First Clarification of the Mechanism of Action of the Apple Glycosyltransferase MdUGT91AJ2 Involved in the Detoxification Metabolism of the Triketone Herbicide Sulcotrione
Abstract
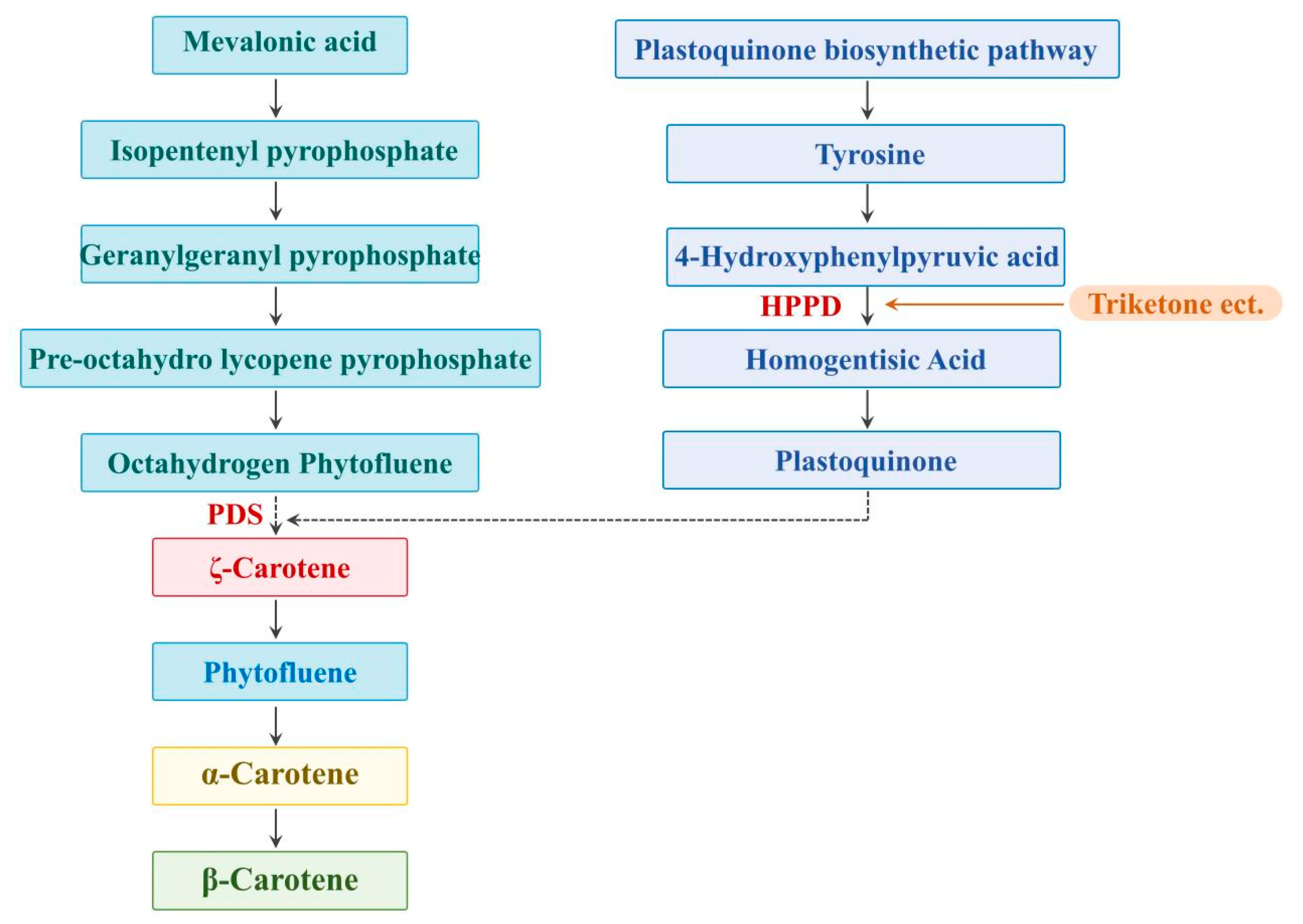
1. Introduction
2. Results
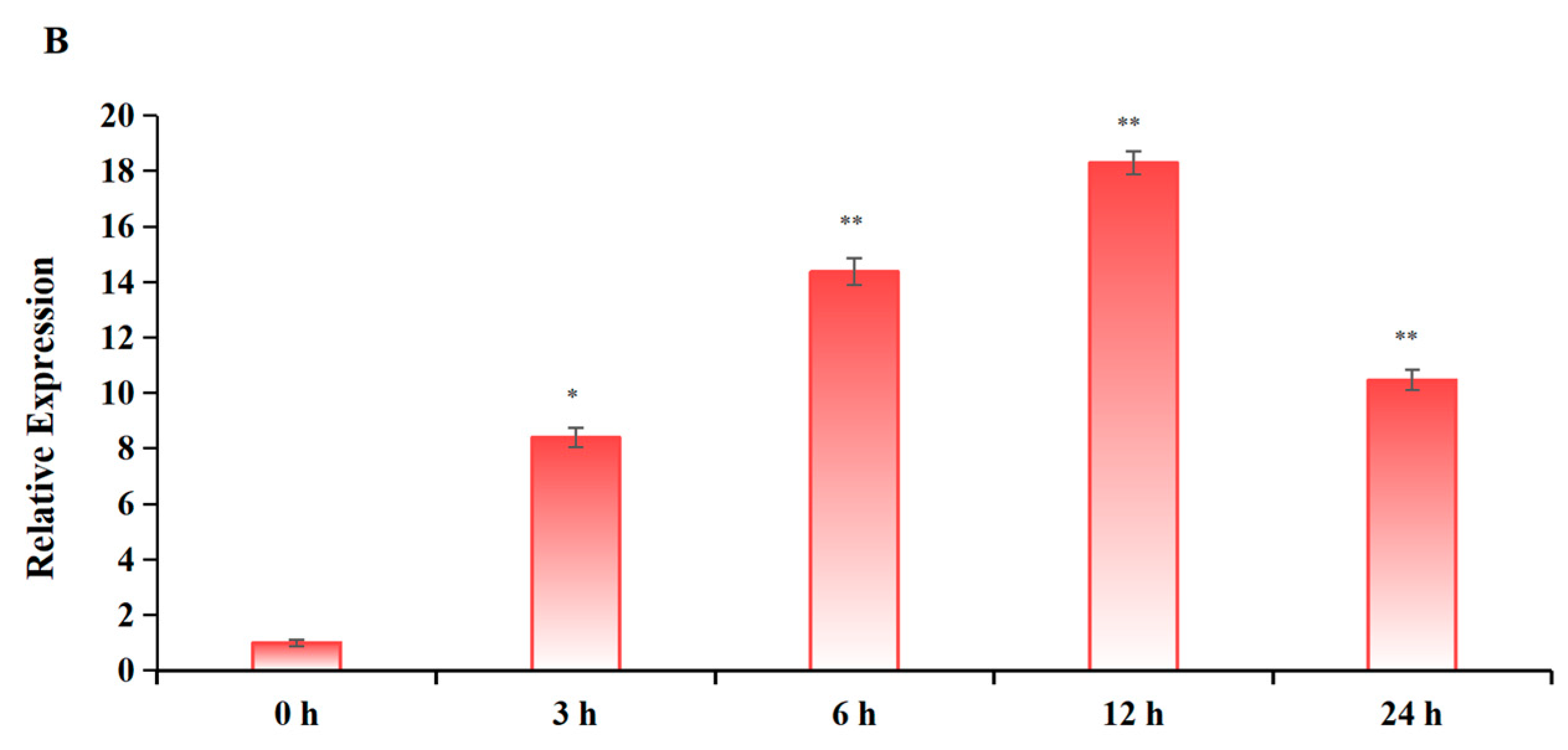
2.1. Induction of mRNA Expression Level of Apple Glycosyltransferase Gene by Triketone Herbicides
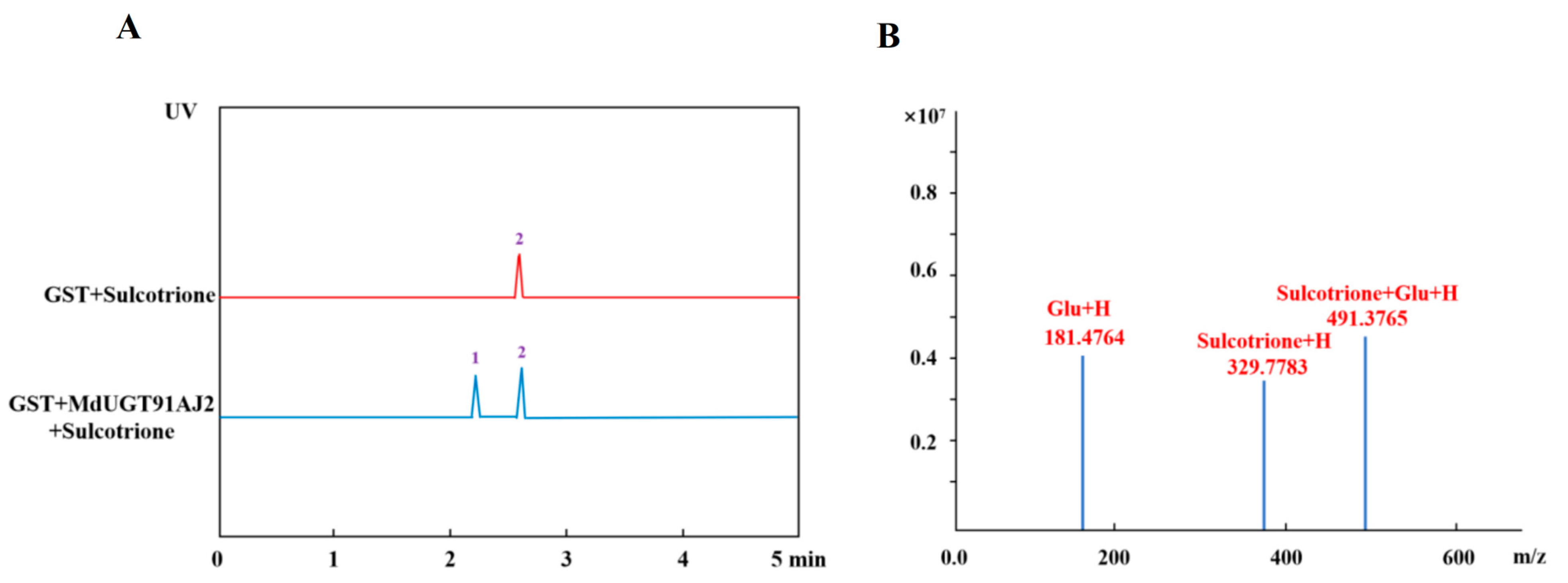
2.2. In Vitro Enzyme Activity Assay of Apple Glycosyltransferase Glycosylated Modified Triketone Herbicides
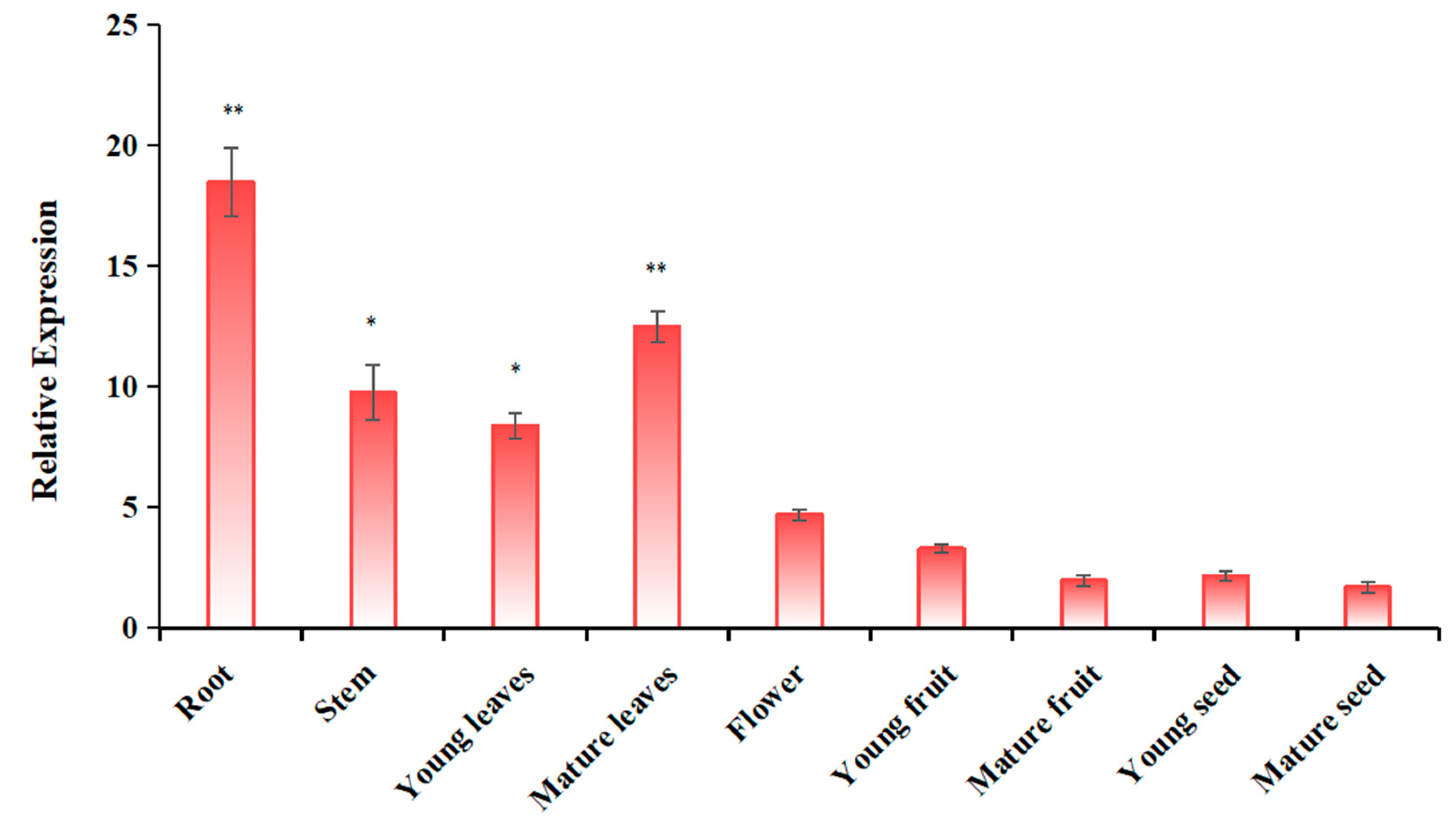
2.3. Analysis of the Expression Pattern of MdUGT91AJ2 in Different Tissue Sites
2.4. Glycosylation of Apple Glycosyltransferase MdUG91AJ2 In Vivo in Plant to Modify Sulforaphane Enzyme Activity Assays
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Exogenous Treatment of Apple Seedlings with Triketone Herbicides
4.3. Real-Time PCR
4.4. Expression Vector Construction
4.5. Glycosyltransferase Protein Purification and In Vitro Enzyme Activation Reaction
4.6. Determination of Triketone Herbicide Glycosides via HPLC
4.7. Transgenic Apple Seedling Screening
4.8. Data Statistics and Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Liu, W.; Jin, T.; Peng, X.; Zhang, L.; Wang, J. Bipyrazone: A new HPPD-inhibiting herbicide in wheat. Sci. Rep. 2020, 10, 5521. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, Q.; Han, H.; Owen, M.J.; Powles, S.B. Evolution of resistance to HPPD-inhibiting herbicides in a wild radish population via enhanced herbicide metabolism. Pest. Manag. Sci. 2020, 76, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiao, Z.; Yao, C.; Sun, S.; Liu, W.; Wang, J. Effects of the novel HPPD-inhibitor herbicide QYM201 on enzyme activity and microorganisms, and its degradation in soil. Ecotoxicology 2021, 30, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bacteria, Fungi, and Enzymes in Soil Treated with Sulcotrione and Terbuthylazine. Int. J. Mol. Sci. 2023, 24, 14469. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, W.; Zhang, L.; He, X.; Fan, Y.; Alam, S.; Yuan, X. Pyrazosulfuron-Methyl on the Photosynthetic Characteristics and Antioxidant Systems of Foxtail Millet. Front. Plant Sci. 2021, 12, 696169. [Google Scholar] [CrossRef] [PubMed]
- Brabham, C.; Norsworthy, J.K.; González-Torralva, F. Presence of the HPPD Inhibitor Sensitive 1 Gene and ALSS653N Mutation in Weedy Oryza sativa Sensitive to Benzobicyclon. Plants 2020, 9, 1576. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Georgelis, N.; Bedair, M.; Hong, Y.J.; Qi, Q.; Larue, C.T.; Sitoula, B.; Huang, W.; Krebel, B.; Shepard, M.; et al. Ectopic expression of a rice triketone dioxygenase gene confers mesotrione tolerance in soybean. Pest. Manag. Sci. 2022, 78, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Mužinić, V.; Katić, A.; Kašuba, V.; Micek, V.; Milić, M.; Želježić, D. Assessment of transplacental and lactational genotoxicity of tembotrione in Wistar rats at different developmental stages by alkaline comet assay. Toxicology 2021, 463, 152983. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Liu, R.; Boyer, G.; Stahlman, P.W. Confirmation of 2,4-D resistance and identification of multiple resistance in a Kansas Palmer amaranth (Amaranthus palmeri) population. Pest Manag. Sci. 2019, 75, 925–2933. [Google Scholar] [CrossRef]
- Šojić Merkulov, D.V.; Despotović, V.N.; Banić, N.D.; Armaković, S.J.; Finčur, N.L.; Lazarević, M.J.; Četojević-Simin, D.D.; Orčić, D.Z.; Radoičić, M.B.; Šaponjić, Z.V.; et al. Photocatalytic decomposition of selected biologically active compounds in environmental waters using TiO2/polyaniline nanocomposites: Kinetics, toxicity and intermediates assessment. Environ. Pollut. 2018, 239, 457–465. [Google Scholar] [CrossRef]
- Chen, P.; Shi, M.; Liu, X.; Wang, X.; Fang, M.; Guo, Z.; Wu, X.; Wang, Y. Comparison of the binding interactions of 4-hydroxyphenylpyruvate dioxygenase inhibitor herbicides with humic acid: Insights from multispectroscopic techniques, DFT and 2D-COS-FTIR. Ecotoxicol. Environ. Saf. 2022, 239, 113699. [Google Scholar] [CrossRef] [PubMed]
- Goujon, E.; Maruel, S.; Richard, C.; Goupil, P.; Ledoigt, G. Transformation of the Herbicide Sulcotrione into a Root Growth Enhancer Compound by Sequential Photolysis and Hydrolysis. J. Agric. Food Chem. 2016, 64, 563–569. [Google Scholar] [CrossRef]
- Goujon, E.; Richard, C.; Goupil, P.; Ledoigt, G. Cytotoxicity on Allium cepa of the two main sulcotrione photoproducts, xanthene-1,9-dione-3,4-dihydro-6-methylsulphonyl and 2-chloro-4-mesylbenzoic acid. Pestic. Biochem. Physiol. 2015, 124, 37–42. [Google Scholar] [CrossRef]
- ter Halle, A.; Lavieille, D.; Richard, C. The effect of mixing two herbicides mesotrione and nicosulfuron on their photochemical reactivity on cuticular wax film. Chemosphere 2010, 79, 482–487. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Halle, A.T.; Richard, C.; Bonnemoy, F.; Bohatier, J. Toxicity of sulcotrione photoproducts mixture towards vibrio fischeri in the aquatic environment. Arch. Environ. Prot. 2011, 37, 15–23. [Google Scholar]
- Rani, R.; Juwarkar, A. Adsorption of Phorate, an Organophosphorus Pesticide, on Vertisol. Arch. Environ. Contam. Toxicol. 2010, 58, 927–934. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Kristanti, R.A. Bioremediation of perfluorochemicals: Current state and the way forward. Bioprocess. Biosyst. Eng. 2022, 45, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Morillo, E.; Madrid, F.; Lara-Moreno, A.; Villaverde, J. Soil bioremediation by cyclodextrins. A review. Int. J. Pharm. 2020, 591, 119943. [Google Scholar] [CrossRef]
- Borthakur, D.; Rani, M.; Das, K.; Shah, M.P.; Sharma, B.K.; Kumar, A. Bioremediation: An alternative approach for detoxification of polymers from the contaminated environment. Lett. Appl. Microbiol. 2022, 75, 744–758. [Google Scholar] [CrossRef]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in soil-plant system: Transport, detoxification and bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Bhat, S.A.; Singh, S.; Singh, J.; Kumar, S.; Bhawana; Vig, A.P. Bioremediation and detoxification of industrial wastes by earthworms: Vermicompost as powerful crop nutrient in sustainable agriculture. Bioresour. Technol. 2018, 252, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef]
- Ferreira, P.; Fernandes, P.A.; Ramos, M.J. The Catalytic Mechanism of the Retaining Glycosyltransferase Mannosylglycerate Synthase. Chemistry 2021, 27, 13998–14006. [Google Scholar] [CrossRef] [PubMed]
- Blomstedt, C.K.; O’Donnell, N.H.; Bjarnholt, N.; Neale, A.D.; Hamill, J.D.; Møller, B.L.; Gleadow, R.M. Metabolic consequences of knocking out UGT85B1, the gene encoding the glucosyltransferase required for synthesis of dhurrin in Sorghum bicolor (L. Moench). Plant Cell Physiol. 2016, 57, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Xu, Z.S.; Peng, R.H.; Tian, Y.S.; Zhao, W.; Han, H.J.; Yao, Q.H.; Wu, A.Z. Phytoremediation of trichlorophenol by Phase II metabolism in transgenic Arabidopsis overexpressing a Populus glucosyltransferase. Environ. Sci. Technol. 2012, 46, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.; Giraudo, M.; Goujon, E.; Halma, M.; Knhili, E.; Stauffert, M.; Batisson, I.; Besse-Hoggan, P.; Bohatier, J.; Bouchard, P.; et al. Fate and ecotoxicological impact of new generation herbicides from the triketone family: An overview to assess the environmental risks. J. Hazard. Mater. 2017, 325, 136–156. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.R. 4-Hydroxyphenylpyruvate dioxygenase. Arch. Biochem. Biophys. 2005, 433, 117–128. [Google Scholar] [CrossRef]
- Matringe, M.; Sailland, A.; Pelissier, B.; Rolland, A.; Zink, O. p-Hydroxyphenylpyruvate dioxygenase inhibitor-resistant plants. Pest Manag. Sci. 2005, 61, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.B.; Gao, S.; Hu, W.; Sheng, B.R.; Shi, J.; Ye, F.; Fu, Y. Design, synthesis and biological activity of novel triketone herbicides containing natural product fragments. Pestic. Biochem. Physiol. 2023, 194, 105493. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef]
- Trivella, A.; Richard, C. New insights into pesticide photoprotection. Environ. Sci. Pollut. Res. Int. 2014, 21, 4828–4836. [Google Scholar] [CrossRef] [PubMed]
- Goujon, E.; Sta, C.; Trivella, A.; Goupil, P.; Richard, C.; Ledoigt, G. Genotoxicity of sulcotrione pesticide and photoproducts on Allium cepa root meristem. Pestic. Biochem. Physiol. 2014, 113, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.X.; Zhao, S.M.; Zhang, Y.Y.; Li, Y.J.; Shen, H.N.; Li, X.; Hou, B.K. A novel UDP-glycosyltransferase 91C1 confers specific herbicide resistance through detoxification reaction in Arabidopsis. Plant Physiol. Biochem. 2021, 159, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, A.; Li, R.; Han, S.; Li, N.; Ji, L.; Lei, K. Identifying a Detoxifying Uridine Diphosphate Glucosyltransferase (UGT), MdUGT83K2, Which Can Glycosylate the Aryloxyphenoxypropionate Herbicide. Agronomy 2023, 13, 306. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, A.; Mu, L.; Teng, X.; Ma, Y.; Li, R.; Lei, K.; Ji, L.; Wang, X.; Li, P. First Clarification of the Involvement of Glycosyltransferase MdUGT73CG22 in the Detoxification Metabolism of Nicosulfuron in Apple. Plants 2024, 13, 1171. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Zheng, P.F.; Ren, Y.R.; Yao, Y.X.; You, C.X.; Wang, X.F.; Hao, Y.J. Apple MdSAT1 encodes a bHLHm1 transcription factor involved in salinity and drought responses. Planta 2021, 253, 46. [Google Scholar] [CrossRef] [PubMed]
- Forcella, M.; Manzoni, M.; Benaglia, G.; Bonanomi, M.; Giacopuzzi, E.; Papini, N.; Bresciani, R.; Fusi, P.; Borsani, G.; Monti, E. Characterization of three sialidases from Danio rerio. Biochimie 2021, 187, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Beihaghi, M.; Marashi, H.; Bagheri, A.; Sankian, M. Transient expression of CCL21as recombinant protein in tomato. Biotechnol. Rep. 2017, 17, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Vicent, C.; Cerezo, M.; Mauch-Mani, B.; Dean, J.; Flors, V. Detection, characterization and quantification of salicylic acid conjugates in plant extracts by ESI tandem mass spectrometric techniques. Plant Physiol. Biochem. 2012, 53, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Attaran, E.; Zeier, T.E.; Griebel, T.; Zeier, J. Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 2009, 21, 954–971. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]


| Name | Substrates | Specific Activity (nkat/mg Protein) |
|---|---|---|
| MdUGT91AJ1 | Sulcotrione | 1.24 ± 0.19 |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | ND | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91AJ2 | Sulcotrione | 3.56 ± 0.25 |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | ND | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91AJ3 | Sulcotrione | ND |
| Tefuryltrione | 0.36 ± 0.11 | |
| Benzobicyclon | ND | |
| Mesotrione | ND | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91AJ4 | Sulcotrione | 0.76 ± 0.20 |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | ND | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91AJ5 | Sulcotrione | ND |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | ND | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91AJ6 | Sulcotrione | ND |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | 0.31 ± 0.04 | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91AJ7 | Sulcotrione | ND |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | 0.45 ± 0.08 | |
| Tembotrione | ND | |
| Bicyclopyrone | ND | |
| MdUGT91C7 | Sulcotrione | ND |
| Tefuryltrione | ND | |
| Benzobicyclon | ND | |
| Mesotrione | 0.39 ± 0.07 | |
| Tembotrione | ND | |
| Bicyclopyrone | ND |
| Chemical Compound | Structure Name | Structural Formula | Manufacturer | CAS |
|---|---|---|---|---|
| Sulcotrione | 2-(2-Chloro-4-(methylsulfonyl)benzoyl)-1,3-cyclohexanedione | 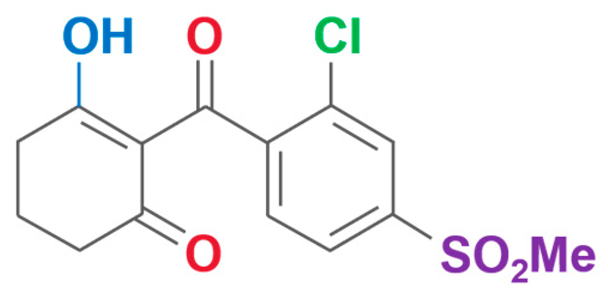 | JLK Pharmaceutical Technology Co., Ltd (Beijing, China) | 99105-77-8 |
| Tefuryltrione | 2-{2-chloro-4-(methylsulfonyl)-3-[(tetrahydrofuran-2-ylmethoxy)methyl]benzoyl}cyclohexane-1,3-dione |  | Bayer CropScience Co., Ltd (Hangzhou, China) | 473278-76-1 |
| Benzobicyclon | 3-[2-chloro-4-(methylsulfonyl)benzoyl]-4-(phenylthio)bicyclo[3.2.1]oct-3-en-2-one | 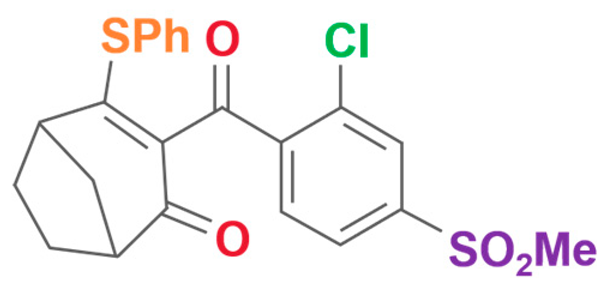 | One Plus One Biotechnology Co., Ltd (Wuhan, China) | 156963-66-5 |
| Mesotrione | 2-(4-Methylsulfonyl-2-nitrobenzoyl)-1,3-cyclohexanedione | 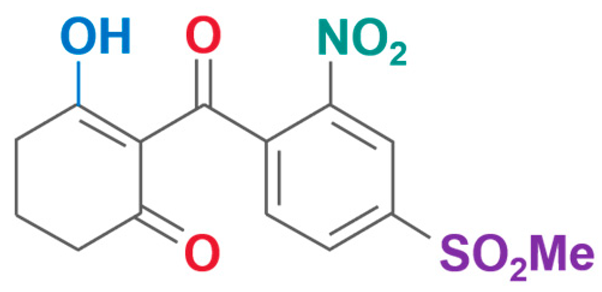 | Xianda Agrochemical Co., Ltd (Binzhou, China) | 104206-82-8 |
| Tembotrione | 2-{2-chloro-4-mesyl-3-[(2,2,2-trifluoroethoxy)methyl]benzoyl}cyclohexane-1,3-dione | 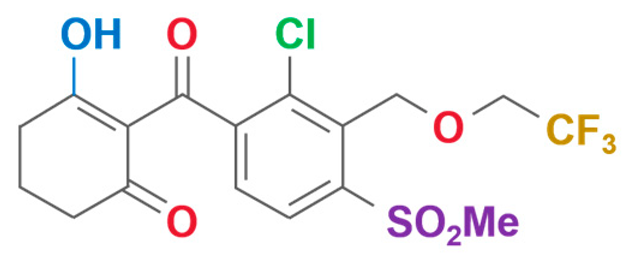 | Bayer CropScience Co., Ltd (Hangzhou, China) | 335104-84-2 |
| Bicyclopyrone | 4-Hydroxy-3-{2-[(2-methoxyethoxy)methyl]-6-(trifluoromethyl)-3-pyridinylcarbonyl}bicyclo[3.2.1]oct-3-en-2-one |  | Huisheng Agricultural Technology Co., Ltd (Beijing, China) | 352010-68-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, A.; Teng, X.; Ma, Y.; Mu, L.; Han, S.; Wang, S.; Lei, K.; Ji, L.; Li, P. First Clarification of the Mechanism of Action of the Apple Glycosyltransferase MdUGT91AJ2 Involved in the Detoxification Metabolism of the Triketone Herbicide Sulcotrione. Plants 2024, 13, 1796. https://doi.org/10.3390/plants13131796
Zhao A, Teng X, Ma Y, Mu L, Han S, Wang S, Lei K, Ji L, Li P. First Clarification of the Mechanism of Action of the Apple Glycosyltransferase MdUGT91AJ2 Involved in the Detoxification Metabolism of the Triketone Herbicide Sulcotrione. Plants. 2024; 13(13):1796. https://doi.org/10.3390/plants13131796
Chicago/Turabian StyleZhao, Aijuan, Xiao Teng, Yingxin Ma, Lijun Mu, Shibo Han, Shumin Wang, Kang Lei, Lusha Ji, and Pan Li. 2024. "First Clarification of the Mechanism of Action of the Apple Glycosyltransferase MdUGT91AJ2 Involved in the Detoxification Metabolism of the Triketone Herbicide Sulcotrione" Plants 13, no. 13: 1796. https://doi.org/10.3390/plants13131796
APA StyleZhao, A., Teng, X., Ma, Y., Mu, L., Han, S., Wang, S., Lei, K., Ji, L., & Li, P. (2024). First Clarification of the Mechanism of Action of the Apple Glycosyltransferase MdUGT91AJ2 Involved in the Detoxification Metabolism of the Triketone Herbicide Sulcotrione. Plants, 13(13), 1796. https://doi.org/10.3390/plants13131796







