Choose Wisely: Great Variation among Genotypes of Promising Paludiculture Crop Phragmites australis
Abstract
1. Introduction
- (1)
- P. australis genotypes differ in biomass productivity, and morphological traits and are thus differently well suited for paludiculture;
- (2)
- Conditions at the extreme ends of the resource availability gradients (macronutrient deficiency/surplus; drought/flooding) cause stress for P. australis and lead to a higher expression of oxidative stress response genes;
- (3)
- Differences in productivity and growth among P. australis genotypes arise from the fact that they follow different strategies in the plant economics spectrum and are indicated by (a) ‘fast’ genotypes outperforming ‘slow’ genotypes under favorable conditions and suffering stronger under stressful conditions, and (b) functional trait differentiation between genotypes according to these plant strategies;
- (4)
- Performance of P. australis genotypes for paludiculture can be predicted using functional traits and plant strategies.
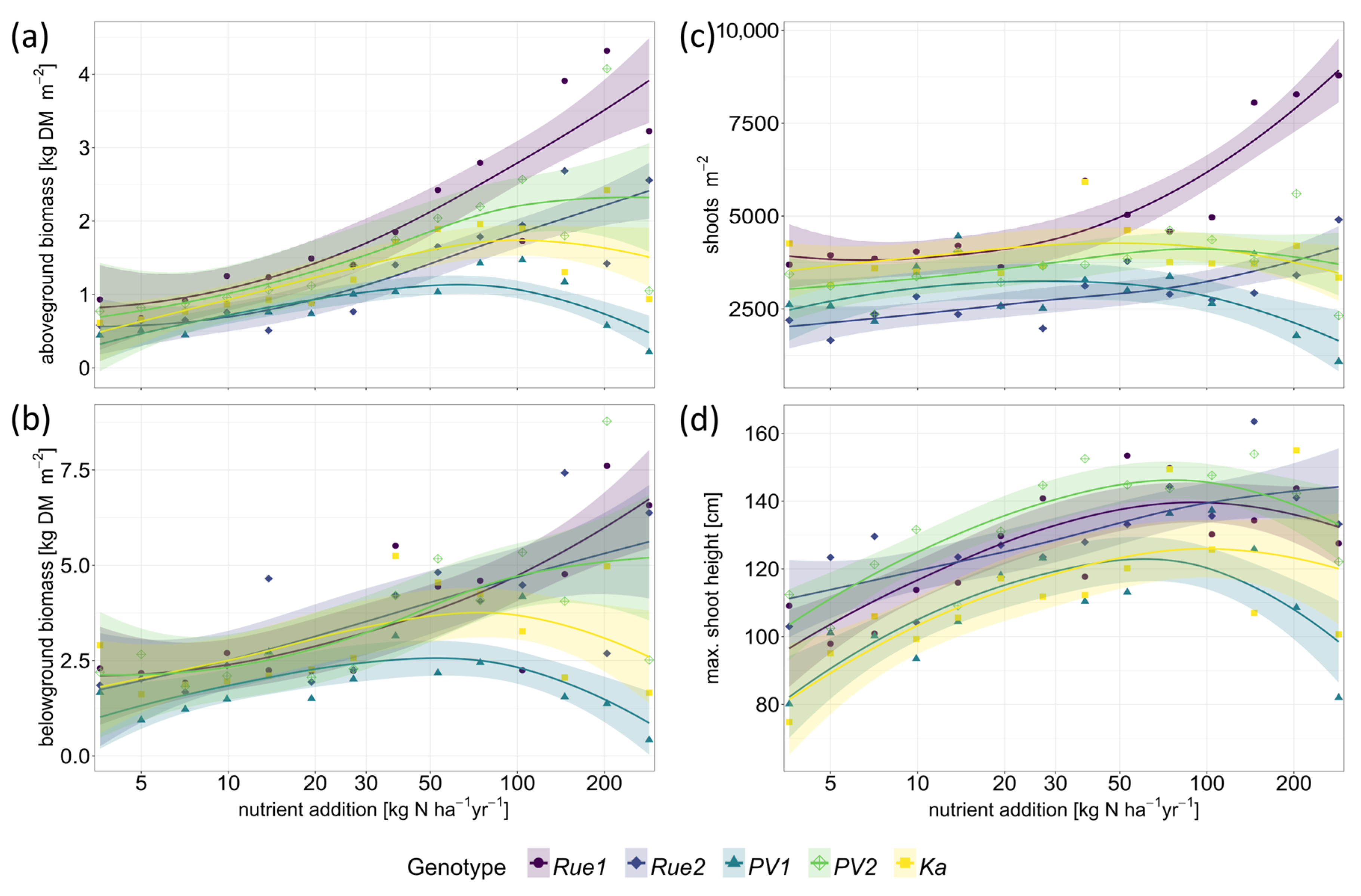
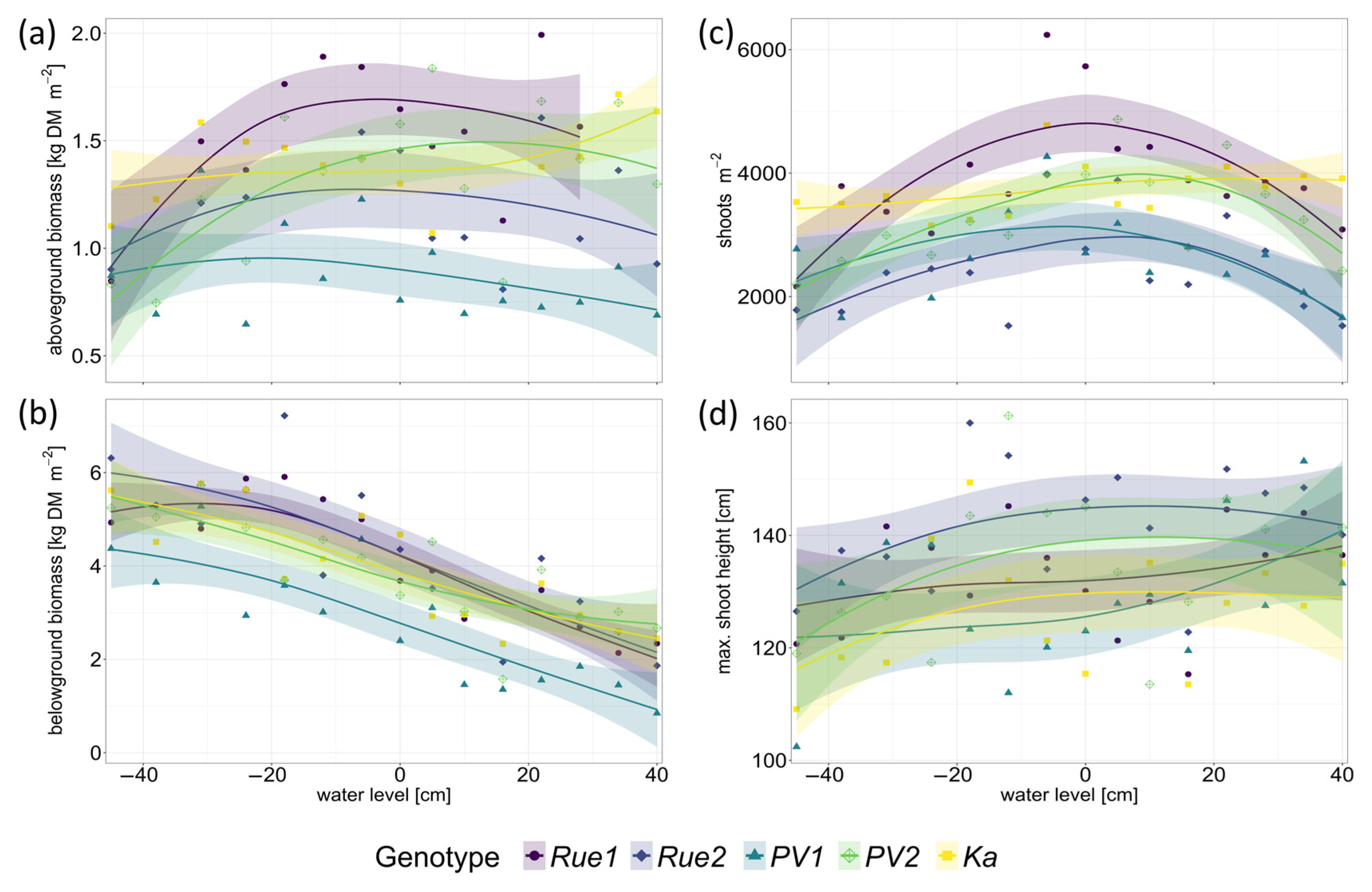
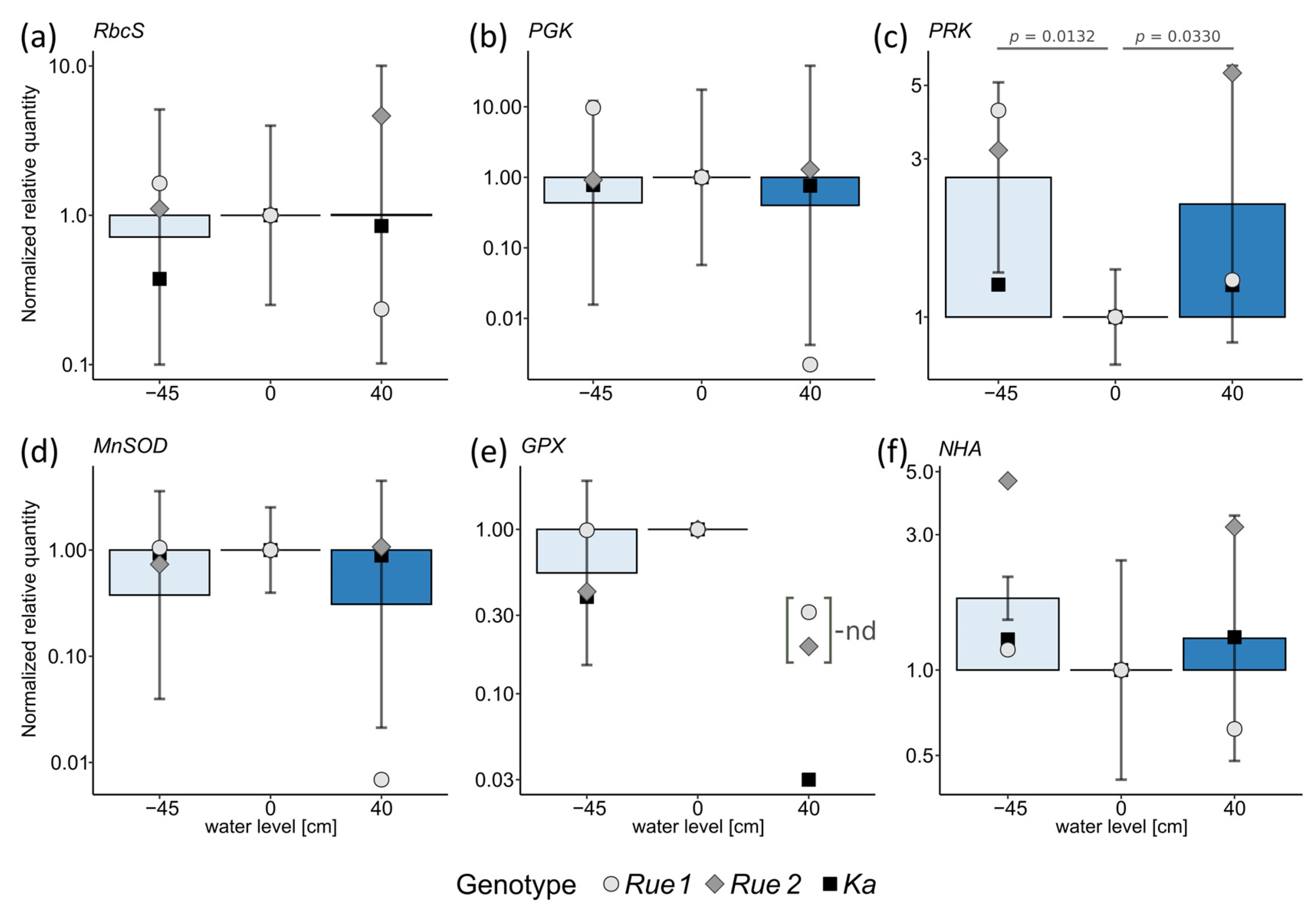
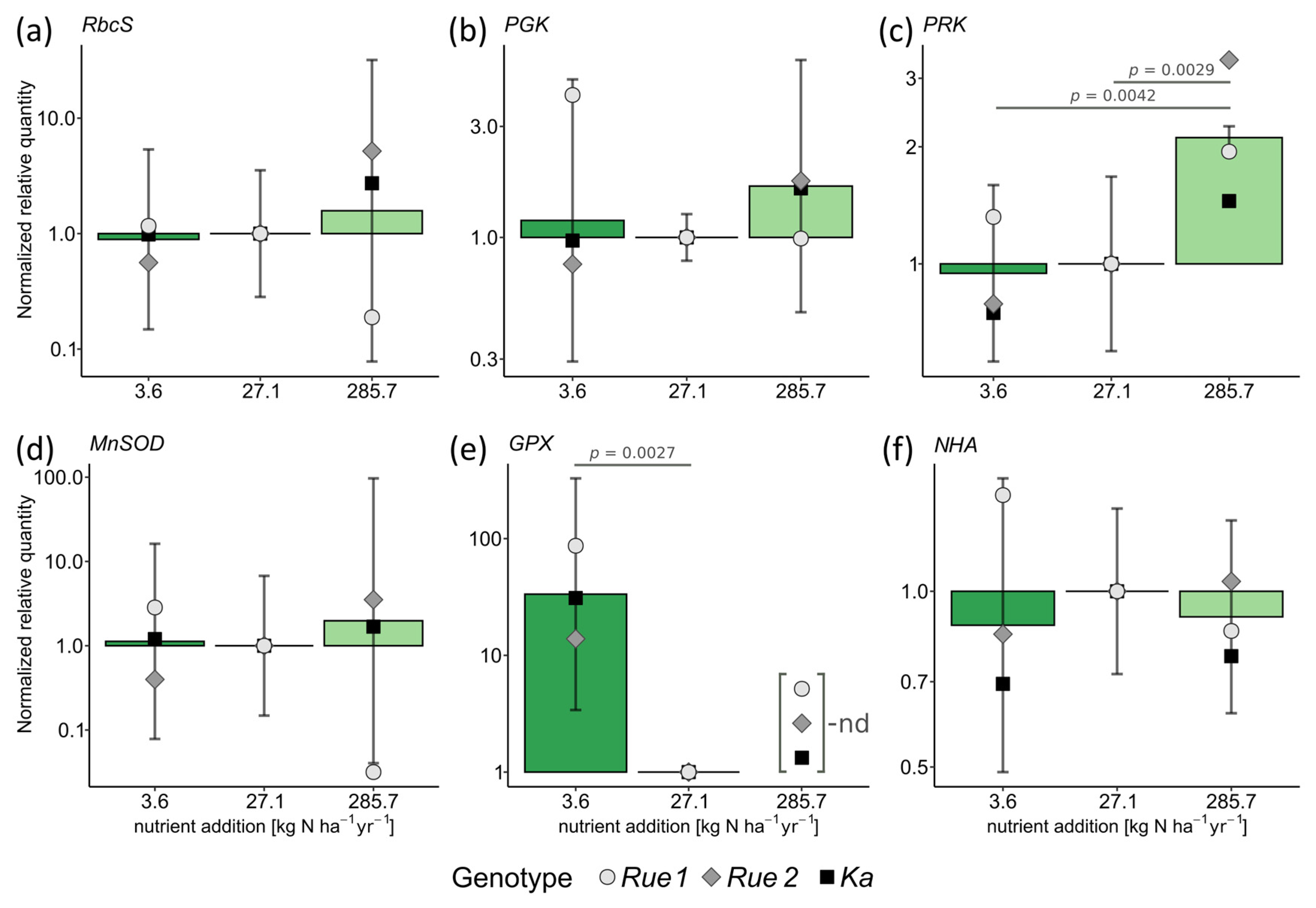
2. Results
3. Discussion
3.1. Genotypes Differ in Productivity and Morphology
3.2. Functional Traits and Performance along Gradients of Resource Availability Do Not Reveal Different Plant Strategies
3.3. Performance of P. australis Genotypes with Regard to Paludiculture Cannot Be Predicted Using the Plant Economics Spectrum
4. Materials and Methods
4.1. Plant Material
4.2. Study Design
4.3. Growth, Morphology, and Biomass
4.4. Photosynthetic Rate and Functional Leaf and Root Traits
4.5. Gene Expression Analysis
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Unit | Method | Value |
|---|---|---|---|
| pH | DIN EN ISO 10523 | 7.39 | |
| NH4+ | [mg L−1] | DIN EN ISO 11732 | 0.014 |
| NO2− | [mg L−1] | DIN EN ISO 13395 | 0.016 |
| NO32− | [mg L−1] | DIN EN ISO 10304-1 | 1.72 |
| PO43− | [mg L−1] | DIN EN ISO 15681-1 | 0.038 |
| K+ | [mg L−1] | DIN EN ISO 11885 | 2.84 |
| Level | Target Amount of N [kg ha−1 yr−1] | Total Amount of Fertilizer Per Pot [g yr−1] | ||
|---|---|---|---|---|
| (NH4)2HPO4 | NH4NO3 | K2CO3 | ||
| 1 | 3.6 | 0.131 | 0.371 | 0.545 |
| 2 | 5.0 | 0.184 | 0.519 | 0.763 |
| 3 | 7.1 | 0.257 | 0.727 | 1.069 |
| 4 | 9.9 | 0.360 | 1.018 | 1.496 |
| 5 | 13.8 | 0.504 | 1.425 | 2.094 |
| 6 | 19.4 | 0.706 | 1.995 | 2.932 |
| 7 | 27.1 | 0.988 | 2.793 | 4.105 |
| 8 | 37.9 | 1.383 | 3.910 | 5.747 |
| 9 | 53.1 | 1.936 | 5.474 | 8.046 |
| 10 | 74.4 | 2.710 | 7.664 | 11.264 |
| 11 | 104.1 | 3.795 | 10.729 | 15.770 |
| 12 | 145.8 | 5.312 | 15.021 | 22.078 |
| 13 | 204.1 | 7.437 | 21.029 | 30.909 |
| 14 | 285.7 | 10.412 | 29.441 | 43.273 |
| Level | Water Level [cm] |
|---|---|
| 1 | −45 |
| 2 | −38 |
| 3 | −31 |
| 4 | −24 |
| 5 | −18 |
| 6 | −12 |
| 7 | −6 |
| 8 | 0 |
| 9 | +5 |
| 10 | +10 |
| 11 | +16 |
| 12 | +22 |
| 13 | +28 |
| 14 | +34 |
| 15 | +40 |
| Gene | Protein | Primer | PCR Efficiency [%] | Product Size [bp] | Primer Sequence (5’–3’) |
|---|---|---|---|---|---|
| RbcS | Ribulose bisphosphate carboxylase small chain | rbcS-fw † rbcS-rev † | 107.5 | 150 | CAG GTG CAT GCA GGT GTG G CCG ACC TTG CTG AAC TCG AGG |
| PGK | Phosphoglycerate kinase | Phgly-fwd † Phgly-rev † | 120.5 | 149 | GTT TGC TGT AGG AAC TGA GGC TGT CAC CTC CCG TTG AAA TGT GGC TCA |
| PRK | Phosphoribulokinase | Phori-fwd † Phori-rev † | 92.0 | 183 | GAC TCT TAC TTC GGC CAT GAG GTA TCA GAA GAG ACC TGT TCC ATT GTT GCT |
| GPX | Glutathione peroxidase | GPX-fwd † GPX-rev † | NA ‡ | 163 | GAA TTC CCT ATT TTT GAC AAG GTT GA GCG CAT AGC GAT CCA CAA C |
| MnSOD | Manganese superoxide dismutase | SOD-fwd † SOD-rev † | 145.7 | 147 | CAA GGA TCT GGA TGG GTG TGG C GTA GTA CGC ATG CTC CCA GAC AT |
| NHA | Na+/H+ antiporter | NaH-fwd † NaH-rev † | 118.0 | 170 | GTG CGG CTT TTG AAT GGT GTG GGG AAC TGG ACA CTG GAC TGT AAA |
| EF1α | Elongation factor 1α | EF1a-fwd EF1a-rev | 107.2 | 109 | TGA GGC TGG TAT CTC CAA GGA AGT GGT GGC RTC CAT CTT GTT GC |
| PP2A4 | Serine/threonine protein phosphatise 4 catalytic subunit-like | PP2A4-fwd PP2A4-rev | 110.6 | 138 | GTG TGC GTA GCT TRG ATC GTG TCC GAT ATG TCC TGY CCA AAA GTG TAG CCA G |
| UBC | Ubiquitine conjugating protein | UBC-fwd † UBC-rev † | 113.1 | 117 | CTT CAA GCC RCC AAA GGT MTC GAT ATT GTC AAA GCA GGG CTC CA |
References
- Barthelmes, A.; Couwenberg, J.; Risager, M.; Tegetmeyer, C.; Joosten, H. Peatlands and Climate in a Ramsar Context: A Nordic-Baltic Perspective; Nordisk Ministerråd: Copenhagen, Denmark, 2015; ISBN 978-92-893-4197-4. [Google Scholar]
- Rocha, A.V.; Goulden, M.L. Why Is Marsh Productivity so High? New Insights from Eddy Covariance and Biomass Measurements in a Typha Marsh. Agr. Forest Meteorol. 2009, 149, 159–168. [Google Scholar] [CrossRef]
- Scharlemann, J.P.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global Soil Carbon: Understanding and Managing the Largest Terrestrial Carbon Pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Joosten, H. The Global Peatland CO2 Picture: Peatland Status and Emissions in All Countries of the World; Wetlands International: Ede, The Netherlands, 2009. [Google Scholar]
- Rydin, H.; Jeglum, J.K.; Hooijer, A. The Biology of Peatlands; The Biology of Habitats; Oxford University Press: Oxford, UK; New York, NY, USA, 2006; ISBN 978-0-19-852872-2. [Google Scholar]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt Rewetting of Drained Peatlands Reduces Climate Warming despite Methane Emissions. Nat. Commun. 2020, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Tanneberger, F.; Abel, S.; Couwenberg, J.; Dahms, T.; Gaudig, G.; Günther, A.; Kreyling, J.; Peters, J.; Pongratz, J.; Joosten, H. Towards Net Zero CO2 in 2050: An Emission Reduction Pathway for Organic Soils in Germany. Mires. Peat. 2021, 27, 5. [Google Scholar] [CrossRef]
- Biancalani, R.; Avagyan, A.; Food and Agriculture Organization of the United Nations (Eds.) Towards Climate-Responsible Peatlands Management; Mitigation of Climate Change in Agriculture Series; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2014; ISBN 978-92-5-108546-2. [Google Scholar]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making; International Peat Society; International Mire Conservation Group: Jyväskylä, Finland; Greifswald, Germany, 2002; ISBN 978-951-97744-8-0. [Google Scholar]
- Packer, J.G.; Meyerson, L.A.; Skálová, H.; Pyšek, P.; Kueffer, C. Biological Flora of the British Isles: Phragmites Australis. J. Ecol. 2017, 105, 1123–1162. [Google Scholar] [CrossRef]
- Brix, H. Genetic Diversity, Ecophysiology and Growth Dynamics of Reed (Phragmites Australis). Aquat. Bot. 1999, 64, 179–184. [Google Scholar] [CrossRef]
- Den Hartog, C.; Květ, J.; Sukopp, H. Reed. A Common Species in Decline. Aquat. Bot. 1989, 35, 1–4. [Google Scholar] [CrossRef]
- Hellings, S.E.; Gallagher, J.L. The Effects of Salinity and Flooding on Phragmites Australis. J. Appl. Ecol. 1992, 29, 41–49. [Google Scholar] [CrossRef]
- Achenbach, L.; Eller, F.; Nguyen, L.X.; Brix, H. Differences in Salinity Tolerance of Genetically Distinct Phragmites Australis Clones. AoB Plants 2013, 5, plt019. [Google Scholar] [CrossRef]
- Richert, M.; Dietrich, O.; Koppisch, D.; Roth, S. The Influence of Rewetting on Vegetation Developement and Decomposition in a Degraded Fen. Restor. Ecol. 2000, 8, 186–195. [Google Scholar] [CrossRef]
- Geurts, J.; Oehmke, C.; Lambertini, C.; Eller, F.; Sorrell, B.K.; Mandiola, S.R.; Grootjans, A.P.; Brix, H.; Wichtmann, W.; Lamers, L.P.M.; et al. Nutrient Removal Potential and Biomass Production by Phragmites Australis and Typha Latifolia on European Rewetted Peat and Mineral Soils. Sci. Total Environ. 2020, 747, 141102. [Google Scholar] [CrossRef]
- Ren, L.; Eller, F.; Lambertini, C.; Guo, W.-Y.; Brix, H.; Sorrell, B.K. Assessing Nutrient Responses and Biomass Quality for Selection of Appropriate Paludiculture Crops. Sci. Total Environ. 2019, 664, 1150–1161. [Google Scholar] [CrossRef]
- Wichtmann, W.; Joosten, H. Paludiculture: Peat Formation and Renewable Resources from Rewetted Peatlands. IMCG Newsl. 2007, 3, 24–28. [Google Scholar]
- Becker, L.; Wichmann, S.; Beckmann, V. Common Reed for Thatching in Northern Germany: Estimating the Market Potential of Reed of Regional Origin. Resources 2020, 9, 146. [Google Scholar] [CrossRef]
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A Review of Unconventional Sustainable Building Insulation Materials. Sustain. Mater. Techno. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Dragoni, F.; Giannini, V.; Ragaglini, G.; Bonari, E.; Silvestri, N. Effect of Harvest Time and Frequency on Biomass Quality and Biomethane Potential of Common Reed (Phragmites Australis) Under Paludiculture Conditions. Bioenerg. Res. 2017, 10, 1066–1078. [Google Scholar] [CrossRef]
- Brix, H.; Ye, S.; Laws, E.A.; Sun, D.; Li, G.; Ding, X.; Yuan, H.; Zhao, G.; Wang, J.; Pei, S. Large-Scale Management of Common Reed, Phragmites Australis, for Paper Production: A Case Study from the Liaohe Delta, China. Ecol. Eng. 2014, 73, 760–769. [Google Scholar] [CrossRef]
- Freeman, C.; Ostle, N.; Kang, H. An Enzymatic “latch” on a Global Carbon Store. Nature 2001, 409, 149. [Google Scholar] [CrossRef]
- Schwieger, S.; Kreyling, J.; Couwenberg, J.; Smiljanić, M.; Weigel, R.; Wilmking, M.; Blume-Werry, G. Wetter Is Better: Rewetting of Minerotrophic Peatlands Increases Plant Production and Moves Them Towards Carbon Sinks in a Dry Year. Ecosystems 2020, 24, 1093–1109. [Google Scholar] [CrossRef]
- Dierßen, K.; Dierßen, B. Moore; Ökosysteme Mitteleuropas aus Geobotanischer Sicht; Ulmer: Stuttgart, Germany, 2001; ISBN 978-3-8001-3245-4. [Google Scholar]
- Timmermann, T.; Katalin, M.; Gábor, T.; Vegelin, K. Restoration of Peat-Forming Vegetation by Rewetting Species-Poor Fen Grasslands. Appl. Veg. Sci. 2006, 9, 241–250. [Google Scholar] [CrossRef]
- Achenbach, L.; Lambertini, C.; Brix, H. Phenotypic Traits of Phragmites Australis Clones Are Not Related to Ploidy Level and Distribution Range. AoB Plants 2012, 2012, pls017. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.L.; Lambertini, C.; Jampeetong, A.; Brix, H. Clone-Specific Differences in Phragmites Australis: Effects of Ploidy Level and Geographic Origin. Aquat. Bot. 2007, 86, 269–279. [Google Scholar] [CrossRef]
- Koppitz, H.; Kühl, H.; Kohl, J.-G. Differences in Morphology and C/N-Balance between Clones of Phragmites Australis within a Plantation at a Degraded Fen. Folia Geobot. 2000, 35, 389–402. [Google Scholar] [CrossRef]
- Rolletschek, H.; Rolletschek, A.; Kühl, H.; Kohl, J.-G. Clone Specific Differences in a Phragmites Australis Stand II. Seasonal Development of Morphological and Physiological Characteristics at the Natural Site and after Transplantation. Aquat. Bot. 1999, 64, 247–260. [Google Scholar] [CrossRef]
- Kühl, H.; Koppitz, H.; Rolletschek, H.; Kohl, J.-G. Clone Specific Differences in a Phragmites Australis Stand I. Morphology, Genetics and Site Description. Aquat. Bot. 1999, 64, 235–246. [Google Scholar] [CrossRef]
- Kuprina, K.; Seeber, E.; Schnittler, M.; Landeau, R.; Lambertini, C.; Bog, M. Genetic Diversity of Common Reed in the Southern Baltic Sea Region—Is There an Influence of Disturbance? Aquat. Bot. 2022, 177, 103471. [Google Scholar] [CrossRef]
- Eller, F.; Skálová, H.; Caplan, J.S.; Bhattarai, G.P.; Burger, M.K.; Cronin, J.T.; Guo, W.-Y.; Guo, X.; Hazelton, E.L.G.; Kettenring, K.M.; et al. Cosmopolitan Species As Models for Ecophysiological Responses to Global Change: The Common Reed Phragmites Australis. Front. Plant Sci. 2017, 8, 1833. [Google Scholar] [CrossRef]
- Hanganu, J.; Mihail, G.; Coops, H. Responses of Ecotypes of Phragmites Australis to Increased Seawater Influence: A Field Study in the Danube Delta, Romania. Aquat. Bot. 1999, 64, 351–358. [Google Scholar] [CrossRef]
- Eller, F.; Brix, H. Different Genotypes of Phragmites Australis Show Distinct Phenotypic Plasticity in Response to Nutrient Availability and Temperature. Aquat. Bot. 2012, 103, 89–97. [Google Scholar] [CrossRef]
- Takahashi, R.; Nishio, T.; Ichizen, N.; Takano, T. High-Affinity K+ Transporter PhaHAK5 Is Expressed Only in Salt-Sensitive Reed Plants and Shows Na+ Permeability under NaCl Stress. Plant Cell Rep. 2007, 26, 1673–1679. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Lu, F.; Liu, Z.; Lan, S.; Han, G. Differentially Expressed Genes Related to Oxidoreductase Activity and Glutathione Metabolism Underlying the Adaptation of Phragmites Australis from the Salt Marsh in the Yellow River Delta, China. PeerJ 2020, 8, e10024. [Google Scholar] [CrossRef]
- Kühl, H.; Woitke, P.; Kohl, J.-G. Strategies of nitrogen cycling of Phragmites australis at two sites differing in nutrient availability. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1997, 82, 57–66. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Aerts, R. Evidence of the “plant Economics Spectrum” in a Subarctic Flora. J. Ecol. 2010, 98, 362–373. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast-Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Ryser, P.; Lambers, H. Root and Leaf Attributes Accounting for the Performance of Fast- and Slow-Growing Grasses at Different Nutrient Supply. Plant Soil 1995, 170, 251–265. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root Tissue Structure Is Linked to Ecological Strategies of Grasses. New Phytol. 2000, 148, 459–471. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Niinemets, Ü. Is There a Species Spectrum within the World-wide Leaf Economics Spectrum? Major Variations in Leaf Functional Traits in the Mediterranean Sclerophyll Quercus Ilex. New Phytol. 2015, 205, 79–96. [Google Scholar] [CrossRef]
- Hu, Y.-K.; Pan, X.; Liu, G.-F.; Li, W.-B.; Dai, W.-H.; Tang, S.-L.; Zhang, Y.-L.; Xiao, T.; Chen, L.-Y.; Xiong, W.; et al. Novel Evidence for Within-Species Leaf Economics Spectrum at Multiple Spatial Scales. Front. Plant Sci. 2015, 6, 901. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Enquist, B.J. Assessing the Causes and Scales of the Leaf Economics Spectrum Using Venation Networks in Populus Tremuloides. J. Ecol. 2013, 101, 981–989. [Google Scholar] [CrossRef]
- Vitt, D.H.; Wieder, R.K.; Scott, K.D.; Faller, S. Decomposition and Peat Accumulation in Rich Fens of Boreal Alberta, Canada. Ecosystems 2009, 12, 360–373. [Google Scholar] [CrossRef]
- Fiala, K. Underground Organs of Phragmites Communis, Their Growth, Biomass and Net Production. Folia Geobot. Phytotx. 1976, 11, 225–259. [Google Scholar] [CrossRef]
- Haslam, S.M. The Reed. Phragmites Australis (Cav.) Trin. Ex Steud; Updated Edition; British Reed Growers Association, Brown & Co.: Norwich, UK, 2009. [Google Scholar]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The Utilisation of Reed (Phragmites Australis): A Review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Wöhler-Geske, A.; Moschner, C.R.; Thiessen, E.; Hartung, E. Non-Destructive Determination of Morphological Properties of Thatching Reed by Image Analysis. In Proceedings of the International Conference of Agricultural Engineering, Zurich, Switzerland, 10 July 2014. [Google Scholar]
- Wichmann, S. Commercial Viability of Paludiculture: A Comparison of Harvesting Reeds for Biogas Production, Direct Combustion, and Thatching. Ecol. Eng. 2017, 103, 497–505. [Google Scholar] [CrossRef]
- Giannini, V.; Oehmke, C.; Silvestri, N.; Wichtmann, W.; Dragoni, F.; Bonari, E. Combustibility of Biomass from Perennial Crops Cultivated on a Rewetted Mediterranean Peatland. Ecol. Eng. 2016, 97, 157–169. [Google Scholar] [CrossRef]
- Eller, F.; Lambertini, C.; Nielsen, M.W.; Radutoiu, S.; Brix, H. Expression of Major Photosynthetic and Salt-resistance Genes in Invasive Reed Lineages Grown under Elevated CO2 and Temperature. Ecol. Evol. 2014, 4, 4161–4172. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Köhn, N. (University of Greifswald, Greifswald, Germany). Personal Communication, 2022.
- Lessmann, J.M.; Brix, H.; Bauer, V.; Clevering, O.A.; Comín, F.A. Effect of Climatic Gradients on the Photosynthetic Responses of Four Phragmites Australis Populations. Aquat. Bot. 2001, 69, 109–126. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root Traits Are Multidimensional: Specific Root Length Is Independent from Root Tissue Density and the Plant Economic Spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Wright, J.P.; Sutton-Grier, A. Does the Leaf Economic Spectrum Hold within Local Species Pools across Varying Environmental Conditions? Funct. Ecol. 2012, 26, 1390–1398. [Google Scholar] [CrossRef]
- Pan, Y.; Cieraad, E.; Bodegom, P.M. Are Ecophysiological Adaptive Traits Decoupled from Leaf Economics Traits in Wetlands? Funct. Ecol. 2019, 33, 1202–1210. [Google Scholar] [CrossRef]
- Oehmke, C.; Abel, S. 3.1. Ausgewählte Paludikulturen. In Paludikultur—Bewirtschaftung Nasser Moore; E. Schweitzerbart’sche Verlagsbuchhandlung (Nägele u. Obermiller): Stuttgart, Germany, 2016; pp. 22–38. ISBN 978-3-510-65282-2. [Google Scholar]
- IPNI, International Plant Names Index. The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium. Available online: http://www.ipni.org (accessed on 2 February 2023).
- Olde Venterink, H.; Pieterse, N.M.; Belgers, J.D.M.; Wassen, M.J.; De Ruiter, P.C. N, P and K Budgets along Nutrient Availability and Productivity Gradients in Wetlands. Ecol. Appl. 2002, 12, 1010–1026. [Google Scholar] [CrossRef]
- Tylová, E.; Steinbachová, L.; Soukup, A.; Gloser, V.; Votrubová, O. Pore Water N:P and NH4+:NO3− Alter the Response of Phragmites Australis and Glyceria Maxima to Extreme Nutrient Regimes. Hydrobiologia 2013, 700, 141–155. [Google Scholar] [CrossRef]
- Paludiculture Pilots and Experiments with Focus on Cattail and Reed in the Netherlands. Technical Report. In CINDERELLA Project. FACCE-JPI ERA-NET Plus on Climate Smart Agriculture; Geurts, J., Fritz, C., Eds.; Radboud University Nijmegen; Department of Aquatic Ecology & Environmental Biology; Institute for Water & Wetland Research (IWWR): Nijmegen, The Netherlands, 2018. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Kimura, K.; Kikuchi, S.; Yamasaki, S. Accurate Root Length Measurement by Image Analysis. Plant Soil 1999, 216, 117–127. [Google Scholar] [CrossRef]
- Pierret, A.; Gonkhamdee, S.; Jourdan, C.; Maeght, J.-L. IJ_Rhizo: An Open-Source Software to Measure Scanned Images of Root Samples. Plant Soil 2013, 373, 531–539. [Google Scholar] [CrossRef]
- Jensen, C.R.; Luxmoore, R.J.; Van Gundy, S.D.; Stolzy, L.H. Root Air Space Measurements by a Pycnometer Method. Agron. J. 1969, 61, 474–475. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 7 June 2019).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: https://www.rstudio.com/ (accessed on 7 June 2019).
- Kreyling, J.; Schweiger, A.H.; Bahn, M.; Ineson, P.; Migliavacca, M.; Morel-Journel, T.; Christiansen, J.R.; Schtickzelle, N.; Larsen, K.S. To Replicate, or Not to Replicate—That Is the Question: How to Tackle Nonlinear Responses in Ecological Experiments. Ecol. Lett. 2018, 21, 1629–1638. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Analytical Methods for Social Research; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; ISBN 978-0-521-86706-1. [Google Scholar]
- Austin, P.C.; Hux, J.E. A Brief Note on Overlapping Confidence Intervals. J. Vasc. Surg. 2002, 36, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Payton, M.E.; Greenstone, M.H.; Schenker, N. Overlapping Confidence Intervals or Standard Error Intervals: What Do They Mean in Terms of Statistical Significance? J. Insect Sci. 2003, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. MiRDeepFinder: A MiRNA Analysis Tool for Deep Sequencing of Plant Small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of Real-Time PCR Gene Expression Data from Independent Biological Replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef]
- Stadtwerke Greifswald GmbH, Trinkwasseranalyse. Available online: https://www.sw-greifswald.de/Energie/Trinkwasser/Trinkwasseranalyse (accessed on 15 March 2019).
| Genotype | Area of Origin | Approximate Collection Coordinates | Haplotype (TrnT-TrnL) |
|---|---|---|---|
| Rue1 | Lieschow peninsula, Rügen | 54.43480° N, 13.18848° E | T4b |
| Rue2 | Lieschow peninsula, Rügen | 54.43574° N, 13.19760° E | T4b |
| PV1 | Lower Peene valley | 53.85401° N, 13.78094° E | T4c |
| PV2 | Lower Peene valley | 53.85589° N, 13.79369° E | T4b |
| Ka | Karrendorfer Wiesen near Greifswald | 54.15396° N, 13.38301° E | T7c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haldan, K.; Kuprina, K.; Haase, M.I.; Kieckhäfer, F.; Schade, L.; Schmoldt, J.; Schock, L.S.; Stein, M.; Wille, A.; Schnittler, M.; et al. Choose Wisely: Great Variation among Genotypes of Promising Paludiculture Crop Phragmites australis. Plants 2023, 12, 1045. https://doi.org/10.3390/plants12051045
Haldan K, Kuprina K, Haase MI, Kieckhäfer F, Schade L, Schmoldt J, Schock LS, Stein M, Wille A, Schnittler M, et al. Choose Wisely: Great Variation among Genotypes of Promising Paludiculture Crop Phragmites australis. Plants. 2023; 12(5):1045. https://doi.org/10.3390/plants12051045
Chicago/Turabian StyleHaldan, Kerstin, Kristina Kuprina, Meike Ingeborg Haase, Fabian Kieckhäfer, Lisa Schade, Joraine Schmoldt, Lina Stella Schock, Marthe Stein, Alexander Wille, Martin Schnittler, and et al. 2023. "Choose Wisely: Great Variation among Genotypes of Promising Paludiculture Crop Phragmites australis" Plants 12, no. 5: 1045. https://doi.org/10.3390/plants12051045
APA StyleHaldan, K., Kuprina, K., Haase, M. I., Kieckhäfer, F., Schade, L., Schmoldt, J., Schock, L. S., Stein, M., Wille, A., Schnittler, M., Bog, M., & Kreyling, J. (2023). Choose Wisely: Great Variation among Genotypes of Promising Paludiculture Crop Phragmites australis. Plants, 12(5), 1045. https://doi.org/10.3390/plants12051045







