A Blind-Identification Test on Criconema annuliferum (de Man, 1921) Micoletzky, 1925 Species Complex Corroborate the Hyper-Cryptic Species Diversity Using Integrative Taxonomy
Abstract
1. Introduction
2. Results
2.1. Blind-Identification Test
2.2. Species Delimitation Using Morphometry by Principal Component Analysis
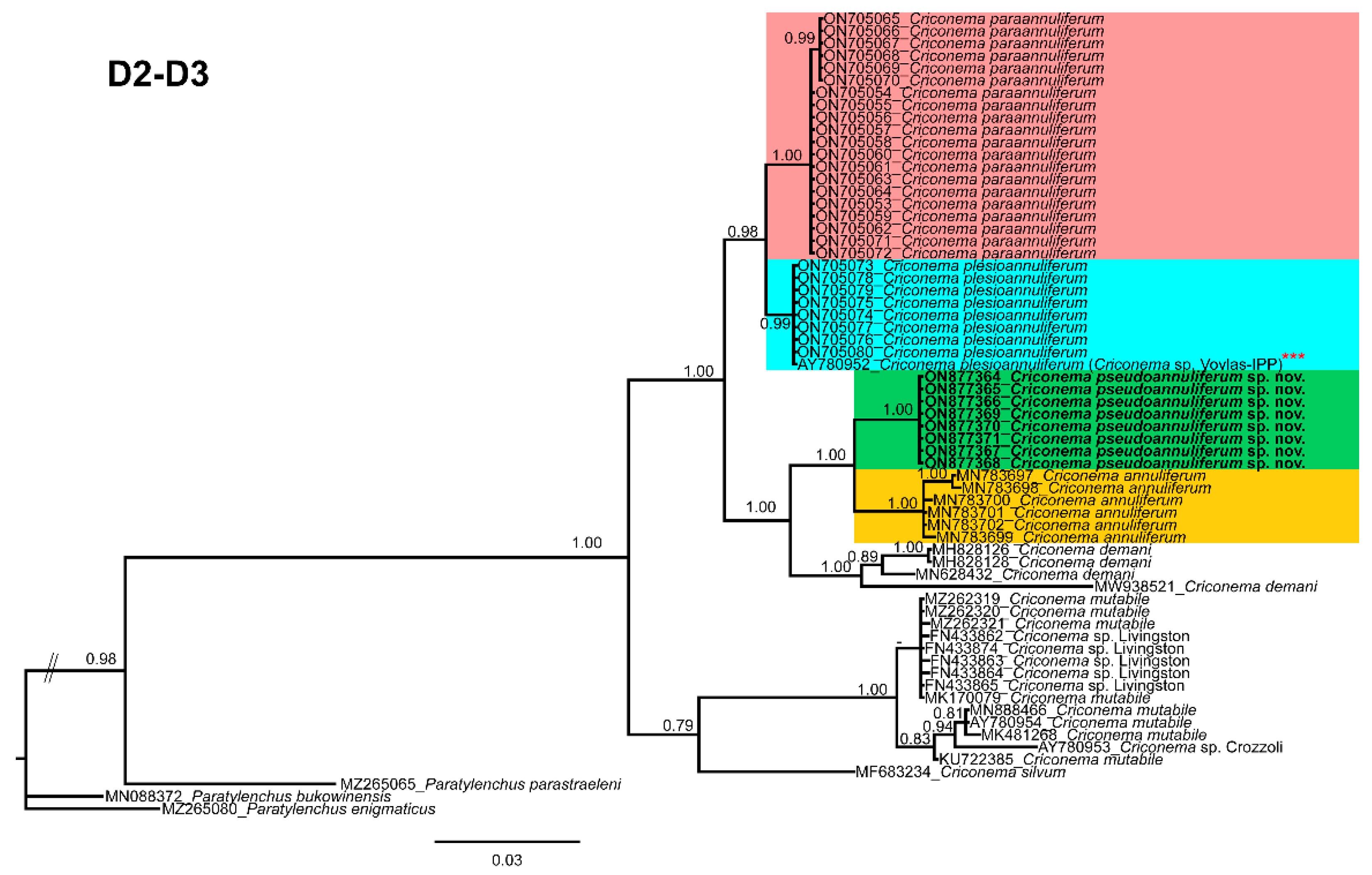
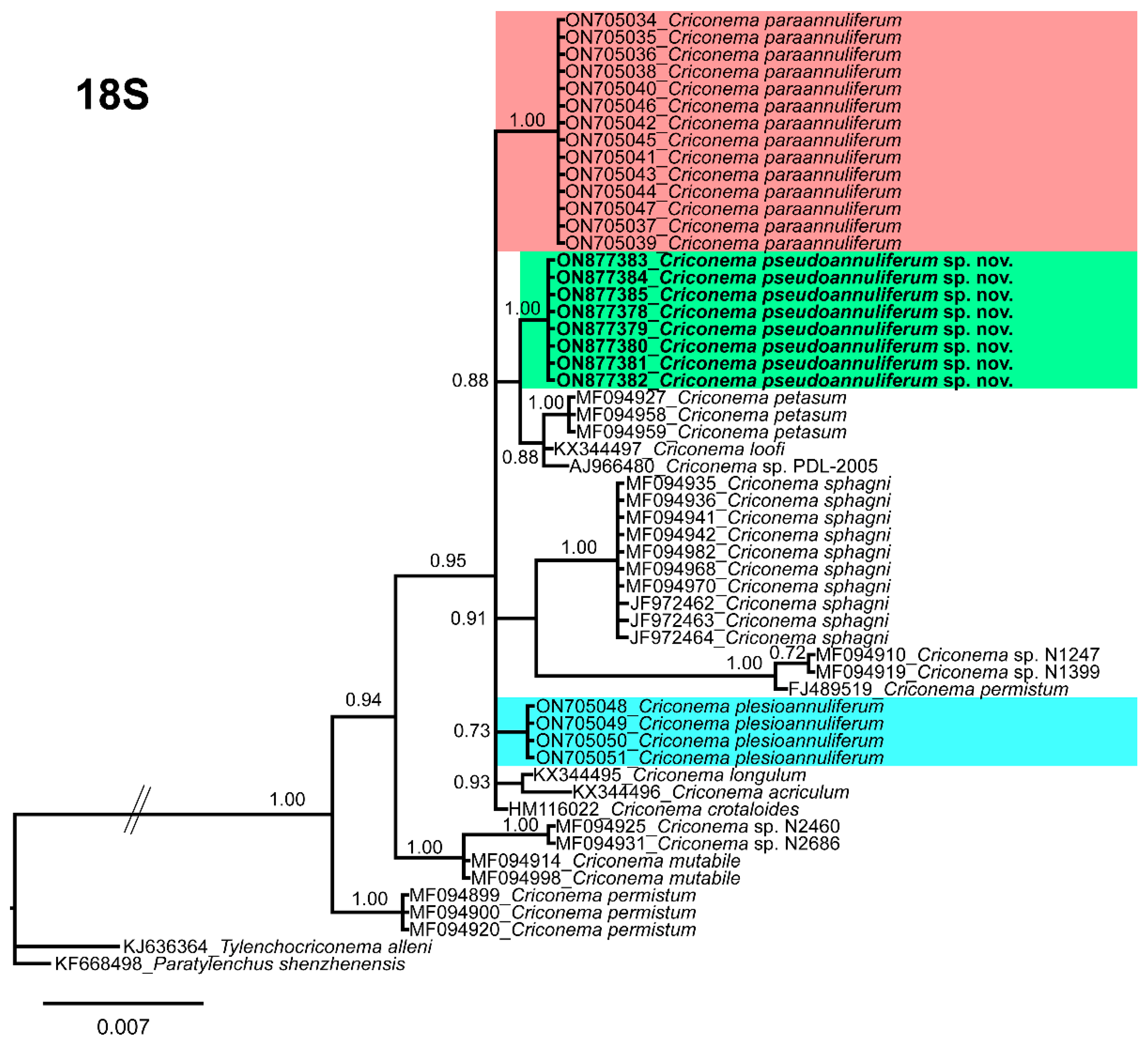
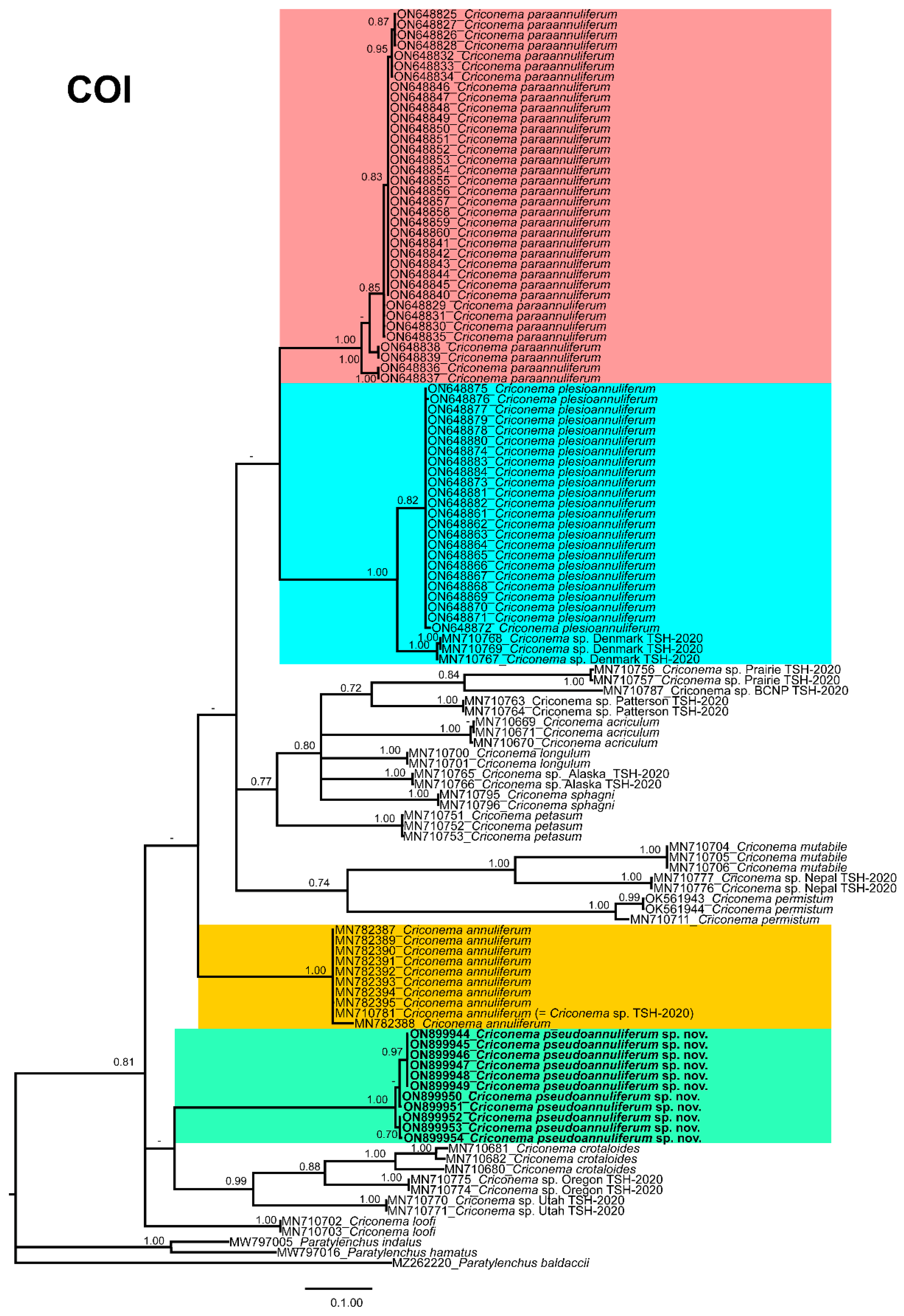
2.3. Species Separation Based on Ribosomal and Mitochondrial DNA
2.4. Ribosomal and Mitochondrial Haplotype Diversity within Criconema annuliferum Species Complex
2.5. Systematics
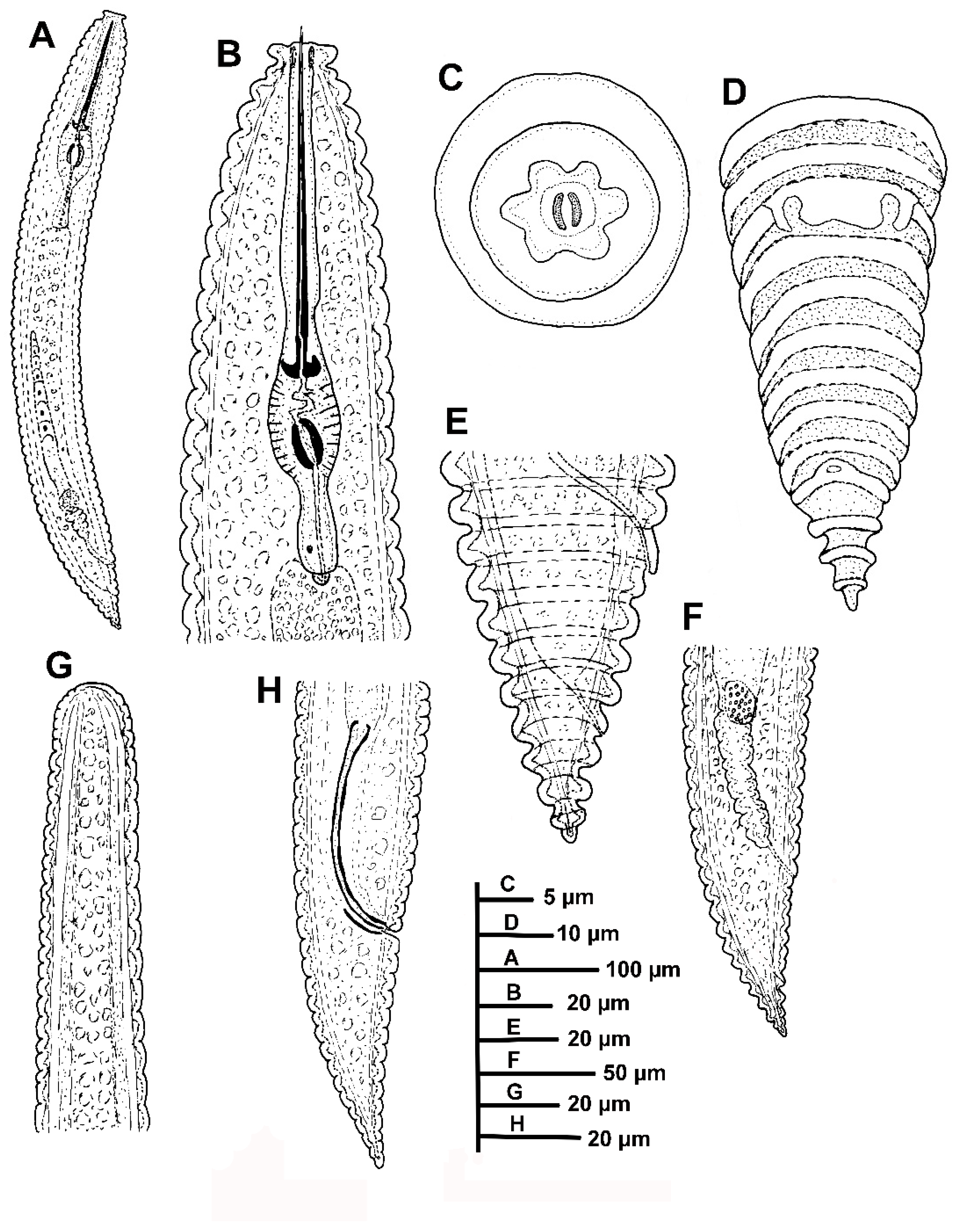
Description of Criconema pseudoannuliferum sp. nov.
Diagnosis and Relationships
Molecular Characterization
Type Habitat and Locality
Etymology
Type Material
2.6. Phylogenetic Analyses of Criconema annuliferum Species Complex
3. Discussion
4. Materials and Methods
4.1. Sampling Sites and Nematode Morphological Identification
4.2. Blind-Identification Test
4.3. DNA Extraction, PCR and Sequencing
4.4. Species Delimitation within Criconema annuliferum Species Complex
4.5. Phylogenetic Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmänner, B.; Menzel, R. Neue arten freilebender Nematoden aus der Schweiz. Zool. Anz. 1914, 441, 80–91. [Google Scholar]
- Geraert, E. The Criconematidae of the World. Identification of the Family Criconematidae (Nematoda); Academia Press: Ghent, Belgium, 2010; 615p. [Google Scholar]
- Ferris, H.; Zheng, L.; Walker, M.A. Resistance of grape rootstocks to plant-parasitic nematodes. J. Nematol. 2012, 4, 377–386. [Google Scholar]
- Westerdahl, B.B.; Duncan, R.A. Peach nematodes. UC Pest Management Guidelines (Updated 9/15). UCIPM University of California Agriculture and Natural Resources, State-Wide Integrated Pest Management Program. 2015. Available online: http://ipm.ucanr.edu/PMG/r602200111.html (accessed on 26 December 2022).
- Braun, A.L.; Mojtahedi, H.; Lownsbery, B.F. Separate and combined effects of Paratylenchus neoamblycephalus and Criconemoides xenoplax on “Myrobalan” plum. Phytopathology 1975, 65, 328–330. [Google Scholar] [CrossRef]
- Raski, D.J.; Valenzuela, A. Descriptions of four new species of Criconematoidea (Tylenchina: Nemata) from Southern Chile. J. Nematol. 1986, 18, 252–266. [Google Scholar]
- Okie, W.R.; Reighard, G.L.; Nyczepir, A.P. Importance of scion cultivar in peach tree short life. J. Am. Pomol. Soc. 2009, 63, 58–63. [Google Scholar]
- Subbotin, S.A.; Vovlas, N.; Crozzoli, R.; Sturhan, D.; Lamberti, F.; Moens, M.; Baldwin, J.G. Phylogeny of Criconematina Siddiqi, 1980 (Nematoda: Tylenchida) based on morphology and D2-D3 expansion segments of the 28S-rRNA gene sequences with application of a secondary structure model. Nematology 2005, 7, 927–944. [Google Scholar]
- Cordero, M.A.; Robbins, R.T.; Szalanski, A.L. Taxonomic and molecular identification of Bakernema, Criconema, Hemicriconemoides, Ogma and Xenocriconemella species (Nematoda: Criconematidae). J. Nematol. 2012, 44, 427–446. [Google Scholar]
- Powers, T.O.; Harris, T.S.; Higgins, R.S.; Mullin, P.G.; Powers, K.S. An 18S rDNA perspective on the classification of Criconematoidea. J. Nematol. 2017, 49, 236–244. [Google Scholar] [CrossRef]
- Etongwe, C.M.; Singh, P.R.; Bert, W.; Subbotin, S.A. Molecular characterisation of some plant-parasitic nematodes (Nematoda: Tylenchida) from Belgium. Russ. J. Nematol. 2020, 28, 1–28. [Google Scholar]
- Powers, T.O.; Harris, T.S.; Higgins, R.S.; Mullin, P.G.; Powers, K.S. Nematode biodiversity assessments need vouchered databases: A BOLD reference library for plant-parasitic nematodes in the superfamily Criconematoidea. Genome 2021, 64, 232–241. [Google Scholar] [CrossRef]
- Clavero-Camacho, I.; Palomares-Rius, J.E.; Cantalapiedra-Navarrete, C.; Castillo, P.; Archidona-Yuste, A. A Proposed new species complex within the cosmopolitan ring nematode Criconema annuliferum (de Man, 1921) Micoletzky, 1925. Plants 2022, 11, 1977. [Google Scholar] [CrossRef]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda, Errantia) II; Pedozoologica Hungarica: Budapest, Hungary, 2007; Volume 4, 496p. [Google Scholar]
- Raski, D.J.; Golden, M.A. Studies on the genus Criconemoides Taylor, 1936 with descriptions of eleven new species and Bakernema variabile n. sp. (Criconematidae: Nematoda). Nematologica 1965, 11, 501–565. [Google Scholar]
- Escuer, M.; Lara, M.P.; Bello, A. Nematodos del suelo de la subfamilia Criconematinae (Nematoda: Criconematidae) en España peninsular. Orsis 1997, 12, 39–63. [Google Scholar]
- Katalan-Gateva, S.; Aleksiev, A.D.; Iliev, I.L.; Ilieva, Z.I. On the species composition and distribution of the family Criconematidae (Taylor, 1936) Thorne, 1949 (Nematoda) in Bulgaria. Acta Zool. Bulg. 1991, 41, 53–57. [Google Scholar]
- Lišková, M.; Vovlas, N.; Sasanelli, N. Criconematidae (Nematoda) in the Slovak Republic. Helminthologia 2004, 41, 161–170. [Google Scholar]
- Zapałowska, A.; Tomasz Skwiercz, A. Populations of parasitic nematodes colonizing Jerusalem artichoke (Helianthus tuberosus L.). Acta Soc. Bot. Pol. 2018, 87, 1–10. [Google Scholar] [CrossRef]
- Bello, A.; Escuer, M.; Lara, M.P. La Familia Criconematidae en las Islas Canarias. Nematol. Mediterr. 1994, 22, 225–232. [Google Scholar]
- Eskandari, A.; Karegar, A.; Pourjam, E.; Alizadeh, A. Three new records of Criconematidae from Iran. Iran. J. Plant Pathol. 2006, 42, 134–157. [Google Scholar]
- Knight, K.W.L. Plant parasitic nematodes associated with six subtropical crops in New Zealand. N. Z. J. Crop Hortic. Sci. 2001, 29, 267–275. [Google Scholar] [CrossRef]
- Van den Berg, E.; Heyns, J. Notes on some plant-parasitic nematodes from South America. Nematologica 1997, 43, 131–144. [Google Scholar] [CrossRef]
- Archidona-Yuste, A.; Wiegand, T.; Castillo, P.; Navas-Cortés, J.A. Spatial structure and soil properties shape local community structure of plant-parasitic nematodes in cultivated olive trees in southern Spain. Agric. Ecosyst. Environ. 2020, 287, 106688. [Google Scholar] [CrossRef]
- Archidona-Yuste, A.; Wiegand, T.; Eisenhauer, N.; Cantalapiedra-Navarrete, C.; Palomares-Rius, J.E.; Castillo, P. Agriculture causes homogenization of plant-feeding nematode communities at the regional scale. J. Appl. Ecol. 2021, 58, 2881–2891. [Google Scholar] [CrossRef]
- Maria, M.; Miao, W.; Cai, R.; Tian, Z.; Castillo, P.; Zheng, J. Species diversity of ring nematodes of the genus Criconemoides (Nematoda: Criconematidae) based on three new species from China, using integrative taxonomy. Eur. J. Plant. Pathol. 2020, 157, 119–139. [Google Scholar] [CrossRef]
- Li, J.; Munawar, M.; Castillo, P.; Zheng, J. Morpho-Molecular and ultrastructural characterization of Discocriconemella parasinensis n. sp. from Zhejiang Province, China. J. Nematol. 2022, 54, e2022-01. [Google Scholar] [CrossRef] [PubMed]
- Rundell, R.J.; Price, T.D. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends Ecol. Evol. 2009, 24, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Raadik, T.A.; Burridge, C.P.; Georges, A. Global biodiversity assessment and hyper-cryptic species complexes: More than one species of elephant in the room? Syst. Biol. 2014, 63, 518–533. [Google Scholar] [CrossRef]
- Wattier, R.; Mamos, T.; Copilaş-Ciocianu, D.; Jelic, M.; Ollivier, A.; Chaumot, A.; Danger, M.; Felten, V.; Piscart, C.; Zganec, K.; et al. Continental-scale patterns of hyper-cryptic diversity within the freshwater model taxon Gammarus fossarum (Crustacea, Amphipoda). Sci. Rep. 2020, 10, 16536. [Google Scholar] [CrossRef]
- Nygren, A.; Parapar, J.; Pons, J.; Meiûner, K.; Bakken, T.; Kongsrud, J.A.; Oug, E.; Gaeva, D.; Sikorski, A.; Johansen, R.A.; et al. A mega-cryptic species complex hidden among one of the most common annelids in the North East Atlantic. PLoS ONE 2018, 13, e0198356. [Google Scholar] [CrossRef]
- Cai, R.; Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Palomares-Rius, J.E.; Castillo, P. New evidence of cryptic speciation in the family Longidoridae (Nematoda: Dorylaimida). J. Zool. Syst. Evol. Res. 2020, 58, 869–899. [Google Scholar] [CrossRef]
- Clavero-Camacho, I.; Palomares-Rius, J.E.; Cantalapiedra-Navarrete, C.; León-Ropero, G.; Martín-Barbarroja, J.; Archidona-Yuste, A.; Castillo, P. Integrative taxonomy reveals hidden cryptic diversity within pin nematodes of the genus Paratylenchus (Nematoda: Tylenchulidae). Plants 2021, 10, 1454. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Archidona-Yuste, A.; Clavero-Camacho, I.; Carreira de la Fuente, J.; Rey, A.; Viñegla, B.; Liebanas, G.; Cantalapiedra-Navarrete, C.; Castillo, P. DNA barcoding and morphometry reveal further cryptic biodiversity of pin nematodes of the genus Paratylenchus (Nematoda: Tylenchulidae). Plants 2022, 11, 3385. [Google Scholar] [CrossRef]
- Hunt, D.J.; Palomares-Rius, J.E. General morphology and morphometries of plant-parasitic nematodes. In Practical Plant Nematology; Biblioteca Básica de Agricultura: Texcoco, Mexico, 2012; pp. 25–64. [Google Scholar]
- Masters, B.C.; Fan, V.; Ross, H.A. Species delimitation a Geneious plugin for the exploration of species boundaries. Mol. Ecol. Resour. 2011, 11, 154–157. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Hendrixson, B.E.; Brewer, M.S.; Bond, J.E. An evaluation of sampling effects on multiple DNA barcoding methods leads to an integrative approach for delimiting species: A case study of the North American tarantula genus Aphonopelma (Araneae, Mygalomorphae, Theraphosidae). Mol. Phylogen. Evol. 2014, 71, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.A.; Murugan, S.; Li, W.L.S. Testing the reliability of genetic methods of species identification via simulation. Syst. Biol. 2008, 57, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.A. Statistical tests for taxonomic distinctiveness from observations of monophyly. Evolution 2007, 61, 317–323. [Google Scholar] [CrossRef]
- Peneva, V.; Neilson, R.; Boag, B.; Brown, D.J.F. Criconematidae (Nematoda) from oak forests in two nature reserves in Russia. Syst. Parasitol. 2000, 46, 191–201. [Google Scholar] [CrossRef]
- Afshar, F.J.; Porjam, E.; Mokhtassi-Bidgoli, A.; Pedram, M. Three new records of criconematids (Criconematidae: Criconematinae) from Iran. J. Crop Prot. 2020, 9, 355–366. [Google Scholar]
- Azimi, S.; Pedram, M. Description of Criconema iranicum n. sp. (Nematoda: Criconematidae) from Iran. J. Crop Prot. 2020, 9, 497–505. [Google Scholar]
- Flis, Ł.; Kornobis, F.W.; Kubicz, M.; Guðmundsson, J. New records of plant-parasitic nematodes from Iceland. Polar Biol. 2020, 43, 1655–1661. [Google Scholar] [CrossRef]
- Van den Berg, E.; Tied, L.R.; Subbotin, S.A. Morphological and molecular characterization of some Criconematidae (Nematoda, Tylenchida): Ogma decalineatus (Chitwood, 1957) Andrassy, 1979, Criconema silvum (Van den Berg, 1984) Raski & Luc, 1985 and Neobakernema variabile (Raski & Golden, 1966) Ebsary, 1981 from South Africa and the USA. Russ. J. Nematol. 2017, 25, 101–119. [Google Scholar]
- Meldal, B.H.; Debenham, N.J.; De Ley, P.; De Ley, I.T.; Vanfleteren, J.R.; Vierstraete, A.R.; Bert, W.; Borgonie, G.; Moens, T.; Tyler, P.A.; et al. An improved molecular phylogeny of the Nematoda with special emphasis on marine taxa. Mol. Phylogenet. Evol. 2007, 42, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Powers, T.O.; Mullin, P.G.; Higgins, R.S.; Harris, T.S.; Powers, K.S. Description of Mesocriconema ericaceum n. sp. (Nematoda: Criconematidae) and notes on other nematode species discovered in an ericaceous heath bald community in Great Smoky Mountains National Park, USA. Nematology 2016, 18, 879–903. [Google Scholar] [CrossRef]
- Pérez-Ponce de León, G.; Poulin, R. Taxonomic distribution of cryptic diversity among metazoans: Not so homogeneous after all. Biol. Lett. 2016, 12, 20160371. [Google Scholar] [CrossRef] [PubMed]
- Fiser, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 27, 613–635. [Google Scholar] [CrossRef]
- Vilas, R.; Criscione, C.D.; Blouin, M.S. A comparison between mitochondrial DNA and the ribosomal internal transcribed regions in prospecting for cryptic species of platyhelminth parasites. Parasitology 2005, 131, 839–846. [Google Scholar] [CrossRef]
- Dey, A.; Chan, C.K.; Thomas, C.G.; Cutter, A.D. Molecular hyperdiversity defines populations of the nematode Caenorhabditis brenneri. Proc. Natl. Acad. Sci. USA 2013, 110, 11056–11060. [Google Scholar] [CrossRef]
- Olson, M.; Harris, T.; Higgins, R.; Mullin, P.; Powers, K.; Olson, S.; Powers, T.O. Species delimitation and description of Mesocriconema nebraskense n. sp. (Nematoda: Criconematidae), a morphologically cryptic, parthenogenetic species from north American grasslands. J. Nematol. 2017, 49, 42–66. [Google Scholar] [CrossRef]
- Cho, M.R.; Robbins, R.T. Morphological variation among 23 Xiphinema americanum populations. J. Nematol. 1991, 23, 134–144. [Google Scholar]
- Archidona-Yuste, A.; Navas-Cortés, J.A.; Cantalapiedra-Navarrete, C.; Palomares-Rius, J.E.; Castillo, P. Cryptic diversity and species delimitation in the Xiphinema americanum-group complex (Nematoda: Longidoridae) as inferred from morphometrics and molecular markers. Zool. J. Linn. Soc. 2016, 176, 231–265. [Google Scholar] [CrossRef]
- Cai, R.; Prior, T.; Lawson, B.; Cantalapiedra-Navarrete, C.; Palomares-Rius, J.E.; Castillo, P.; Archidona-Yuste, A. An integrative taxonomic study of the needle nematode complex Longidorus goodeyi Hooper, 1961 (Nematoda: Longidoridae) with description of a new species. Eur. J. Plant Pathol. 2020, 158, 59–81. [Google Scholar] [CrossRef]
- Gittenberger, E. What about non-adaptive radiation? Biol. J. Linn. Soc. 1991, 3, 263–272. [Google Scholar] [CrossRef]
- Gissi, C.; Iannelli, F.; Pesole, G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity 2008, 101, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Karssen, G.; Coureur, M.; Subbotin, S.; Bert, W. Integrative taxonomy and molecular phylogeny of the plant-parasitic nematode genus Paratylenchus (Nematoda: Paratylenchinae): Linking species with molecular barcodes. Plants 2021, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Zink, R.M.; Barrowclough, G.F. Mitochondrial DNA under siege in avian phylogeography. Mol. Ecol. 2008, 17, 2107–2121. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Cipriano, F.; Hare, M.P. Predicting nuclear gene coalescence from mitochondrial data: The three-times rule. Evolution 2001, 55, 859–868. [Google Scholar] [CrossRef]
- Munawar, M.; Powers, T.O.; Tian, Z.; Harris, T.; Higgins, R.; Zheng, J. Description and distribution of three criconematid nematodes from Hangzhou, Zhejiang Province, China. J. Nematol. 2017, 50, 1–24. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, T.D.; Le, T.M.L.; Trinh, Q.P.; Bert, W. Remarks on phylogeny and molecular variations of criconematid species (Nematoda: Criconematidae) with case studies from Vietnam. Sci. Rep. 2022, 12, 14832. [Google Scholar] [CrossRef]
- Derycke, S.; Remerie, T.; Backeljau, T.; Vierstraete, A.; Vanfleteren, J.; Vincx, M.; Moens, T. Phylogeography of the Rhabditis (Pellioditis) marina species complex: Evidence for long-distance dispersal, and for range expansions and restricted gene flow in the northeast Atlantic. Mol. Ecol. 2008, 17, 3306–3322. [Google Scholar] [CrossRef]
- Clavero-Camacho, I.; Cantalapiedra-Navarrete, C.; Archidona-Yuste, A.; Castillo, P.; Palomares-Rius, J.E. Remarkable cryptic diversity of Paratylenchus spp. (Nematoda: Tylenchulidae) in Spain. Animals 2021, 11, 1161. [Google Scholar] [CrossRef]
- Coolen, W.A. Methods for extraction of Meloidogyne spp. and other nematodes from roots and soil. In Root-Knot Nematodes (Meloidogyne Species). Systematics, Biology and Control; Lamberti, F., Taylor, C.E., Eds.; Academic Press: New York, NY, USA, 1979; pp. 317–329. [Google Scholar]
- Seinhorst, J.W. Killing nematodes for taxonomic study with hot F.A. 4:1. Nematologica 1966, 12, 178. [Google Scholar] [CrossRef]
- De Grisse, A.T. Redescription ou modifications de quelques techniques utilisées dans l’étude de nématodes phytoparasitaires. Meded. Rijksfac. Landbouwwet. Gent. 1969, 34, 315–359. [Google Scholar]
- Abolafia, J. A low-cost technique to manufacture a container to process meiofauna for scanning electron microscopy. Microsc. Res. Tech. 2015, 78, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Palomares-Rius, J.E.; Clavero-Camacho, I.; Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; León-Ropero, G.; Braun Miyara, S.; Karssen, G.; Castillo, P. Global distribution of the reniform nematode genus Rotylenchulus with the synonymy of Rotylenchulus macrosoma with Rotylenchulus borealis. Plants 2021, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- De Ley, P.; Felix, M.A.; Frisse, L.; Nadler, S.; Sternberg, P.; Thomas, W.K. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology 1999, 1, 591–612. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Vierstraete, A.; De Ley, P.; Rowe, J.; Waeyenberge, L.; Moens, M.; Vanfleteren, J.R. Phylogenetic relationships within the cyst-forming nematodes (Nematoda, Heteroderidae) based on analysis of sequences from the ITS regions of ribosomal DNA. Mol. Phylogenet. Evol. 2001, 21, 1–16. [Google Scholar] [CrossRef]
- Powers, T.O.; Bernard, E.C.; Harris, T.; Higgins, R.; Olson, M.; Lodema, M.; Mullin, P.; Sutton, L.; Powers, K.S. COI haplotype groups in Mesocriconema (Nematoda: Criconematidae) and their morphospecies associations. Zootaxa 2014, 3827, 101–146. [Google Scholar] [CrossRef]
- Holterman, M.; Van der Wurff, A.; Van den Elsen, S.; Van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1800. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; 990p. [Google Scholar]
- Micoletzky, H. Die freilebende Süsswasser- und Moornematoden Dänemarks. Nebst Anhang: Über Amöbosporidien und andere Parasiten bei freilebenden Nematoden. K. Danske Vidensk. Selsk. Skr. 1925, 10, 57–310. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Introduction to Linear Regression Analysis; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Revelle, W. psych: Procedures for Personality and Psychological Research; Northwestern University, Evanston, Illinois. R Package Version 2.2.5. 2022. Available online: https://CRAN.R-project.org/package=psychTools (accessed on 30 December 2022).
- Quadros, A. emstreeR: Tools for Fast Computing and Plotting Euclidean Minimum Spanning Trees. R Package Version 3.0.0. 2022. Available online: https://CRAN.R-project.org/package=emstreeR (accessed on 30 December 2022).
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 30 December 2022).
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program forWindows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Tan, G.; Muffato, M.; Ledergerber, C.; Herrero, J.; Goldman, N.; Gil, M.; Dessimoz, C. Current methods for automated filtering of multiple sequence alignments frequently worsen single-gene phylogenetic inference. Syst. Biol. 2015, 64, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v.1.4.3, A Graphical Viewer of Phylogenetic Trees. 2016. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 28 September 2022).
- Clement, M.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K. TCS: Estimating Gene Genealogies. In Proceedings of the 6th International Parallel and Distributed Processing Symposium, Fort Lauderdale, FL, USA, 15–19 April 2002; IEEE Computer Society: Fort Lauderdale, FL, USA, 2002; p. 184. [Google Scholar]
- Leigh, J.W.; Bryant, D. POPART: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Castillo, P.; Palomares-Rius, J.E. Molecular phylogenetic analysis and comparative morphology reveals the diversity and distribution of needle nematodes of the genus Longidorus (Dorylaimida: Longidoridae) from Spain. Contrib. Zool. 2019, 88, 1–41. [Google Scholar] [CrossRef]






| Character/Ratio b | MLC1 | MLC2 | MLC3 |
|---|---|---|---|
| L | 0.86 | 0.25 | 0.30 |
| Stylet length | 0.66 | 0.00 | 0.36 |
| R | 0.63 | 0.25 | 0.57 |
| RV | −0.06 | 0.85 | 0.20 |
| Ran | −0.22 | 0.90 | −0.19 |
| a | 0.67 | 0.03 | −0.04 |
| b | 0.61 | −0.33 | 0.16 |
| c′ | 0.13 | 0.81 | −0.57 |
| V | 0.24 | −0.13 | 0.64 |
| SS loadings | 2.52 | 2.43 | 1.38 |
| % of total variance | 0.28 | 0.27 | 0.15 |
| Cumulative % of total variance | 0.28 | 0.55 | 0.70 |
| Species | Gene | Intra/Inter a | P ID (Liberal) b | Clade Support c | Rosenberg’s PAB d |
|---|---|---|---|---|---|
| Criconema annuliferum | D2-D3 | 0.19 | 0.95 (0.85, 1.0)e | 1.00 | 5.1 × 10−5 |
| ITS | - | - | - | - | |
| COI | 0.02 | 1.00 (0.95, 1.0) | 1.00 | 2.1 × 10−5 | |
| Criconema paraannuliferum | D2-D3 | 0.14 | 0.98 (0.95, 1.0) | 1.00 | 7.1 × 10−9 |
| ITS | 0.05 | 0.99 (0.97, 1.0) | 1.00 | 2.5 × 10−11 | |
| COI | 0.05 | 1.00 (0.97, 1.0) | 1.00 | 6.5 × 10−20 | |
| Criconema plesioannuliferum | D2-D3 | 0.09 | 0.98 (0.93, 1.0) | 0.98 | 7.1 × 10−9 |
| ITS | 0.02 | 1.00 (0.96, 1.0) | 1.00 | 2.5 × 10−11 | |
| COI | 0.07 | 1.00 (0.98, 1.0) | 1.00 | 2.6 × 10−5 | |
| Criconema pseudoannuliferum sp. nov. | D2-D3 | 0.05 | 0.99 (0.93, 1.0) | 1.00 | 5.1 × 10−5 |
| ITS | 0.01 | 0.98 (0.88, 1.0) | 1.00 | 1.1 × 10−8 | |
| COI | 0.02 | 1.00 (0.95, 1.0) | 1.00 | 3.6 × 10−6 |
| Character/Ratio a | Holotype | Female Paratypes | Males | Fourth-Stage Juveniles |
|---|---|---|---|---|
| n | 1 | 20 | 2 | 4 |
| L | 641 | 633.6 ± 54.1 (526–715) | (486, 597) | 503.4 ± 14.4 (486–520) |
| R | 70 | 71.5 ± 2.8 (67–79) | - | 73.3 ± 2.8 (70–76) |
| Rst | 16 | 14.0 ± 1.5 (12–16) | - | 17.0 ± 1.8 (15–19) |
| Roes | 21 | 19.3 ± 1.6 (17–22) | - | 21.5 ± 1.3 (20–23) |
| Rex | 22 | 21.0 ± 1.4 (19–23) | - | 23.0 ± 1.2 (22–24) |
| RV | 9 | 9.6 ± 0.7 (9–11) | - | - |
| Rvan | 5 | 4.7 ± 0.6 (4–6) | - | - |
| Ran | 4 | 4.8 ± 0.5 (4–6) | - | 4.5 ± 0.6 (4–5) |
| O | 9.1 | 9.3 ± 0.9 (7.5–11.0) | - | 10.0 ± 0.6 (9.2–10.5) |
| a | 9.3 | 11.4 ± 1.8 (8.6–14.2) | (16,8, 23.9) | 9.8 ± 1.5 (8.5–11.8) |
| b | 3.9 | 3.9 ± 0.3 (3.4–4.4) | (3.8, 4.9) | 3.5 ± 0.1 (3.4–3.6) |
| c | 25.6 | 24.7 ± 3.2 (19.6–29.5) | (14.6, 16.2) | 18.4 ± 1.0 (17.0–19.4) |
| c′ | 1.0 | 1.2 ± 0.1 (1.0–1.4) | (1.4, 2.1) | 1.2 ± 0.1 (1.0–1.2) |
| V or T | 87.7 | 89.3 ± 1.1 (87.7–91.7) | (28.6, 37.0) | - |
| VL/VB | 1.4 | 1.6 ± 0.2 (1.3–1.9) | - | - |
| First annulus | 23.0 | 21.8 ± 1.1 (20.0–23.5) | - | 19.3 ± 1.0 (18.0–20.0) |
| Second annulus | 21.0 | 20.0 ± 1.0 (18.0–21.5) | - | 17.0 ± 0.8 (16.0–18.0) |
| Stylet | 110.0 | 109.7 ± 3.0 (105.0–116.0) | - | 96.0 ± 2.9 (93.0–99.0) |
| Conus | 90.0 | 91.0 ± 2.3 (87.0–97.0) | - | 80.3 ± 1.7 (78.0–82.0) |
| Pharynx | 165.0 | 164.2 ± 7.3 (152–177) | (121, 127) | 145.0 ± 3.7 (140–149) |
| Max. body width | 69.0 | 56.5 ± 7.0 (43.0–69.0) | (25.0, 29.0) | 52.0 ± 14.4 (43.0–59.0) |
| Anal body diam. | 24.0 | 22.6 ± 2.6 (18.0–27.0) | (20.0, 21.5) | 23.6 ± 2.3 21.0–26.0) |
| Vulva to anus distance | 43.0 | 42.2 ± 4.6 (34.0–50.0) | - | - |
| Tail | 25.0 | 26.0 ± 3.1 (20.0–32.0) | (30.0, 41.0) | 27.5 ± 2.1 (25.0–30.0) |
| Spicules | - | - | (47.0, 48.0) | - |
| Gubernaculum | - | - | (9.0, 10.0) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archidona-Yuste, A.; Palomares-Rius, J.E.; Clavero-Camacho, I.; Cantalapiedra-Navarrete, C.; Liébanas, G.; Castillo, P. A Blind-Identification Test on Criconema annuliferum (de Man, 1921) Micoletzky, 1925 Species Complex Corroborate the Hyper-Cryptic Species Diversity Using Integrative Taxonomy. Plants 2023, 12, 1044. https://doi.org/10.3390/plants12051044
Archidona-Yuste A, Palomares-Rius JE, Clavero-Camacho I, Cantalapiedra-Navarrete C, Liébanas G, Castillo P. A Blind-Identification Test on Criconema annuliferum (de Man, 1921) Micoletzky, 1925 Species Complex Corroborate the Hyper-Cryptic Species Diversity Using Integrative Taxonomy. Plants. 2023; 12(5):1044. https://doi.org/10.3390/plants12051044
Chicago/Turabian StyleArchidona-Yuste, Antonio, Juan Emilio Palomares-Rius, Ilenia Clavero-Camacho, Carolina Cantalapiedra-Navarrete, Gracia Liébanas, and Pablo Castillo. 2023. "A Blind-Identification Test on Criconema annuliferum (de Man, 1921) Micoletzky, 1925 Species Complex Corroborate the Hyper-Cryptic Species Diversity Using Integrative Taxonomy" Plants 12, no. 5: 1044. https://doi.org/10.3390/plants12051044
APA StyleArchidona-Yuste, A., Palomares-Rius, J. E., Clavero-Camacho, I., Cantalapiedra-Navarrete, C., Liébanas, G., & Castillo, P. (2023). A Blind-Identification Test on Criconema annuliferum (de Man, 1921) Micoletzky, 1925 Species Complex Corroborate the Hyper-Cryptic Species Diversity Using Integrative Taxonomy. Plants, 12(5), 1044. https://doi.org/10.3390/plants12051044







