive biomonitoring to assess the degree of environmental pollution by selected elements (Mn, Fe, Ni, Cu, Zn, Cd, and Pb).
- (6)
- Medicinal/herbal plants should only be taken from potentially clean (unpolluted) areas, unencumbered by human anthropogenic activity, for therapeutic/food purposes.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zajęcka, E.; Świercz, A. Biomonitoring of the Urban Environment of Kielce and Olsztyn (Poland) Based on Studies of Total and Bioavailable Lead Content in Soils and Common Dandelion (Taraxacum officinale Agg.). Minerals 2021, 11, 52. [Google Scholar] [CrossRef]
- Świsłowski, P.; Rajfur, M. Mushrooms as Biomonitors of Heavy Metals Contamination in Forest Areas. Ecol. Chem. Eng. S 2018, 25, 557–568. [Google Scholar] [CrossRef]
- Salinitro, M.; Tassoni, A.; Casolari, S.; de Laurentiis, F.; Zappi, A.; Melucci, D. Heavy Metals Bioindication Potential of the Common Weeds Senecio vulgaris L., Polygonum aviculare L. and Poa annua L. Molecules 2019, 24, 2813. [Google Scholar] [CrossRef] [PubMed]
- Isinkaralar, O.; Świsłowski, P.; Isinkaralar, K.; Rajfur, M. Moss as a Passive Biomonitoring Tool for the Atmospheric Deposition and Spatial Distribution Pattern of Toxic Metals in an Industrial City. Environ. Monit. Assess. 2024, 196, 513. [Google Scholar] [CrossRef]
- Levei, L.; Kovacs, E.; Hoaghia, M.A.; Ozunu, A. Accumulation of Heavy Metals in Plantago Major Grown in Urban and Post-Industrial Areas. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 87–98. [Google Scholar] [CrossRef]
- Jonczak, J.; Parzych, A. Bioaccumulation of Macronutrients in Herbaceous Plants of the Sławno Glaciolacustrine Plain, Northern Poland. Acta Fytotech. Zootech. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Petrović, J.V.; Alagić, S.; Milić, S.M.; Tošić, S.B.; Bugarin, M.M. Chemometric Characterization of Heavy Metals in Soils and Shoots of the Two Pioneer Species Sampled near the Polluted Water Bodies in the Close Vicinity of the Copper Mining and Metallurgical Complex in Bor (Serbia): Phytoextraction and Biomonitoring Cont. Chemosphere 2021, 262, 127808. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, N.; Pezo, L.; Terzić, A.; Šerbula, S.; Kovačević, R. The Biometrics Techniques for the Assessment of the Degree of Adoption of Toxic and Essential Elements. Zast. Mater. 2018, 59, 56–66. [Google Scholar] [CrossRef]
- Golia, E.E.; Dimirkou, A.; Floras, S.A. Spatial Monitoring of Arsenic and Heavy Metals in the Almyros Area, Central Greece. Statistical Approach for Assessing the Sources of Contamination. Environ. Monit. Assess. 2015, 187, 399. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Nathanail, P.; Tian, L.; Ma, Y. Integrated GIS and Multivariate Statistical Analysis for Regional Scale Assessment of Heavy Metal Soil Contamination: A Critical Review. Environ. Pollut. 2017, 231, 1188–1200. [Google Scholar] [CrossRef]
- Ryzhenko, N.; Yastrebtsova, N.I.; Ryzhenko, D.I. Cd and Pb in the “Soil-Plant” System of Holosiyiv Green Park Area in Kyiv. Polish J. Soil Sci. 2020, 53, 199–210. [Google Scholar]
- Bielińska, E.J. Soil Enzymes Activity in the Rhizosphere of the Dandelion As an Indicator of the Ecochemical Condition of Urban Soils. J. Res. Appl. Agric. Eng. 2007, 52, 10–14. [Google Scholar]
- Angelova, V.; Ivanov, K. Heavy Metal Content in Dandelion (TARAXACUM OFFICINALE WEB.). Agric. Sci. 2018, 10, 55–61. [Google Scholar] [CrossRef]
- Velimirović, D.; Kaličanin, B.; Stojković, M.; Tošić, S. Determining the Content of Cd, Cu, Pb AND Zn in the Leaves of Dandelion (Taraxacum officinale webb.) and in the Soil by icp-Oes. Acta Medica Median. 2020, 59, 23–30. [Google Scholar] [CrossRef]
- Chemerys, I.; Myslyuk, O.; Chemerys, V. Effect of Vehicle Emissions on the Morphological and Physiological Changes of Taraxacum officinale Web. Ukr. J. Ecol. 2020, 10, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Szabela, D.; Lisowska, K.; Wolf, W.M. Hysteresis of Heavy Metals Uptake Induced in Taraxacum officinale by Thiuram. Sci. Rep. 2021, 11, 20151. [Google Scholar] [CrossRef] [PubMed]
- Levei, L.; Andrei, M.L.; Hoaghia, M.A.; Ozunu, A. Assessment of Metals Content in Dandelion (Taraxacum officinale) Leaves Grown on Mine Tailings. AIP Conf. Proc. 2017, 1917, 020001. [Google Scholar] [CrossRef]
- Romeh, A.A.A. Risk Assessment of Heavy Metals Pollution at Zagazig University, Zagazig, Egypt. Int. J. Environ. Sci. Technol. 2018, 15, 1393–1410. [Google Scholar] [CrossRef]
- Gómez-Arroyo, S.; Cortés-Eslava, J.; Loza-Gómez, P.; Arenas-Huertero, F.; Grutter de la Mora, M.; Morton Bermea, O. In Situ Biomonitoring of Air Quality in Rural and Urban Environments of Mexico Valley through Genotoxicity Evaluated in Wild Plants. Atmos. Pollut. Res. 2018, 9, 119–125. [Google Scholar] [CrossRef]
- Isinkaralar, O.; Isinkaralar, K.; Ambade, B. Assessment of Societal Health Risks: Spatial Distribution and Potential Hazards of Toxic Metals in Street Dust Across Diverse Communities. Water. Air. Soil Pollut. 2024, 235, 302. [Google Scholar] [CrossRef]
- Stančić, Z.; Fiket, Ž.; Vujević, D. Can Urban Grassland Plants Contribute to the Phytoremediation of Soils Contaminated with Heavy Metals. Molecules 2022, 27, 6558. [Google Scholar] [CrossRef]
- Giacomino, A.; Malandrino, M.; Colombo, M.L.; Miaglia, S.; Maimone, P.; Blancato, S.; Conca, E.; Abollino, O. Metal Content in Dandelion (Taraxacum officinale) Leaves: Influence of Vehicular Traffic and Safety upon Consumption as Food. J. Chem. 2016, 2016, 9842987. [Google Scholar] [CrossRef]
- Lidija, B.; Dinko, P.; Vlatka, G.; Domagoj, V.; Dragana, J.; Zdenko, L.; Ada, P.; Eda, P.; Marina, V.; Ida, P.; et al. Presence of War Related Elements in Dandelion (Taraxacum officinale) as a Possible Consequence of Military Activities in East Croatia. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 264–272. [Google Scholar] [CrossRef]
- Pieczka, M.; Świsłowski, P.; Rajfur, M. Heavy Metal Pollution in Matricaria chamomilla L. and Plantago lanceolata L. Proc. ECOpole 2019, 13, 135–143. [Google Scholar] [CrossRef]
- Rajfur, M. Heavy Metals Phytocumulation in Selected Species of Herbs. Proc. ECOpole 2015, 9, 14–16. [Google Scholar] [CrossRef]
- Lisiak-Zielińska, M.; Borowiak, K.; Budka, A.; Kanclerz, J.; Janicka, E.; Kaczor, A.; Żyromski, A.; Biniak-Pieróg, M.; Podawca, K.; Mleczek, M.; et al. How Polluted Are Cities in Central Europe?—Heavy Metal Contamination in Taraxacum officinale and Soils Collected from Different Land Use Areas of Three Representative Cities. Chemosphere 2021, 266, 129113. [Google Scholar] [CrossRef]
- Nadgórska–Socha, A.; Kandziora-Ciupa, M.; Trzęsicki, M.; Barczyk, G. Air Pollution Tolerance Index and Heavy Metal Bioaccumulation in Selected Plant Species from Urban Biotopes. Chemosphere 2017, 183, 471–482. [Google Scholar] [CrossRef]
- Gómez-Arroyo, S.; Barba-García, A.; Arenas-Huertero, F.; Cortés-Eslava, J.; de la Mora, M.G.; García-Martínez, R. Indicators of Environmental Contamination by Heavy Metals in Leaves of Taraxacum officinale in Two Zones of the Metropolitan Area of Mexico City. Environ. Sci. Pollut. Res. 2018, 25, 4739–4749. [Google Scholar] [CrossRef]
- Borowiak, K.; Lisiak, M.; Kanclerz, J.; Budka, A.; Mleczek, M.; Niedzielski, P.; Adamska, A.; Janicka, E. Relations between Rare Earth Elements Accumulation in Taraxacum officinale L. and Land Use in an Urban Area—A Preliminary Study. Ecol. Indic. 2018, 94, 22–27. [Google Scholar] [CrossRef]
- The, T.E.P.A.T.C.O. European Union Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC). 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1272/oj (accessed on 20 May 2024).
- The European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Królak, E.; Marciniuk, J.; Popijantus, K.; Wasilczuk, P.; Kasprzykowski, Z. Environmental Factors Determining the Accumulation of Metals: Cu, Zn, Mn and Fe in Tissues of Taraxacum Sp. Sect. Taraxacum. Bull. Environ. Contam. Toxicol. 2018, 101, 68–74. [Google Scholar] [CrossRef]
- Odigie, K.O.; Rojero, J.; Hibdon, S.A.; Flegal, A.R. Natural Lead Levels in Dandelions (Taraxacum officinale): A Weed, Folk Medicine, and Biomonitor. Environ. Sci. Technol. 2019, 53, 954–962. [Google Scholar] [CrossRef]
- Gjorgieva, D.; Kadifkova-Panovska, T.; Bačeva, K.; Stafilov, T. Assessment of Heavy Metal Pollution in Republic of Macedonia Using a Plant Assay. Arch. Environ. Contam. Toxicol. 2011, 60, 233–240. [Google Scholar] [CrossRef]
- Ligocki, M.; Tarasewicz, Z.; Zygmunt, A.; Aniśko, M. The Common Dandelion (Taraxacum officinale) as an Indicator of Anthropogenic Toxic Metal Marek Ligocki, Zofia Tarasewicz, Aneta Zygmunt, Mieczysław Aniśko. Acta Sci. Pol. Zootech. 2011, 10, 73–82. [Google Scholar]
- Trzyna, A.; Rybak, J.; Górka, M.; Olszowski, T.; Kamińska, J.A.; Węsierski, T.; Majder-Łopatka, M. Comparison of Active and Passive Methods for Atmospheric Particulate Matter Collection: From Case Study to a Useful Biomonitoring Tool. Chemosphere 2023, 334, 139004. [Google Scholar] [CrossRef]
- Balabanova, B.; Stafilov, T.; Bačeva, K.; Šajn, R. Biomonitoring of Atmospheric Pollution with Heavy Metals in the Copper Mine Vicinity Located near Radovis, Republic of Macedonia. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2010, 45, 1504–1518. [Google Scholar] [CrossRef]
- Meteoblue Historical Climate and Weather Data for Ozimek. Available online: https://www.meteoblue.com/pl/pogoda/historyclimate/climatemodelled/ozimek_polska_3089583 (accessed on 18 May 2024).
- Bajraktari, N.; Morina, I.; Demaku, S. Assessing the Presence of Heavy Metals in the Area of Glloogoc (Kosovo) by Using Mosses as a Bioindicator for Heavy Metals. J. Ecol. Eng. 2019, 20, 135–140. [Google Scholar] [CrossRef]
- Pullanikkatil, D.; Palamuleni, L.G.; Ruhiiga, T.M. Land Use/Land Cover Change and Implications for Ecosystems Services in the Likangala River Catchment, Malawi. Phys. Chem. Earth 2016, 93, 96–103. [Google Scholar] [CrossRef]
- Ozimek Commune. Available online: https://ozimek.pl/pl/184-gmina/32239-informator.html (accessed on 12 May 2024).
- Wikipedia Ozimek. Available online: https://pl.wikipedia.org/wiki/Ozimek (accessed on 12 May 2024).
- Kozioł, W.; Borcz, A.; Machniak, Ł. Status of Resources and Exploitation of Rock Deposits in the Opolskie Province. Nowocz. Bud. Inżynieryjne. Krus. Rap. 2014, 3, 84–88. (In Polish) [Google Scholar]
- Gabzdyl, W. Geology of Deposits; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 1999. (In Polish) [Google Scholar]
- Respondek, Z.; Jerz, D.; Świsłowski, P.; Rajfur, M. Active Biomonitoring of Heavy Metal Concentrations in Aquatic Environment Using Mosses and Algae. Water 2022, 14, 3335. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Inc. ICE 3000 Series AA Spectrometers Operator’s Manual; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2011; Volume 44, pp. 1-1–7-18. [Google Scholar]
- Świsłowski, P.; Nowak, A.; Rajfur, M. Is Your Moss Alive during Active Biomonitoring Study? Plants 2021, 10, 2389. [Google Scholar] [CrossRef]
- Rochel, R.; Kowol, J.; Zielonka, M.; Librowska, G.; Suchecka, D. Ecotoxicological Characteristic of Environment in Range of the Influence Point Source of Nickel Emission. JEcolHealth 2011, 15, 13–18. (In Polish) [Google Scholar]
- Isinkaralar, O.; Isinkaralar, K.; Bayraktar, E.P. Monitoring the Spatial Distribution Pattern According to Urban Land Use and Health Risk Assessment on Potential Toxic Metal Contamination via Street Dust in Ankara, Türkiye. Environ. Monit. Assess. 2023, 195, 1085. [Google Scholar] [CrossRef]
- Nekhoroshkov, P.; Peshkova, A.; Zinicovscaia, I.; Vergel, K.; Kravtsova, A. Assessment of the Atmospheric Deposition of Heavy Metals and Other Elements in the Mountain Crimea Using Moss Biomonitoring Technique. Atmosphere 2022, 13, 573. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive Statistics and Normality Tests for Statistical Data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Chaligava, O.; Shetekauri, S.; Badawy, W.M.; Frontasyeva, M.V.; Zinicovscaia, I.; Shetekauri, T.; Kvlividze, A.; Vergel, K.; Yushin, N. Characterization of Trace Elements in Atmospheric Deposition Studied by Moss Biomonitoring in Georgia. Arch. Environ. Contam. Toxicol. 2021, 80, 350–367. [Google Scholar] [CrossRef]
| Plant—Site A | |||
|---|---|---|---|
| Element | Min–Max | Mean | Median |
| Mn | 9.46–240 | 49.1 | 28.4 |
| Fe | 82.0–207 | 133 | 138 |
| Ni | 2.63–4.94 | 3.79 | 3.79 |
| Cu | 2.44–9.88 | 6.52 | 7.05 |
| Zn | 25.7–161 | 68.1 | 58.2 |
| Cd | 1.04–1.66 | 1.35 | 1.35 |
| Pb | 3.61–7.00 | 5.14 | 4.81 |
| Plant—Site B | |||
| Element | Min–Max | Mean | Median |
| Mn | 15.9–52.4 | 31.1 | 31.9 |
| Fe | 102–635 | 281 | 257 |
| Ni | 2.55–2.55 | 2.55 | 2.55 |
| Cu | 3.80–9.78 | 7.50 | 7.98 |
| Zn | 14.2–143 | 57.0 | 49.7 |
| Cd | 0.830–5.91 | 2.48 | 1.96 |
| Pb | 3.70–20.3 | 8.39 | 6.96 |
| Soil—Site A | |||
|---|---|---|---|
| Element | Min–Max | Mean | Median |
| Mn | 86.6–605 | 264 | 220 |
| Fe | 1150–1804 | 1482 | 1408 |
| Ni | 3.56–138 | 22.1 | 8.61 |
| Cu | 6.96–64.3 | 19.9 | 14.2 |
| Zn | 34.9–388 | 147 | 107 |
| Cd | n.d. | n.d. | n.d. |
| Pb | 10.4–48.8 | 30.9 | 34.0 |
| Soil—Site B | |||
| Element | Min–Max | Mean | Median |
| Mn | 204–752 | 382 | 347 |
| Fe | 1366–1998 | 1670 | 1780 |
| Ni | 7.23–37.1 | 16.7 | 10.4 |
| Cu | 8.70–27.0 | 17.0 | 15.7 |
| Zn | 61.5–353 | 147 | 119 |
| Cd | 0.890–3.42 | 1.91 | 1.61 |
| Pb | 5.89–54.1 | 20.3 | 20.4 |
| Plant | ||
|---|---|---|
| Element | Test | p |
| Mn | B-C | *** |
| Fe | S-T | *** |
| Cu | M-T | n.s. |
| Zn | S-T | n.s. |
| Pb | B-C | *** |
| Soil | ||
| Element | Test | p |
| Mn | M-T | ** |
| Fe | S-T | * |
| Ni | M-T | n.s. |
| Cu | M-T | n.s. |
| Zn | M-T | n.s. |
| Pb | M-T | * |
| Element—Area A | BCF Value [-] | Description |
|---|---|---|
| Mn | 0.221 | medium |
| Fe | 0.092 | weak |
| Ni | 0.405 | medium |
| Cu | 0.473 | |
| Zn | 0.785 | |
| Pb | 0.249 | |
| Element—Area B | BCF Value [-] | Description |
| Mn | 0.088 | weak |
| Fe | 0.171 | medium |
| Ni | 0.148 | |
| Cu | 0.483 | |
| Zn | 0.452 | |
| Cd | 3.220 | intensive |
| Pb | 0.637 | medium |
| Metal | IDL | IQL |
|---|---|---|
| Mn | 0.0016 | 0.020 |
| Fe | 0.0043 | 0.050 |
| Ni | 0.0043 | 0.050 |
| Cu | 0.0045 | 0.033 |
| Zn | 0.0033 | 0.010 |
| Cd | 0.0028 | 0.013 |
| Pb | 0.0130 | 0.070 |
| BCR-482 lichen | AAS | Rel. ** | |||
|---|---|---|---|---|---|
| Metal | Concentration | ±Uncertainty | Mean | ±SD * | |
| [mg/kg d.m.] | [%] | ||||
| Mn | 33.0 | 0.5 | 31.7 | 0.68 | −3.9 |
| Fe | 804 | 160 | 771 | 154 | −4.1 |
| Ni | 2.47 | 0.07 | 2.16 | 0.32 | −13 |
| Cu | 7.03 | 0.19 | 6.63 | 0.17 | −5.7 |
| Zn | 100.6 | 2.2 | 95.1 | 2.3 | −5.5 |
| Cd | 0.56 | 0.02 | 0.53 | 0.03 | −5.3 |
| Pb | 40.9 | 1.4 | 38.2 | 1.0 | −6.6 |
| BCR-414 plankton | AAS | Rel. ** | |||
| Metal | Concentration | ±Uncertainty | Mean | ±SD * | |
| [mg/kg d.m.] | [%] | ||||
| Mn | 299 | 12 | 284 | 13 | −5.0 |
| Fe | 1.85 | 0.19 | 1.79 | 0.20 | −3.2 |
| Ni | 18.8 | 0.8 | 18.2 | 0.9 | −3.2 |
| Cu | 29.5 | 1.3 | 28.4 | 1.6 | −3.7 |
| Zn | 112 | 3.0 | 107 | 3 | −4.5 |
| Cd | 0.383 | 0.014 | 0.371 | 0.018 | −3.1 |
| Pb | 3.97 | 0.19 | 3.75 | 0.21 | −5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Respondek, Z.; Isinkaralar, O.; Świsłowski, P.; Isinkaralar, K.; Rajfur, M. Biomonitoring with the Use of the Herbal Plant Taraxacum officinale as a Source of Information on Environmental Contamination. Plants 2024, 13, 1805. https://doi.org/10.3390/plants13131805
Respondek Z, Isinkaralar O, Świsłowski P, Isinkaralar K, Rajfur M. Biomonitoring with the Use of the Herbal Plant Taraxacum officinale as a Source of Information on Environmental Contamination. Plants. 2024; 13(13):1805. https://doi.org/10.3390/plants13131805
Chicago/Turabian StyleRespondek, Zuzanna, Oznur Isinkaralar, Paweł Świsłowski, Kaan Isinkaralar, and Małgorzata Rajfur. 2024. "Biomonitoring with the Use of the Herbal Plant Taraxacum officinale as a Source of Information on Environmental Contamination" Plants 13, no. 13: 1805. https://doi.org/10.3390/plants13131805
APA StyleRespondek, Z., Isinkaralar, O., Świsłowski, P., Isinkaralar, K., & Rajfur, M. (2024). Biomonitoring with the Use of the Herbal Plant Taraxacum officinale as a Source of Information on Environmental Contamination. Plants, 13(13), 1805. https://doi.org/10.3390/plants13131805








