Integrative Transcriptomics and Proteomics Analysis of a Cotton Mutant yl1 with a Chlorophyll-Reduced Leaf
Abstract
1. Introduction
2. Results
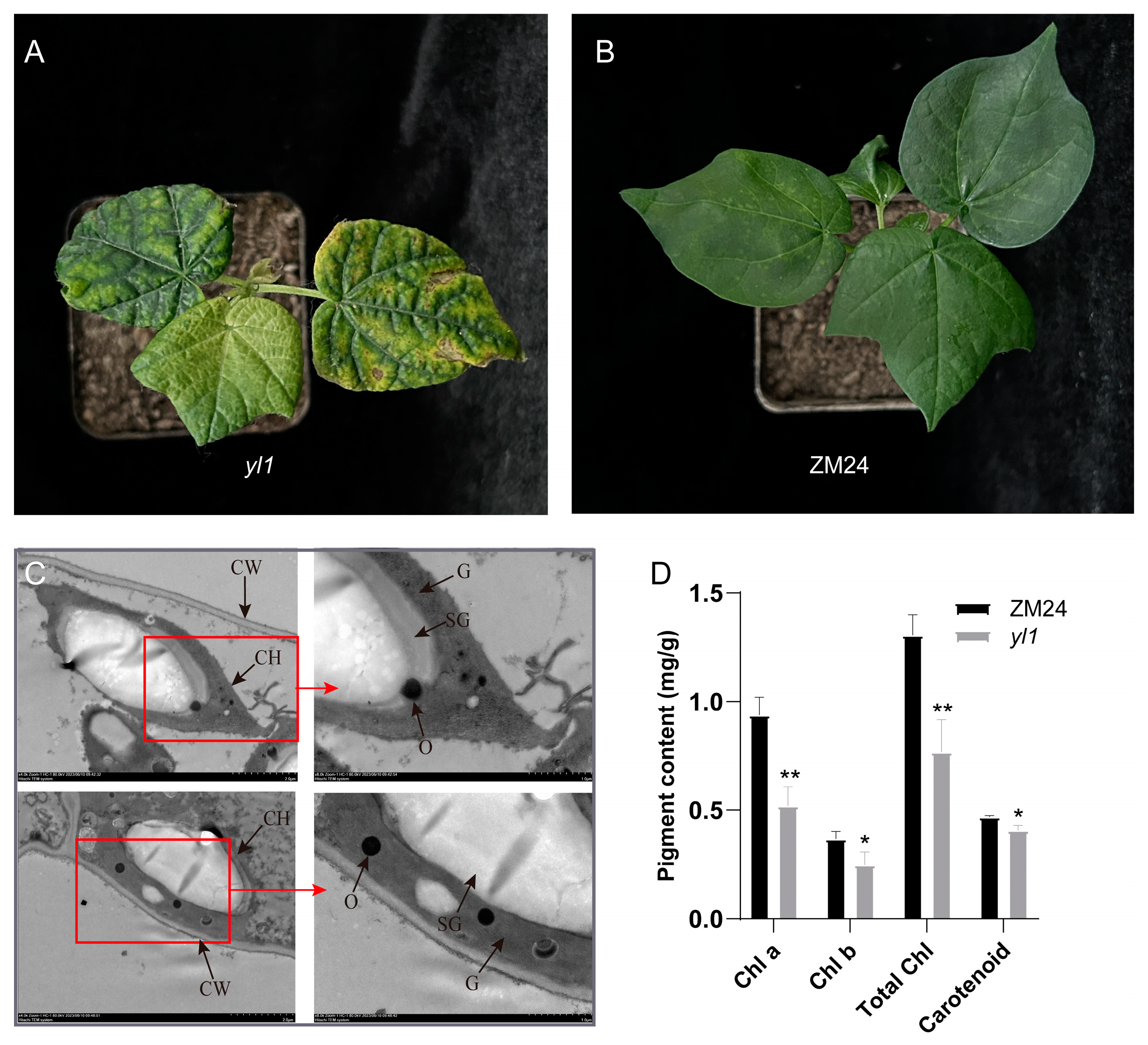
2.1. Characterization of the Chlorophyll-Reduced Mutant yl1
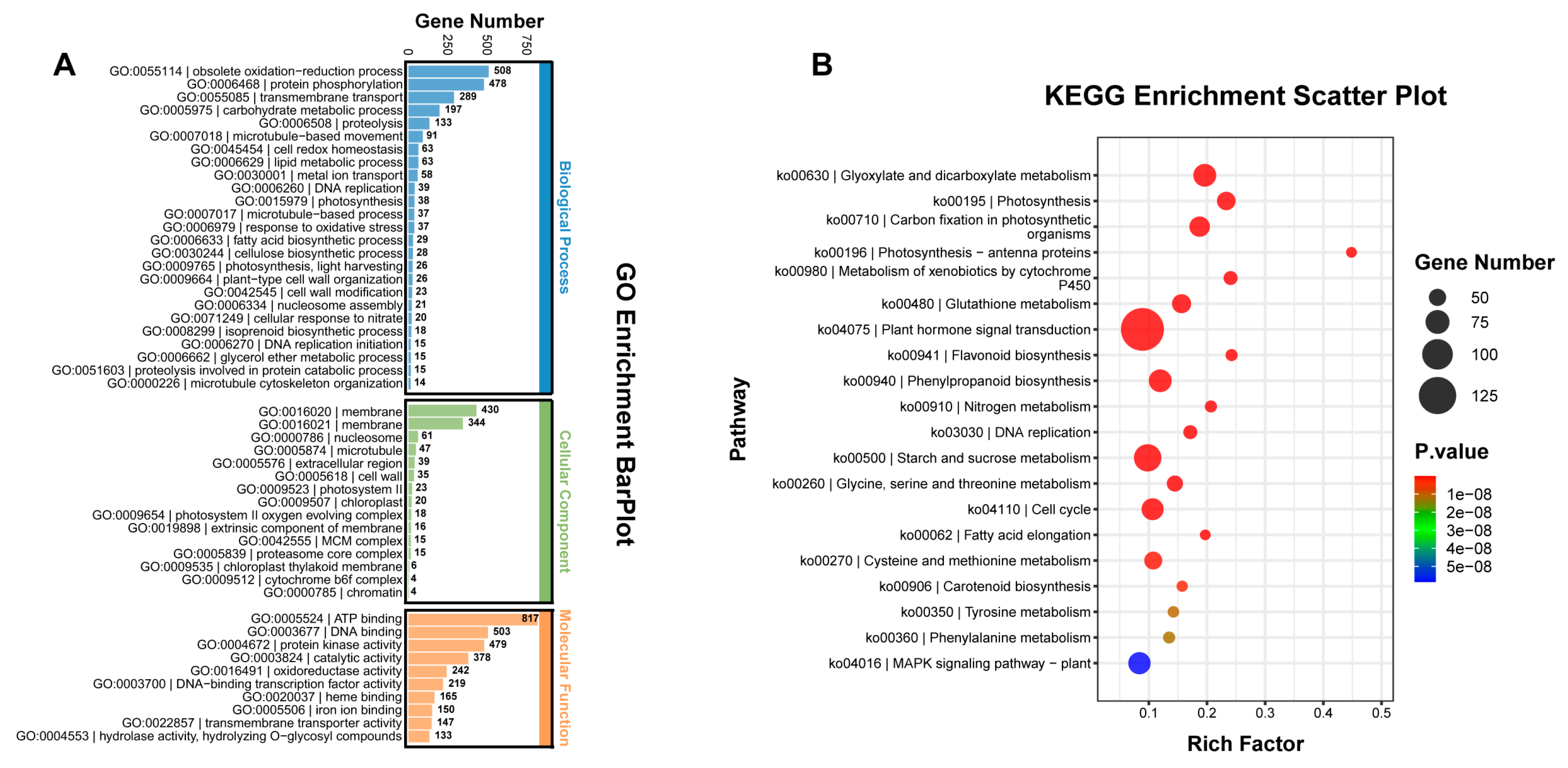
2.2. Differentially Expressed Gene Analysis between yl1 and ZM24
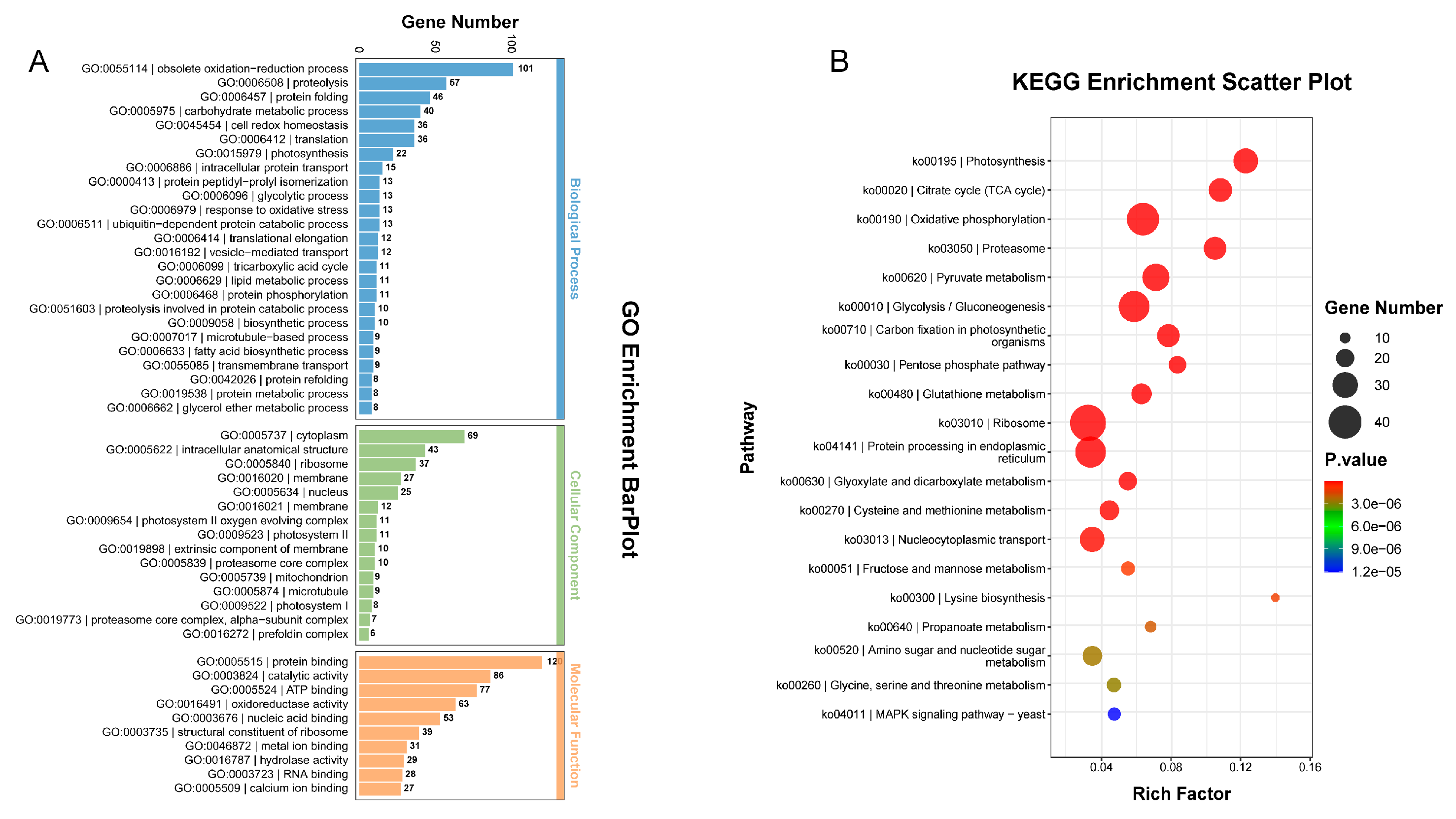
2.3. Differentially Accumulated Protein Analysis between yl1 and ZM24
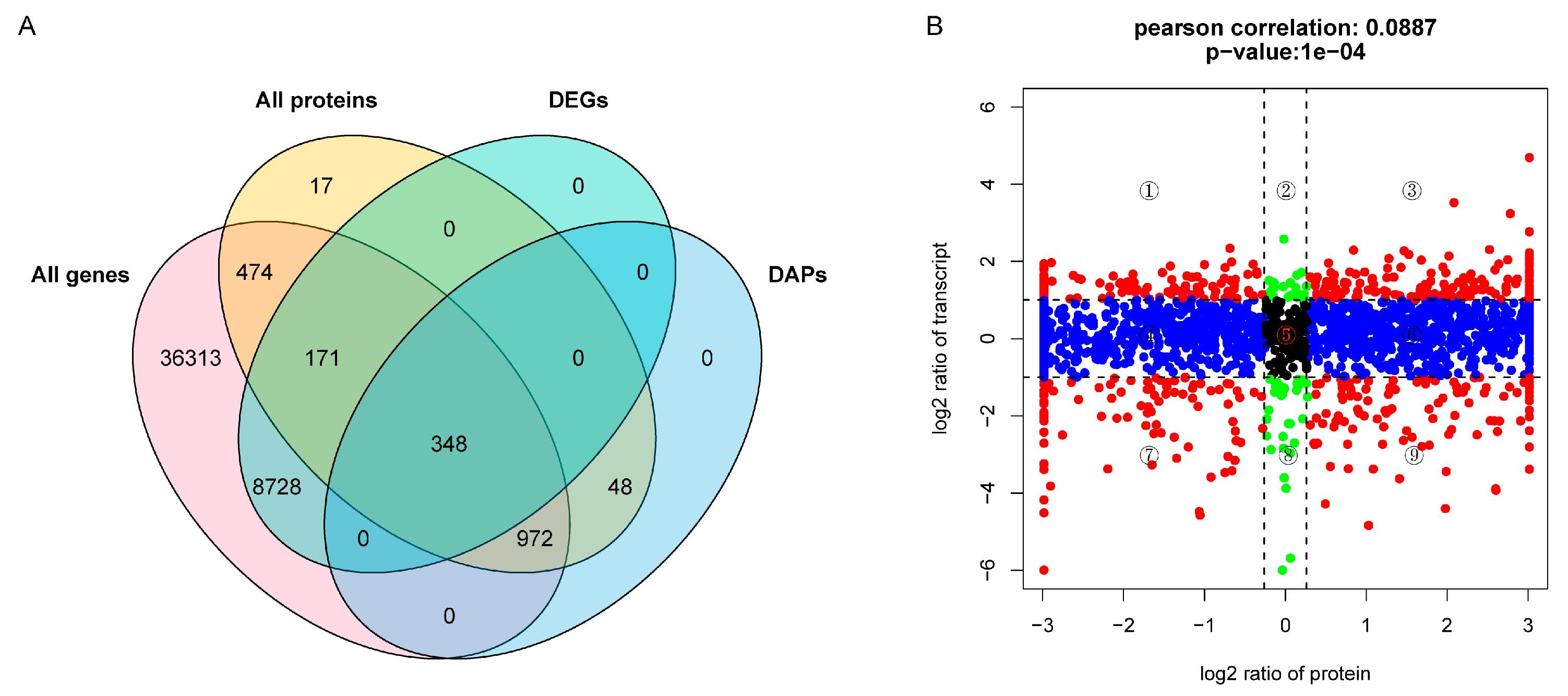
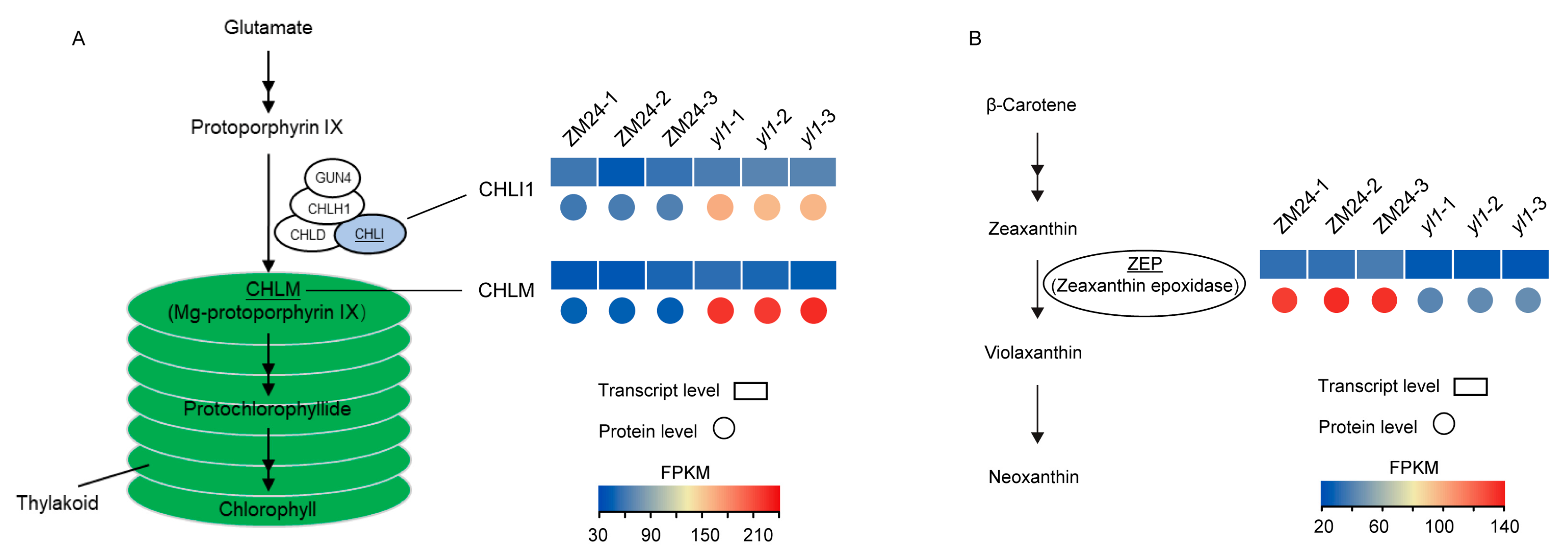
2.4. Integrated Analysis of Transcriptome and Proteome Data
2.5. Analysis of DEGs and DAPs Involved in Photosynthesis and Light Harvesting
2.6. Silencing of GhPPD1 Can Induce Leaf Yellowing
3. Discussion
4. Materials and Methods
4.1. Plants Materials
4.2. Pigment Determination
4.3. Cytological Observation
4.4. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS) and Database Search
4.5. Functional Analysis of Protein
4.6. RNA Sequencing and Data Analysis
4.7. Virus-Induced Gene Silencing of GhPPD1 in Gossypium hirsutum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Chen, Y.; Liu, J.; Wang, Z.; Li, F.; Ge, X. Recent advances and future perspectives in early-maturing cotton research. New Phytol. 2023, 237, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Xu, J.; Yang, Z.; Yang, X.; Wang, Y.; Chen, Y.; Wang, P.; Li, F. Efficient genotype-independent cotton genetic transformation and genome editing. J. Integr. Plant Biol. 2023, 65, 907–917. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Wang, P.; Gao, L.; Yang, R.; Li, Y. Label-free comparative proteomic and physiological analysis provides insight into leaf color variation of the golden-yellow leaf mutant of Lagerstroemia indica. J. Proteom. 2020, 228, 103942. [Google Scholar] [CrossRef]
- He, L.; Li, M.; Qiu, Z.; Chen, D.; Zhang, G.; Wang, X.; Chen, G.; Hu, J.; Gao, Z.; Dong, G.; et al. Primary leaf-type ferredoxin 1 participates in photosynthetic electron transport and carbon assimilation in rice. Plant J. 2020, 104, 44–58. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, S.; Qiu, Z.; Zhao, J.; Nie, W.; Lin, H.; Zhu, Z.; Zeng, D.; Qian, Q.; Zhu, L. FRUCTOKINASE-LIKE PROTEIN 1 interacts with TRXz to regulate chloroplast development in rice. J. Integr. Plant Biol. 2018, 60, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Yang, H.; Chen, C.; Ren, T.; Li, Z.; Tan, F.; Luo, P. Phenotypic characterization of the wheat temperature-sensitive leaf color mutant and physical mapping of mutant gene by reduced-representation sequencing. Plant Sci. Int. J. Exp. Plant Biol. 2023, 330, 111657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Mao, Y.; Yin, Y.; Shen, X. Physiological analysis and transcriptome sequencing of a delayed-green leaf mutant ‘Duojiao’ of ornamental crabapple (Malus sp.). Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2022, 28, 1833–1848. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Meng, F.; Mo, F.; Qi, H.; Wang, P.; Chen, X.; Liu, J.; Ghanizadeh, H.; Zhang, H.; Wang, A. Slym1 control the color etiolation of leaves by facilitating the decomposition of chlorophyll in tomato. Plant Sci. Int. J. Exp. Plant Biol. 2022, 324, 111457. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Zhang, Y.; Zhou, W.; Zhang, A.; Lu, C. Pentatricopeptide repeat protein PHOTOSYSTEM I BIOGENESIS FACTOR2 is required for splicing of ycf3. J. Integr. Plant Biol. 2020, 62, 1741–1761. [Google Scholar] [CrossRef]
- Lu, Y. Identification and Roles of Photosystem II Assembly, Stability, and Repair Factors in Arabidopsis. Front. Plant Sci. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plant. 2011, 142, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Yokono, M.; Takabayashi, A.; Kishimoto, J.; Fujita, T.; Iwai, M.; Murakami, A.; Akimoto, S.; Tanaka, A. The PSI-PSII Megacomplex in Green Plants. Plant Cell Physiol. 2019, 60, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Schiphorst, C.; Achterberg, L.; Gómez, R.; Koehorst, R.; Bassi, R.; van Amerongen, H.; Dall’Osto, L.; Wientjes, E. The role of light-harvesting complex I in excitation energy transfer from LHCII to photosystem I in Arabidopsis. Plant Physiol. 2022, 188, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka-Nishimura, M. Close Relationships Between the PSII Repair Cycle and Thylakoid Membrane Dynamics. Plant Cell Physiol. 2016, 57, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; Suorsa, M.; Rokka, A.; Allahverdiyeva, Y.; Paakkarinen, V.; Saleem, A.; Battchikova, N.; Rintamäki, E. Dynamics of photosystem II: A proteomic approach to thylakoid protein complexes. J. Exp. Bot. 2005, 56, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Perrine, Z.; Friedland, N.; Kumar, A.; Tokutsu, R.; Minagawa, J.; Berg, H.; Barry, A.N.; Govindjee, G.; Sayre, R. Light regulation of light-harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae(†). Plant J. Cell Mol. Biol. 2020, 103, 584–603. [Google Scholar] [CrossRef]
- Kirst, H.; García-Cerdán, J.G.; Zurbriggen, A.; Melis, A. Assembly of the light-harvesting chlorophyll antenna in the green alga Chlamydomonas reinhardtii requires expression of the TLA2-CpFTSY gene. Plant Physiol. 2012, 158, 930–945. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Wang, C.; Lin, W.; Huang, C.; Fan, C.; Han, D.; Lu, D.; Xu, X.; Sui, S.; et al. Regulatory dynamics of the higher-plant PSI-LHCI supercomplex during state transitions. Mol. Plant 2023, 16, 1937–1950. [Google Scholar] [CrossRef]
- Pan, X.; Ma, J.; Su, X.; Cao, P.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure of the maize photosystem I supercomplex with light-harvesting complexes I and II. Science 2018, 360, 1109–1113. [Google Scholar] [CrossRef]
- You, X.; Zhang, X.; Cheng, J.; Xiao, Y.; Ma, J.; Sun, S.; Zhang, X.; Wang, H.W.; Sui, S.F. In situ structure of the red algal phycobilisome-PSII-PSI-LHC megacomplex. Nature 2023, 616, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hargett, S.R.; Frankel, L.K.; Bricker, T.M. The PsbP protein, but not the PsbQ protein, is required for normal thylakoid architecture in Arabidopsis thaliana. FEBS Lett. 2009, 583, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Roose, J.L.; Frankel, L.K.; Bricker, T.M. The PsbP domain protein 1 functions in the assembly of lumenal domains in photosystem I. J. Biol. Chem. 2014, 289, 23776–23785. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Shi, L.; Shen, P.; Li, Y. Leaf color formation mechanisms in Alternanthera bettzickiana elucidated by metabolite and transcriptome analyses. Planta 2022, 255, 59. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Wang, X.; Li, J.; Zhu, W.; Cui, J.; Zhang, K. Regulatory mechanisms of leaf color change in Acer pictum subsp. mono. Genome 2019, 62, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Huang, Z.; Zhao, W.; Lin, N.; Wang, Y.; Shang, F. Mechanisms for leaf color changes in Osmanthus fragrans ‘Ziyan Gongzhu’ using physiology, transcriptomics and metabolomics. BMC Plant Biol. 2023, 23, 453. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Ge, X.; Lu, L.; Qin, W.; Qanmber, G.; Liu, L.; Wang, Z.; Li, F. Brassinosteroids regulate cotton fiber elongation by modulating very-long-chain fatty acid biosynthesis. Plant Cell 2023, 35, 2114–2131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qanmber, G.; Wang, Z.; Yang, Z.; Li, F. Gossypium Genomics: Trends, Scope, and Utilization for Cotton Improvement. Trends Plant Sci. 2020, 25, 488–500. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Z.; Chen, W.; Liang, C.; Li, X.; Liu, Z.; Cui, Q.; Ma, Y.; Zou, X. Fine-mapping and transcriptome analysis of the photosensitive leaf -yellowing gene CaLY1 in pepper (Capsicum annuum L.). Hortic. Plant J. 2023, 9, 122–132. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, C.; Liu, H.; Chen, J.; Han, Z.; Yan, Q.; Liu, S.; Liu, H. Effect of chlorophyll biosynthesis-related genes on the leaf color in Hosta (Hosta plantaginea Aschers) and tobacco (Nicotiana tabacum L.). BMC Plant Biol. 2021, 21, 45. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Nie, Z.; Gai, J.; Bhat, J.A.; Kong, J.; Zhao, T. Double mutation of two homologous genes YL1 and YL2 results in a leaf yellowing phenotype in soybean [Glycine max (L.) Merr]. Plant Mol. Biol. 2020, 103, 527–543. [Google Scholar] [CrossRef]
- Wang, L.; Cao, H.; Chen, C.; Yue, C.; Hao, X.; Yang, Y.; Wang, X. Complementary transcriptomic and proteomic analyses of a chlorophyll-deficient tea plant cultivar reveal multiple metabolic pathway changes. J. Proteom. 2016, 130, 160–169. [Google Scholar] [CrossRef]
- Yang, P.; Li, Y.; He, C.; Yan, J.; Zhang, W.; Li, X.; Xiang, F.; Zuo, Z.; Li, X.; Zhu, Y.; et al. Phenotype and TMT-based quantitative proteomics analysis of Brassica napus reveals new insight into chlorophyll synthesis and chloroplast structure. J. Proteom. 2020, 214, 103621. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, N.; Shen, S. iTRAQ-Based Quantitative Proteomics Analysis Reveals the Mechanism of Golden-Yellow Leaf Mutant in Hybrid Paper Mulberry. Int. J. Mol. Sci. 2021, 23, 127. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Lu, Q.; Wen, X.; Chen, F.; Peng, L.; Zhang, L.; Lu, C. PsbP-domain protein1, a nuclear-encoded thylakoid lumenal protein, is essential for photosystem I assembly in Arabidopsis. Plant Cell 2012, 24, 4992–5006. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Froehlich, J.E.; Strand, D.D.; Buck, S.M.; Larkin, R.M. GUN4-Porphyrin Complexes Bind the ChlH/GUN5 Subunit of Mg-Chelatase and Promote Chlorophyll Biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1449–1467. [Google Scholar] [CrossRef] [PubMed]
- Pontier, D.; Albrieux, C.; Joyard, J.; Lagrange, T.; Block, M.A. Knock-out of the magnesium protoporphyrin IX methyltransferase gene in Arabidopsis. Effects on chloroplast development and on chloroplast-to-nucleus signaling. J. Biol. Chem. 2007, 282, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Sahara, T.; Misawa, N. Violaxanthin: Natural function and occurrence, biosynthesis, and heterologous production. Appl. Microbiol. Biotechnol. 2021, 105, 6133–6142. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, H.; Liu, J.; Bian, Y.; Ma, Q.; Mao, G.; Wang, H.; Wu, A.; Zhang, J.; Chen, P.; et al. Non-functional GoFLA19s are responsible for the male sterility caused by hybrid breakdown in cotton (Gossypium spp.). Plant J. 2021, 107, 1198–1212. [Google Scholar] [CrossRef]
- Wang, B.; Wu, W.; Wang, X.; Wang, Z.; Qian, Y. Proteomic Analysis of Pollen–Stigma Interaction between Vitis rotundifolia and Vitis vinifera. J. Am. Soc. Hortic. Sci. 2022, 147, 152–160. [Google Scholar] [CrossRef]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Pang, J.; Zhu, Y.; Li, Q.; Liu, J.; Tian, Y.; Liu, Y.; Wu, J. Development of Agrobacterium-mediated virus-induced gene silencing and performance evaluation of four marker genes in Gossypium barbadense. PLoS ONE 2013, 8, e73211. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Xiao, Y.; Liu, Y.; Zhang, J.; Zhao, Y. Integrative Transcriptomics and Proteomics Analysis of a Cotton Mutant yl1 with a Chlorophyll-Reduced Leaf. Plants 2024, 13, 1789. https://doi.org/10.3390/plants13131789
Lu H, Xiao Y, Liu Y, Zhang J, Zhao Y. Integrative Transcriptomics and Proteomics Analysis of a Cotton Mutant yl1 with a Chlorophyll-Reduced Leaf. Plants. 2024; 13(13):1789. https://doi.org/10.3390/plants13131789
Chicago/Turabian StyleLu, Hejun, Yuyang Xiao, Yuxin Liu, Jiachen Zhang, and Yanyan Zhao. 2024. "Integrative Transcriptomics and Proteomics Analysis of a Cotton Mutant yl1 with a Chlorophyll-Reduced Leaf" Plants 13, no. 13: 1789. https://doi.org/10.3390/plants13131789
APA StyleLu, H., Xiao, Y., Liu, Y., Zhang, J., & Zhao, Y. (2024). Integrative Transcriptomics and Proteomics Analysis of a Cotton Mutant yl1 with a Chlorophyll-Reduced Leaf. Plants, 13(13), 1789. https://doi.org/10.3390/plants13131789




