Cross-Talk and Physiological Role of Jasmonic Acid, Ethylene, and Reactive Oxygen Species in Wound-Induced Phenolic Biosynthesis in Broccoli
Abstract
1. Introduction
2. Results and Discussion
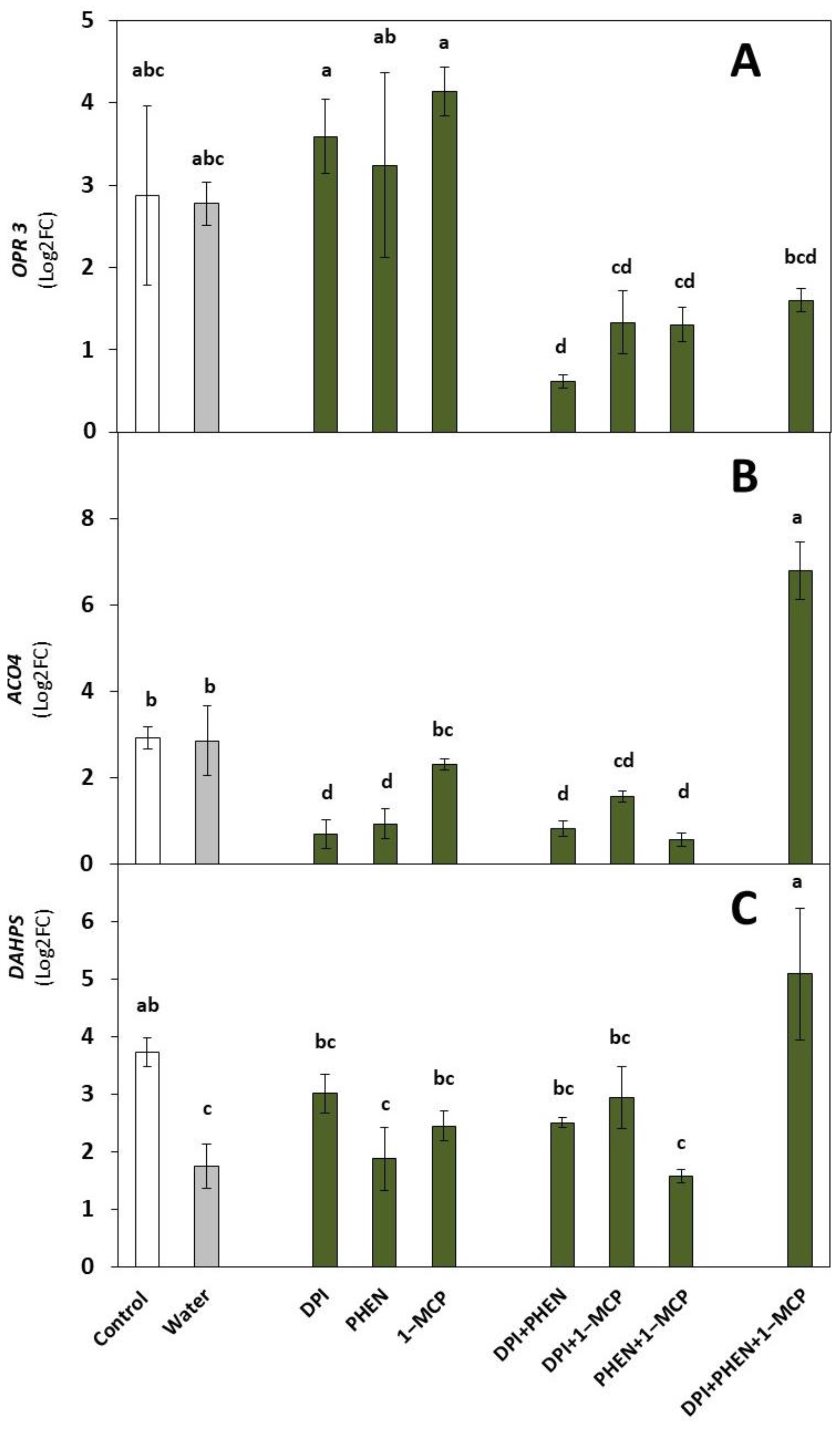
2.1. Role of ROS, ET, and JA Inhibitors on the Wound-Induced Activation of Stress-Signaling Molecules and Primary Metabolites Biosynthetic Genes
2.1.1. Stress Signaling Molecules’ Biosynthetic Genes
2.1.2. Primary Metabolites Biosynthetic Gene
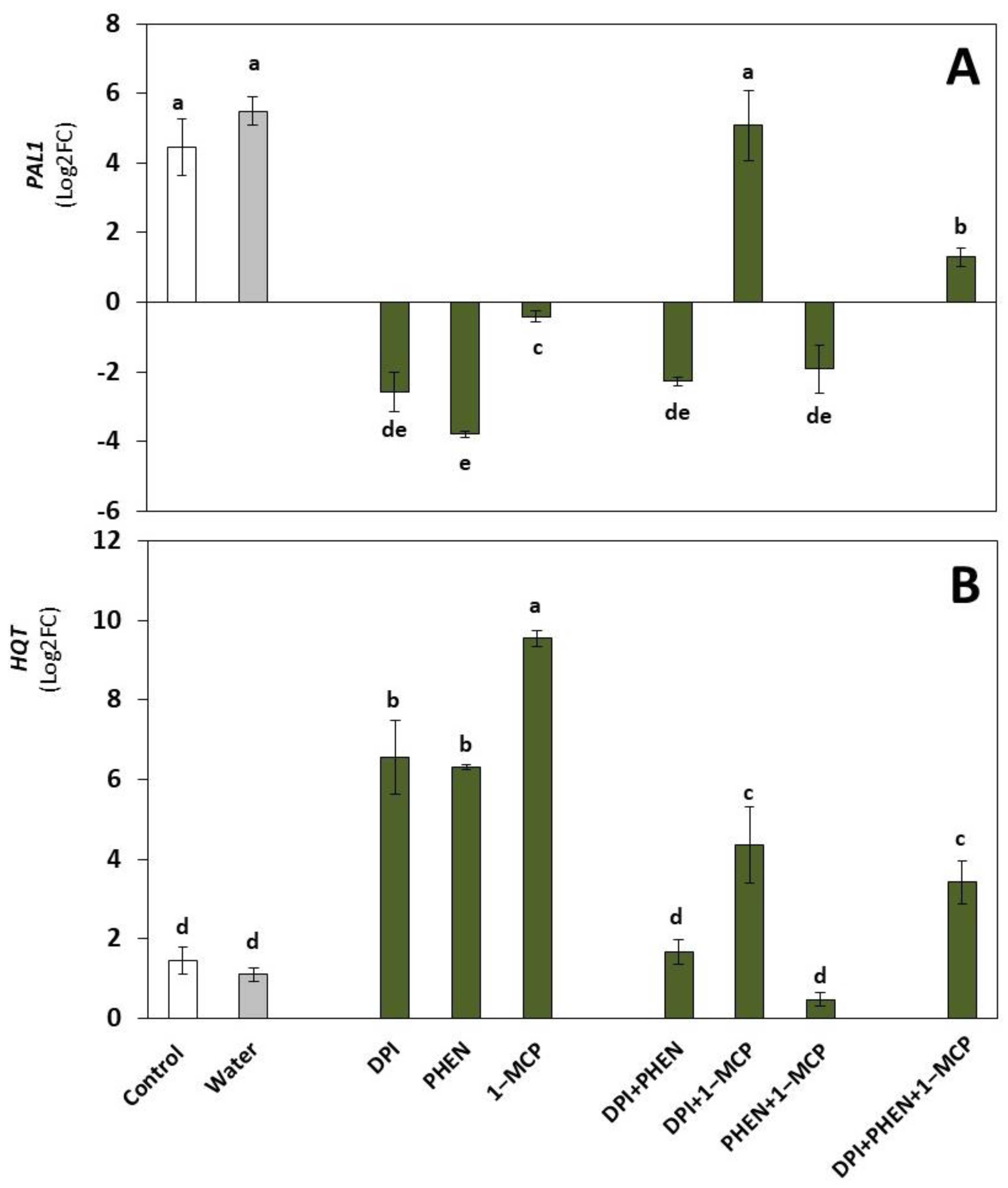
2.2. Role of ROS, ET, and JA Inhibitors on the Wound-Induced Activation of Phenolic Biosynthetic Genes and Individual Phenolic Accumulation
2.2.1. Phenolic Biosynthetic Genes
2.2.2. Identification of Individual Phenolic Compounds
2.2.3. Accumulation of Individual Phenolic Compounds
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material, Processing, and Storage of Broccoli Samples
3.3. Application of Stress-Signaling Molecules Inhibitors
3.4. RNA Extraction and Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR)
3.5. Phenolic Compounds Analysis
3.6. Statistical Analysis
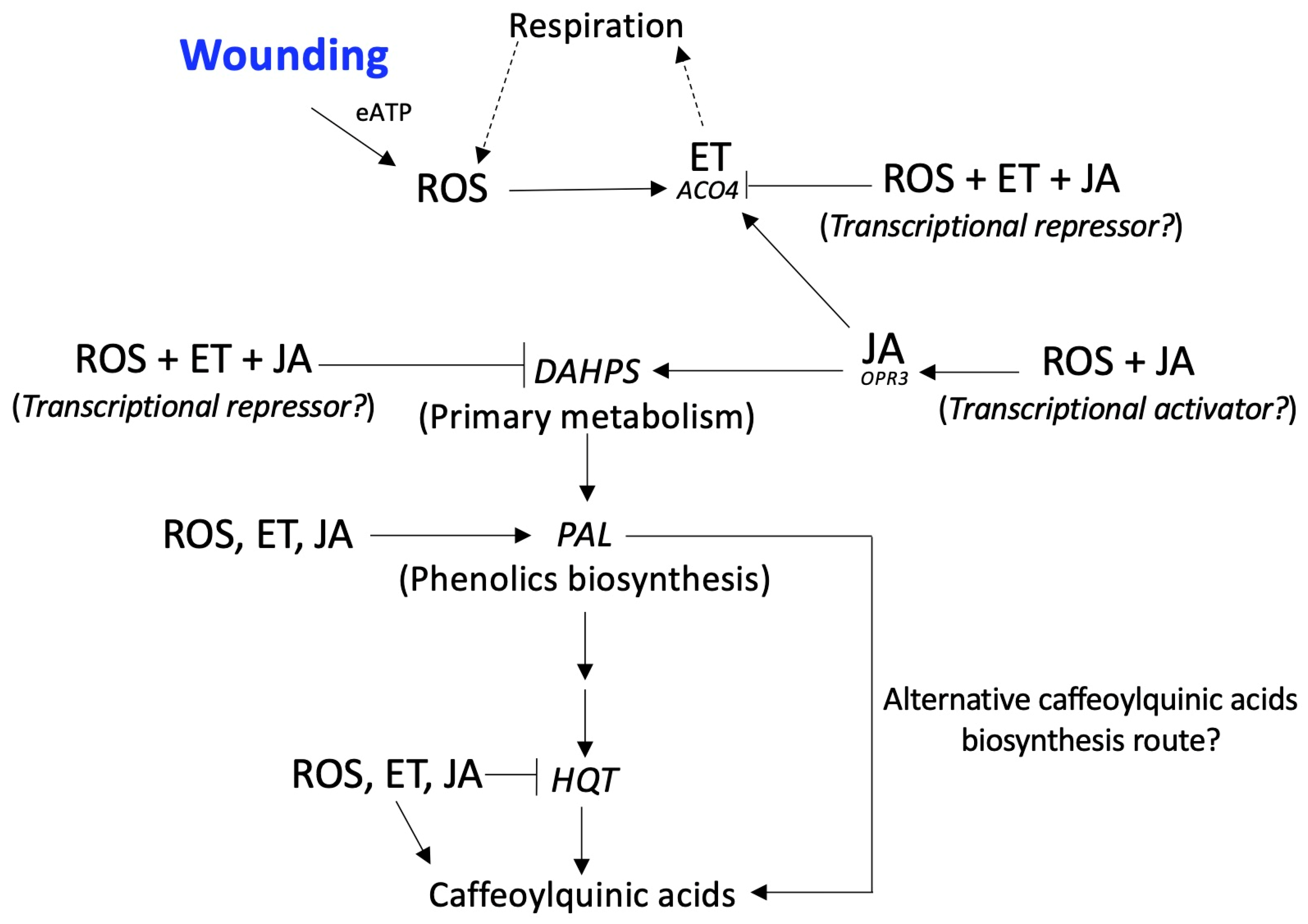
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plumb, G.W.; Price, K.R.; Modes, M.J.; Williamson, G. Antioxidant properties of the major polyphenolic compounds in broccoli. Free Radical Res. 1997, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L.; Jacobo-Velaázquez, D.A. Controlled abiotic stresses revisited: From homeostasis through hormesis to extreme stresses and the impact on nutraceuticals and quality during pre-and postharvest applications in horticultural crops. J. Agric. Food Chem. 2020, 68, 11877–11879. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Velázquez, D.A. Definition of biofortification revisited. ACS Food Sci. Technol. 2022, 2, 782–783. [Google Scholar] [CrossRef]
- Villarreal-García, D.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Postharvest stress-induced accumulation of phenolic compounds and glucosinolates in broccoli subjected to wounding stress and exogenous phytohormones. Front. Plant Sci. 2016, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Torres-Contreras, A.M.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Stability of bioactive compounds in broccoli as affected by cutting styles and storage time. Molecules 2017, 22, 636. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Zhao, M.; Yu, J.; Ji, Y.; Sarengaowa; Yang, X.; Feng, K. The effect of cutting style on the biosynthesis of phenolics and cellular antioxidant capacity in wounded broccoli. Food Res. Int. 2020, 137, 109565. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Yang, X.; Ji, Y.; Feng, K.; Sarengaowa. Metabolomics and physiological analyses validates previous findings on the mechanism of response to wounding stress of different intensities in broccoli. Food Res. Int. 2021, 140, 110058. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Sarengaowa; Ji, Y.; Yang, X.; Feng, K. Proteomic analysis validates previous findings on wounding-responsive plant hormone signaling and primary metabolism contributing to the biosynthesis of secondary metabolites based on metabolomic analysis in harvested broccoli (Brassica oleracea L. var. italica). Food Res. Int. 2021, 145, 110388. [Google Scholar] [CrossRef]
- Song, C.J.; Steinebrunner, I.; Wang, X.; Stout, S.C.; Roux, S.J. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol. 2006, 140, 1222–1232. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martínez-Herández, G.B.; Rodriguez, S.C.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Gastélum-Estrada, A.; Hurtado-Romero, A.; Santacruz, A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Sanitizing after fresh-cutting carrots reduces the wound-induced accumulation of phenolic antioxidants compared to sanitizing before fresh-cutting. J. Sci. Food Agric. 2020, 100, 4995–4998. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Physiological role of reactive oxygen species, ethylene, and jasmonic acid on UV light induced phenolic biosynthesis in wounded carrot tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Nair, V.; Senés-Guerrero, C.; Pacheco, A.; González-Agüero, M.; Ramos-Parra, P.A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chemical genetics applied to elucidate the physiological role of stress-signaling molecules on the wound-induced accumulation of glucosinolates in broccoli. Plants 2021, 10, 2660. [Google Scholar] [CrossRef]
- Blackwell, H.E.; Zhao, Y. Chemical genetic approaches to plant biology. Plant Physiol. 2003, 133, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Greiner, S.; Meimberg, H.; Krüger, R.; Noyer, J.-L.; Heubl, G.; Linsenmair, E.; Boland, W. Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 2004, 430, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Engelberth, J. Selective inhibition of jasmonic acid accumulation by a small α, β-unsaturated carbonyl and phenidone reveals different modes of octadecanoid signalling activation in response to insect elicitors and green leaf volatiles in Zea mays. BMC Res. Notes 2011, 4, 377. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Dong, C.; Terry, L.A.; Watkins, C.B.; Yu, Z.; Cheng, Z.-M. Meta-analysis of the effects of 1-methylcyclopropene (1-MCP) treatment on climacteric fruit ripening. Hort. Res. 2020, 7, 208. [Google Scholar] [CrossRef]
- Torres-Contreras, A.M.; Senés-Guerrero, C.; Pacheco, A.; González-Agüero, M.; Ramos-Parra, P.A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Genes differentially expressed in broccoli as an early and late response to wounding stress. Postharvest Biol. Technol. 2018, 145, 172–182. [Google Scholar] [CrossRef]
- Chotikacharoensuk, T.; Arteca, R.N.; Arteca, J.M. Use of differential display for the identification of touch-induced genes from an ethylene-insensitive Arabidopsis mutant and partial characterization of these genes. J. Plant Physiol. 2006, 163, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Ruduś, I.; Sasiak, M.; Kępczyński, J. Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol. Plant. 2013, 35, 295–307. [Google Scholar] [CrossRef]
- Thirugnanasambantham, K.; Durairaj, S.; Saravanan, S.; Karikalan, K.; Muralidaran, S.; Islam, V.I. Role of ethylene response transcription factor (ERF) and its regulation in response to stress encountered by plants. Plant Mol. Biol. Rep. 2015, 33, 347–357. [Google Scholar] [CrossRef]
- Fijimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box–mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [CrossRef]
- Petersen, M. Hydroxycinnamoyltransferases in plant metabolism. Phytochem. Rev. 2016, 15, 699–727. [Google Scholar] [CrossRef]
- Rommens, C.M.; Richael, C.M.; Yan, H.; Navarre, D.A.; Ye, J.; Krucker, M.; Swords, K. Engineered native pathways for high kaempferol and caffeoylquinate production in potato. Plant Biotechnol. J. 2008, 6, 870–886. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; He, H.; Wang, H.; Wu, H. Correlation of the temporal and spatial expression patterns of HQT with the biosynthesis and accumulation of chlorogenic acid in Lonicera japonica flowers. Hort. Res. 2019, 6, 73. [Google Scholar] [CrossRef]
- Wan, C.Y.; Wilkins, T.A. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal. Biochem. 1994, 223, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Salzman, R.A.; Brady, J.A.; Finlayson, S.A.; Buchanan, C.D.; Summer, E.J.; Sun, F.; Klein, P.E.; Klein, R.R.; Pratt, L.H.; Cordinnier-Pratt, M.-M.; et al. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005, 138, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
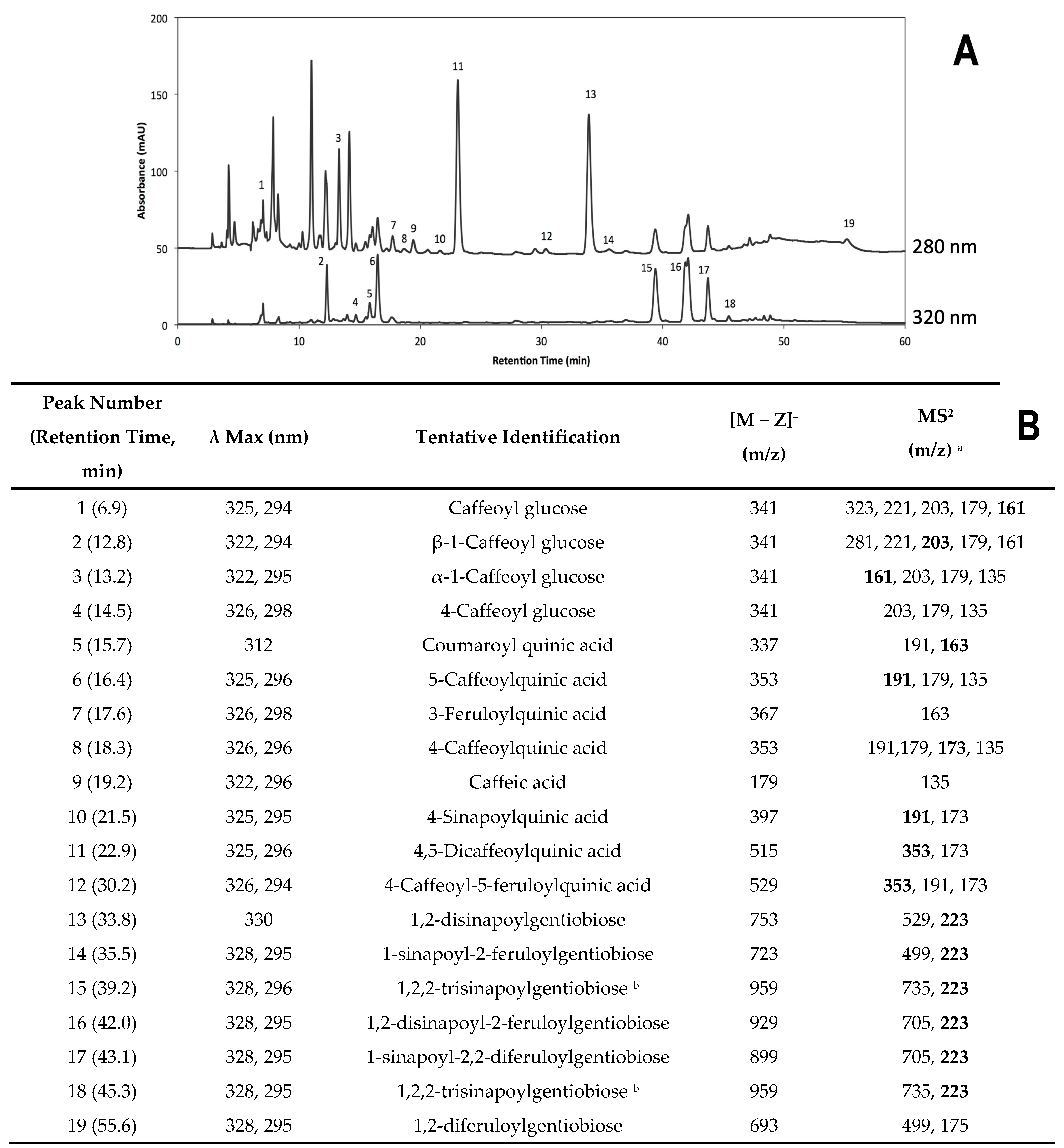
| Treatment | Individual Phenolic Compounds’ Concentration (mg kg−1 DW) 1, 2, 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-glu | β-1-C-glu | α-1-C-glu | 4-C-glu | Coumaroyl-QA | 5-CQA | 3-FQA | ||||||||
| 0 h | 26.59 ± 4.67 | bc | 69.88 ± 11.15 | cde | 105.05 ± 4.35 | d | 16.91 ± 0.05 | f | 12.83 ± 1.14 | g | 76.43 ± 3.62 | fg | 25.19 ± 0.89 | f |
| 0 h-water | 20.38 ± 1.58 | c | 52.19 ± 2.59 | e | 104.45 ± 9.21 | d | 16.59 ± 0.41 | f | 14.65 ± 0.23 | fg | 74.92 ± 2.58 | g | 27.64 ± 3.62 | ef |
| 21 h | 41.20 ± 10.64 | b | 96.30 ± 19.21 | a | 175.31 ± 13.15 | bc | 19.63 ± 0.46 | de | 67.05 ± 2.42 | b | 104.11 ± 2.97 | bc | 51.94 ± 5.05 | cd |
| 21 h-water | 38.67 ± 10.45 | bc | 93.42 ± 6.10 | ab | 145.71 ± 17.59 | c | 23.19 ± 0.76 | bc | 76.39 ± 3.97 | a | 95.33 ± 6.19 | cde | 101.44 ± 8.43 | b |
| DPI | 61.08 ± 10.56 | a | 61.04 ± 4.07 | de | 143.33 ± 11.15 | c | 21.23 ± 1.25 | cd | 16.12 ± 1.23 | fg | 76.95 ± 3.57 | fg | 36.39 ± 1.98 | ef |
| PHEN | 31.37 ± 3.79 | bc | 84.03 ± 3.46 | abc | 181.41 ± 12.50 | b | 18.18 ± 0.16 | ef | 25.79 ± 1.36 | e | 86.50 ± 3.30 | ef | 35.69 ± 1.16 | ef |
| 1-MCP | 22.24 ± 4.20 | bc | 93.85 ± 4.48 | ab | 232.88 ± 13.59 | a | 22.20 ± 0.30 | bc | 49.41 ± 4.95 | c | 99.78 ± 3.04 | cd | 53.26 ± 2.19 | c |
| DPI + PHEN | 23.37 ± 1.92 | bc | 80.10 ± 4.33 | abcd | 187.65 ± 3.69 | b | 21.29 ± 0.36 | cd | 36.90 ± 1.65 | d | 91.24 ± 3.92 | de | 274.92 ± 7.45 | a |
| DPI + 1-MCP | 25.19 ± 3.91 | bc | 75.24 ± 3.04 | abcd | 156.52 ± 10.53 | bc | 29.72 ± 1.09 | a | 18.91 ± 0.77 | efg | 84.10 ± 1.66 | efg | 40.27 ± 4.51 | de |
| PHEN + 1-MCP | 21.79 ± 5.93 | c | 78.33 ± 0.77 | abcd | 173.51 ± 1.50 | bc | 18.50 ± 0.68 | ef | 19.95 ± 1.51 | ef | 121.33 ± 4.43 | a | 33.14 ± 2.11 | ef |
| DPI + PHEN + 1-MCP | 36.91 ± 5.57 | bc | 72.98 ± 3.26 | bcde | 157.20 ± 12.83 | bc | 23.29 ± 0.77 | b | 55.73 ± 2.89 | c | 114.26 ± 5.66 | ab | 36.36 ± 2.38 | ef |
| Treatment | Individual phenolic compounds’ concentration (mg kg−1 DW) | |||||||||||||
| 4-CQA | CA | 4-SQA | 4,5-DiCQA | 4-C-5-FQA | 1,2-DSG | 1-S-2-FG | ||||||||
| 0 h | 8.37 ± 2.67 | d | 43.63 ± 1.42 | a | 15.17 ± 0.17 | abcd | 30.38 ± 0.85 | e | n.d. | n.d. | n.d. | |||
| 0 h-water | 11.99 ± 3.48 | cd | 41.84 ± 2.15 | a | 13.17 ± 0.37 | cd | 24.40 ± 2.14 | e | n.d. | n.d. | n.d. | |||
| 21 h | 14.45 ± 0.27 | bc | 23.85 ± 2.15 | c | 16.18 ± 0.73 | ab | 52.75 ± 3.03 | e | n.d. | n.d. | 20.77 ± 0.26 | b | ||
| 21 h-water | 15.43 ± 0.65 | bc | 27.20 ± 1.90 | c | 16.47 ± 0.87 | a | 51.01 ± 4.04 | e | n.d. | n.d. | 16.78 ± 5.55 | b | ||
| DPI | 10.90 ± 2.61 | cd | 46.35 ± 5.79 | a | 13.33 ± 0.41 | bcd | 44.13 ± 6.71 | e | n.d. | n.d. | 23.48 ± 1.28 | b | ||
| PHEN | 22.85 ± 0.78 | a | 22.93 ± 0.81 | c | 12.84 ± 2.23 | d | 660.30 ± 44.89 | c | 100.57 ± 14.91 | a | n.d. | 18.00 ± 1.96 | b | |
| 1-MCP | 10.90 ± 1.00 | cd | 24.08 ± 0.99 | c | 15.92 ± 0.47 | abc | 53.31 ± 2.62 | e | n.d. | n.d. | n.d. | |||
| DPI + PHEN | 14.09 ± 2.40 | bc | 26.74 ± 0.27 | c | 15.08 ± 0.18 | abcd | 1552.34 ± 53.73 | a | n.d. | 692.47 ± 11.36 | b | 2189.58 ± 66.79 | a | |
| DPI + 1-MCP | 12.40 ± 1.16 | cd | 46.49 ± 1.72 | a | 14.45 ± 0.45 | abcd | 65.97 ± 2.11 | e | 40.58 ± 0.97 | c | 23.11 ± 0.06 | d | 32.94 ± 0.75 | b |
| PHEN + 1-MCP | 18.94 ± 1.37 | ab | 25.47 ± 1.34 | c | 13.58 ± 2.00 | abcd | 871.30 ± 58.67 | b | 71.05 ± 10.26 | b | 1443.67 ± 10.26 | a | 10.98 ± 0.78 | b |
| DPI + PHEN + 1-MCP | 13.34 ± 1.08 | cd | 34.38 ± 1.84 | b | 12.71 ± 0.45 | d | 447.12 ± 26.18 | d | 19.39 ± 0.76 | d | 389.02 ± 12.30 | c | 23.02 ± 2.64 | b |
| Treatment | Individual phenolic compounds’ concentration (mg kg−1 DW) | |||||||||||||
| 1,2,2-TSG * | 1,2-DS-2-FG | 1-S-2,2-DFG | 1,2,2-TSG * | 1,2-DFG | ||||||||||
| 0 h | 177.91 ± 41.36 | a | 282.15 ± 62.34 | ab | 90.39 ± 18.76 | ab | 15.68 ± 2.13 | f | n.d. | |||||
| 0 h-water | 105.55 ± 5.00 | b | 180.34 ± 8.55 | c | 58.26 ± 2.04 | b | 16.94 ± 0.26 | ef | n.d. | |||||
| 21 h | 173.44 ± 50.26 | a | 290.35 ± 8.55 | a | 94.38 ± 25.00 | ab | 18.87 ± 0.63 | cde | 22.07 ± 1.39 | ab | ||||
| 21 h-water | 140.25 ± 7.44 | a | 186.35 ± 38.81 | bc | 81.85 ± 3.85 | ab | 20.98 ± 0.66 | abcd | 22.01 ± 1.04 | ab | ||||
| DPI | 124.53 ± 9.43 | ab | 208.26 ± 15.49 | abc | 88.02 ± 23.75 | ab | 18.03 ± 0.86 | def | 16.76 ± 5.72 | b | ||||
| PHEN | 158.74 ± 7.00 | ab | 282.80 ± 13.97 | ab | 103.06 ± 6.22 | a | 24.14 ± 0.73 | a | 22.60 ± 1.45 | ab | ||||
| 1-MCP | 130.06 ± 7.57 | ab | 222.39 ± 13.75 | abc | 70.63 ± 4.60 | ab | 18.52 ± 0.86 | def | 22.92 ± 1.50 | a | ||||
| DPI + PHEN | 146.89 ± 5.62 | ab | 264.01 ± 9.82 | abc | 95.00 ± 5.54 | a | 23.26 ± 0.39 | ab | 21.95 ± 1.67 | ab | ||||
| DPI + 1-MCP | 144.34 ± 5.64 | ab | 251.86 ± 8.65 | abc | 90.80 ± 4.10 | ab | 22.26 ± 0.40 | abc | 24.11 ± 0.83 | a | ||||
| PHEN + 1-MCP | 161.79 ± 2.33 | ab | 268.93 ± 4.51 | abc | 98.66 ± 2.26 | a | 20.86 ± 2.08 | bcd | 21.33 ± 1.88 | ab | ||||
| DPI + PHEN + 1-MCP | 145.02 ± 5.50 | ab | 240.60 ± 9.74 | abc | 85.65 ± 4.18 | ab | 20.56 ± 0.38 | bcd | 23.53 ± 0.43 | a | ||||
| Gene | Description According to GenBank | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| BoOPR3 | 12-oxophytodienoate reductase 3 | CGATAGGAGCGAGTAAAGTTGG | TTGAGCAAGTCAACCACGGCTA | 109 |
| BoACO4 | 1-aminocyclopropane-1-carboxylate oxidase | TTGAGGTGATAACCAATGGGAAG | TCCAGGGTTGTAGAATGATGCA | 104 |
| BoPAL1 | Phenylalanine ammonia-lyase 1 | TGGCAGCAATCTCGACCCTTG | CCATAACTATCGGTGCCTTTGC | 124 |
| BoHQT | hydroxycinnamoyl-CoA:quinate hydroxycinnamoyltransferase | GCTGGGTCAGATTACCAATTTAC | GCTGCCATCATTTGTAGGACTT | 125 |
| BoDAHPS | 3-deoxy-D-arabino-heptulosonate synthase | CCGTCAAGCAAGCTTCTCCT | CTCCGGTGTCCATTTGGATT | 145 |
| BoACT2 | Actin 2 | GTCGCTATTCAAGCTGTTCTCT | GAGAGCTTCTCCTTGATGTCTC | 251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Contreras, A.M.; Nair, V.; Senés-Guerrero, C.; Pacheco, A.; González-Agüero, M.; Ramos-Parra, P.A.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Cross-Talk and Physiological Role of Jasmonic Acid, Ethylene, and Reactive Oxygen Species in Wound-Induced Phenolic Biosynthesis in Broccoli. Plants 2023, 12, 1434. https://doi.org/10.3390/plants12071434
Torres-Contreras AM, Nair V, Senés-Guerrero C, Pacheco A, González-Agüero M, Ramos-Parra PA, Cisneros-Zevallos L, Jacobo-Velázquez DA. Cross-Talk and Physiological Role of Jasmonic Acid, Ethylene, and Reactive Oxygen Species in Wound-Induced Phenolic Biosynthesis in Broccoli. Plants. 2023; 12(7):1434. https://doi.org/10.3390/plants12071434
Chicago/Turabian StyleTorres-Contreras, Ana Mariel, Vimal Nair, Carolina Senés-Guerrero, Adriana Pacheco, Mauricio González-Agüero, Perla A. Ramos-Parra, Luis Cisneros-Zevallos, and Daniel A. Jacobo-Velázquez. 2023. "Cross-Talk and Physiological Role of Jasmonic Acid, Ethylene, and Reactive Oxygen Species in Wound-Induced Phenolic Biosynthesis in Broccoli" Plants 12, no. 7: 1434. https://doi.org/10.3390/plants12071434
APA StyleTorres-Contreras, A. M., Nair, V., Senés-Guerrero, C., Pacheco, A., González-Agüero, M., Ramos-Parra, P. A., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2023). Cross-Talk and Physiological Role of Jasmonic Acid, Ethylene, and Reactive Oxygen Species in Wound-Induced Phenolic Biosynthesis in Broccoli. Plants, 12(7), 1434. https://doi.org/10.3390/plants12071434








