Identification of Attractants from Three Host Plants and How to Improve Attractiveness of Plant Volatiles for Monochamus saltuarius
Abstract
1. Introduction
2. Results
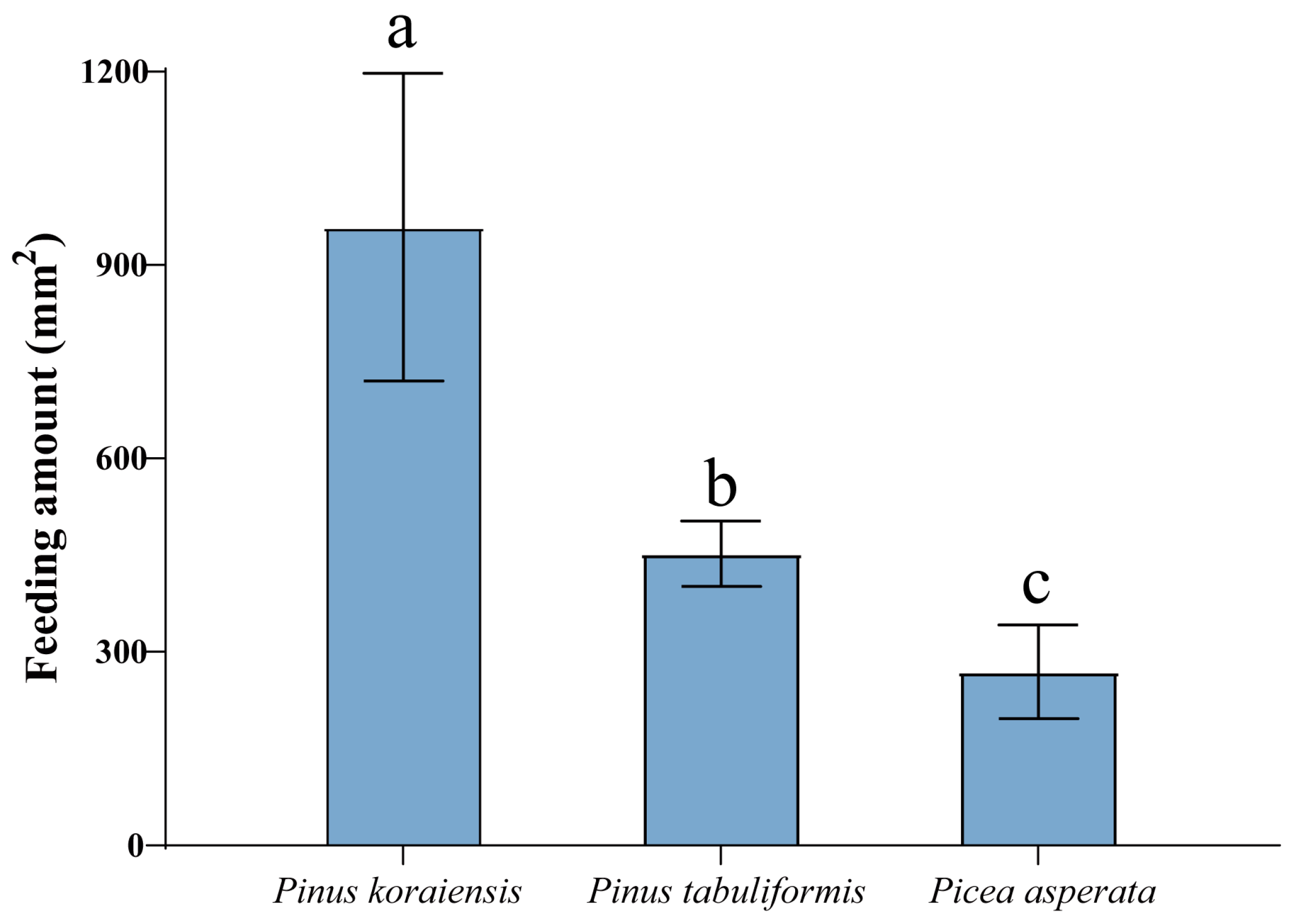
2.1. Feeding Preferences of M. saltuarius
2.2. Volatiles Identification of 3 Host Plants
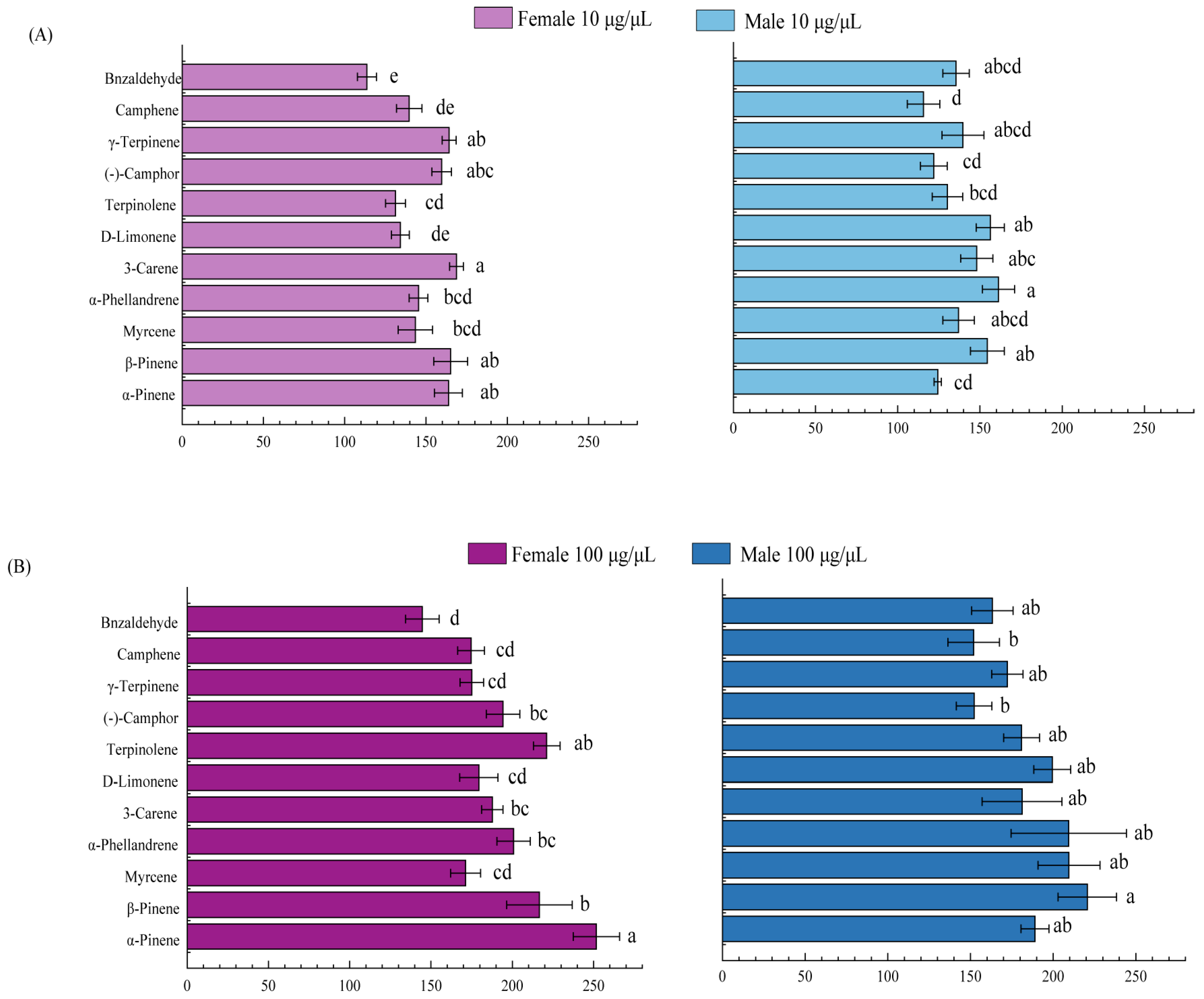
2.3. Electrophysiological Responses of M. saltuarius Adults to Volatiles in 3 Host Plants
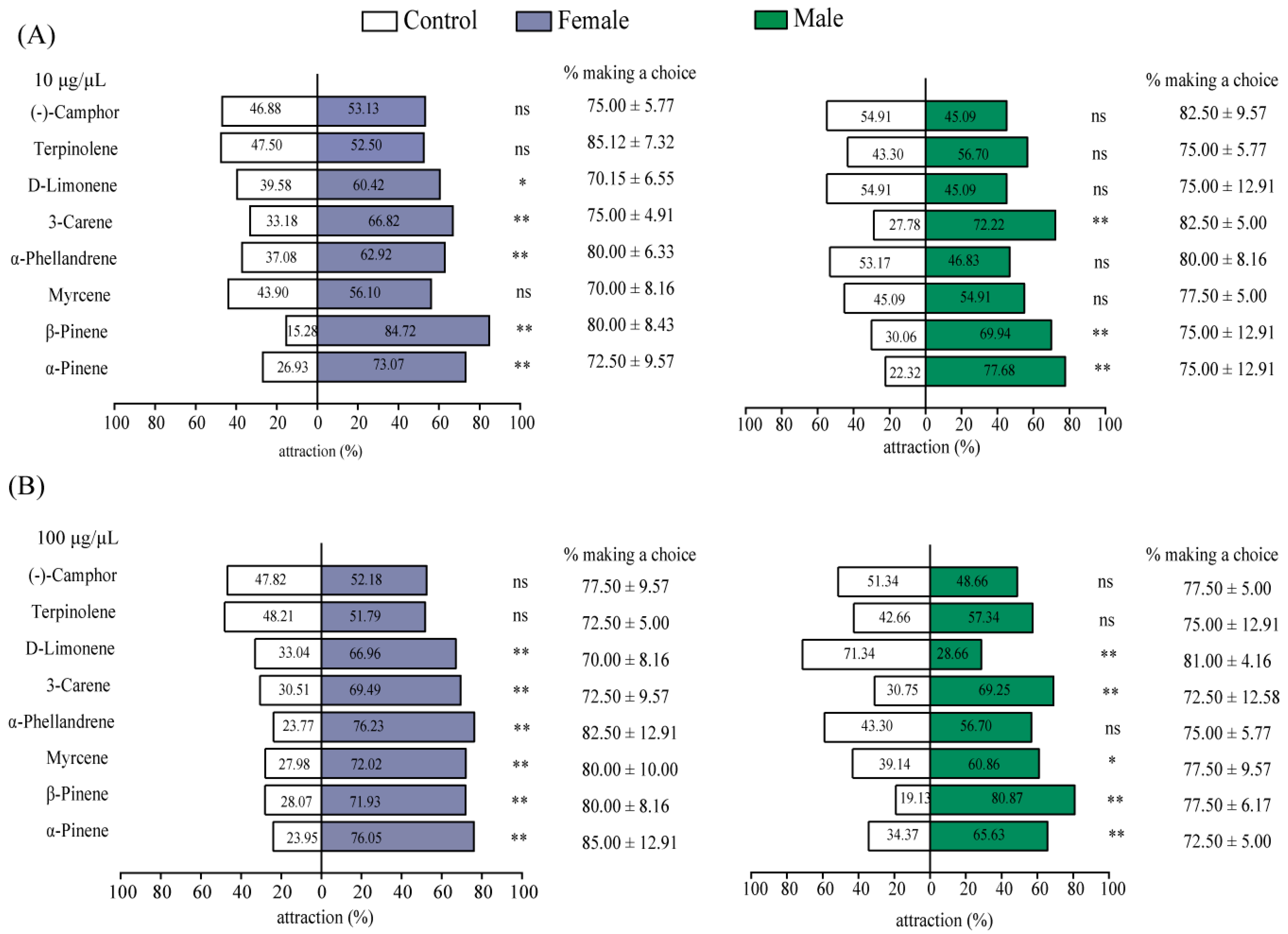
2.4. Olfaction Selection Preference on Antennal-Active Host Volatiles by Y-Tube Assay
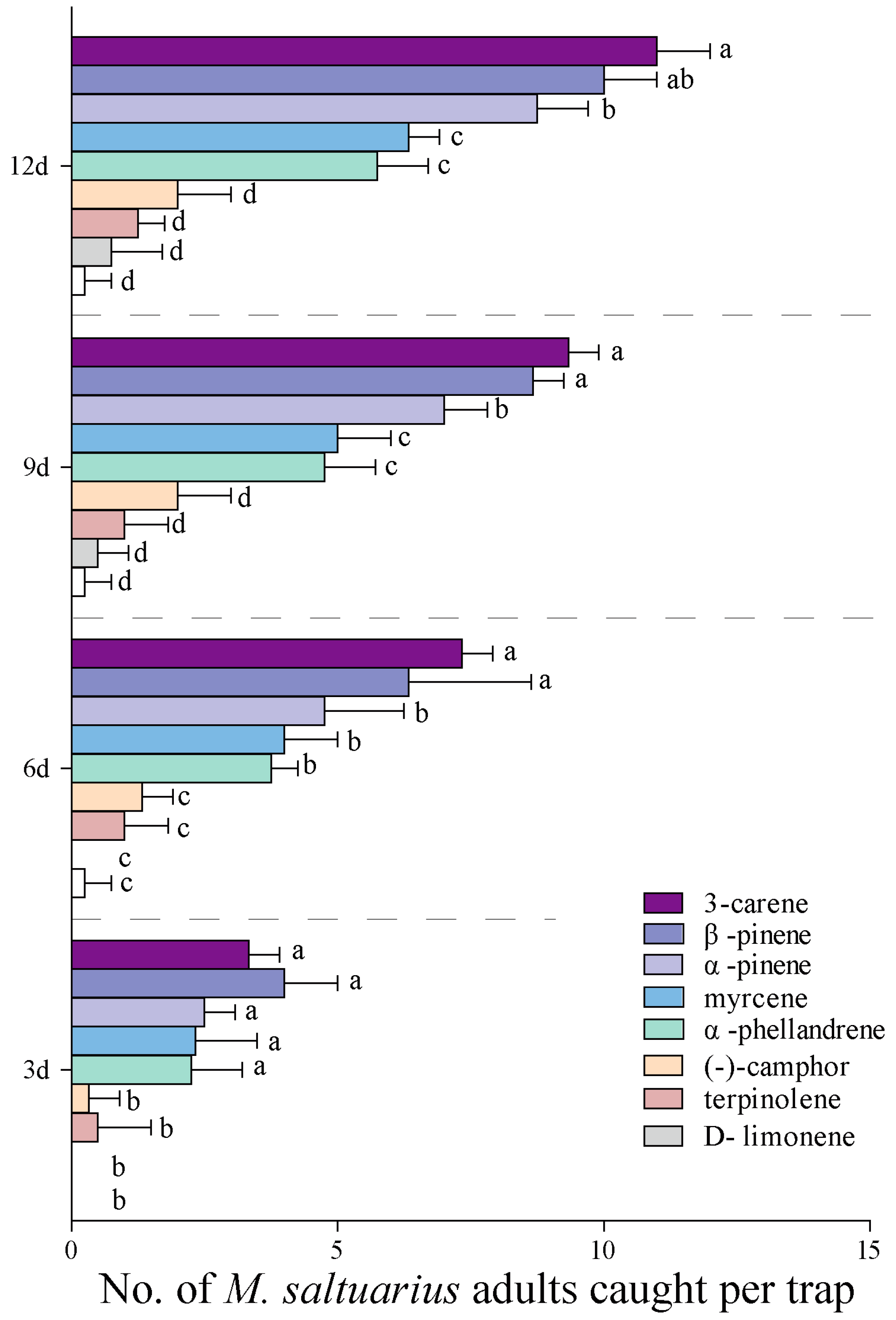
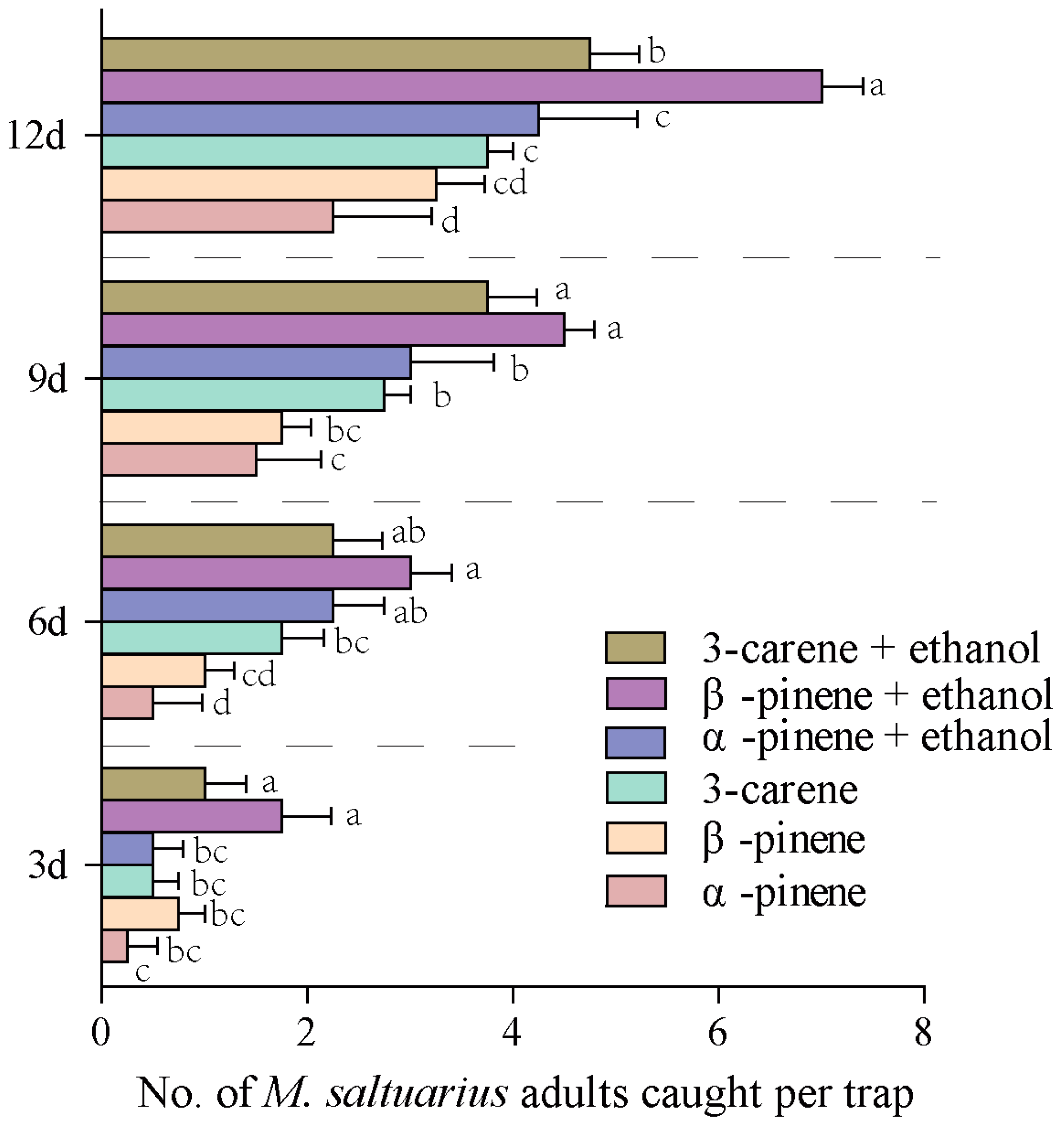
2.5. Forest Trapping Effects of 8 Active Volatiles to M. saltuarius Adults
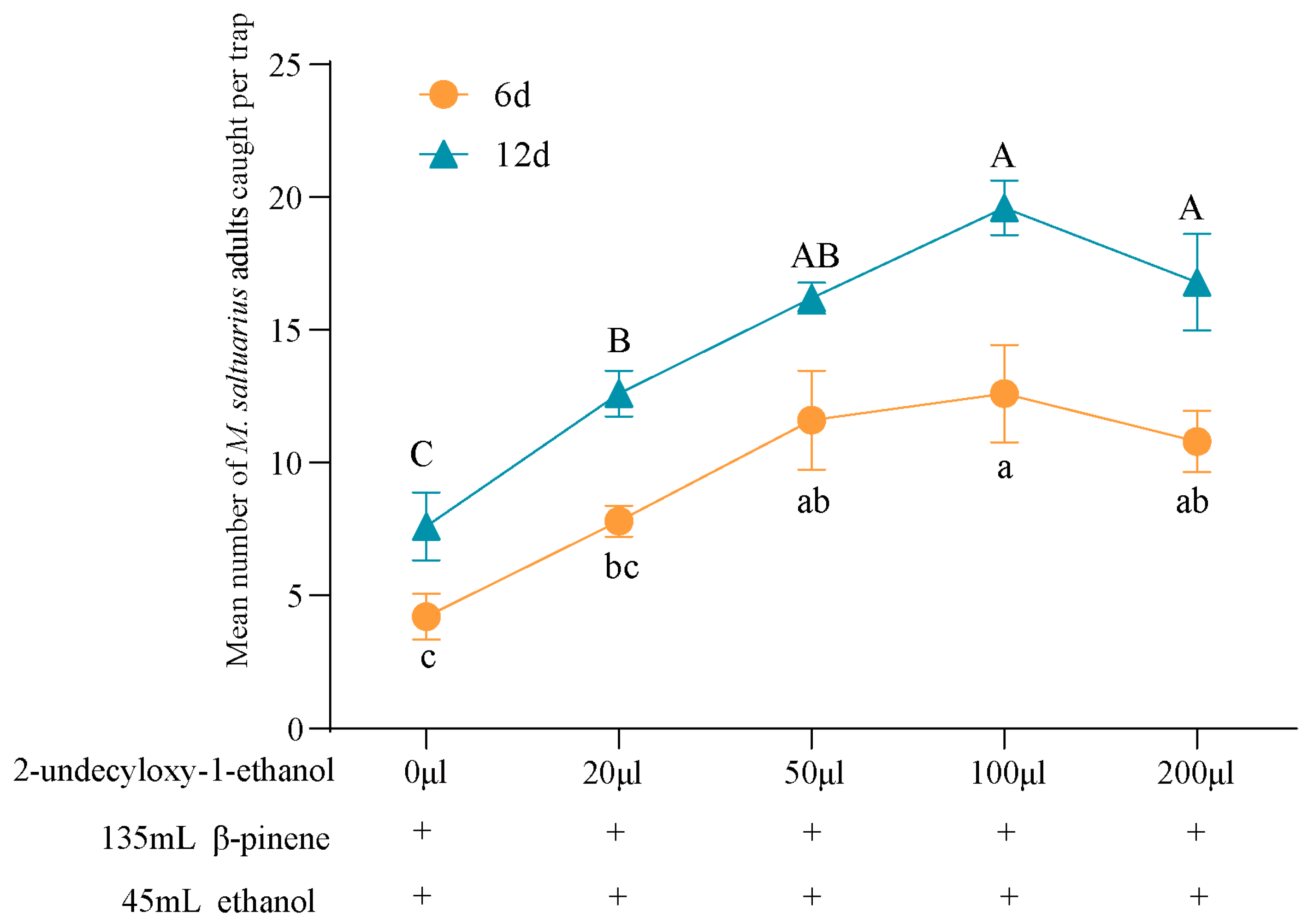
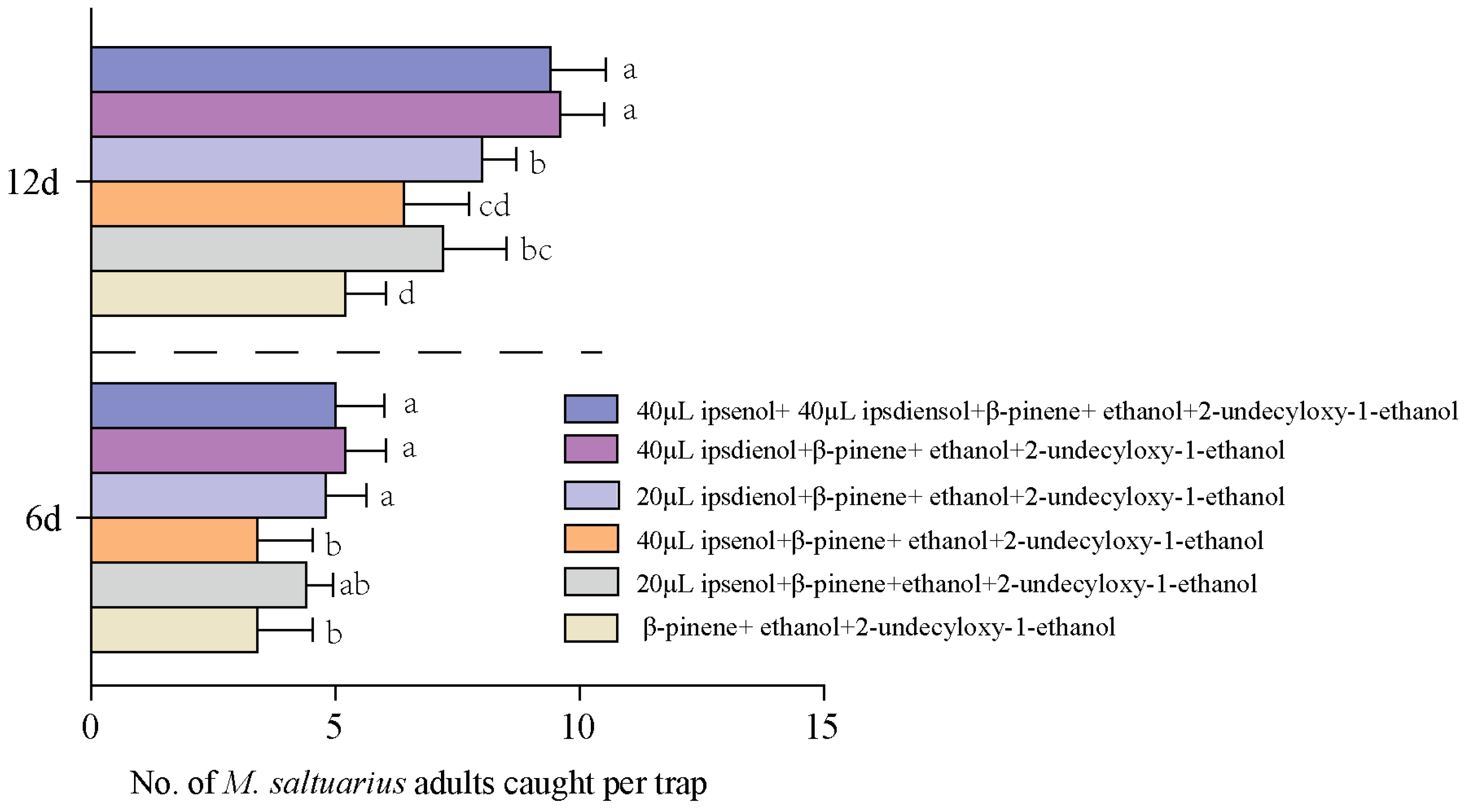
2.6. Field Trapping Effects of Blends on M. saltuarius Adults
2.6.1. Responses of M. saltuarius Adults to Blends of Single Volatile and Ethanol
2.6.2. Responses of M. saltuarius Adults to Blends of β-Pinene, Ethanol and 2-Undecyloxy-1-ethanol
2.6.3. Responses of M. saltuarius Adults to Blends of β-Pinene, Ethanol, 2-Undecyloxy-1-ethanol, and Pheromones of Bark Beetles
3. Discussion
4. Materials and Methods
4.1. Insects and Plants
4.2. Chemicals
4.3. Host Plant Preference Assays of M. saltuarius
4.4. Volatile Analysis of Three Host Plants
4.5. Electroantennography
4.6. Olfactory Behavioral Responses to Synthetic Active Volatiles
4.7. Field Experiments
4.7.1. Trapping Experiments of Single Compounds in Forests
4.7.2. The Synergies of Ethanol on the Trapping Effect of M. saltuarius in the Forest
4.7.3. Trapping Assays on the Synergistic Effect of Aggregation Pheromones of M. saltuarius
4.7.4. Field Attraction Tests to Explore Synergies of Bark Beetle Pheromones—Ipsdienol and Ipsenol
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ge, S.X.; Li, J.X.; Jiang, Z.H.; Zong, S.X.; Ren, L.L. Cradle for the newborn Monochamus saltuarius: Microbial associates to ward off entomopathogens and disarm plant defense. Insect Sci. 2023, 30, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, S.; Zheng, Y. Feeding Preferences and Responses of Monochamus saltuarius to Volatile Components of Host Pine Trees. Insects 2022, 13, 888. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y.; Lee, S. Diel rhythmicity of field responses to synthetic pheromone lures in the pine sawyer Monochamus saltuarius. Insects 2021, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fu, N.; Ren, L.; Luo, Y. Identification and Validation of Reference Genes for Gene Expression Analysis in Monochamus saltuarius Under Bursaphelenchus xylophilus Treatment. Front. Physiol. 2022, 13, 882792. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Shi, F.; Ge, S.; Tao, J.; Ren, L.; Wu, H.; Zong, S. Comparative transcriptome analysis of the newly discovered insect vector of the pine wood nematode in China, revealing putative genes related to host plant adaptation. BMC Genom. 2021, 22, 189. [Google Scholar] [CrossRef]
- Kishi, Y. Pine Wood Nematode and the Japanese Pine Sawyer; Thomas Company Limited: Tokyo, Japan, 1995. [Google Scholar]
- Kwon, T.S.; Lim, J.H.; Sim, S.J.; Kwon, Y.D.; Son, S.K.; Lee, K.Y.; Kim, Y.T.; Park, J.W.; Shin, C.H.; Ryu, S.B. Distribution patterns of Monochamus alternatus and M. saltuarius (Coleoptera: Cerambycidae) in Korea. J. Korean Soc. For. Sci. 2006, 95, 543–550. [Google Scholar]
- Li, M.; Li, H.; Sheng, R.C.; Sun, H.; Sun, S.H.; Chen, F.M. The first record of Monochamus saltuarius (Coleoptera; Cerambycidae) as vector of Bursaphelenchus xylophilus and its new potential hosts in China. Insects 2020, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Linit, M. Nemtaode-vector relationships in the pine wilt disease system. J. Nematol. 1988, 20, 227. [Google Scholar]
- Buszewski, B.; Bukowska, M.; Ligor, M.; Staneczko-Baranowska, I. A holistic study of neonicotinoids neuroactive insecticides properties, applications, occurrence, and analysis. Environ. Sci. Pollut. Res. 2019, 26, 34723–34740. [Google Scholar] [CrossRef]
- Moon, J.H.; Won, S.J.; Maung, C.E.H.; Choi, J.H.; Choi, S.I.; Ajuna, H.B.; Ahn, Y.S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef]
- Bordat, A.; Marchand, G.; Langlade, N.B.; Pouilly, N.; Muños, S.; Dechamp-Guillaume, G.; Vincourt, P.; Bret-Mestries, E. Different genetic architectures underlie crop responses to the same pathogen: The {Helianthus annuus* Phoma macdonaldii} interaction case for black stem disease and premature ripening. BMC Plant Biol. 2017, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Ma, M.; Li, B.; Zhou, Y.; Pan, Y.; Fan, Y.; Yi, B.; Tu, J. Improvement of resistance to clubroot disease in the Ogura CMS restorer line R2163 of Brassica napus. Plants 2022, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Jiang, Q.; Liu, L.; Zhang, S.; Liu, C.; Chen, H.; Ding, W.; Zhang, Y. Exploring the key microbial changes in the rhizosphere that affect the occurrence of tobacco root-knot nematodes. AMB Express 2020, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, T.; Kandul, N.P.; Bui, M.; Gamez, S.; Raban, R.; Bennett, J.; Sánchez, C.H.M.; Lanzaro, G.C.; Schmidt, H. Development of a confinable gene drive system in the human disease vector Aedes aegypti. eLife 2020, 9, e51701. [Google Scholar] [CrossRef] [PubMed]
- Pilz-Junior, H.L.; de Lemos, A.B.; de Almeida, K.N.; Corção, G.; Schrekker, H.S.; Silva, C.E.; da Silva, O.S. Microbiota potentialized larvicidal action of imidazolium salts against Aedes aegypti (Diptera: Culicidae). Sci. Rep. 2019, 9, 16164. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Hu, J.; Zhang, Y.; Xie, K.; Wang, B.; Tuyen, L.Q.; Song, Z.; Wu, H.; Liu, Y.; et al. Detection of Quantitative Trait Loci (QTLs) for Resistances to Small Brown Planthopper and Rice Stripe Virus in Rice Using Recombinant Inbred Lines. Int. J. Mol. Sci. 2013, 14, 8406–8421. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kamala Jayanthi, P.D.; Kempraj, V.; Aurade, R.M.; Venkataramanappa, R.K.; Nandagopal, B.; Verghese, A.; Bruce, T.J. Specific volatile compounds from mango elicit oviposition in gravid Bactrocera dorsalis females. J. Chem. Ecol. 2014, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Light, D.M.; Gee, W.S. Electroantennographic bioassay as a screening tool for host plant volatiles. J. Vis. Exp. 2012, 63, e3931. [Google Scholar] [CrossRef]
- Pérez-Aparicio, A.; Torres-Vila, L.M.; Gemeno, C. EAG responses of adult Lobesia botrana males and females collected from Vitis vinifera and Daphne gnidium to larval host-plant volatiles and sex pheromone. Insects 2019, 10, 281. [Google Scholar] [CrossRef]
- Fettköther, R.; Reddy, G.V.; Noldt, U.; Dettner, K. Effect of host and larval frass volatiles on behavioural response of the old house borer, Hylotrupes bajulus (L.) (Coleoptera: Cerambycidae), in a wind tunnel bioassay. Chemoecology 2000, 10, 1–10. [Google Scholar] [CrossRef]
- Das, A.; Lee, S.H.; Hyun, T.K.; Kim, S.W.; Kim, J.Y. Plant volatiles as method of communication. Plant Biotechnol. Rep. 2013, 7, 9–26. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, J.; Yan, Z.; Ma, Y.; Yang, M.; Zhang, M.; Zhang, Z.; Qin, L.; Cao, Q. Host preference and performance of the yellow peach moth (Conogethes punctiferalis) on chestnut cultivars. PLoS ONE 2016, 11, e0157609. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, H.B.; Yu, J.L.; Pan, D.; Tao, Y.; Lei, Q.; Chen, Y.; Liu, Z.; Wang, J.J. Two odorant receptors regulate 1-octen-3-ol induced oviposition behavior in the oriental fruit fly. Commun. Biol. 2023, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, J.; Yi, T.; Li, Y.-Y.; Xu, T.; Chen, L.; Xu, H. A green leaf volatile, (Z)-3-hexenyl-acetate, mediates differential oviposition by Spodoptera frugiperda on maize and rice. BMC Biol. 2023, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, R.G.; Westlind, D.J. Attraction of red turpentine beetle and other SS colytinae to ethanol, 3-carene or ethanol+ 3-carene in an oregon pine forest. Agric. For. Entomol. 2018, 20, 272–278. [Google Scholar] [CrossRef]
- Ikeda, T.; Oda, K. The occurrence of attractiveness for Monochamus alternatus Hope (Coleoptera: Cerambycidae) in nematode-infected pine trees. J. Jpn. For. Soc. 1980, 62, 432–434. [Google Scholar]
- Ikeda, T.; Enda, N.; Yamane, A.; Oda, K.; Toyoda, T. Attractants for the Japanese pine sawyer, Monochamus alternatus hope (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1980, 15, 358–361. [Google Scholar] [CrossRef]
- Millar, J.; Hanks, L. Chemical Ecology of Cerambycid Beetles. In Cerambycidae of the World: Biology and Pest Management; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2016. [Google Scholar]
- Löbl, I.; Löbl, D. Catalogue of Palaearctic Coleoptera. Volume 1: Archostemata-Myxophaga-Adephaga; Brill Academic Publishers: Leiden, The Netherlands, 2018. [Google Scholar]
- Teale, S.A.; Wickham, J.D.; Zhang, F.; Su, J.; Chen, Y.; Xiao, W.; Hanks, L.M.; Millar, J.G. A male-produced aggregation pheromone of Monochamus alternatus (Coleoptera: Cerambycidae), a major vector of pine wood nematode. J. Econ. Entomol. 2011, 104, 1592–1598. [Google Scholar] [CrossRef]
- Lee, H.R.; Lee, S.C.; Lee, D.H.; Choi, W.S.; Jung, C.S.; Jeon, J.H.; Kim, J.E.; Park, I.K. Identification of the aggregation-sex pheromone produced by male Monochamus saltuarius, a major insect vector of the pine wood nematode. J. Chem. Ecol. 2017, 43, 670–678. [Google Scholar] [CrossRef]
- Allison, J.D.; McKenney, J.L.; Millar, J.G.; Mcelfresh, J.S.; Mitchell, R.F.; Hanks, L.M. Response of the woodborers Monochamus carolinensis and Monochamus titillator (Coleoptera: Cerambycidae) to known cerambycid pheromones in the presence and absence of the host plant volatile α-pinene. Environ. Entomol. 2012, 41, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Hanks, L.M.; Millar, J.G. Sex and aggregation-sex pheromones of cerambycid beetles: Basic science and practical applications. J. Chem. Ecol. 2016, 42, 631–654. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ma, L.; Zhao, J.; Xue, Z.; Yan, X.; Hao, C. Electrophysiological and behavioral responses of Plutella xylostella (Lepidoptera: Plutellidae) to volatiles from a non-host plant, Geranium, Pelargonium × hortorum (Geraniaceae). J. Agric. Food Chem. 2022, 70, 5982–5992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Zhao, J.R.; Mao, L.J.; Shi, Z.H. Effects of the volatiles from different tomato varieties on host selection behavior of B-biotype Bemisia tabaci. J. Appl. Ecol. 2012, 23, 2509–2514. [Google Scholar]
- Yang, X.; Fan, F.; An, L.; Li, M.; Wei, G. Oviposition preferences in Grapholita molesta: The relative importance of visual and olfactory cues. Entomol. Exp. Et Appl. 2019, 167, 722–728. [Google Scholar] [CrossRef]
- Piesik, D.; Wenda-Piesik, A. Sitophilus granarius responses to blends of five groups of cereal kernels and one group of plant volatiles. J. Stored Prod. Res. 2015, 62, 36–39. [Google Scholar] [CrossRef]
- Fürstenau, B.; Rosell, G.; Guerrero, A.; Quero, C. Electrophysiological and behavioral responses of the black-banded oak borer, Coroebus florentinus, to conspecific and host-plant volatiles. J. Chem. Ecol. 2012, 38, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the chemical ecology of the spotted wing drosophila (Drosophila suzukii) and its applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Piesik, D.; Nowak, A.; Wawrzyniak, M. Tribolium confusum responses to blends of cereal kernels and plant volatiles. J. Appl. Entomol. 2016, 140, 558–563. [Google Scholar] [CrossRef]
- Chénier, J.; Philogene, B. Field responses of certain forest Coleoptera to conifer monoterpenes and ethanol. J. Chem. Ecol. 1989, 15, 1729–1745. [Google Scholar] [CrossRef]
- Nehme, M.; Keena, M.; Zhang, A.; Baker, T.; Xu, Z.; Hoover, K. Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. Environ. Entomol. 2010, 39, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Comparative analysis of volatile compounds from different host plants of Anoplophora glabripennis (Motsch.). J. Beijing For. Univ. 2002, 24, 165–169. [Google Scholar]
- Zhu, N.; Zhang, D.Y.; Wu, L.P.; Hu, Q.; Fan, J.T. Attractiveness of aggregation pheromones and host plant volatiles to Anoplophora glabripennis and A. chinensis (Coleoptera: Cerambycidae). Acta Entomol. Sin. 2017, 60, 421–430. [Google Scholar]
- Fan, L.Q.; Yan, S.C.; Sun, Z.H.; Meng, Z.J. EAG and behavioral responses of Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) to plant volatiles. Chin. J. Ecol. 2013, 32, 142–148. [Google Scholar]
- Li, S.; Gao, W.; Cheng, X.; Zhou, Y.; Cui, W. Electroantennogram response of Anoplophora glabripennis (Motsch.) to Acer negundo volatiles. For. Pest Dis. 2016, 35, 9–14. [Google Scholar]
- Nehme, M.; Keena, M.; Zhang, A.; Baker, T.; Hoover, K. Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. Environ. Entomol. 2009, 38, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Z.; Qin, H.; Wang, D.; Shi, J. Effects of lures to trap Anoplophora glabripennis (Motschulsky)(Coleoptera: Cerambycidae) in the coastal protection forest in Zhejiang, China. J. Environ. Entomol. 2017, 39, 694–700. [Google Scholar]
- Cavaletto, G.; Faccoli, M.; Ranger, C.M.; Rassati, D. Ambrosia beetle response to ethanol concentration and host tree species. J. Appl. Entomol. 2021, 145, 800–809. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Persad, A.B.; Herms, D.A. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177–185. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Gandhi, K.J.; Oliver, J.B.; Schultz, P.B.; Cañas, L.; Herms, D.A. Species dependent influence of (−)-α-pinene on attraction of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-baited traps in nursery agroecosystems. J. Econ. Entomol. 2011, 104, 574–579. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Narvaez, T.I.; Carrillo, D. Comparison of trap designs for detection of Euwallacea nr. fornicatus and other Scolytinae (Coleoptera: Curculionidae) that vector fungal pathogens of avocado trees in Florida. J. Econ. Entomol. 2020, 113, 980–987. [Google Scholar] [PubMed]
- Lyu, F.; Hai, X.; Wang, Z. A review of the host plant location and recognition mechanisms of Asian longhorn beetle. Insects 2023, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Guo, X.Q.; Li, X.; Jiang, X.J.; Jiang, X.G.; Ni, W.I.; Qu, M.J. Plant volatiles increase sex pheromone attraction of Holotrichia parallela (Coleoptera: Scarabaeoidea). J. Chem. Ecol. 2017, 43, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Koczor, S.; Tóth, M. Field attraction of Eurydema ornata (Hemiptera: Pentatomidae) to allyl isothiocyanate. Sci. Rep. 2023, 13, 11063. [Google Scholar] [CrossRef] [PubMed]
- Pajares, J.; Ibeas, F.; Diez, J.; Gallego, D. Attractive responses by Monochamus galloprovincialis (Col., Cerambycidae) to host and bark beetle semiochemicals. J. Appl. Entomol. 2004, 128, 633–638. [Google Scholar] [CrossRef]
- Fan, J.T.; Zheng, K.W.; Dong, Y.F.; Gu, Y.T.; Wickham, J.D. Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge. Insects 2023, 14, 911. [Google Scholar] [CrossRef]
| No. | Compounds | Category | No. CAS | Relative Content (%) | ||
|---|---|---|---|---|---|---|
| Pinus koraiensis | Pinus tabuliformis | Picea asperata | ||||
| 1 | Nonane | Alkane | 111-84-2 | 0.34 ± 0.05 | 0 | 0 |
| 2 | α-Pinene | Terpenes | 7785-26-4 | 11.41 ± 0.61 b | 21.36 ± 5.61 a | 9.49 ± 3.30 b |
| 3 | 4-Carene | Terpenes | 29050-33-7 | 1.36 ± 0.81 a | 1.24 ± 0.21 a | 0 |
| 4 | Camphene | Terpenes | 79-92-5 | 0.54 ± 0.04 | 0 | 0 |
| 5 | Benzaldehyde | Aldehyde | 100-52-7 | 0 | 0 | 2.02 ± 1.47 |
| 6 | β-Pinene | Terpenes | 18172-67-3 | 7.20 ± 0.65 b | 34.62 ± 6.71 a | 12.92 ± 3.49 b |
| 7 | β-Myrcene | Terpenes | 123-35-3 | 22.59 ± 2.67 a | 0 | 11.57 ± 2.89 b |
| 8 | α-Phellandrene | Terpenes | 99-83-2 | 3.01 ± 0.53 | 0 | 0 |
| 10 | β-Thujene | Terpenes | 28634-89-1 | 0.24 ± 0.06 | 0 | 0 |
| 11 | γ-Terpinene | Terpenes | 99-85-4 | 4.68 ± 2.95 a | 0.97 ± 0.39 a | 0 |
| 12 | β-Terpinene | Terpenes | 99-84-3 | 0 | 7.57 ± 3.25 | 0 |
| 13 | 3-Carene | Terpenes | 13466-78-9 | 22.75 ± 3.74 a | 0 | 20.76 ± 6.18 b |
| 14 | D-Limonene | Terpenes | 7705-14-8 | 28.00 ± 3.85 b | 25.43 ± 6.46 b | 40.73 ± 8.14 a |
| 15 | Terpinolene | Terpenes | 586-62-9 | 0.54 ± 0.11 a | 0.23 ± 0.09 a | 0 |
| 16 | Undecane | Alkane | 1120-21-4 | 0 | 4.32 ± 0.14 | 0 |
| 17 | (-)-Camphor | Ketones | 464-48-2 | 0.77 ± 0.25 b | 0 | 3.93 ± 1.60 a |
| 18 | Bornyl acetate | Ester | 76-49-3 | 0.1 ± 0.06 | 0 | 0 |
| No. | Compounds | CAS | Purity | Sources |
|---|---|---|---|---|
| 1 | α-Pinene | 7785-26-4 | 98% | J&K SCIENTIFIC |
| 2 | β-Pinene | 18172-67-3 | 98% | J&K SCIENTIFIC |
| 3 | Myrcene | 123-35-3 | 90% | J&K SCIENTIFIC |
| 4 | 3-Carene | 13466-78-9 | 90% | TCI AMERICA |
| 5 | α-Phellandrene | 99-83-2 | 98% | J&K SCIENTIFIC |
| 6 | D-Limonene | 5989-27-5 | 95% | J&K SCIENTIFIC |
| 7 | (-)-Camphor | 464-48-2 | 96% | MACKLIN |
| 8 | Terpinolene | 586-62-9 | 95% | J&K SCIENTIFIC |
| 9 | Camphene | 79-92-5 | 78% | TCI AMERICA |
| 10 | γ-Terpinene | 99-85-4 | 95% | J&K SCIENTIFIC |
| 11 | Benzaldehyde | 100-52-7 | 98% | J&K SCIENTIFIC |
| 12 | Paraffin liquid | 8012-95-1 | 99% | HUSHI |
| Serial Number | Formulations |
|---|---|
| 1 | 135 mL β-pinene + 45 mL ethanol |
| 2 | 135 mL β-pinene + 45 mL ethanol + 20 μL 2-undecyloxy-1-ethanol |
| 3 | 135 mL β-pinene + 45 mL ethanol + 50 μL 2-undecyloxy-1-ethanol |
| 4 | 135 mL β-pinene + 45 mL ethanol + 100 μL 2-undecyloxy-1-ethanol |
| 5 | 135 mL β-pinene + 45 mL ethanol + 200 μL 2-undecyloxy-1-ethanol |
| Serial Number | Formulations |
|---|---|
| 1 | 135 mL α-pinene + 45 mL ethanol + 2-undecyloxy-1-ethanol |
| 2 | 135 mL β-pinene + 45 mL ethanol + 2-undecyloxy-1-ethanol |
| 3 | 135 mL β-pinene + 45 mL ethanol + 100 μL 2-undecyloxy-1-ethanol + 20 μL ipsdienol |
| 4 | 135 mL β-pinene + 45 mL ethanol + 100 μL 2-undecyloxy-1-ethanol + 20 μL ipsenol |
| 5 | 135 mL β-pinene + 45 mL ethanol + 100 μL 2-undecyloxy-1-ethanol + 40 μL ipsdienol |
| 6 | 135 mL β-pinene + 45 mL ethanol + 100 μL 2-undecyloxy-1-ethanol + 40 μL ipsenol |
| 7 | 135 mL β-pinene + 45 mL ethanol + 100 μL 2-undecyloxy-1-ethanol + 40 μL ipsenol + 40 μL ipsdienol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Chen, D.; Zhou, S.; Mao, Z.; Fan, J. Identification of Attractants from Three Host Plants and How to Improve Attractiveness of Plant Volatiles for Monochamus saltuarius. Plants 2024, 13, 1732. https://doi.org/10.3390/plants13131732
Dong Y, Chen D, Zhou S, Mao Z, Fan J. Identification of Attractants from Three Host Plants and How to Improve Attractiveness of Plant Volatiles for Monochamus saltuarius. Plants. 2024; 13(13):1732. https://doi.org/10.3390/plants13131732
Chicago/Turabian StyleDong, Yifan, Dongping Chen, Siye Zhou, Zhengyi Mao, and Jianting Fan. 2024. "Identification of Attractants from Three Host Plants and How to Improve Attractiveness of Plant Volatiles for Monochamus saltuarius" Plants 13, no. 13: 1732. https://doi.org/10.3390/plants13131732
APA StyleDong, Y., Chen, D., Zhou, S., Mao, Z., & Fan, J. (2024). Identification of Attractants from Three Host Plants and How to Improve Attractiveness of Plant Volatiles for Monochamus saltuarius. Plants, 13(13), 1732. https://doi.org/10.3390/plants13131732






