Effect of Biotic Elicitors on the Growth, Antioxidant Activity and Metabolites Accumulation in In Vitro Propagated Shoots of Pueraria tuberosa
Abstract
1. Introduction
2. Results
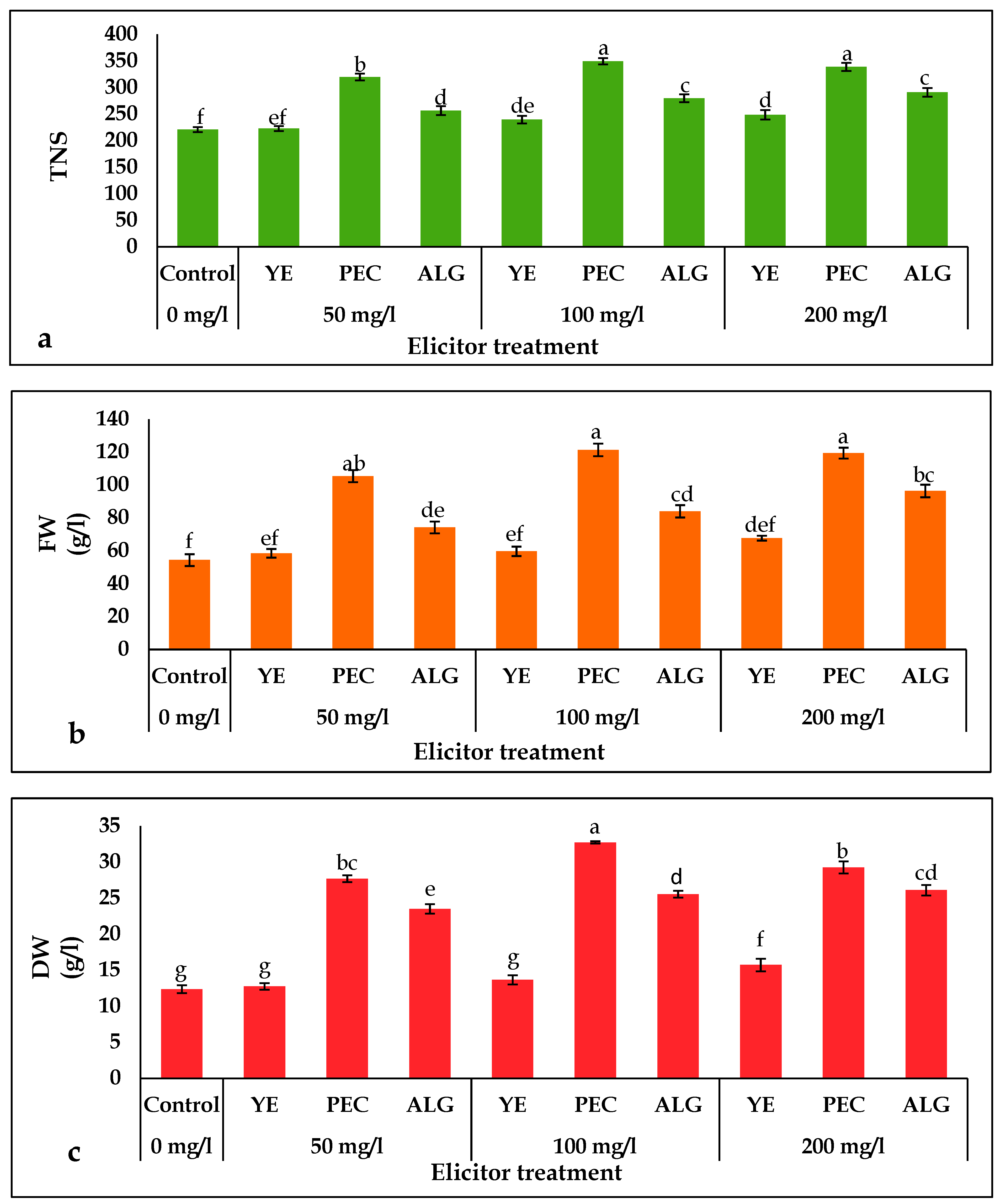
2.1. Effect of Elicitor Treatments on Growth Parameters
2.2. Effect of Elicitor Treatments on Chlorophyll, Protein, and Carbohydrate Content
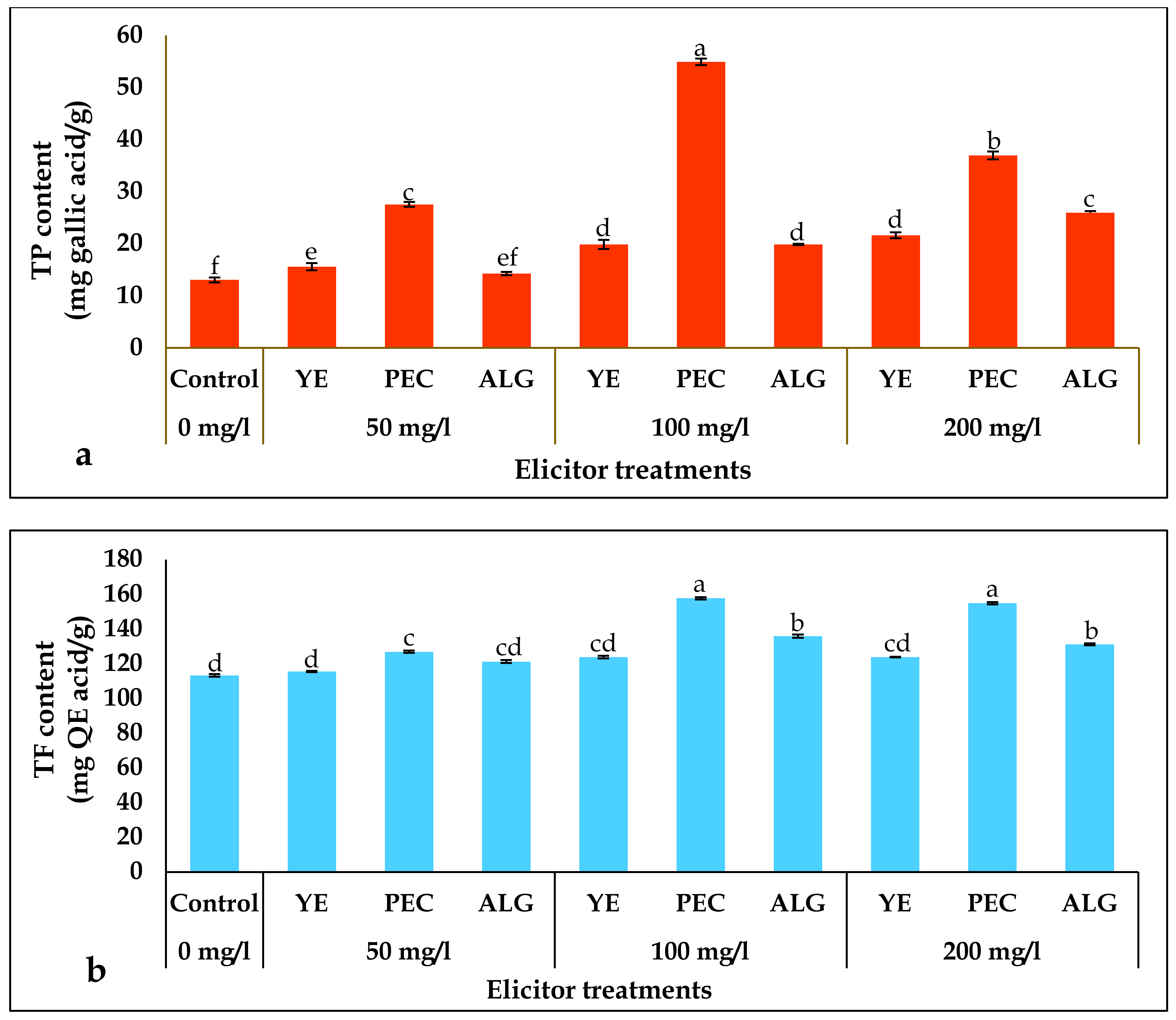
2.3. Effect of Elicitor Treatments on Total Phenolics (TP) and Total Flavonoids (TF) Contents
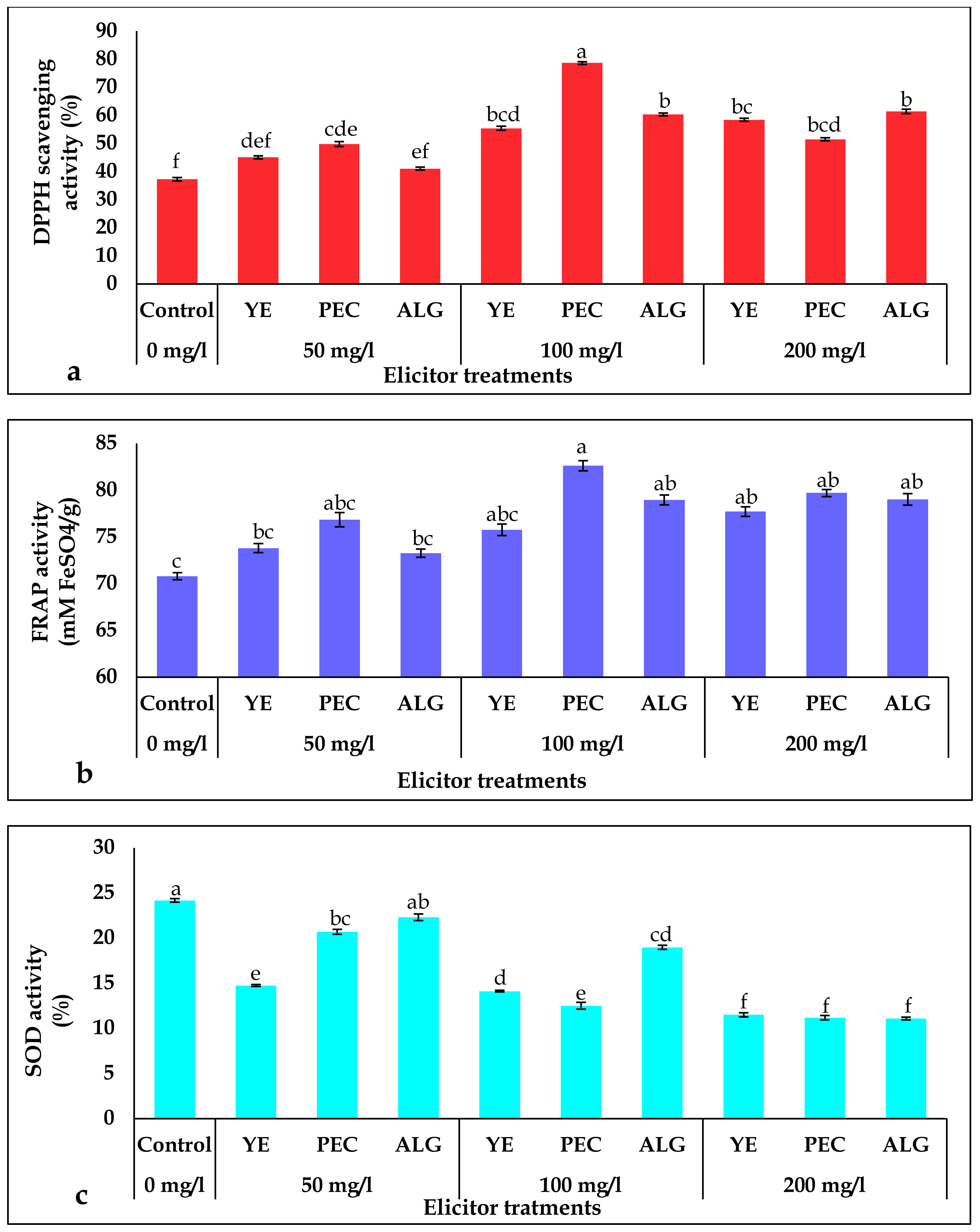
2.4. Effect of Elicitor Treatments on Antioxidant Activity
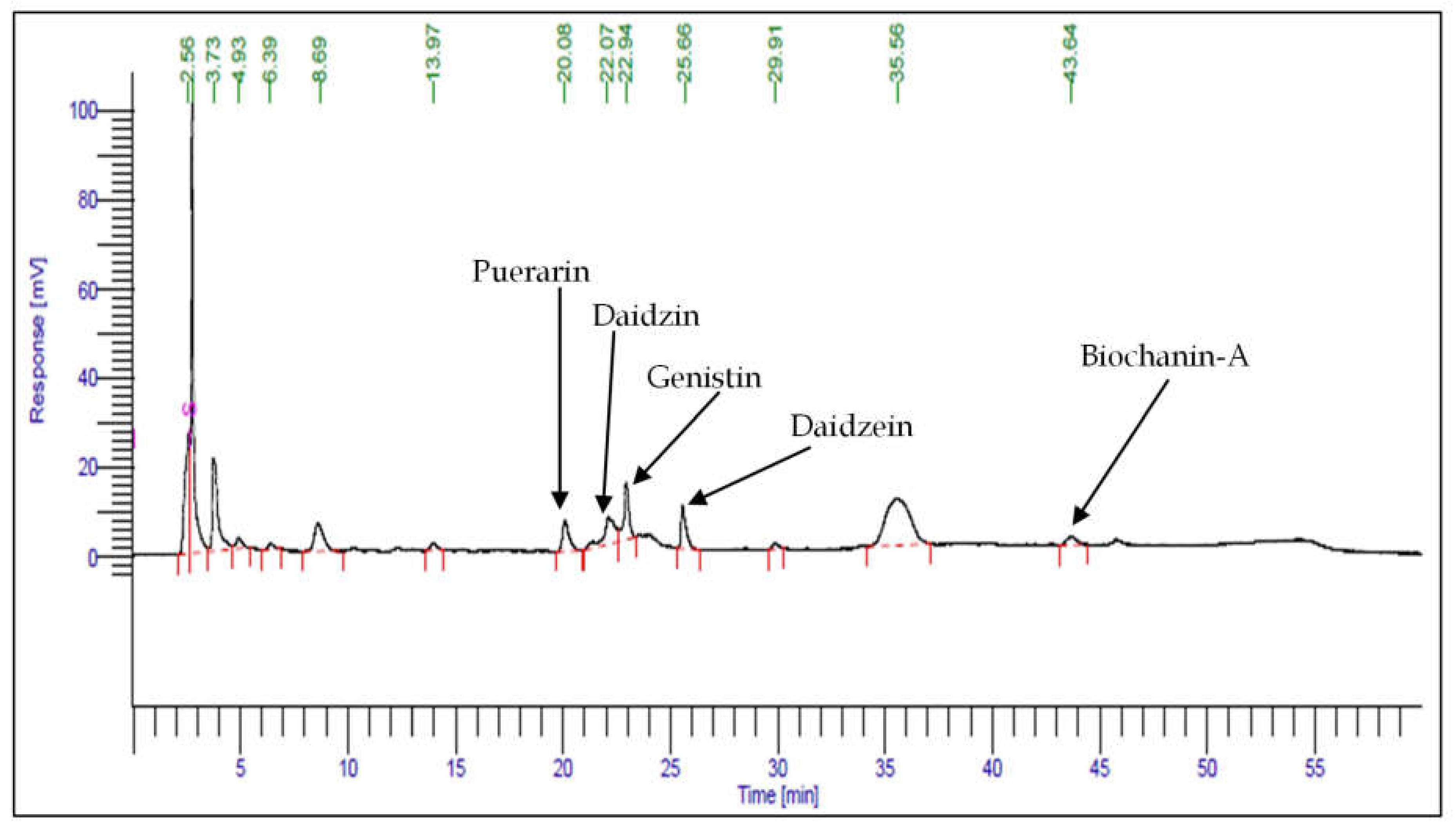
2.5. HPLC Analysis
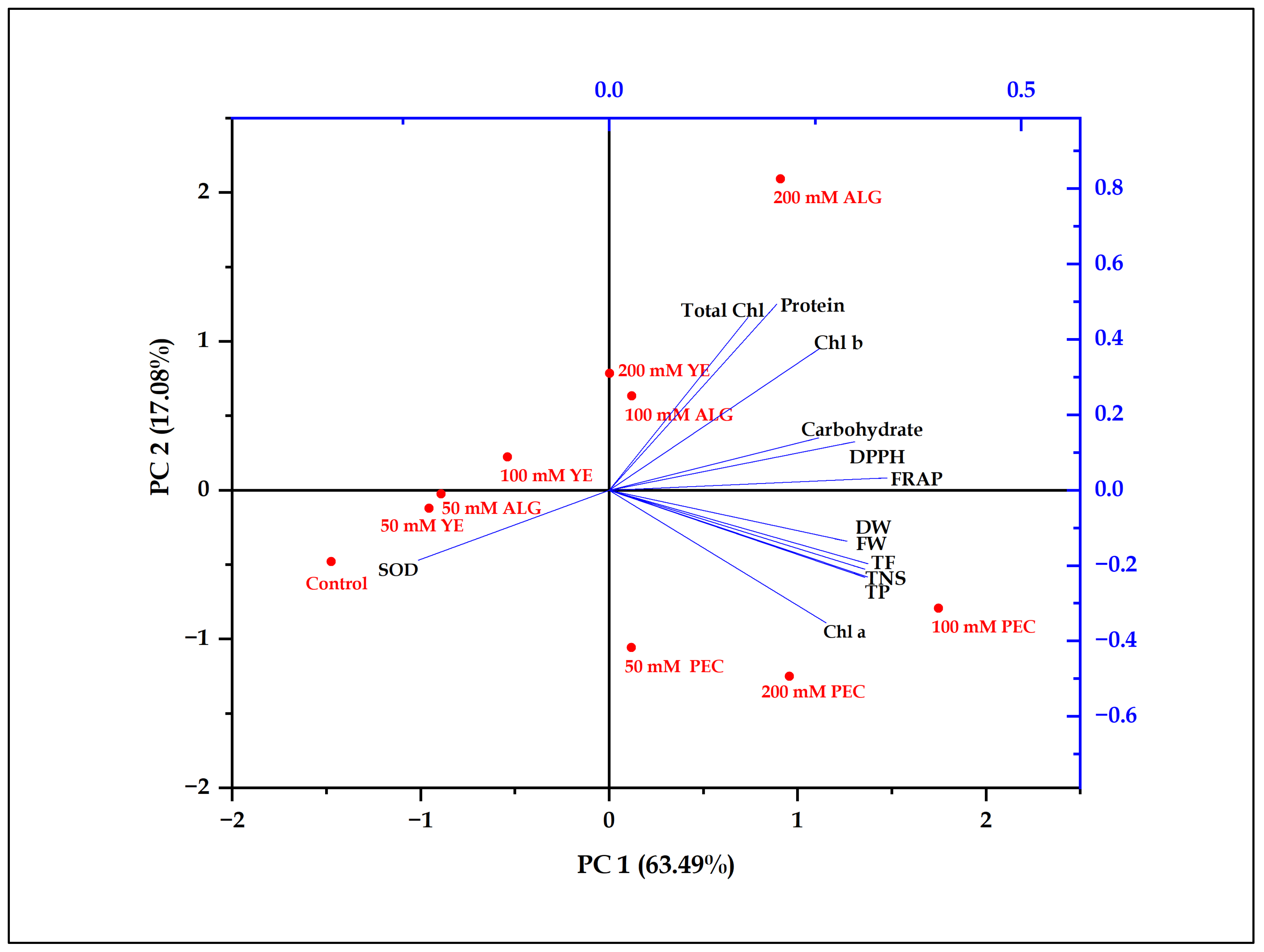
2.6. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of Elicitors
4.3. Elicitor Treatment and Culture Conditions
4.4. Study of Growth
4.5. Estimation of the Chlorophyll, Protein and Carbohydrate Content
4.6. Determination of TP, TF Content and Antioxidant Activity
4.6.1. Sample Preparation
4.6.2. Determination of TP Content
4.6.3. Determination of TF Content
4.6.4. DPPH Radical Scavenging Activity
4.6.5. SOD Activity
4.6.6. FRAP Assay
4.7. HPLC Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanthaliya, B.; Joshi, A.; Arora, J. Evaluation of isoflavonoid content in context to tuber size and seed biology study of Pueraria tuberosa (Roxb. ex. Willd.) DC: A vulnerable medicinal plant. Vegetos 2019, 32, 247–253. [Google Scholar] [CrossRef]
- Bharti, R.; Chopra, B.S.; Raut, S.; Khatri, N. Pueraria tuberosa: A review on traditional uses, pharmacology, and phytochemistry. Front. Pharmacol. 2021, 11, 582506. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones derived from plant raw materials: Bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 2022, 63, 261–287. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Ramawat, K.G.; Arora, J. Medicinal Plants Domestication, Cultivation, Improvement, and Alternative Technologies for the Production of High Value Therapeutics: An Overview. In Medicinal Plants; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Sustainable Development and Biodiversity; Springer: Cham, Switzerland, 2021; Volume 28, pp. 1–29. [Google Scholar]
- Gandhi, S.G.; Mahajan, V.; Bedi, Y.S. Changing trends in biotechnology of secondary metabolism in medicinal and aromatic plants. Planta 2015, 241, 303–317. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Oliveira, M.E.; Cardoso, F.D.C. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Miladinova-Georgieva, K.; Geneva, M.; Stancheva, I.; Petrova, M.; Sichanova, M.; Kirova, E. Effects of Different Elicitors on Micropropagation, Biomass and Secondary Metabolite Production of Stevia rebaudiana Bertoni—A Review. Plants 2023, 12, 153. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Liu, H.; Kang, Y.; Zhao, X.; Liu, Y.; Zhang, X.; Zhang, S. Effects of elicitation on bioactive compounds and biological activities of sprouts. J. Funct. Foods 2019, 53, 136–145. [Google Scholar] [CrossRef]
- Khan, H.; Khan, T.; Ahmad, N.; Zaman, G.; Khan, T.; Ahmad, W.; Batool, S.; Hussain, Z.; Drouet, S.; Hano, C.; et al. Chemical elicitors-induced variation in cellular biomass, biosynthesis of secondary cell products, and antioxidant system in callus cultures of Fagonia indica. Molecules 2021, 26, 6340. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Choudhary, S.; Zehra, A.; Naeem, M.; Aftab, T. Natural Polysaccharides: Novel Plant Growth Regulators. In Plant Growth Regulators; Aftab, T., Hakeem, K.R., Eds.; Springer: Cham, Switzerland, 2021; pp. 335–354. [Google Scholar]
- Zheng, F.; Chen, L.; Zhang, P.; Zhou, J.; Lu, X.; Tian, W. Carbohydrate polymers exhibit great potential as effective elicitors in organic agriculture: A review. Carbohydr. Polym. 2020, 230, 115637. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Kim, K.M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Bhaskar, R.; Xavier, L.S.E.; Udayakumaran, G.; Kumar, D.S.; Venkatesh, R.; Nagella, P. Biotic elicitors: A boon for the in-vitro production of plant secondary metabolites. Plant Cell Tissue Organ Cult. 2022, 149, 7–24. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Perez-Matas, E.; Escrich, A.; Cusido, R.M.; Palazon, J.; Bonfill, M. Biotic elicitors in adventitious and hairy root cultures: A review from 2010 to 2022. Molecules 2022, 27, 5253. [Google Scholar] [CrossRef]
- Twaij, B.M.; Taha, A.J.; Bhuiyan, F.H.; Hasan, M.N. Effect of saccharides on secondary compounds production from stem derived callus of Datura inoxia. Biotechnol. Rep. 2022, 33, e00701. [Google Scholar] [CrossRef]
- Udomsin, O.; Yusakul, G.; Kraithong, W.; Udomsuk, L.; Kitisripanya, T.; Juengwatanatrakul, T.; Putalun, W. Enhanced accumulation of high-value deoxymiroestrol and isoflavonoids using hairy root as a sustainable source of Pueraria candollei var. mirifica. Plant Cell Tissue Organ Cult. 2019, 136, 141–151. [Google Scholar] [CrossRef]
- Li, Y.; Saravana Kumar, P.; Liu, Y.; Qiu, J.; Ran, Y.; Yuan, M.; Fang, X.; Tan, X.; Zhao, R.; He, M. Tailoring enhanced production and identification of isoflavones in the callus cultures of Pueraria thomsonii Benth and its model verification using response surface methodology (RSM): A combined in vitro and statistical optimization. Beni-Suef Uni. J. Basic Appl. Sci. 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Lee, E.; Park, T.H. Isoflavones and biotransformed dihydrodaidzein in hairy roots of Korean wild arrowroot. J. Plant Biotechnol. 2016, 43, 125–131. [Google Scholar] [CrossRef]
- Rathore, M.S.; Shekhawat, N.S. Micropropagation of Pueraria tuberosa (Roxb. Ex Willd.) and determination of puerarin content in different tissues. Plant Cell Tissue Organ Cult. 2009, 99, 327–334. [Google Scholar] [CrossRef]
- Bindu, T.K.; Sheema, D.P.; Udayan, P.S.; Raghu, A.V.; Rahul, R.N. In vitro propagation of Pueraria tuberosa (Roxb. ex Willd.) DC. Trop. Plant Res. 2017, 4, 480–485. [Google Scholar] [CrossRef]
- Patel, I.C.; Hakim, M.; Prajapati, R.; Solanki, A.; Panigrahi, J. In Vitro Approach and Quantification of “Puerarin and Genistein”: Valuable Antidiabetic Compounds from Pueraria tuberosa. In Biotechnology of Anti-diabetic Medicinal Plants; Gantait, S., Verma, S.K., Sharangi, A.B., Eds.; Springer: Singapore, 2021; pp. 1–29. [Google Scholar]
- Goyal, S.; Ramawat, K.G. Increased isoflavonoids accumulation in cell suspension cultures of Pueraria tuberosa by elicitors. Indian J. Biotechnol. 2008, 7, 378–382. [Google Scholar]
- Goyal, S.; Sharma, V.; Ramawat, K.G. Marked effect of Cuscuta on puerarin accumulation in cell cultures of Pueraria tuberosa grown in shake flasks and a bioreactor. Plant Biotechnol. Rep. 2011, 5, 121–126. [Google Scholar] [CrossRef]
- Asgari-Targhi, G.; Iranbakhsh, A.; Ardebili, Z.O. Potential benefits and phytotoxicity of bulk and nano-chitosan on the growth, morphogenesis, physiology, and micropropagation of Capsicum annuum. Plant Physiol. Biochem. 2018, 127, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hassini, I.; Rios, J.J.; Garcia-Ibañez, P.; Baenas, N.; Carvajal, M.; Moreno, D.A. Comparative effect of elicitors on the physiology and secondary metabolites in broccoli plants. J. Plant Physiol. 2019, 239, 1–9. [Google Scholar] [CrossRef]
- Kanthaliya, B.; Joshi, A.; Meena, S.; Arora, J. Biology and Biotechnological Strategies for Conservation Management of Pueraria tuberosa, a Traditionally Established Medicinal Liana. In Medicinal Plants; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 693–719. [Google Scholar]
- Arora, J.; Goyal, S.; Ramawat, K.G. Enhanced stilbene production in cell cultures of Cayratia trifolia through co-treatment with abiotic and biotic elicitors and sucrose. Vitr. Cell. Dev. Biol. 2010, 46, 430–436. [Google Scholar] [CrossRef]
- Bayraktar, M.; Naziri, E.; Akgun, I.H.; Karabey, F.; Ilhan, E.; Akyol, B.; Bedir, E.; Gurel, A. Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana. Plant Cell Tissue Organ Cult. 2016, 127, 289–300. [Google Scholar] [CrossRef]
- Arora, J.; Joshi, A.; Kanthaliya, B.; Khan, F. Effect of biotic elicitors on polyphenol production in Cayratia trifolia cell suspension cultures analyzed by HPLC. BioTechnologia 2020, 101, 35–43. [Google Scholar] [CrossRef]
- Kandoudi, W.; Nemeth-Zamborine, E. Stimulating secondary compound accumulation by elicitation: Is it a realistic tool in medicinal plants in vivo? Phytochem. Rev. 2022, 21, 2007–2025. [Google Scholar] [CrossRef]
- Khan, T.; Abbasi, B.H.; Khan, M.A. The interplay between light, plant growth regulators and elicitors on growth and secondary metabolism in cell cultures of Fagonia indica. J. Photochem. Photobiol. B Biol. 2018, 185, 153–160. [Google Scholar] [CrossRef]
- Andújar, I.; González, M.; García-Ramos, J.C.; Hajari, E.; Bogdanchikova, N.; Pestryakov, A.; Concepción, O.; Lorenzo, J.C.; Escalona, M. Are silver nanoparticles the “silver bullet” to promote diterpene production in Stevia rebaudiana? Plant Cell Tissue Organ Cult. 2023, 30, 1–7. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, B. Pectin drives cell wall morphogenesis without turgor pressure. Trends Plant Sci. 2020, 25, 719–722. [Google Scholar] [CrossRef]
- Voragen, A.G.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Shin, Y.; Chane, A.; Jung, M.; Lee, Y. Recent advances in understanding the roles of pectin as an active participant in plant signaling networks. Plants 2021, 10, 1712. [Google Scholar] [CrossRef]
- Haas, K.T.; Wightman, R.; Meyerowitz, E.M.; Peaucelle, A. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science 2020, 367, 1003–1007. [Google Scholar] [CrossRef]
- Baskaran, P.; Kumari, A.; Van Staden, J. Analysis of the effect of plant growth regulators and organic elicitors on antibacterial activity of Eucomis autumnalis and Drimia robusta ex vitro-grown biomass. Plant Growth Regul. 2018, 85, 143–151. [Google Scholar] [CrossRef]
- Quang, H.T.; Thi, P.T.D.; Sang, D.N.; Tram, T.T.N.; Huy, N.D.; Dung, T.Q.; The, Q.T.T. Effects of Plant Elicitors on Growth and Gypenosides Biosynthesis in Cell Culture of Giao co lam (Gynostemma pentaphyllum). Molecules 2022, 27, 2972. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Meller, E.; Soból, M. Oligo-alginate with low molecular mass improves growth and physiological activity of Eucomis autumnalis under salinity stress. Molecules 2018, 23, 812. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Uddin, M.; Corpas, F.J. Irradiated chitosan (ICH): An alternative tool to increase essential oil content in lemongrass (Cymbopogon flexuosus). Acta Physiol. Plantarum. 2022, 44, 1–15. [Google Scholar] [CrossRef]
- Naeem, M.; Nabi, A.; Aftab, T.; Khan, M.M.A. Oligomers of carrageenan regulate functional activities and artemisinin production in Artemisia annua L. exposed to arsenic stress. Protoplasma 2020, 257, 871–887. [Google Scholar] [CrossRef]
- Tehranian, A.S.; Askari, H.; Rezadoost, H. The effect of alginate as an elicitor on transcription of steviol glycosides biosynthesis pathway related key genes and sweeteners content in in vitro cultured Stevia rebaudiana. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; He, X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Joshi, A.; Rajput, V.D.; Verma, K.K.; Minkina, T.; Ghazaryan, K.; Arora, J. Potential of Suaeda nudiflora and Suaeda fruticosa to Adapt to High Salinity Conditions. Horticulturae 2023, 9, 74. [Google Scholar] [CrossRef]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Gadzovska Simic, S.; Tusevski, O.; Maury, S.; Delaunay, A.; Joseph, C.; Hagège, D. Effects of polysaccharide elicitors on secondary metabolite production and antioxidant response in Hypericum perforatum L. shoot cultures. Sci. World J. 2014, 2014, 609649. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell. Dev. Biol. 2022, 58, 615–629. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Naik, P.M. Elicitor-induced production of biomass and pharmaceutical phenolic compounds in cell suspension culture of date palm (Phoenix dactylifera L.). Molecules 2020, 25, 4669. [Google Scholar] [CrossRef]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current state of knowledge on the antioxidant effects and mechanisms of action of polyphenolic compounds. Nat. Product Commun. 2021, 16, 1934578X211027745. [Google Scholar] [CrossRef]
- Ahn, S.; Halake, K.; Lee, J. Antioxidant and ion-induced gelation functions of pectins enabled by polyphenol conjugation. Int. J. Biol. Macromol. 2017, 101, 776–782. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Laura, A.; Alvarez-Parrilla, E. Effect of pectin on the interactions among phenolic compounds determined by antioxidant capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Joshi, A.; Kanthaliya, B.; Arora, J. Evaluation of growth and antioxidant activity in Suaeda monoica and Suaeda nudiflora callus cultures under sequential exposure to saline conditions. Curr. Biotechnol. 2019, 8, 42–52. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Ullah, M.A.; Nadeem, M.; Tungmunnithum, D.; Hano, C. Exogenous application of salicylic acid and gibberellic acid on biomass accumulation, antioxidant and anti-inflammatory secondary metabolites production in multiple shoot culture of Ajuga integrifolia Buch. Ham. ex D. Don. Ind. Crops Prod. 2020, 145, 112098. [Google Scholar] [CrossRef]
- Ullah, M.A.; Gul, F.Z.; Khan, T.; Bajwa, M.N.; Drouet, S.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Liu, C.; Hano, C.; Abbasi, B.H. Differential induction of antioxidant and anti-inflammatory phytochemicals in agitated micro-shoot cultures of Ajuga integrifolia Buch. Ham. ex D. Don with biotic elicitors. AMB Express 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Devi, M.A.; Kumar, G.; Giridhar, P. Effect of biotic and abiotic elicitors on isoflavone biosynthesis during seed development and in suspension cultures of soybean (Glycine max L.). 3 Biotech 2020, 10, 1–14. [Google Scholar] [CrossRef]
- García-Calderón, M.; Pérez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Márquez, A.J. Flavonoids and isoflavonoids biosynthesis in the model legume Lotus japonicus; connections to nitrogen metabolism and photorespiration. Plants 2020, 9, 774. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Apola, A.; Ekiert, H.; Szopa, A. Impacts of elicitors on metabolite production and on antioxidant potential and tyrosinase inhibition in watercress microshoot cultures. Appl. Microbiol. Biotechnol. 2022, 106, 619–633. [Google Scholar] [CrossRef]
- Rani, D.; Meelaph, T.; De-Eknamkul, W.; Vimolmangkang, S. Yeast extract elicited isoflavonoid accumulation and biosynthetic gene expression in Pueraria candollei var. mirifica cell cultures. Plant Cell Tissue Organ Cult. 2020, 141, 661–667. [Google Scholar] [CrossRef]
- Rasouli, D.; Werbrouck, S.; Maleki, B.; Jafary, H.; Schurdi-Levraud, V. Elicitor-induced in vitro shoot multiplication and steviol glycosides production in Stevia rebaudiana. S. Afr. J. Bot. 2021, 137, 265–271. [Google Scholar] [CrossRef]
- Arnon, D. Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Farkas, G.L.; Kiraaly, Z. Role of phenolic compounds in the physiology of plant diseases and disease resistance. J. Phytopathol. 1962, 44, 105–150. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Jain, P.K.; Ravichandran, V.; Agrawal, R.K. Antioxidant and free radical scavenging properties of traditionally used three Indian medicinal plants. Curr. Trends Biotechnol. Pharm. 2008, 2, 538–547. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
| Concentration | Elicitor | Chlorophyll (mg/g) | Protein (mg/g) | Carbohydrate (mg/g) | ||
|---|---|---|---|---|---|---|
| Chl a | Chl b | Total Chl | ||||
| 0 mg/L | Control | 17.4 ± 0.35 cde | 11.96 ± 0.47 de | 29.36 ± 0.41 fg | 8.29 ± 0.78 e | 6.55 ± 0.33 d |
| 50 mg/L | YE | 17.81 ± 0.56 cd | 11.25 ± 0.13 e | 28.06 ± 0.74 gh | 9.52 ± 0.64 de | 9.18 ± 0.14 bc |
| PEC | 22.58 ± 0.47 b | 12.59 ± 0.15 de | 30.17 ± 0.19 ef | 10.74 ± 0.58 cd | 9.83 ± 0.17 ab | |
| ALG | 12.26 ± 0.36 f | 13.58 ± 0.19 d | 32.45 ± 0.57 cd | 10.29 ± 0.37 cd | 7.01 ± 0.12 d | |
| 100 mg/L | YE | 15.82 ± 0.45 e | 13.31 ± 0.85 d | 27.12 ± 0.52 h | 10.71 ± 0.29 cd | 10.04 ± 0.57 ab |
| PEC | 27.55 ± 0.15 a | 16.71 ± 0.21 bc | 34.26 ± 0.51 c | 12.87 ± 0.62 b | 11.1 ± 0.18 ab | |
| ALG | 16.16 ± 0.25 de | 16.02 ± 0.14 bc | 42.17 ± 0.46 b | 12.21 ± 0.22 bc | 7.63 ± 0.78 cd | |
| 200 mg/L | YE | 18.84 ± 0.77 c | 17.64 ± 0.47 ab | 31.16 ± 0.91 de | 11.25 ± 0.91 bcd | 11.1 ± 0.78 ab |
| PEC | 26.02 ± 0.35 a | 15.46 ± 0.29 c | 31.48 ± 0.34 de | 9.53 ± 0.42 de | 10.22 ± 0.78 ab | |
| ALG | 18.28 ± 0.41 c | 18.87 ± 0.09 a | 48.15 ± 0.39 a | 18.66 ± 0.17 a | 11.46 ± 0.78 a | |
| Traits | PC1 | PC2 |
|---|---|---|
| TNS | 0.311 | −0.231 |
| FW | 0.310 | −0.210 |
| DW | 0.289 | −0.135 |
| Chl a | 0.263 | −0.352 |
| Chl b | 0.256 | 0.377 |
| Total Chl | 0.168 | 0.458 |
| Protein | 0.203 | 0.494 |
| Carbohydrates | 0.254 | 0.138 |
| TP | 0.313 | −0.229 |
| TF | 0.314 | −0.195 |
| DPPH | 0.298 | 0.129 |
| FRAP | 0.337 | 0.032 |
| SOD | −0.231 | −0.186 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanthaliya, B.; Joshi, A.; Arora, J.; Alqahtani, M.D.; Abd_Allah, E.F. Effect of Biotic Elicitors on the Growth, Antioxidant Activity and Metabolites Accumulation in In Vitro Propagated Shoots of Pueraria tuberosa. Plants 2023, 12, 1300. https://doi.org/10.3390/plants12061300
Kanthaliya B, Joshi A, Arora J, Alqahtani MD, Abd_Allah EF. Effect of Biotic Elicitors on the Growth, Antioxidant Activity and Metabolites Accumulation in In Vitro Propagated Shoots of Pueraria tuberosa. Plants. 2023; 12(6):1300. https://doi.org/10.3390/plants12061300
Chicago/Turabian StyleKanthaliya, Bhanupriya, Abhishek Joshi, Jaya Arora, Mashael Daghash Alqahtani, and Elsayed Fathi Abd_Allah. 2023. "Effect of Biotic Elicitors on the Growth, Antioxidant Activity and Metabolites Accumulation in In Vitro Propagated Shoots of Pueraria tuberosa" Plants 12, no. 6: 1300. https://doi.org/10.3390/plants12061300
APA StyleKanthaliya, B., Joshi, A., Arora, J., Alqahtani, M. D., & Abd_Allah, E. F. (2023). Effect of Biotic Elicitors on the Growth, Antioxidant Activity and Metabolites Accumulation in In Vitro Propagated Shoots of Pueraria tuberosa. Plants, 12(6), 1300. https://doi.org/10.3390/plants12061300







