Effect of Harvest Time on Growth and Bioactive Compounds in Salvia miltiorrhiza
Abstract
1. Introduction
2. Results
2.1. Plant Growth Index, Leaf SPAD Values, Shoot Number, and Root Number
2.2. Shoot Fresh Weight, Shoot Dry Weight, Maximum Root Length, and Maximum Root Diameter
2.3. Root Fresh Weight and Root Dry Weight
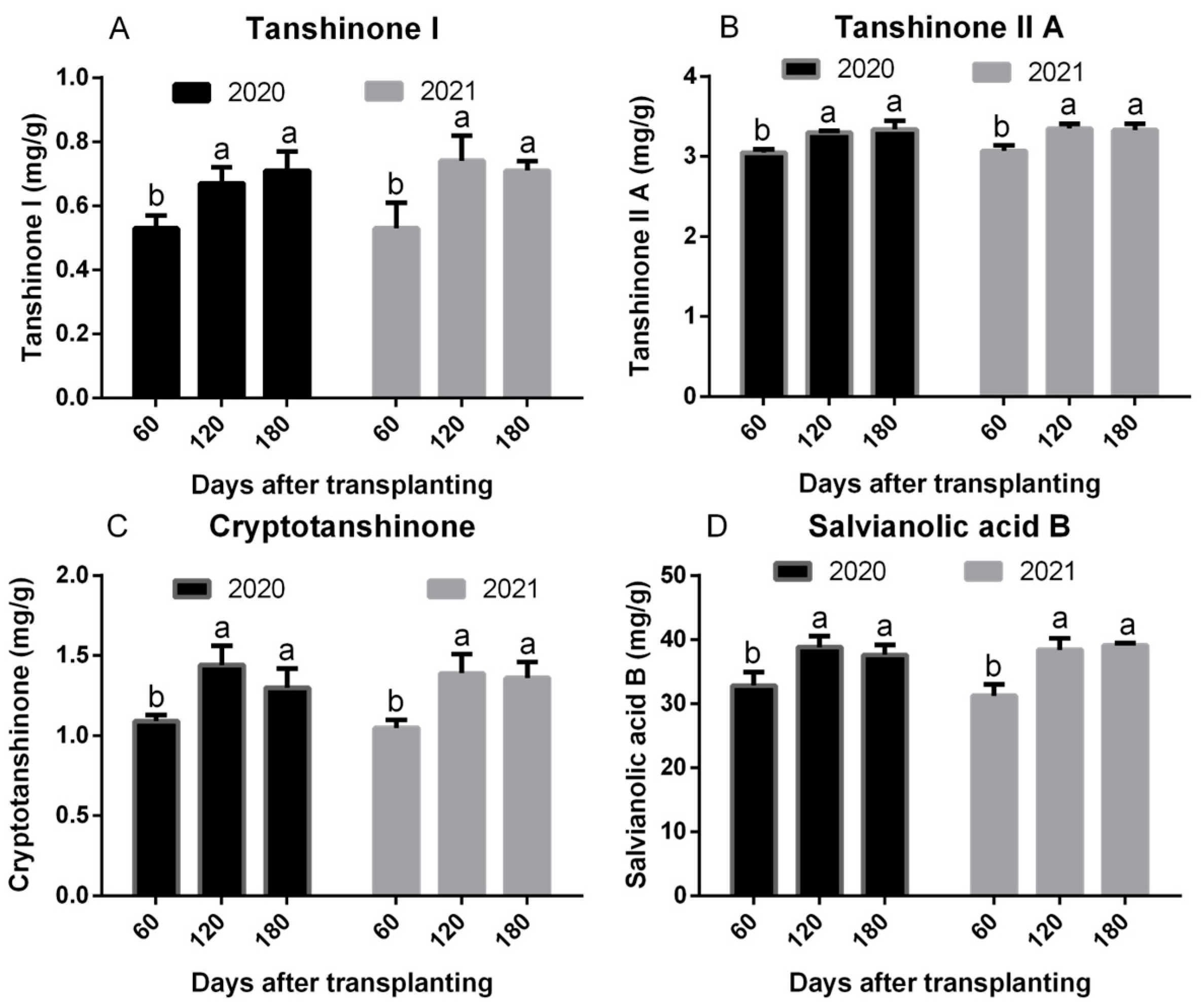
2.4. Tanshinone I, Tanshinone IIA, Cryptotanshinone, and Salvianolic Acid B
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation
4.2. Plant Growth
4.3. Preparation of Danshen Extract
4.4. Analysis of Tanshinone I, Tanshinone IIA, Cryptotanshinone, and Salvianolic Acid B
4.5. Linear Regression and Linear Range
4.6. Reagent and Standards
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Z.Q.; Li, X.F.; Wang, H.G.; Wang, J.H. Genetic Diversity and Population Structure of Salvia miltiorrhiza Bunge in China Revealed by ISSR and SRAP. Genetica 2010, 138, 241–249. [Google Scholar] [CrossRef]
- He, C.E.; Wei, J.; Jin, Y.; Chen, S. Bioactive Components of the Roots of Salvia miltiorrhizae: Changes Related to Harvest Time and Germplasm Line. Ind. Crops Prod. 2010, 32, 313–317. [Google Scholar] [CrossRef]
- Wang, B.Q. Salvia miltiorrhiza: Chemical and Pharmacological Review of a Medicinal Plant. J. Med. Plant Res. 2010, 4, 2813–2820. [Google Scholar]
- Fu, S.; Zhang, J.; Gao, X.; Xia, Y.; Ferrelli, R.; Fauci, A.; Guerra, R.; Hu, L. Clinical Practice of Traditional Chinese Medicines for Chronic Heart Failure. Heart Asia 2010, 2, 24–27. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Zhang, J.; Sun, C.; Yang, R.; Sheng, M.; Hu, J.; Kai, J.; Han, B. Adjuvant Role of Salvia miltiorrhiza Bunge in Cancer Chemotherapy: A Review of Its Bioactive Components, Health-Promotion Effect and Mechanisms. J. Ethnopharmacol. 2023, 318, 117022. [Google Scholar] [CrossRef]
- Li, Z.M.; Xu, S.W.; Liu, P.Q. Salvia miltiorrhiza Burge (Danshen): A Golden Herbal Medicine in Cardiovascular Therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, L.; Zhang, X.; Long, Y.; Zou, F.; Yan, C.; Zou, W. Protective Effects and Active Ingredients of Salvia miltiorrhiza Bunge Extracts on Airway Responsiveness, Inflammation and Remodeling in Mice with Ovalbumin-Induced Allergic Asthma. Phytomedicine 2019, 52, 168–177. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, S.; Tian, X.Y. The Effect of Salvianolic Acid on Vascular Protection and Possible Mechanisms. Oxid. Med. Cell. Longev. 2020, 2020, 5472096. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Liu, C.; Liu, H.; Zhu, R.; Guo, S.; Tang, M.; Li, Y.; Niu, J.; Fu, M.; et al. Salvia miltiorrhiza: A Potential Red Light to the Development of Cardiovascular Diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef]
- Wang, L.S.; Yen, P.T.; Weng, S.F.; Hsu, J.H.; Yeh, J.L. Clinical Patterns of Traditional Chinese Medicine for Ischemic Heart Disease Treatment: A Population-Based Cohort Study. Medicina 2022, 58, 879. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 66193. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, M.; Sun, P.; Liang, W.; Hornbeck, R.G.; Che, X.; Rao, C.; Zhao, Y.; Guo, L.; Huang, Y.; et al. Market Access for Chinese Herbal Medicinal Products in Europe—A Ten-Year Review of Relevant Products, Policies, and Challenges. Phytomedicine 2022, 103, 154237. [Google Scholar] [CrossRef]
- Kum, K.Y.; Kirchhof, R.; Luick, R.; Heinrich, M. Danshen (Salvia miltiorrhiza) on the Global Market: What Are the Implications for Products’ Quality? Front. Pharmacol. 2021, 12, 609. [Google Scholar] [CrossRef]
- Golpîra, H. Optimal Integration of the Facility Location Problem into the Multi-Project Multi-Supplier Multi-Resource Construction Supply Chain Network Design Under the Vendor Managed Inventory Strategy. Expert Syst. Appl. 2020, 139, 112841. [Google Scholar] [CrossRef]
- Fong, H.H. Integration of Herbal Medicine into Modern Medical Practices: Issues and Prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef]
- Choudhary, S.; Zehra, A.; Mukarram, M.; Wani, K.I.; Naeem, M.; Hakeem, K.R.; Aftab, T. Potential Uses of Bioactive Compounds of Medicinal Plants and Their Mode of Action in Several Human Diseases. In Medicinal and Aromatic Plants: Healthcare and Industrial Applications; Springer: Cham, Switzerland, 2021; pp. 143–158. [Google Scholar]
- Shen, B.; Zhang, Z.; Shi, Q.; Du, J.; Xue, Q.; Li, X. Active Compound Analysis of Ziziphus Jujuba cv. Jinsixiaozao in Different Developmental Stages Using Metabolomic and Transcriptomic Approaches. Plant Physiol. Biochem. 2022, 189, 14–23. [Google Scholar] [CrossRef]
- Zhang, Y. Study on the Optimal Harvesting Time of Salva miltiorrhiza. Res. Pract. Chin. Med. 2008, 01, 12–14. [Google Scholar]
- Song, S.Y.; Park, D.H.; Seo, S.W.; Park, K.M.; Bae, C.S.; Son, H.S.; Kim, H.G.; Lee, G.H.; Yoon, G.; Shim, J.H.; et al. Effects of Harvest Time on Phytochemical Constituents and Biological Activities of Panax Ginseng Berry Extracts. Molecules 2019, 24, 3343. [Google Scholar] [CrossRef]
- Das, P.P.; Singh, K.R.; Nagpure, G.; Mansoori, A.; Singh, R.P.; Ghazi, I.A.; Kumar, A.; Singh, J. Plant-Soil-Microbes: A Tripartite Interaction for Nutrient Acquisition and Better Plant Growth for Sustainable Agricultural Practices. Environ. Res. 2022, 214, 113821. [Google Scholar] [CrossRef]
- Gupta, S.; Schillaci, M.; Walker, R.; Smith, P.M.; Watt, M.; Roessner, U. Alleviation of Salinity Stress in Plants by Endophytic Plant-Fungal Symbiosis: Current Knowledge, Perspectives and Future Directions. Plant Soil 2021, 461, 219–244. [Google Scholar] [CrossRef]
- Lundgren, M.R.; Des Marais, D.L. Life History Variation as a Model for Understanding Trade-Offs in Plant–Environment Interactions. Curr. Biol. 2020, 30, R180–R189. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The Relationship Between Leaf Area Growth and Biomass Accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 130011. [Google Scholar] [CrossRef]
- Subaedah, S.T.; Edy, E.; Mariana, K. Growth, Yield, and Sugar Content of Different Varieties of Sweet Corn and Harvest Time. Int. J. Agron. 2021, 2021, 8882140. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Gendy, A.S.H.; Said-Al Ahl, H.A.H.; Grulova, D.; Astatkie, T.; Abdelrazik, T.M. Impacts of Harvest Time and Water Stress on the Growth and Essential Oil Components of Horehound (Marrubium vulgare). Sci. Hortic. 2018, 232, 139–144. [Google Scholar] [CrossRef]
- Poethig, R.S. Vegetative Phase Change and Shoot Maturation in Plants. Curr. Top. Dev. Biol. 2013, 105, 125–152. [Google Scholar]
- Meng, Z.; Zhou, Y.; Yang, K.; Li, W.; Zong, S.; Liu, F.; Ma, H.; Zhu, Y.; Zhu, J.; Song, X.; et al. Effects of Delayed Harvest on SPAD and Burnt-Sweet Mellow-Sweet Style of Flue-cured Tobacco Upper Six Leaves in Yongzhou. J. Henan Agric. Sci. 2022, 51, 142. [Google Scholar]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of Reduced Chlorophyll Content on Photosystem Functions and Photosynthetic Electron Transport Rate in Rice Leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef]
- Zhang, J.L.; Li, X.G.; Xu, X.H.; Chen, H.P.; Li, Y.L.; Guy, R.D. Leaf Morphology, Photosynthesis and Pigments Change with Age and Light Regime in Savin Juniper. Plant Biol. 2021, 23, 1097–1108. [Google Scholar] [CrossRef]
- Donnelly, A.; Yu, R.; Rehberg, C.; Meyer, G.; Young, E.B. Leaf Chlorophyll Estimates of Temperate Deciduous Shrubs During Autumn Senescence Using a Spad-502 Meter and Calibra-Tion with Extracted Chlorophyll. Ann. For. Sci. 2020, 77, 30. [Google Scholar] [CrossRef]
- Sheng, S. Cultivation and Quality Studies of Danshen (Salvia miltiorrhiza) in Australia. Ph.D. Thesis, Chinese Medicine Discipline Melbourne, RMIT University Australia, Melbourne, Australia, 2007. Volume 369. [Google Scholar]
- Amzad Hossain, M. Effects of Harvest Time on Shoot Biomass and Yield of Turmeric (Curcuma longa L.) in Okinawa, Japan. Plant Prod. Sci. 2010, 13, 97–103. [Google Scholar] [CrossRef]
- Fageria, N.K.; Moreira, A. The Role of Mineral Nutrition on Root Growth of Crop Plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]
- Schurr, U.; Walter, A.; Rascher, U. Functional Dynamics of Plant Growth and Photosynthesis–From Steady-State to Dynamics–From Homogeneity to Heteroge-Neity. Plant Cell Environ. 2006, 29, 340–352. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.; Bergeron, Y. Influence of Environmental Variability on Root Dynamics in Northern Forests. Crit. Rev. Plant Sci. 2009, 28, 179–197. [Google Scholar] [CrossRef]
- Osano, S.N.; Mwea, S.K. The Effect of Strain Rate and Specimen Length on the Stress-Strain Relationship of Vegetation Roots Used in Slope Stabilization. Icastor J. Eng. 2015, 8, 1. [Google Scholar]
- Kim, Y.G.; Kim, K.S.; Chang, Y.H.; Yu, H.S. Effects of Harvesting Time on Growth and Root Yield in Astragalus membranaceus Bunge. Korean J. Med. Crop Sci. 1996, 4, 329–332. [Google Scholar]
- Tate, H.T.; Page, T. Cutting Propagation of Santalum austrocaledonicum: The Effect of Genotype, Cutting Source, Cutting Size, Propagation Me-Dium, Iba and Irradiance. New For. 2018, 49, 551–570. [Google Scholar] [CrossRef]
- Sui, C. Salvia miltiorrhiza Resources, Cultivation, and Breeding. In The Salvia miltiorrhiza Genome; Springer: Cham, Switzerland, 2019; pp. 17–32. [Google Scholar]
- Yu, W.; Yu, Y.; Wang, C.; Zhang, Z.; Xue, Z. Mechanism by which salt stress induces physiological responses and regulates tanshinone synthesis. Plant Physiol. Biochem. 2021, 164, 10–20. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, S.; Fan, W.; Lilan, L.; Hui, N.; Xia, W.; Wei, J. Expression patterns of some genes involved in tanshinone biosynthesis in Salvia miltiorrhiza roots. Ind. Crops Prod. 2019, 130, 606–614. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Yang, D.; Hou, Z.; Liang, Z. Transcriptional Profiles of Smwrky Family Genes and Their Putative Roles in the Biosynthesis of Tanshinone and Phenolic Acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 1593. [Google Scholar] [CrossRef]
- Golizadeh, F.; Kumleh, H.H. Physiological Responses and Expression Changes of Fatty Acid Metabolism–Related Genes in Wheat (Triticum aestivum) Under Cold Stress. Plant Mol. Biol. Rep. 2019, 37, 224–236. [Google Scholar] [CrossRef]
- Kharel, B.; Rusalepp, L.; Bhattarai, B.; Kaasik, A.; Kupper, P.; Lutter, R.; Mänd, P.; Rohula-Okunev, G.; Rosenvald, K.; Tullus, A. Effects of air humidity and soil moisture on secondary metabolites in the leaves and roots of Betula pendula of different competitive status. Oecologia 2023, 202, 193–210. [Google Scholar] [CrossRef]
- Li, L.; Wang, D.; Zhou, L.; Yu, X.; Yan, X.; Zhang, Q.; Li, B.; Liu, Y.; Zhou, W.; Cao, X.; et al. JA-Responsive Transcription Factor SmMYB97 Promotes Phenolic Acid and Tanshinone Accumulation in Salvia miltiorrhiza. J. Agric. Food Chem. 2020, 68, 14850–14862. [Google Scholar] [CrossRef]
- Heiduk, A.; Haenni, J.P.; Meve, U.; Schulz, S.; Dötterl, S. Flower Scent of Ceropegia stenantha: Electrophysiological Activity and Synthesis of Novel Components. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2019, 205, 301–310. [Google Scholar] [CrossRef]
- He, C.E.; Lu, L.L.; Jin, Y.; Wei, J.H.; Christie, P. Effects of Nitrogen on Root Development and Contents of Bioactive Compounds in Salvia miltiorrhiza Bunge. Crop. Sci. 2013, 53, 2028–2039. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 439237. [Google Scholar] [CrossRef]
- Yu, Z.X.; Zhang, Y.Y.; Zhao, X.X.; Yu, L.; Chen, X.B.; Wan, H.T.; He, Y.; Jin, W.F. Simultaneous Optimization of Ultrasonic-Assisted Extraction of Danshen for Maximal Tanshinone II A and Salvianolic Acid B Yields and Antioxidant Activity: A Comparative Study of the Response Surface Methodology and Artificial Neural Network. Ind. Crops Prod. 2021, 161, 113199. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China 2020; China Medical Science Press: Beijing, China, 2020; pp. 76–77. [Google Scholar]
- Ren, J.; Jiang, T.; Li, C.; Gu, L.H.; Li, J.M. Content Determination of Tanshinone and Salvianolic acid B in Zhongfeng Huichun Capsule by HPLC. Pharm. Today 2021, 31, 32–34. [Google Scholar]
| Harvest Time (Days after Transplanting) | PGI x | SPAD | Shoot Number (per Plant) | Root Number (per Plant) y | ||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| 60 | 27.5 ± 2.6 c z | 31.5 ± 1.6 b | 29.8 ± 1.9 a | 30.9 ± 1.3 a | 3.2 ± 0.4 c | 3.6 ± 0.5 c | 11.8 ± 1.3 c | 10.4 ± 1.1 c |
| 120 | 37.3 ± 2.2 b | 38.1 ± 2.8 a | 31.9 ± 1.9 a | 33.5 ± 2.2 a | 5.6 ± 0.4 b | 6.8 ± 0.8 b | 19.4 ± 2.3 b | 17.4 ± 1.1 b |
| 180 | 44.5 ± 3.8 a | 40.1 ± 3.8 a | 23.9 ± 2.2 b | 24.1 ± 2.5 b | 7.2 ± 0.8 a | 8.4 ± 1.0 a | 41.4 ± 3.1 a | 32.0 ± 4.5 a |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Harvest Time (Days after Transplanting) | Shoot Fresh Weight (g per Plant) | Shoot Dry Weight (g per Plant) | Maximum Root Length (cm) x | Maximum Root Diameter (mm) y | ||||
|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| 60 | 57.6 ± 5.2 c z | 55.5 ± 4.1 c | 18.4 ± 1.6 c | 18.2 ± 1.2 c | 23.6 ± 3.1 c | 19.2 ± 0.8 c | 5.0 ± 0.4 c | 6.3 ± 0.5 b |
| 120 | 96.3 ± 8.4 b | 93.3 ± 4.8 b | 24.1 ± 1.8 b | 22.7 ± 0.9 b | 28.8 ± 2.8 b | 34.6 ± 2.8 b | 7.2 ± 0.3 b | 7.3 ± 1.1 b |
| 180 | 120.1 ± 6.5 a | 115.0 ± 4.4 a | 36.4 ± 3.1 a | 37.1 ± 3.1 a | 41.2 ± 2.7 a | 40.6 ± 3.1 a | 10.9 ± 0.9 a | 10.7 ± 0.9 a |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Harvest Time (Days after Transplanting) | Root Fresh Weight (g per Plant) | Root Dry Weight (g per Plant) | ||
|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | |
| 60 | 46.8 ± 4 c z | 30.8 ± 2.2 c | 3.9 ± 0.4 c | 2.7 ± 0.2 c |
| 120 | 146.3 ± 10.8 b | 143.3 ± 10.1 b | 29.8 ± 2.3 b | 30 ± 6.4 b |
| 180 | 266.2 ± 5.2 a | 265.5 ± 11.5 a | 84.6 ± 2.5 a | 82.9 ± 3.5 a |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Compounds | Linear Regression | Linear Range (mg/mL) | R2 |
|---|---|---|---|
| Cryptotanshinone | Y = 13,958X − 91.38 | 0.038–0.188 | 0.9994 |
| Tanshinone I | Y = 3586.7X − 2.70 | 0.038–0.188 | 0.9999 |
| Tanshinone IIA | Y = 6460.6X − 27.31 | 0.083–0.417 | 0.9998 |
| Salvianolic Acid B | Y = 16,128X − 36.10 | 0.375–0.625 | 0.9994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Bi, G.; Li, T.; Zhang, Q.; Knight, P.R. Effect of Harvest Time on Growth and Bioactive Compounds in Salvia miltiorrhiza. Plants 2024, 13, 1788. https://doi.org/10.3390/plants13131788
Xing Z, Bi G, Li T, Zhang Q, Knight PR. Effect of Harvest Time on Growth and Bioactive Compounds in Salvia miltiorrhiza. Plants. 2024; 13(13):1788. https://doi.org/10.3390/plants13131788
Chicago/Turabian StyleXing, Zhiheng, Guihong Bi, Tongyin Li, Qianwen Zhang, and Patricia R. Knight. 2024. "Effect of Harvest Time on Growth and Bioactive Compounds in Salvia miltiorrhiza" Plants 13, no. 13: 1788. https://doi.org/10.3390/plants13131788
APA StyleXing, Z., Bi, G., Li, T., Zhang, Q., & Knight, P. R. (2024). Effect of Harvest Time on Growth and Bioactive Compounds in Salvia miltiorrhiza. Plants, 13(13), 1788. https://doi.org/10.3390/plants13131788






