Morphological and Molecular Diversity among Pin Nematodes of the Genus Paratylenchus (Nematoda: Paratylenchidae) from Florida and Other Localities and Molecular Phylogeny of the Genus
Abstract
1. Introduction
2. Results
2.1. Species Identification and Delimitation
2.2. Taxonomic Studies
2.2.1. Paratylenchus borealis sp. n.
| Reference | Etongwe et al. [29] | Singh et al. [14] | This Study | Total Range | |
|---|---|---|---|---|---|
| Population | Zwijnaarde, Belgium | Merendree, Belgium | Alaska, USA (CD3781) | ||
| Character a | ♀ (Fixed) | ♀ (Fixed) | ♀ (Fixed) | ♀ | |
| Holotype | Paratypes | ||||
| n | 3 | 17 | 1 | 5 | 25 |
| L | 330–350 | 300 ± 21.1 (264–339) | 320 | 347.3 ± 13.6 (325.7–362.3) | 264–362 |
| Stylet length (St) | 27.9–29.7 | 27.6 ± 1.2 (25.3–29.6) | 30.5 | 31.0 ± 0.5 (30.6–31.6) | 25–32 |
| Conus length | 19.9–21.8 | 18.5 ± 0.9 (17.1–20.3) | 20.5 | 20.6 ± 0.7 (20.0–21.8) | 17–22 |
| Stylet shaft + knob height | 5.6–6.5 | - | 10.0 | 10.4 ± 0.7 (9.8–11.6) | 5.5–11.5 |
| Knob width | 3.9–4.9 | 3.6 ± 0.3 (3.3–4.1) | 4.5 | 4.7 ± 0.2 (4.5–4.9) | 3.5–5.0 |
| Knob height | 1.5–1.8 | - | 2.0 | 2.2 ± 0.3 (2.0–2.6) | 1.5–2.5 |
| DGO | 3.6–4.6 | - | 4.5 | 4.7 ± 0.2 (4.5–4.9) | 3.5–5.0 |
| Median bulb valve length | - | - | 6.5 | 7.5 ± 0.5 (7.0–8.0) | 7–8 |
| Median bulb width | - | - | 8.0 | 9.6 ± 0.5 (8.7–10.0) | 8–10 |
| Isthmus length | - | - | 15.5 | 14.2 ± 1.3 (13.2–16.5) | 13–17 |
| Pharynx length | 86.1–91.2 | 74.8 ± 4.9 (67.7–83.1) | 80 | 85.7 ± 4.0 (80.5–89.1) | 68–91 |
| Anterior end to excretory pore (Ep) | 71.2–78.0 | 63.0 ± 5.4 (51.5–70.6) | 67 | 79.1 ± 5.8 (71.0–85.1) | 51–85 |
| Max body width | 16.7–17.0 | 14.8 ± 1.1 (13.3–16.6) | 14.0 | 16.4 ± 1.5 (15.0–18.8) | 13–19 |
| Body width at vulva | - | - | 12.0 | 13.8 ± 1.0 (13.0–15.5) | 13–16 |
| Body width at anus | - | 9.0 ± 0.6 (8.0–9.9) | 8.5 | 9.5 ± 0.5 (8.9–10.2) | 8–10 |
| Anterior end to median bulb base | - | - | 51 | 58.0 ± 1.5 (56.4–60.3) | 56–60 |
| Lateral field width | - | - | 3.5 | 4.4 ± 0.4 (4.0–4.9) | 3.5–5.0 |
| Anterior end-vulva distance | - | 217 ± 17.2 (217–278) | 260 | 282.3 ± 15.5 (257.4–300.0) | 217–300 |
| Vulva-tail terminus distance | - | - | 59 | 65.0 ± 4.2 (60.0–70.2) | 60–70 |
| Genital tract length | - | - | 89 | 151.9 ± 53.3 (104.9–223.7) | 89–224 |
| Vulva-anus distance | 30.0–38.5 | - | 35 | 40.4 ± 3.8 (35.3–44.5) | 30–45 |
| Tail length | 22.3–25.3 | 23.1 ± 2.4 (20.0–26.5) | 22.5 | 24.6 ± 1.0 (23.0–25.7) | 20–27 |
| Stylet base (Stb) to median bulb valve base (v) | - | - | 16.0 | 17.9 ± 0.7 (16.8–18.9) | 16–19 |
| PERCENTAGES | |||||
| V | 81–83 | 82 ± 0.7 (80.9–83.5) | 81 | 81.2 ± 1.6 (79.0–82.8) | 79–84 |
| G or T | - | - | 28 | 43.4 ± 13.9 (29.6–61.7) | 28–62 |
| Stb-v/St | - | - | 52 | 57.6 ± (54.7–61.7) | 52–62 |
| St/L | 8.0–9.0 | - | 9.5 | 8.9 ± 0.4 (8.4–9.4) | 8–9 |
| Ep/L | 21.0–22.3 | 21.0 ± 1.6 (18.6–24.2) | 20.9 | 22.7 ± 1.0 (21.7–24.0) | 19–24 |
| RATIOS | |||||
| a | 19.4–20.8 | 20.3 ± 1.3 (18.4–23.0) | 23.1 | 21.2 ± 1.2 (19.2–22.5) | 18–23 |
| b | 3.7–4.0 | 4.0 ± 0.3 (3.7–4.6) | 4.0 | 4.0 ± 0.2 (3.8–4.3) | 3.5–4.5 |
| c | 13.8–15.4 | 12.9 ± 0.8 (11.8–14.0) | 14.3 | 14.1 ± 0.9 (13.2–15.7) | 12–16 |
| c′ | 2.1–2.5 | 2.6 ± 0.2 (2.3–2.9) | 2.7 | 2.6 ± 0.2 (2.2–2.8) | 2–3 |
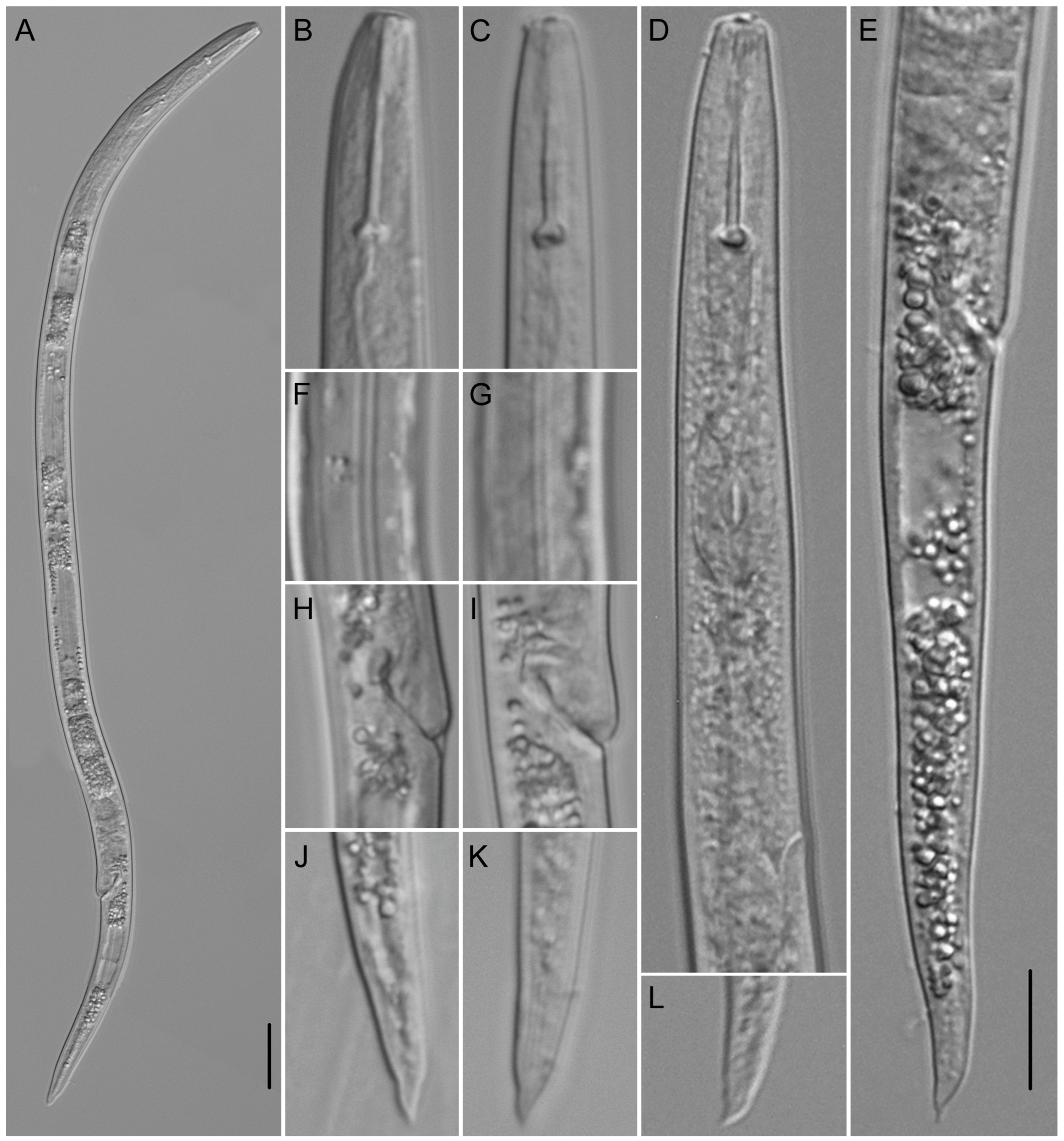
Description
Type Habitat and Locality
Other Habitats and Localities
Etymology
Type Material
Molecular Characterization
Diagnosis (Based on All Known Populations) and Relationships
Remarks
2.2.2. Paratylenchus hawaiiensis sp. n.
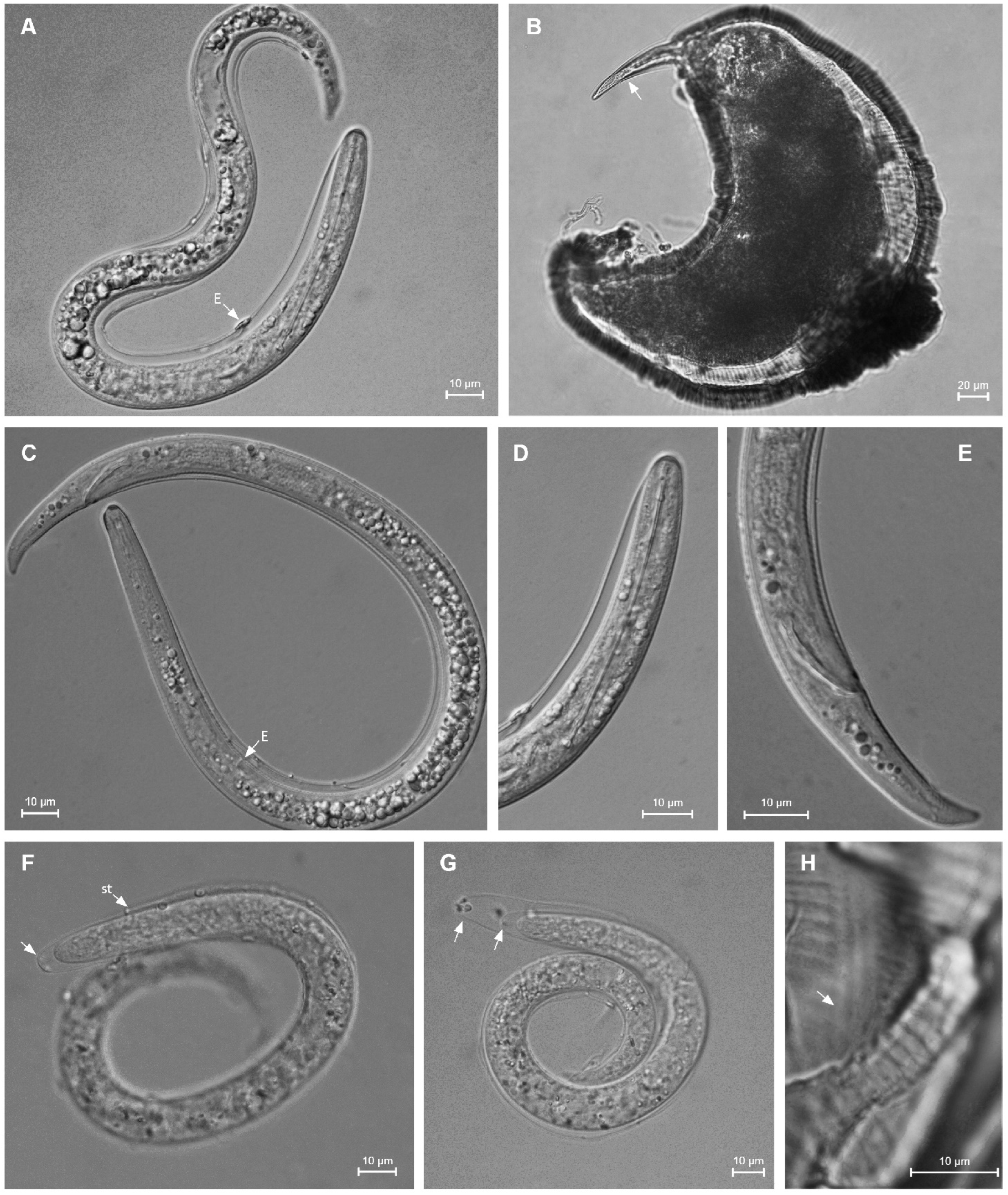
Description
Type Habitat and Locality
Other Hosts and Localities
Etymology
Type Material
Molecular Characterization
Diagnosis and Relationships
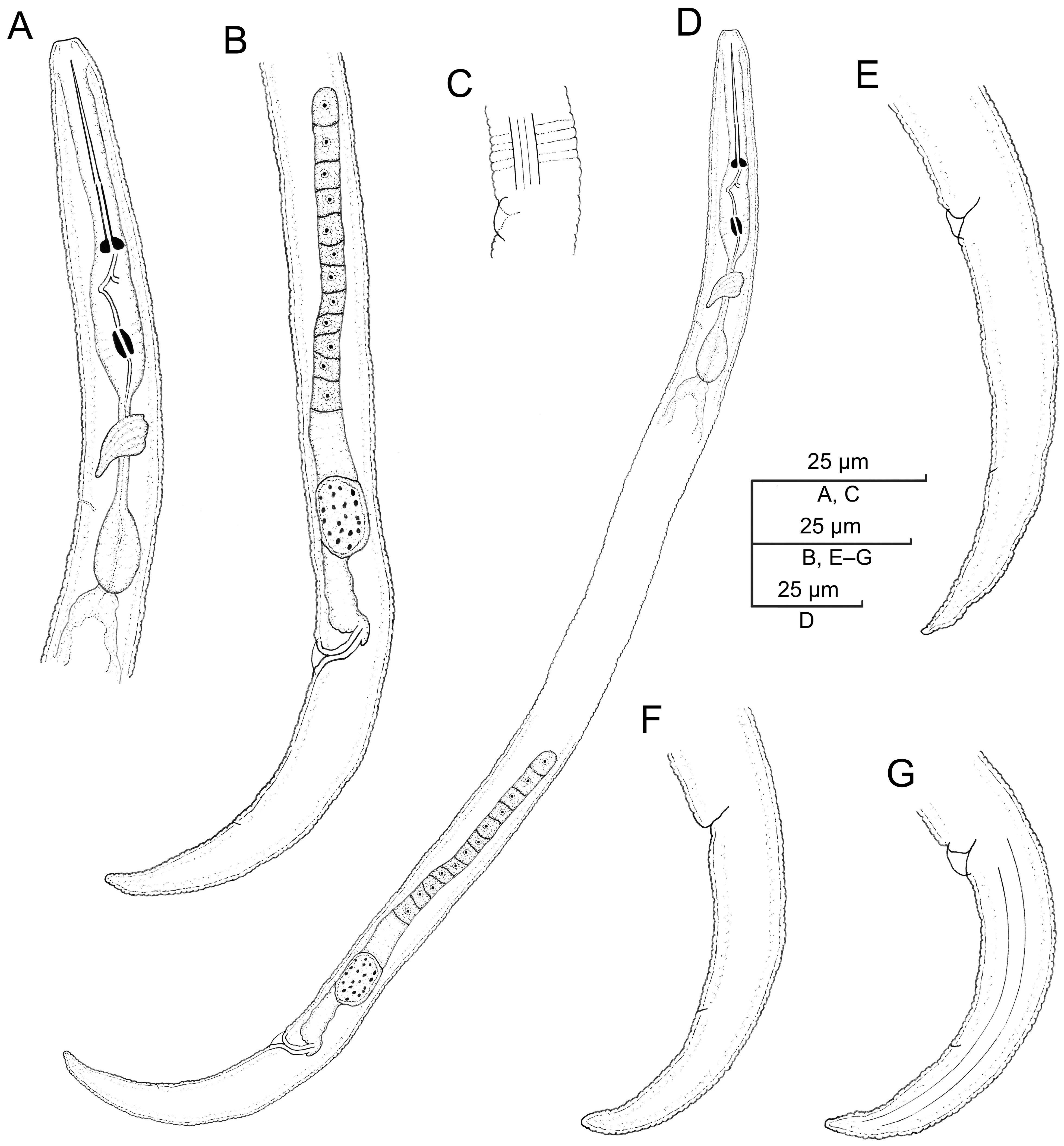
2.2.3. Paratylenchus roboris sp. n.
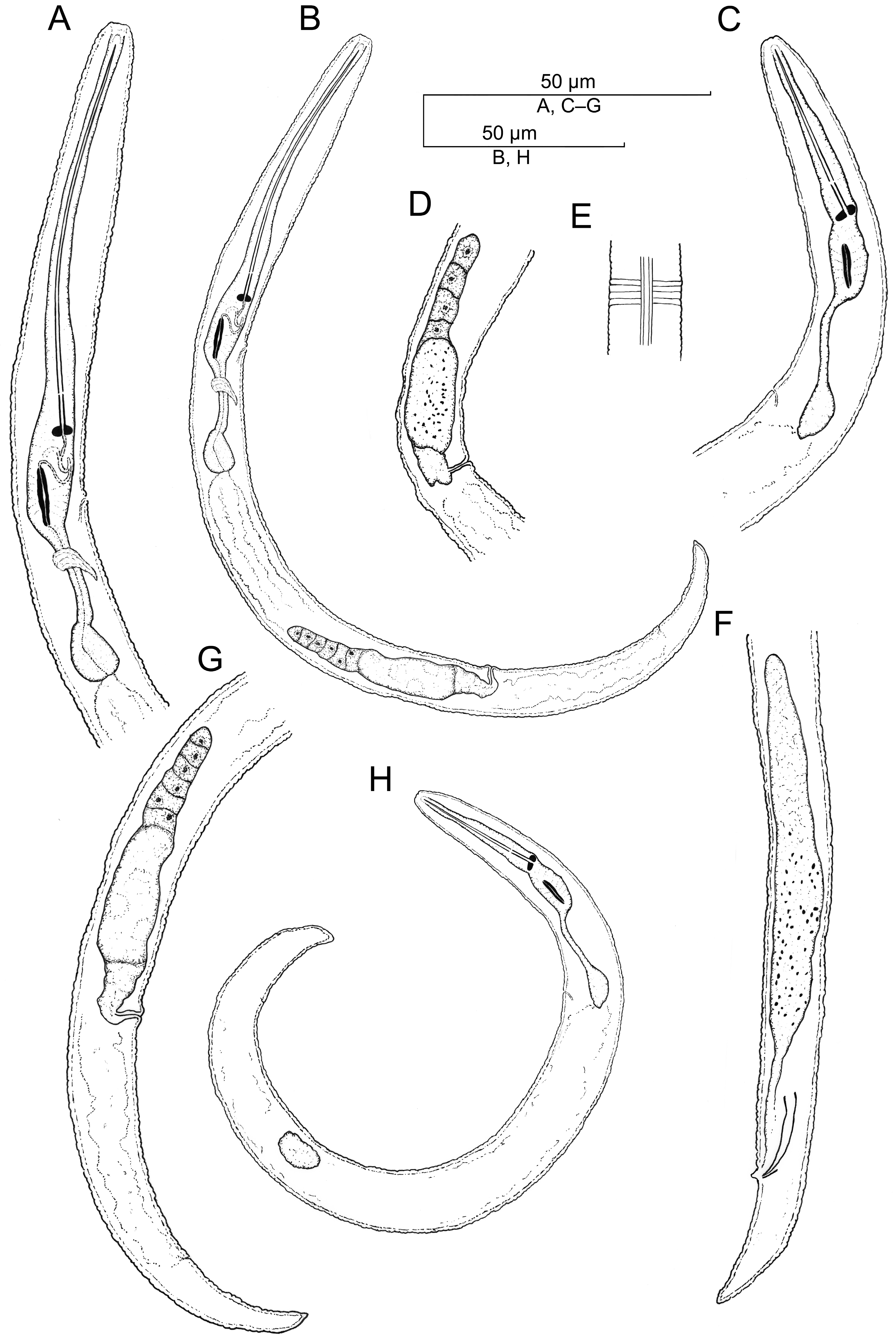
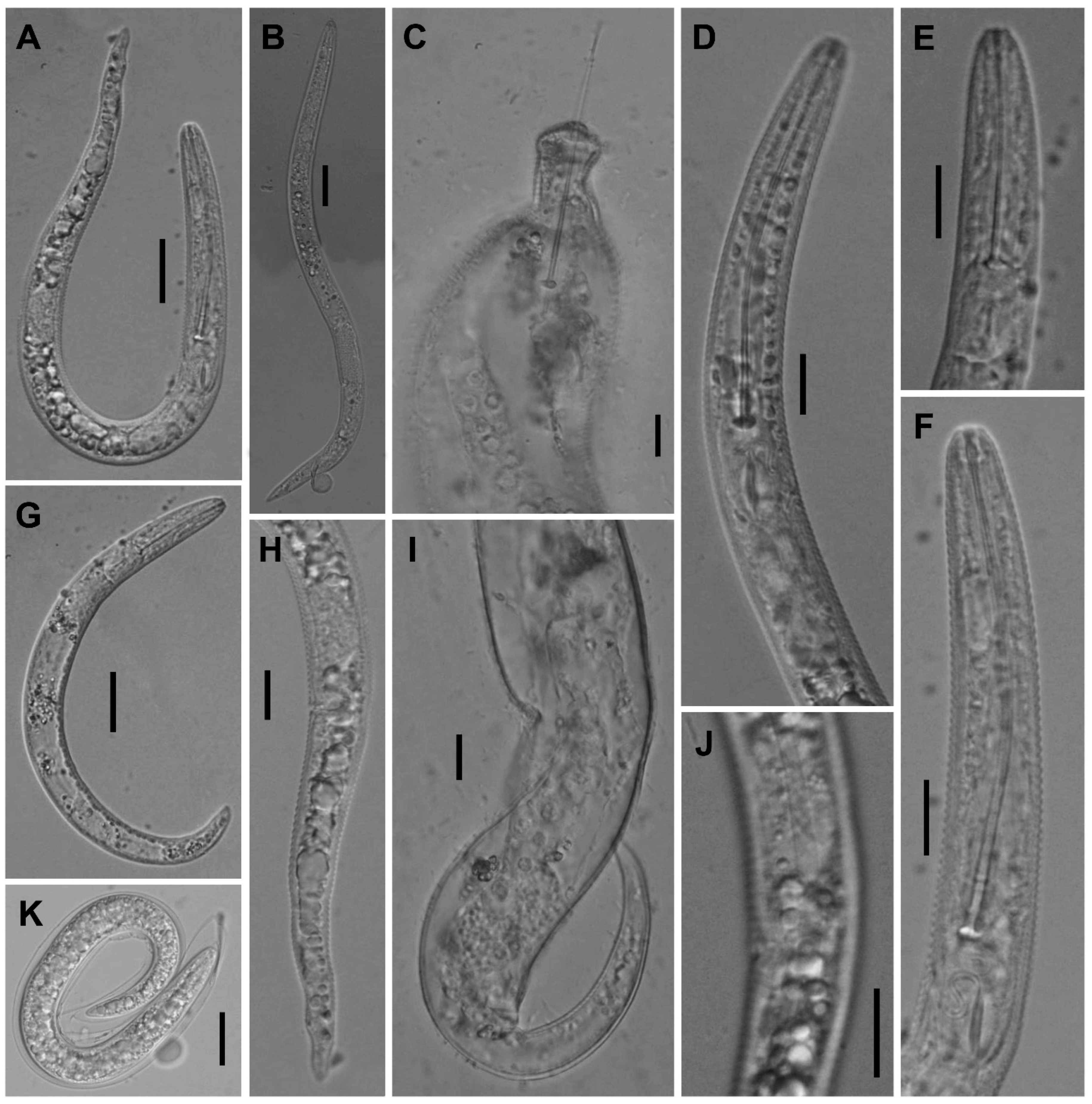
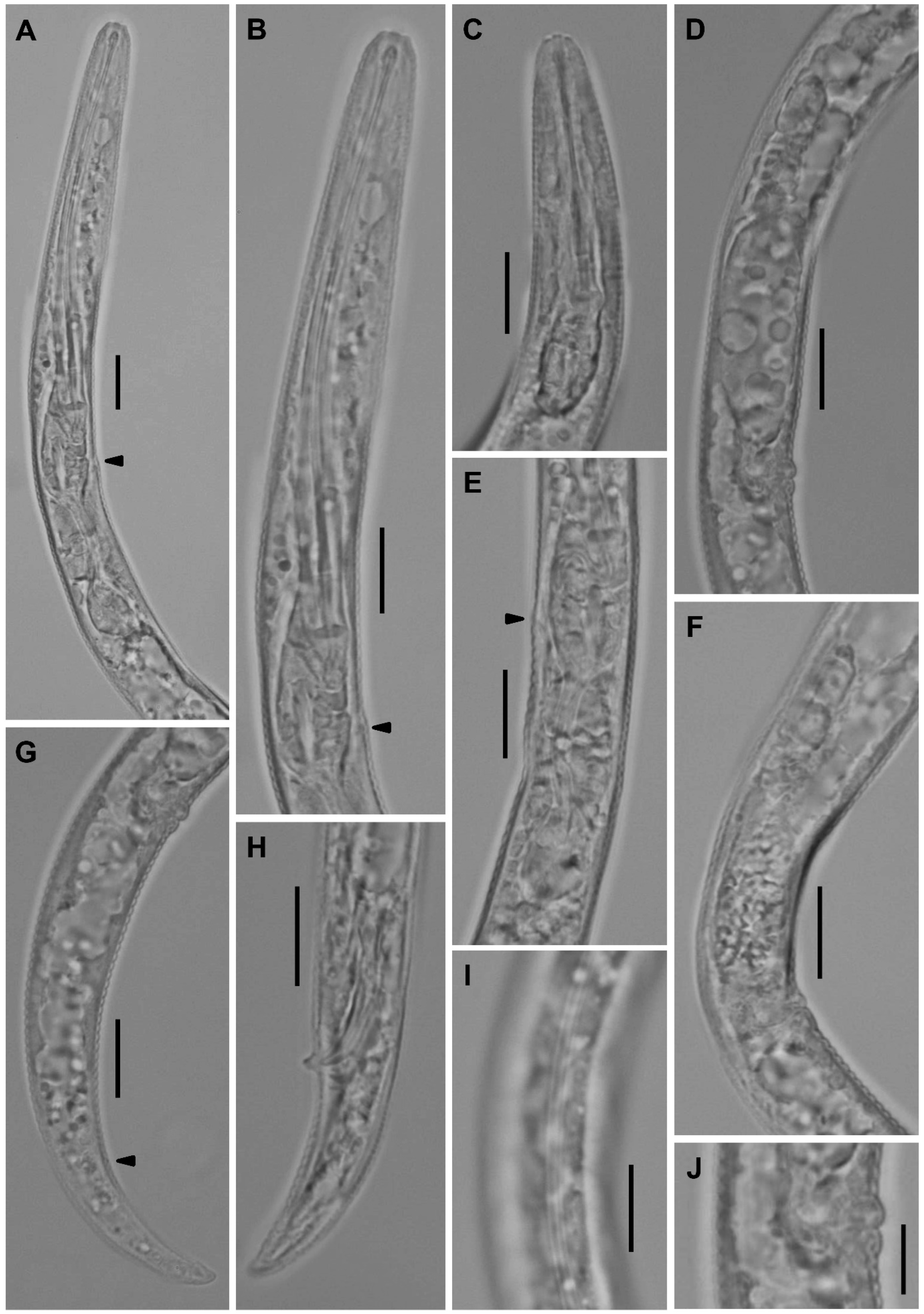

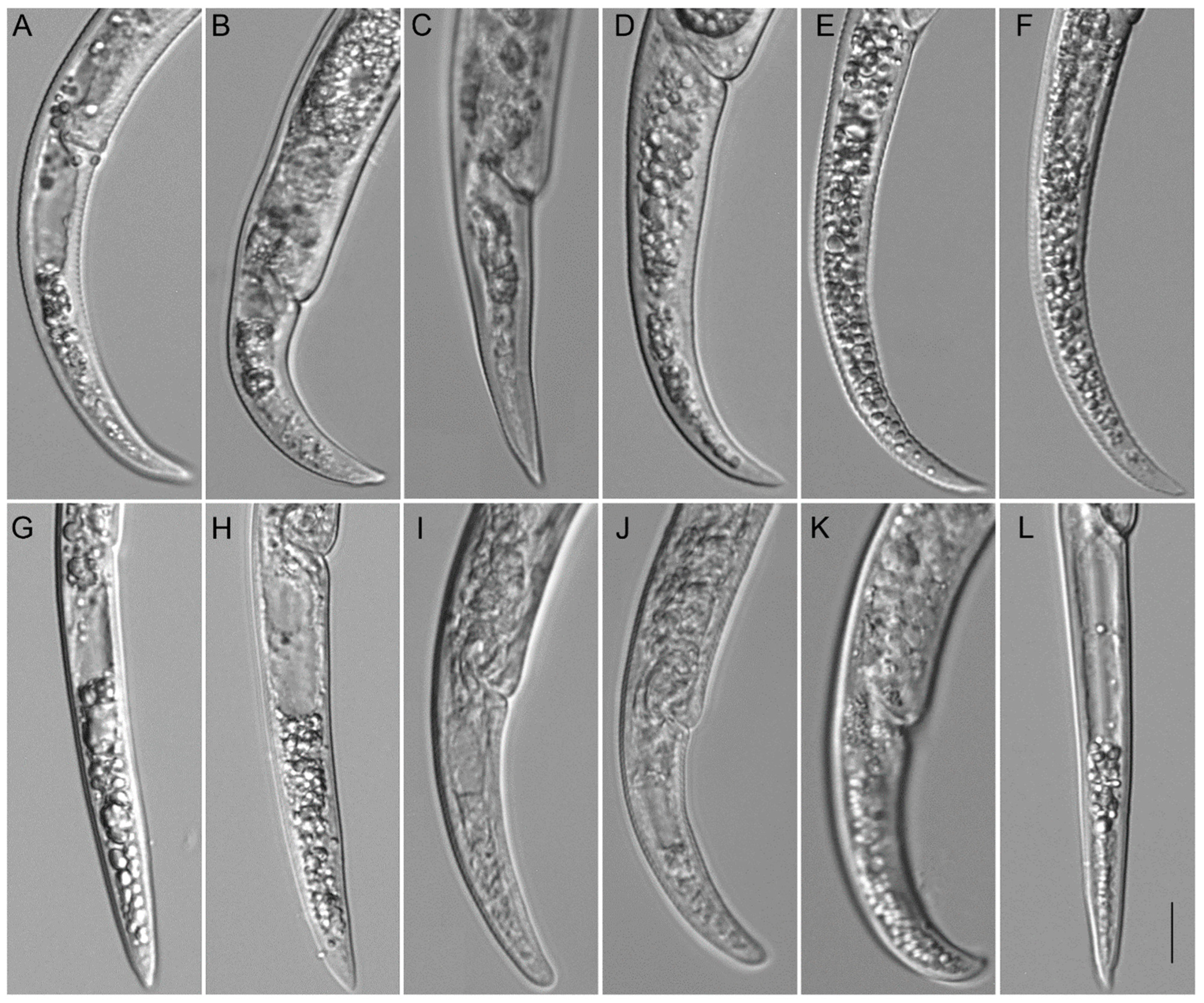
Description
| Population | High Springs, Alachua County (CD3450—N21-00211) | ||||
|---|---|---|---|---|---|
| Character | Vermiform ♀ (Live) | Vermiform ♀ (Fixed) | Total Range Vermiform ♀ | Obese ♀ (Dead) | |
| Holotype | Paratypes | ||||
| n | 7 | 1 | 9 | 17 | 5 |
| L | 262.7 ± 6.7 (257.0–275.2) | 283.8 | 275.4 ± 7.6 (261.0–283.0) | 257.0–283.8 | 266.8 ± 17.7 (243.0–288.0) |
| Stylet length (St) | 65.3 ± 1.1 (63.3–66.8) | 71 | 66.9 ± 2.3 (64.0–70.0) | 63.3–71.0 | 64.0 (n = 1) |
| Conus length | 57.5 ± 0.7 (56.4–58.4) | 63 | 59.4 ± 1.9 (57.0–62.0) | 56.4–63.0 | - |
| Stylet shaft + knob height | 7.7 ± 1.0 (6.1–9.2) | 8 | 7.4 ± 0.5 (7.0–8.0) | 6.1–9.2 | - |
| Knob width | 3.9 ± 0.9 (3.5–4.0) | 3.5 | 3.0 ± 0.1 (3.0–3.2) | 3.0–4.0 | - |
| Knob height | 1.9 ± 0.0 (1.9–2.0) | 1.5 | 1.5 ± 0.0 (1.5–1.5) | 1.5–2.0 | - |
| DGO | 7.6 ± 0.4 (7.0–8.0) | 7.1 | 7.12 ± 0.13 (7.00–7.30) | 7.0–8.0 | - |
| Median bulb valve length | 9.8 ± 0.4 (8.9–10.0) | 8.5 | 8.8 ± 0.3 (8.5–9.0) | 7.0–10.0 | 9.0 (n = 1) |
| Median bulb width | 9.7 ± 0.7 (9.0–10.8) | 15 | 14.8 ± 0.4 (14.0–15.5) | 8.5–10.8 | 15.0, 20.0 (n = 2) |
| Isthmus length | 14.1 ± 0.5 (13.6–15.0) | 3.5 | 3.4 ± 0.2 (3.0–3.5) | 13.6–15.5 | - |
| Pharynx length | 108.6 ± 3.0 (103.9–113.8) | 117 | 108.3 ± 3.4 (102.0–114.0) | 102.0–117.0 | - |
| Anterior end to excretory pore (Ep) | 74.9 ± 2.3 (72.0–78.2) | 83.5 | 75.8 ± 2.8 (72.0–81.0) | 72.0–83.5 | - |
| Max body width | 14.5 ± 0.6 (13.3–15.3) | 14 | 14.2 ± 0.6 (13.0–15.0) | 13.0–15.3 | 44.2 ± 11.8 (30.0–65.0) |
| Body width at vulva | 12.6 ± 0.3 (12.2–13.0) | 12.5 | 12.4 ± 0.5 (11.5–13.0) | 11.5–13.0 | 20.0 ± 2.9 (17.0–25.0) |
| Body width at anus | 7.2 ± 0.3 (6.9–7.9) | 6 | 6.2 ± 0.7 (5.5–7.0) | 5.5–7.9 | - |
| Anterior end to median bulb base | 82.2 ± 3.4 (77.7–89.1) | 90 | 84.3 ± 2.2 (81.0–87.0) | 77.7–90.0 | - |
| Lateral field width | 2.8 ± 0.2 (2.5–3.0) | 1.8 | 1.8 ± 0.0 (1.8–1.9) | 1.8–3.0 | 4.3 ± 0.4 (4.0–5.0) |
| Anterior end-vulva distance | 189.9 ± 4.1 (186.0–197.0) | 211.2 | 200.9 ± 7.2 (185.0–207.1) | 185.0–211.2 | 199.6 ± 11.9 (252.0–288.0) |
| Vulva-tail terminus distance | 72.9 ± 3.1 (68.3–78.2) | 72.7 | 74.8 ± 4.0 (68.2–82.2) | 68.2–82.2 | 67.2 ± 5.9 (60.0–75.0) |
| Genital tract length | 51.8 ± 3.8 (48.0–60.3) | 54.5 | 50.0 ± 4.8 (45.0–60.0) | 45.0–60.3 | - |
| Vulva-anus distance | 48.5 ± 5.9 (39.0–57.4) | 48 | 47.2 ± 6.0 (40.0–59.0) | 39.0–59.0 | - |
| Tail length | 20.7 ± 1.3 (18.8–22.7) | 21 | 20.4 ± 1.3 (19.0–23.0) | 18.8–23.0 | - |
| Stylet base (Stb) to median bulb valve base (v) | 15.6 ± 1.5 (14.0–17.8) | 15 | 14.2 ± 0.4 (14.0–15.0) | 14.0–17.8 | - |
| PERCENTAGES | |||||
| V | 72.2 ± 0.6 (71.4–73.4) | 74.4 | 72.9 ± 1.4 (70.9–74.6) | 70.9–74.6 | 74.1 ± 1.3 (71.7–75.3) |
| G or T | 19.5 ± 1.5 (18.4–22.9) | 19.2 | 18.2 ± 1.9 (15.9–22.5) | 15.9–22.9 | - |
| Stb-v/St | 23.9 ± 2.6 (21.4–28.1) | 21.1 | 21.3 ± 1.0 (20.0–23.1) | 20.0–28.1 | - |
| St/L | 24.8 ± 0.9 (23.0–25.9) | 25.0 | 24.3 ± 0.8 (22.6–25.1) | 22.6–25.9 | - |
| Ep/L | 28.6 ± 1.3 (26.4–30.4) | 29.4 | 27.6 ± 1.2 (25.8–29.1) | 25.8–30.4 | - |
| RATIOS | |||||
| a | 18.1 ± 0.8 (17.2–19.3) | 20.3 | 19.5 ± 0.6 (18.7–20.2) | 17.2–20.3 | 6.5 ± 1.6 (4.0–8.5) |
| b | 2.4 ± 0.1 (2.2–2.4) | 2.4 | 2.5 ± 0.1 (2.4–2.7) | 2.2–2.7 | - |
| c | 12.7 ± 0.9 (11.9–14.6) | 13.5 | 13.5 ± 1.1 (11.3–14.9) | 11.3–14.9 | - |
| c′ | 2.8 ± 0.2 (2.4–3.1) | 3.5 | 3.3 ± 0.3 (2.9–3.8) | 2.4–3.8 | - |
| Population | High Springs, Alachua County (CD3450—N21-00211) | ||
|---|---|---|---|
| Character | ♂ (Live) | ♂ (Fixed)—Paratypes | Total Range ♂ |
| n | 4 | 8 | 12 |
| L | 299.0 ± 6.2 (291.0–305.9) | 258.5 ± 12.7 (245.0–281.0) | 245.0–305.9 |
| Pharynx length | 88.1 ± 5.1 (82.1–96.0) | 71.6 ± 2.7 (68.0–76.0) | 68.0–96.0 |
| Anterior end to excretory pore (Ep) | 77.8 ± 2.9 (74.2–82.1) | 63.3 ± 2.2 (60.0–66.0) | 60.0–82.1 |
| Max body width | 12.6 ± 0.4 (12.0–13.0) | 10.6 ± 0.6 (9.5–11.0) | 9.5–13.0 |
| Body width at anus | 10.0 ± 0.5 (9.4–10.8) | 7.8 ± 0.5 (7.0–8.0) | 7.0–8.0 |
| Genital tract length | 105.9 ± 8.8 (96.0–118.8) | 100.9 ± 4.6 (96.6–109.2) | 96.6–118.8 |
| Tail length | 27.2 ± 1.1 (25.7–28.7) | 20.8 ± 0.7 (19.5–22.0) | 19.5–28.7 |
| Spicule | 17.6 ± 0.2 (17.4–17.8) | 16.4 ± 0.4 (16.0–17.0) | 16.0–17.8 |
| Gubernaculum | 4.1 ± 0.1 (4.0–4.2) | 3.4 ± 0.2 (3.0–3.5) | 3.0–4.2 |
| PERCENTAGES | |||
| G or T | 35.4 ± 3.5 (31.5–40.2) | 39.6 ± 2.9 (35.4–44.6) | 31.5–44.6 |
| Ep/L | 26.0 ± 0.6 (25.4–27.0) | 24.5 ± 0.9 (23.1–25.6) | 23.1–27.0 |
| RATIOS | |||
| a | 23.7 ± 1.1 (22.3–25.4) | 24.5 ± 1.8 (22.3–26.5) | 22.3–26.5 |
| b | 3.4 ± 0.2 (3.1–3.5) | 3.6 ± 0.1 (3.4–3.7) | 3.1–3.7 |
| c | 11.0 ± 0.3 (10.6–11.3) | 12.4 ± 0.6 (11.7–13.3) | 10.6–13.3 |
| c′ | 2.7 ± 0.2 (2.4–3.0) | 2.7 ± 0.2 (2.4–3.0) | 2.4–3.0 |
Type Habitat and Locality
| Population | High Springs, Alachua County (CD3450—N21-00211) | ||
|---|---|---|---|
| Character | J2 (Live) | J2 (Fixed) | Total Range J2 |
| n | 6 | 4 | 10 |
| L | 210.2 ± 7.1 (200.0–219.7) | 251.8 ± 5.5 (246.8–258.3) | 200.0–258.3 |
| Stylet length (St) | 32.9 ± 0.7 (32.1–34.1) | 31.8 ± 0.3 (31.5–32.0) | 31.5–34.1 |
| Conus length | 27.1 ± 0.8 (26.5–28.8) | 26.3 ± 0.3 (26.0–26.5) | 26.0–28.8 |
| Stylet shaft + knob height | 5.8 ± 0.5 (5.3–6.6) | 5.5 ± 0.0 (5.5–5.5) | 5.3–6.6 |
| Knob width | 3.8 ± 0.9 (3.5–3.9) | 3.5 ± 0.0 (3.5–3.5) | 3.5–3.9 |
| Knob height | 1.7 ± 0.1 (1.5–1.9) | 1.5 ± 0.0 (1.5–1.5) | 1.5–1.9 |
| DGO | 4.8 ± 0.1 (4.7–5.0) | 4.5, 4.7 (n = 2) | 4.5–5.0 |
| Median bulb valve length | 4.9 ± 0.1 (4.7–5.0) | 5.0 ± 0.0 (5.0–5.0) | 4.7–5.0 |
| Median bulb width | 8.4 ± 0.5 (7.5–8.9) | 6.5 ± 0.5 (6.0–7.0) | 6.0–8.9 |
| Isthmus length | 12.3 ± 2.3 (8.0–15.5) | 12.7 ± 3.2 (9.0–15.0) | 8.0–15.5 |
| Pharynx length | 84.1 ± 4.1 (79.0–91.5) | 73.3 ± 0.5 (73.0–74.0) | 73.0–91.5 |
| Anterior end to excretory pore (Ep) | 65.5 ± 3.5 (60.0–70.3) | 63.0 ± 1.0 (62.0–64.0) | 60.0–70.3 |
| Max body width | 13.4 ± 0.3 (13.0–13.9) | 15.4 ± 0.3 (15.0–15.5) | 13.0–15.5 |
| Body width at anus | 8.3 ± 0.3 (7.8–8.8) | 8.6 ± 0.3 (8.5–9.0) | 7.8–9.0 |
| Anterior end to median bulb base | 56.0 ± 3.4 (50.0–61.3) | 48.4 ± 1.3 (47.0–50.0) | 47.0–61.3 |
| Genital primordium length | 12.6 ± 1.9 (10.0–15.0) | 11.5, 13.0 (n = 2) | 10.0–15.0 |
| Tail length | 18.8 ± 1.2 (17.5–21.3) | 19.3 ± 0.5 (19.0–20.0) | 17.5–21.3 |
| Stylet base (Stb) to median bulb valve base (v) | 14.9 ± 1.9 (12.0–17.8) | 14.5 ± 1.9 (13.0–17.0) | 12.0–17.8 |
| PERCENTAGES | |||
| Stb-v/St | 45.2 ± 5.3 (37.2–52.9) | 45.7 ± 6.2 (40.6–54.0) | 37.2–54.0 |
| St/L | 15.6 ± 0.6 (14.8–16.5) | 12.6 ± 0.4 (12.2–13.0) | 12.2–16.5 |
| Ep/L | 30.9 ± 1.4 (28.5–32.2) | 24.9 ± 0.4 (24.4–25.2) | 24.4–32.2 |
| RATIOS | |||
| a | 15.7 ± 0.4 (15.0–16.2) | 16.4 ± 0.5 (15.9–17.0) | 15.0–17.0 |
| b | 2.5 ± 0.1 (2.0–2.5) | 3.4 ± 0.1 (3.4–3.5) | 2.0–3.5 |
| c | 11.2 ± 0.5 (10.2–11.7) | 13.1 ± 0.6 (12.3–13.6) | 10.2–13.6 |
| c′ | 2.2 ± 0.2 (2.0–2.5) | 2.2 ± 0.0 (2.2–2.2) | 2.0–2.5 |
Etymology
Type Material
Molecular Characterization
Diagnosis and Relationships
Remarks
2.2.4. Paratylenchus acti Eroshenko, 1978 [15]
Description
Habitat and Locality
Molecular Characterization
Diagnosis (Based on Specimens Examined)
Remarks
2.2.5. Paratylenchus aquaticus (type C) Merny, 1966 [16]

| Population | Okeechobee, Okeechobee County (CD3480—N21-00445) | ||
|---|---|---|---|
| Character | ♀ (Live) | ♂ (Live) | J2 (Live) |
| n | 8 | 4 | 3 |
| L | 364 ± 26.3 (321.7–409.8) | 340.5 ± 15.8 (314.8–355.4) | 225.0 ± 22.4 (196.0–250.4) |
| Stylet length (St) | 16.9 ± 0.3 (16.2–17.0) | - | 16.5 ± 0.6 (16.2–17.5) |
| Conus length | 10.2 ± 0.4 (9.5–10.5) | - | 10.0 ± 0.1 (9.9–10.2) |
| Stylet shaft + knob height | 6.7 ± 0.3 (6.3–7.1) | - | 6.2 ± 0.1 (6.0–6.3) |
| Knob width | 3.5 ± 0.9 (3.2–3.8) | - | 3.3 ± 0.9 (3.1–3.5) |
| Knob height | 1.8 ± 0.1 (1.6–1.9) | - | 1.5 ± 0.0 (1.5–1.5) |
| DGO | 6.7 ± 0.3 (6.1–7.0) | - | 5.0 ± 0.1 (4.9–5.2) |
| Median bulb valve length | 6.9 ± 0.3 (6.2–7.0) | - | 4.8 ± 0.2 (4.5–5.0) |
| Median bulb width | 8.0 ± 1.2 (6.9–9.9) | - | 6.9 ± 0.3 (6.5–7.1) |
| Isthmus length | 17.3 ± 2.0 (14.0–19.5) | - | 14.0 ± 2.2 (12.0–17.0) |
| Pharynx length | 90.6 ± 3.9 (85.0–97.0) | 88.9 ± 2.4 (87.1–93.0) | 72.4 ± 6.3 (67.0–81.2) |
| Anterior end to excretory pore (Ep) | 78.1 ± 5.1 (71.3–87.1) | 71.6 ± 1.8 (70.0–74.2) | 57.3 ± 1.4 (55.0–58.4) |
| Max body width | 13.1 ± 2.3 (11.0–17.8) | 10.6 ± 0.2 (10.4–10.8) | 9.9 ± 0.5 (9.3–10.5) |
| Body width at vulva | 11.1 ± 1.9 (9.0–14.5) | - | - |
| Body width at anus | 8.1 ± 1.0 (6.4–8.9) | 9.1 ± 0.4 (8.8–9.9) | 6.0 ± 0.9 (5.0–7.0) |
| Anterior end to median bulb base | 58.6 ± 2.4 (55.4–62.3) | - | 46.8 ± 3.5 (43.0–51.4) |
| Lateral field width | 3.8 ± 0.3 (3.5–4.0) | - | - |
| Anterior end-vulva distance | 296.0 ± 23.2 (257.4–324.7) | - | - |
| Vulva-tail terminus distance | 68.2 ± 7.7 (60.0–87.1) | - | - |
| Genital tract length | 125.9 ± 45.9 (90.0–202.5) | 96.7 ± 13.6 (75.2–112.8) | - |
| Vulva-anus distance | 44.4 ± 3.6 (40.0–53.0) | - | - |
| Tail length | 21.7 ± 1.9 (17.0–23.7) | 24.1 ± 3.0 (19.8–28.0) | 22.6 ± 1.9 (20.0–24.5) |
| Stylet base (Stb) to median bulb valve base (v) | 32.6 ± 2.3 (30.0–35.6) | - | 22.6 ± 1.8 (21.0–25.2) |
| Spicule length | - | 17.4 ± 0.4 (17.0–17.9) | - |
| Gubernaculum length | - | 3.9 ± 0.1 (3.8–4.0) | - |
| PERCENTAGES | |||
| V | 80.6 ± 1.7 (78.5–84.3) | - | - |
| G or T | 34.4 ± 11.3 (23.0–53.0) | 28.4 ± 4.0 (22.0–31.7) | - |
| Stb-v/St | 1.9 ± 0.1 (1.7–2.1) | - | 1.3 ± 0.1 (1.2–1.4) |
| St/L | 4.6 ± 0.4 (4.1–5.2) | - | 7.4 ± 0.6 (7.0–8.2) |
| Ep/L | 21.2 ± 0.7 (19.8–22.1) | 21.3 ± 0.7 (20.5–22.2) | 25.5 ± 3.0 (22.9–29.7) |
| RATIOS | |||
| a | 26.9 ± 3.8 (21.7–32.1) | 31.7 ± 1.2 (29.9–32.9) | 22.6 ± 1.8 (21.0–25.2) |
| b | 4.0 ± 0.4 (3.3–4.4) | 3.8 ± 0.1 (3.6–4.0) | 3.1 ± 0.2 (2.9–3.3) |
| c | 16.9 ± 2.3 (15.2–22.3) | 14.3 ± 1.1 (12.6–15.8) | 18.2 ± 1.3 (16.3–19.2) |
| c′ | 2.7 ± 0.5 (1.9–3.5) | 2.6 ± 0.3 (2.3–3.1) | 2.2 ± 0.4 (1.7–2.6) |
Description
Habitat and Locality
Molecular Characterization
Diagnosis (Based on Specimens Examined)
Remarks
2.2.6. Paratylenchus goldeni Raski, 1975 [17] Species Complex
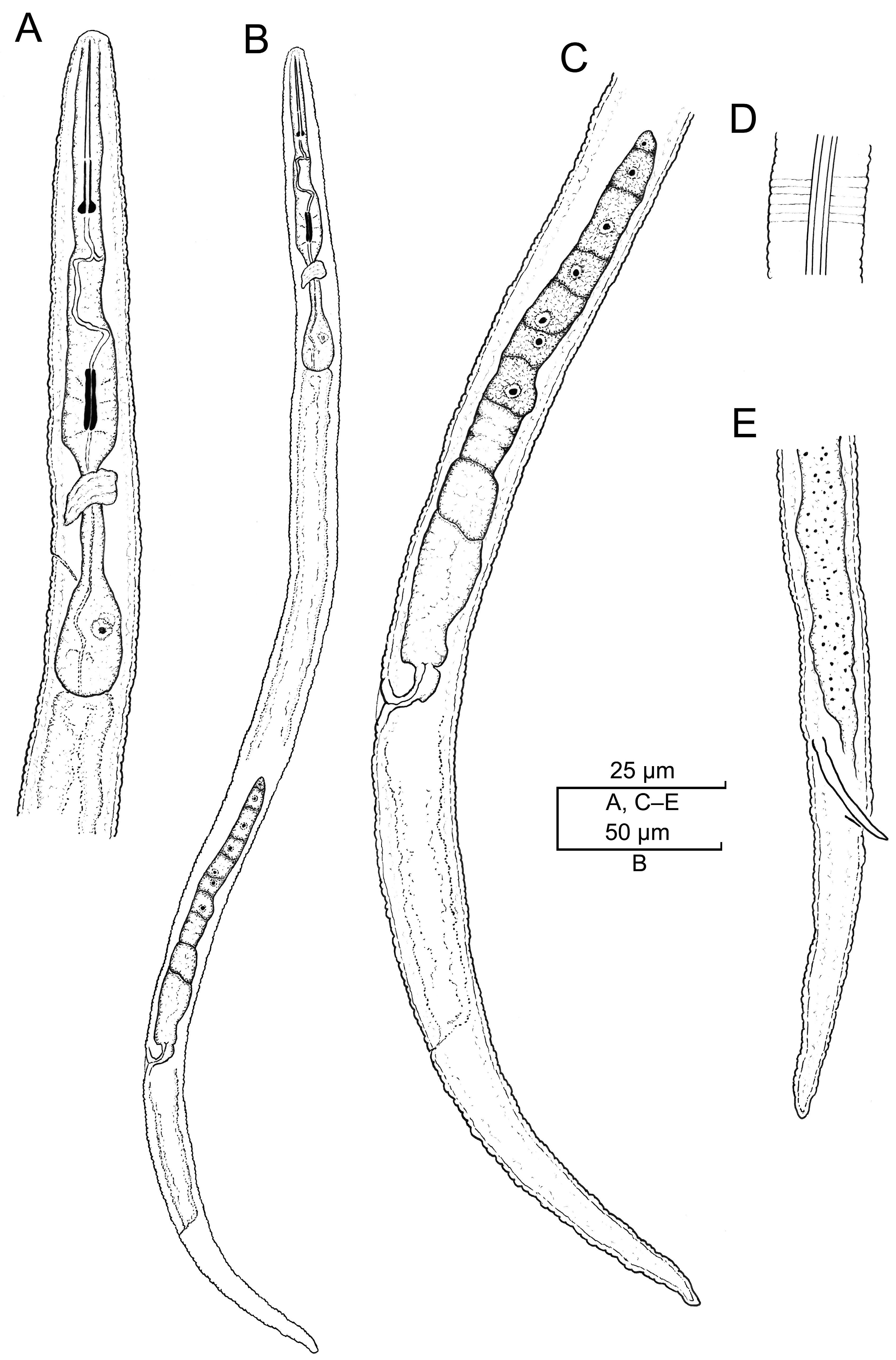

| Population | Madison, Madison County (CD3651—N21-01426-4) | North Carolina Zeng et al. [54] | ||
|---|---|---|---|---|
| Character | Vermiform ♀ (Live) | Egg-Laying ♀ (Live) | Vermiform ♀ (Fixed) | Vermiform ♀ (Fixed) |
| n | 10 | 2 | 5 | 10 |
| L | 434.6 ± 31.5 (385.1–478.1) | 520, 488 | 373.5 ± 42.1 (309.7–414.8) | 374.9 ± 17.1 (358.9–399.8) |
| Stylet length (St) | 26.8 ± 0.7 (25.7–27.7) | 25.2, 27.2 | 25.2 ± 0.8 (24.0–26.0) | 19.5 ± 0.8 (18.2–20.2) |
| Conus length | 16.9 ± 0.4 (16.3–17.5) | 16.9, 16.5 | 17.2 ± 0.4 (17.0–18.0) | - |
| Stylet shaft + knob height | 9.9 ± 0.6 (8.8–11.0) | 8.3, 10.7 | 8.0 ± 0.7 (7.0–8.7) | - |
| Knob width | 3.7 ± 0.9 (3.1–4.0) | 3.9, 3.9 | 3.0 ± 0.0 (3.0–3.0) | - |
| Knob height | 1.9 ± 0.1 (1.7–2.0) | 1.9, 1.7 | 1.6 ± 0.1 (1.5–1.7) | - |
| DGO | 7.5 ± 0.6 (6.3–8.4) | 7.8, 6.9 | 6.5 ± 0.4 (6.0–7.0) | - |
| Median bulb valve length | 9.7 ± 0.4 (9.0–10.0) | 9.4, 9.4 | 8.4 ± 0.2 (8.0–8.5) | - |
| Median bulb width | 9.8 ± 0.6 (8.8–10.8) | 14.9, 14.8 | 8.0 ± 0.7 (7.0–8.5) | - |
| Isthmus length | 16.8 ± 1.0 (15.0–18.0) | 18.6, 20.0 | 15.8 ± 0.8 (15.0–17.0) | - |
| Pharynx length | 113.7 ± 5.8 (101.0–122.7) | 101.9, 109.8 | 93.1 ± 3.2 (89.5–98.0) | 90.6 ± 4.7 (82.9–94.8) |
| Anterior end to excretory pore (Ep) | 90.1 ± 6.1 (75.0–96.0) | 97.0, 98.0 | 69.2 ± 4.6 (65.0–75.0) | 74.8 ± 4.1 (68.5–79.2) |
| Max body width | 16.1 ± 0.9 (14.5–17.8) | 24.7, 21.2 | 14.4 ± 2.4 (11.0–16.5) | 17.5 ± 0.4 (16.9–18.1) |
| Body width at vulva | 13.7 ± 0.8 (12.4–14.8) | 18.0, 16.5 | 11.3 ± 1.7 (9.0–13.0) | 14.1 ± 0.6 (13.5–15.0) |
| Body width at anus | 9.8 ± 0.7 (8.9–11.0) | 12.3, 9.9 | 9.1 ± 1.5 (7.0–10.5) | 9.6 ± 0.4 (9.1–10.3) |
| Anterior end to median bulb base | 76.6 ± 3.0 (71.3–82.1) | 74.2, 77.2 | 61.2 ± 2.6 (57.0–64.0) | - |
| Lateral field width | 3.4 ± 0.4 (3.0–4.0) | - | 2.9 ± 0.3 (2.5–3.0) | - |
| Anterior end-vulva distance | 334.8 ± 22.3 (302.0–363.3) | 416.7, 386.1 | 281.0 ± 29.2 (238.8–310.9) | - |
| Vulva-tail terminus distance | 99.0 ± 11.6 (83.1–115.6) | 103.9, 101.9 | 93.3 ± 12.7 (72.0–104.0) | - |
| Genital tract length | 104.9 ± 16.0 (85.0–136.6) | 297.0, 261.3 | 88.5 ± 13.3 (69.0–99.0) | - |
| Vulva-anus distance | 45.9 ± 4.7 (40.0–55.4) | 54.0, 51.5 | 43.1 ± 5.2 (38.5–49.0) | 40.3 ± 2.8 (37.6–44.4) |
| Tail length | 52.9 ± 4.8 (44.5–58.4) | 49.9, 50.4 | 56.5 ± 4.7 (53.0–63.0) | 39.0 ± 3.2 (34.2–43.0) |
| Stylet base (Stb) to median bulb valve base (v) | 38.8 ± 2.4 (34.0–42.5) | 35.6, 37.6 | 29.9 ± 2.1 (27.5–32.0) | - |
| PERCENTAGES | ||||
| V | 77.2 ± 1.3 (75.7–79.4) | 79.1, 80.1 | 75.3 ± 1.0 (74.6–77.1) | 78.9 ± 0.9 (78.1–80.3) |
| G or T | 24.0 ± 2.7 (20.6–28.7) | 57.1, 53.5 | 22.6 ± 2.4 (19.5–24.8) | - |
| Stb-v/St | 1.4 ± 0.1 (1.2–1.6) | 1.4, 1.3 | 118.4 ± 7.2 (109.8–128.0) | - |
| St/L | 6.2 ± 0.4 (5.5–6.6) | 4.8, 5.5 | 6.8 ± 0.6 (6.3–7.7) | - |
| Ep/L | 20.7 ± 1.2 (18.7–22.4) | 18.6, 20.0 | 18.7 ± 1.9 (15.7–21.0) | - |
| RATIOS | ||||
| a | 27.1 ± 1.9 (24.4–30.1) | 21.0, 23.0 | 27.5 ± 3.6 (24.2–32.2) | 21.4 ± 1.3 (20.2–23.6) |
| b | 3.8 ± 0.3 (3.4–4.2) | 5.1, 4.4 | 4.0 ± 0.4 (3.5–4.3) | 4.1 ± 0.2 (3.8–4.4) |
| c | 8.3 ± 0.7 (6.8–9.6) | 10.4, 9.4 | 6.9 ± 0.5 (6.3–7.4) | 9.7 ± 0.9 (8.9–11.2) |
| c′ | 5.3 ± 0.6 (4.0–6.5) | 4.0, 5.2 | 6.3 ± 0.9 (5.6–7.6) | 4.1 ± 0.4 (3.6–4.7) |
| Population | Ilex sp. Morriston, Levy County (CD3443—N21-00015-4) | Enydra sp. Milton, Santa Rosa County (CD3453—N21-00238-7) | Paratypes North Carolina Raski [17] | ||
|---|---|---|---|---|---|
| Character | ♀ (Live) | ♂ (Live) | ♀ (Live) | ♀ (Fixed) | ♂ (Fixed) |
| n | 4 | 1 | 5 | 24 | 7 |
| L | 389.2 ± 19.2 (367.1–413.8) | 396.9 | 369.6 ± 34.1 (308.0–409.8) | 380.0 (350.0–430.0) | 330.0 (300.0–380.0) |
| Stylet length (St) | 17.5 ± 0.2 (17.3–17.8) | - | 19.6 ± 1.0 (18.3–21.2) | 17.0 (14.0–19.0) | - |
| Conus length | 10.0 ± 0.4 (9.5–10.5) | - | 11.2 ± 0.6 (10.5–11.9) | 11.0 (10.0–11.0) | - |
| Stylet shaft + knob height | 7.5 ± 0.5 (6.8–8.1) | - | 8.4 ± 0.5 (7.8–9.3) | - | - |
| Knob width | 2.4 ± 0.9 (2.3–2.5) | - | 2.9 ± 0.9 (2.4–3.3) | - | - |
| Knob height | 1.4 ± 0.2 (1.1–1.6) | - | 1.7 ± 0.2 (1.4–2.0) | - | - |
| DGO | 6.8 ± 0.7 (6.0–7.9) | - | 8.7 ± 0.6 (8.0–9.5) | 5.0 | - |
| Median bulb valve length | 8.7 ± 0.4 (8.0–8.9) | - | 9.4 ± 0.4 (9.0–9.9) | - | - |
| Median bulb width | 9.1 ± 0.5 (8.7–9.9) | - | 10.6 ± 1.0 (9.0–11.8) | - | - |
| Isthmus length | 14.8 ± 1.4 (12.8–16.2) | - | 14.3 ± 0.7 (13.3–15.5) | - | - |
| Pharynx length | 98.2 ± 1.7 (96.0–99.9) | 90.0 | 107.6 ± 2.7 (105.0–112.8) | 93.0 | - |
| Anterior end excretory pore (Ep) | 79.3 ± 3.9 (75.0–85.6) | 74.7 | 80.8 ± 8.6 (65.0–89.1) | 77.0 (67.0–83.0) | 63.0 (54.0–66.0) |
| Max body width | 15.1 ± 0.4 (14.8–15.8) | 13.8 | 17.1 ± 1.6 (15.3–19.9) | 13.3 | - |
| Body width at vulva | 12.7 ± 0.2 (12.3–12.8) | - | 13.6 ± 1.3 (12.0–15.5) | - | - |
| Body width at anus | 8.9 ± 0.0 (8.8–8.9) | 10.8 | 8.7 ± 1.0 (7.5–9.9) | - | - |
| Anterior end to median bulb base | 65.1 ± 1.3 (64.3–67.3) | - | 59.6 ± 3.6 (54.4–65.0) | - | - |
| Lateral field width | 3.4 ± 0.4 (3.0–3.7) | - | 4.6 ± 0.2 (4.4–4.8) | - | - |
| Anterior end-vulva distance | 300.7 ± 14.2 (282.1–316.8) | - | 295.7 ± 29.1 (243.0–331.6) | 320 | - |
| Vulva-tail terminus distance | 88.7 ± 5.5 (82.2–97.0) | - | 74.0 ± 5.5 (65.0–79.2) | 80 | - |
| Genital tract length | 101.2 ± 4.7 (97.0–108.9) | 96 | 118.2 ± 12.5 (99.0–136.6) | 140 | - |
| Vulva-anus distance | 41.3 ± 2.2 (37.6–43.5) | - | 38.4 ± 3.3 (33.1–42.5) | - | - |
| Tail length | 37.0 ± 1.9 (34.0–39.0) | 50.4 | 34.7 ± 2.8 (30.5–38.6) | - | - |
| Stylet base (Stb) to median bulb valve base (v) | 37.4 ± 1.3 (35.6–38.6) | - | 38.2 ± 2.5 (35.0–42.0) | - | - |
| Spicule length | - | 21.3 | - | - | 22.0 (21.0–23.0) |
| Gubernaculum length | - | 4.9 | - | - | 4.0 (3.0–5.0) |
| PERCENTAGES | |||||
| V | 77.2 ± 0.6 (76.5–78.0) | - | 74.1 ± 1.3 (71.7–75.3) | 80.0 (78.0–81.0) | - |
| G or T | 25.7 ± 0.4 (25.1–26.3) | 24.2 | 31.9 ± 1.1 (30.1–33.3) | 35 | 32.0 (29.0–36.0) |
| Stb-v/St | 2.1 ± 0.1 (2.0–2.2) | - | 2.0 ± 0.2 (1.7–2.2) | - | - |
| St/L | 4.5 ± 0.2 (4.2–4.8) | - | 5.3 ± 0.3 (5.0–5.9) | - | - |
| Ep/L | 20.3 ± 0.6 (19.2–20.8) | 18.8 | 23.0 ± 3.2 (20.4–29.3) | - | - |
| RATIOS | |||||
| a | 25.7 ± 0.7 (24.8–26.7) | 26.8 | 21.6 ± 1.6 (19.3–23.3) | 27.0 (19.0–33.0) | 29.0 (27.0–29.0) |
| b | 3.9 ± 0.2 (3.7–4.1) | 4.4 | 3.4 ± 0.3 (2.8–3.6) | 4.2 (3.9–5.0) | 5.4 |
| c | 10.5 ± 0.2 (10.1–10.7) | 7.8 | 10.7 ± 0.8 (9.4–11.9) | - | 9.0 (8.0–10.0) |
| c′ | 4.2 ± 0.2 (3.8–4.4) | 4.6 | 3.9 ± 0.3 (3.5–4.3) | - | 2.3 ± 0.2 |
Description
Habitat and Locality
Molecular Characterization
Diagnosis (Based on Specimens Examined)
Remarks
2.2.7. Paratylenchus minutus Linford, Oliveira & Ishi, 1949 [22]
Description (Based on the Hawaiian (from Roots of Coffea sp.) and Florida (from Roots of Hemerocallis sp.) Populations)
Habitats and Localities
| Population | Coffea sp. (CD3538—N21-01001) Hawaii | Hemerocallis sp. (CD3465—N21-00389) Florida | |
|---|---|---|---|
| Character | ♀ (Live) | ♀ (Live) | ♂ (Live) |
| n | 4 | 8 | 5 |
| L | 228.5 ± 9.7 (220.6–244.5) | 253.3 ± 12.7 (239.5–279.5) | 242.5 ± 20.7 (210.8–269.2) |
| Stylet length (St) | 17.5 ± 1.2 (16.3–19.0) | 18.0 ± 0.3 (16.9–18.8) | - |
| Conus length | 11.0 ± 0.6 (10.3–12.0) | 10.8 ± 0.3 (10.0–11.2) | - |
| Stylet shaft + knob height | 6.5 ± 0.7 (5.6–7.3) | 7.2 ± 0.5 (6.6–8.0) | - |
| Knob width | 3.3 ± 0.9 (3.1–3.5) | 3.5 ± 0.9 (3.1–3.9) | - |
| Knob height | 1.5 ± 0.0 (1.4–1.6) | 1.6 ± 0.2 (1.3–1.8) | - |
| DGO | 4.2 ± 0.1 (4.0–4.3) | 4.4 ± 0.2 (4.0–4.7) | - |
| Median bulb valve length | 5.1 ± 0.4 (4.5–5.5) | 5.3 ± 0.2 (5.0–5.5) | - |
| Median bulb width | 7.5 ± 0.9 (6.5–8.9) | 8.2 ± 1.1 (7.0–9.9) | - |
| Isthmus length | 13.5 ± 2.2 (11.3–16.5) | 12.4 ± 2.0 (8.0–14.8) | - |
| Pharynx length | 71.0 ± 1.7 (69.0–73.0) | 73.8 ± 3.1 (70.3–79.2) | 70.7 ± 3.6 (64.3–74.2) |
| Anterior end to excretory pore (Ep) | 56.8 ± 1.6 (54.5–59.0) | 60.7 ± 4.3 (52.0–65.3) | 56.6 ± 3.1 (50.5–59.4) |
| Max body width | 11.5 ± 1.1 (10.3–13.0) | 14.1 ± 1.8 (12.3–16.8) | 9.7 ± 0.4 (9.2–10.3) |
| Body width at vulva | 9.9 ± 0.7 (9.0–10.5) | 10.2 ± 0.8 (9.0–11.8) | - |
| Body width at anus | 6.7 ± 1.0 (5.5–8.0) | 7.3 ± 0.5 (6.4–8.0) | 7.5 ± 0.4 (6.9–8.0) |
| Anterior end to median bulb base | 45.5 ± 2.3 (43.0–49.0) | 41.3 ± 1.7 (39.6–45.0) | - |
| Anterior end-vulva distance | 187.8 ± 6.5 (182.0–198.0) | 212.0 ± 11.7 (198.0–238.0) | - |
| Vulva-tail terminus distance | 40.8 ± 3.3 (38.5–46.5) | 41.3 ± 2.6 (38.0–47.5) | - |
| Genital tract length | 68.1 ± 9.4 (60.0–81.2) | 99.0 ± 22.6 (29.0–135.6) | 86.5 ± 14.9 (62.0–105.9) |
| Vulva-anus distance | 30.4 ± 3.7 (27.1–35.6) | 23.5 ± 2.1 (21.0–27.7) | - |
| Tail length | 17.3 ± 2.5 (15.0–21.4) | 17.5 ± 1.5 (15.8–21.0) | 17.7 ± 2.6 (15.0–21.7) |
| Stylet base (Stb) to median bulb valve base (v) | 21.0 ± 0.1 (20.8–21.0) | 23.2 ± 2.1 (20.7–27.0) | - |
| Spicule length | - | - | 17.4 ± 0.5 (16.8–18.1) |
| Gubernaculum length | - | - | 3.0 ± 0.1 (3.0–3.2) |
| PERCENTAGES | |||
| V | 82.2 ± 0.7 (81.0–82.8) | 83.7 ± 1.0 (82.0–85.1) | - |
| G or T | 30.4 ± 3.7 (27.2–35.6) | 38.7 ± 8.2 (24.3–50.4) | 34.9 ± 4.5 (29.4–42.4) |
| Stb-v/St | 1.2 ± 0.0 (1.1–1.2) | 1.3 ± 0.2 (1.1–1.5) | - |
| St/L | 7.6 ± 0.7 (6.7–8.3) | 7.1 ± 0.5 (6.4–7.7) | - |
| Ep/L | 24.9 ± 0.7 (24.1–25.9) | 23.9 ± 1.2 (21.7–25.8) | 23.4 ± 1.3 (21.6–25.2) |
| RATIOS | |||
| a | 20.1 ± 0.1 (20.8–21.0) | 18.2 ± 2.1 (14.8–21.3) | 24.8 ± 1.3 (22.9–27.1) |
| b | 3.2 ± 0.1 (3.0–3.3) | 3.4 ± 0.2 (3.2–3.9) | 3.4 ± 0.2 (3.1–3.7) |
| c | 13.4 ± 1.3 (11.5–15.2) | 14.5 ± 0.8 (13.3–15.4) | 13.8 ± 1.3 (12.4–15.6) |
| c′ | 2.6 ± 0.4 (2.0–3.0) | 2.4 ± 0.2 (2.1–2.8) | 2.3 ± 0.4 (2.0–2.8) |
Molecular Characterization
Diagnosis (Based on Specimens Examined)
Remarks
On the Identity of P. shenzhenensis
Emended Diagnosis of Paratylenchus minutus (=Paratylenchus shenzhenensis syn. n.)
2.2.8. Paratylenchus paralatescens (Munawar, Cai, Ye, Powers & Zheng, 2018) Munawar, Miao, Castillo, Zheng, 2020 [23,24]
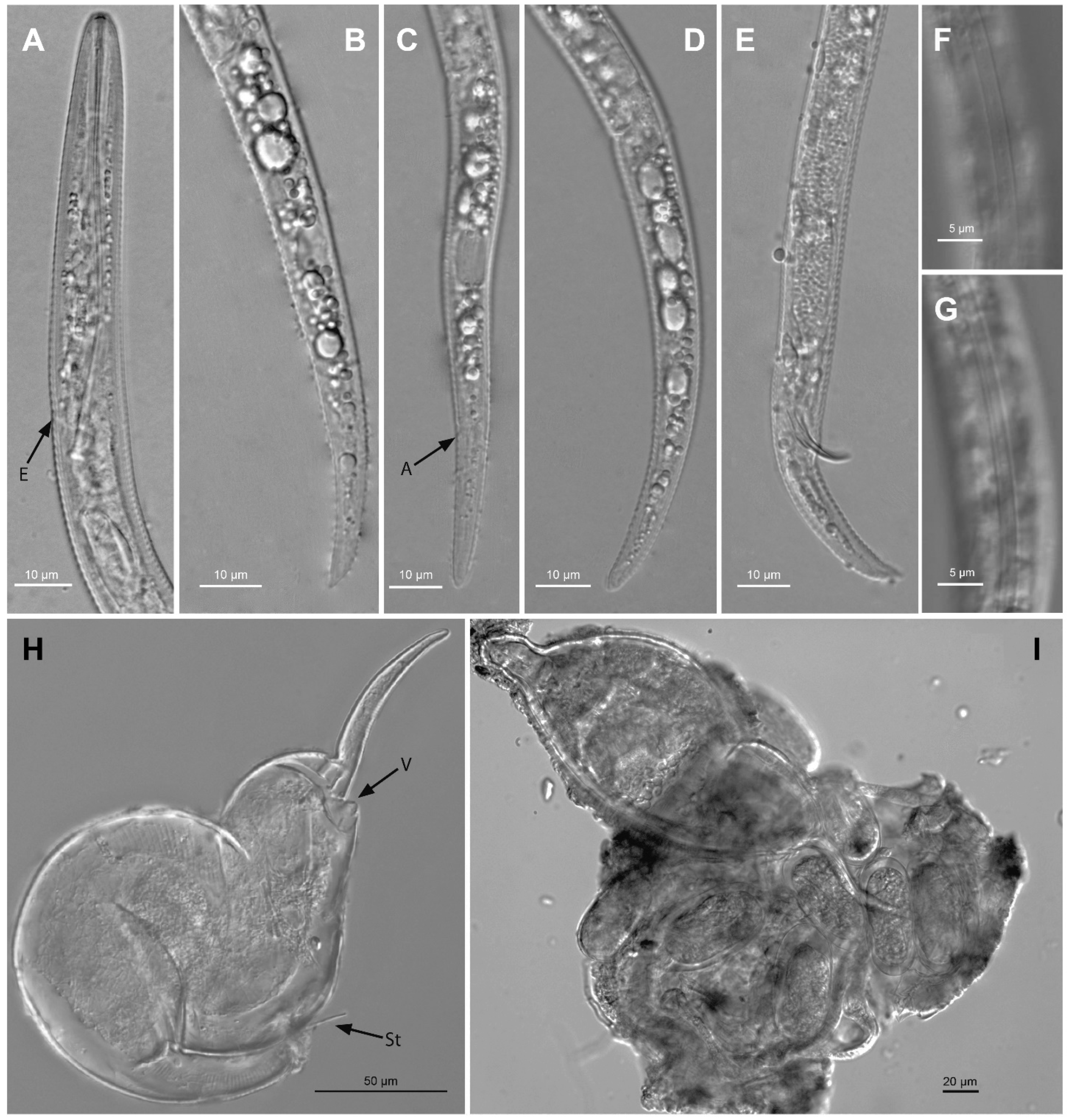
| Population | Phyllostachys nigra (CD3396—N20-01262-1) Florida | Phyllostachys bambusoides (as P. latescens) Troccoli et al. [8] Florida | |
|---|---|---|---|
| Character | ♀ (Live) | ♀ (Fixed) | ♀ (Fixed) |
| n | 7 | 2 | 15 |
| L | 294.7 ± 16.7 (277.2–326.6) | 260.0, 294.0 | 279.0 ± 10.9 (264.0–300.0) |
| Stylet length (St) | 74.6 ± 3.4 (67.0–77.7) | 71.0, 80.0 | 72.0 ± 2.8 (67.5–76.5) |
| Conus length | 68.2 ± 3.1 (61.5–71.2) | 65.5, 74.5 | 66.0 ± 3.3 (59.5–70.0) |
| Stylet shaft + knob height | 6.4 ± 08 (5.5–8.1) | 5.5, 5.5 | 6.0 ± 0.9 (4.5–7.5) |
| Knob width | 3.1 ± 0.9 (2.9–2.4) | 2.5, 2.1 | 2.5 ± 0.4 (2.3–5.0) |
| Knob height | 1.5 ± 0.2 (1.3–1.9) | 1.3, 1.2 | - |
| Median bulb valve length | 9.8 ± 0.6 (9.0–10.5) | 9.9, 9.4 | 9.0 ± 0.7 (7.5–10.0) |
| Median bulb width | 9.1 ± 0.6 (8.0–9.9) | 9.0, 10.0 | 8.0 ± 0.4 (7.5–8.5) |
| Isthmus length | 15.5 ± 1.6 (13.8–17.8) | 15.0, 14.0 | 16.0 ± 1.7 (12.0–18.5) |
| Pharynx length | 130.6 ± 4.6 (123.7–135.6) | 115.8, 134.5 | 127.0 ± 4.3 (120.0–132.0) |
| Anterior end to excretory pore (Ep) | 68.7 ± 3.9 (61.3–72.9) | 65.4, 69.3 | 68.0 ± 3.5 (64.0–78.0) |
| Max body width | 13.6 ± 0.4 (13.0–14.3) | 12.0, 12.6 | 11.5 ± 0.6 (11.0–13.0) |
| Body width at vulva | 12.0 ± 0.5 (11.1–12.8) | 11.0, 11.8 | - |
| Body width at anus | 6.6 ± 0.4 (5.9–7.1) | 6.4, 6.9 | 6.0 ± 0.4 (5.5–6.5) |
| Anterior end to median bulb base | 100.7 ± 3.1 (94.0–103.9) | 90.0, 103.1 | - |
| Lateral field width | 2.4 ± 0.4 (2.0–2.9) | 1.9, 2.2 | 2.2 ± 0.3 (2.0–2.5) |
| Anterior end-vulva distance | 207.7 ± 11.3 (197.0–230.6) | 182.0, 201.0 | 198 ± 9.2 (185.0–218.0) |
| Vulva-tail terminus distance | 87.0 ± 6.0 (79.2–96.0) | 78.0, 98.0 | 87.2 ± 1.7 (73.0–132.0) |
| Genital tract length | 44.0 ± 6.0 (41.6–45.5) | 37.0, 38.0 | 37.0 ± 3.5 (31.0–42.0) |
| Vulva-anus distance | 60.7 ± 5.6 (53.5–71.2) | 54.0, 58.0 | 55.0 ± 3.1 (47.0–59.0) |
| Tail length | 25.2 ± 0.7 (23.7–26.0) | 26.2, 24.7 | 24.5 ± 2.5 (21.5–31.5) |
| Stylet base (Stb) to median bulb valve base (v) | 22.6 ± 3.6 (18.0–29.7) | 17.8, 15.8 | - |
| PERCENTAGES | |||
| V | 70.5 ± 0.8 (68.6–71.9) | 70.0, 68.4 | 71.0 ± 1.4 (69.0–74.0) |
| G or T | 14.9 ± 0.7 (13.9–16.0) | 14.2, 12.9 | 13.0 ± 1.4 (11.0–15.0) |
| Stb-v/St | 29.7 ± 5.6 (22.5–42.3) | 23.0, 22.6 | - |
| St/L | 25.3 ± 1.4 (23.6–26.9) | 27.3, 27.2 | - |
| Ep/L | 23.3 ± 0.9 (21.8–24.5) | 25.1, 23.5 | - |
| RATIOS | |||
| a | 21.7 ± 1.4 (19.7–24.3) | 21.6, 23.3 | 23.9 ± 1.8 (21.5–27.3) |
| b | 2.3 ± 0.1 (2.1–2.5) | 2.2, 2.2 | 2.2 ± 0.1 (2.1–2.4) |
| c | 11.7 ± 0.5 (11.0–12.5) | 10.0, 11.9 | 11.7 ± 1.2 (8.4–14.0) |
| c′ | 3.8 ± 0.3 (3.4–4.3) | 4.0, 3.5 | 4.1 ± 0.4 (3.5–4.8) |
Description
Habitat and Locality
Molecular Characterization
Diagnosis (Based on Florida Specimens Examined)
Remarks
2.2.9. Paratylenchus straeleni (De Coninck, 1931) Oostenbrink, 1960 [27,28]
Description
| Population | Ocala, Marion County (CD3633—N21-01260-3) | |||
|---|---|---|---|---|
| Character | ♀ (Live) | ♂ (Live) | J2 (Live) | J4 (Live) |
| n | 10 | 7 | 2 | 4 |
| L | 333.7 ± 9.3 (317.7–352.0) | 362.2 ± 14.2 (305.0–347.4) | 226.7, 207.0 | 287.6 ± 48.1 (245.5–355.0) |
| Stylet length (St) | 54.6 ± 2.2 (51.4–57.9) | - | 38.6, 37.6 | 44.0 ± 0.8 (43.5–44.5) |
| Conus length | 42.5 ± 1.1 (41.0–44.5) | - | 28.7, 27.7 | 33.4 ± 0.8 (32.6–34.1) |
| Stylet shaft + knob height | 12.1 ± 1.4 (10.4–14.4) | - | 9.9 (n = 2) | 10.7 ± 0.3 (10.4–10.9) |
| Knob width | 3.7 ± 0.9 (3.1–3.9) | - | 2.8, 2.9 | 3.0 ± 0.9 (2.9–3.1) |
| Knob height | 1.8 ± 0.1 (1.5–1.9) | - | 1.1, 1.3 | 1.3 ± 0.1 (1.2–1.3) |
| DGO | 6.0 ± 0.5 (5.4–6.9) | - | - | 5.0 |
| Median bulb valve length | 6.2 ± 0.5 (5.5–7.0) | - | 4.5 (n = 2) | 5.0 ± 0.0 (4.9–5.0) |
| Median bulb width | 9.2 ± 0.6 (8.3–9.9) | - | 8.0 (n = 2) | 7.3 ± 0.4 (7.0–7.9) |
| Isthmus length | 11.6 ± 1.6 (9.0–13.8) | - | - | 10.5 ± 0.6 (9.9–11.0) |
| Pharynx length | 104.4 ± 5.0 (95.0–111.8) | 90.0 ± 1.4 (88.6–93.2) | 77.0, 89.0 | 87.6 ± 0.5 (87.0–88.1) |
| Anterior end to excretory pore (Ep) | 85.4 ± 3.7 (80.0–90.9) | 77.9 ± 1.8 (75.2–80.2) | 63.0, 60.4 | 74.2 ± 6.4 (68.3–83.0) |
| Max body width | 15.8 ± 1.0 (14.8–18.3) | 12.3 ± 0.3 (11.9–12.8) | 11.0, 12.0 | 14.9 ± 1.6 (12.9–17.3) |
| Body width at vulva | 13.8 ± 0.8 (12.8–14.8) | - | - | - |
| Body width at anus | 9.0 ± 0.6 (8.0–9.8) | 9.0 ± 0.4 (8.1–9.4) | 7.0, 7.4 | 7.9 |
| Anterior end to median bulb base | 77.7 ± 2.6 (72.3–81.2) | - | 49.5 (n = 1) | 63.2 ± 2.2 (61.0–65.3) |
| Lateral field width | 3.5 ± 0.2 (3.2–4.0) | - | - | - |
| Anterior end-vulva distance | 274.8 ± 9.4 (257.4–291.0) | - | - | - |
| Vulva-tail terminus distance | 58.9 ± 2.0 (54.0–61.0) | - | - | - |
| Genital tract length | 110.5 ± 19.8 (89.0–141.5) | 96.4 ± 9.1 (84.1–113.8) | - | - |
| Vulva-anus distance | 31.7 ± 2.7 (27.0–35.6) | - | - | - |
| Tail length | 23.4 ± 1.6 (21.0–25.2) | 26.3 ± 2.1 (24.7–30.0) | 18.0 (n = 2) | 19.4 ± 0.6 (18.8–20.0) |
| Stylet base (Stb) to median bulb valve base (v) | 15.6 ± 1.8 (13.0–17.8) | - | 10.0 (n = 1) | 12.4 ± 2.4 (10.0–14.8) |
| Spicule length | - | 19.8 ± 0.3 (19.3–20.2) | - | - |
| Gubernaculum length | - | 4.6 ± 0.2 (4.5–5.0) | - | - |
| PERCENTAGES | ||||
| V | 82.3 ± 0.8 (81.0–83.8) | - | - | - |
| G or T | 33.0 ± 5.6 (26.4–40.7) | 29.5 ± 2.5 (26.8–34.5) | - | - |
| Stb-v/St | 28.6 ± 3.0 (23.4–33.6) | - | 26.5 (n = 1) | 28.2 ± 5.8 (22.4–34.0) |
| St/L | 16.3 ± 0.6 (15.2–17.1) | - | 17.0, 18.6 | 17.4 ± 0.4 (17.0–17.7) |
| Ep/L | 25.5 ± 1.0 (24.0–27.6) | 24.6 ± 1.7 (22.9–28.2) | 27.7, 29.1 | 26.1 ± 2.0 (23.3–27.8) |
| RATIOS | ||||
| a | 21.1 ± 1.2 (18.4–22.7) | 26.5 ± 0.5 (25.6–27.1) | 20.6, 17.3 | 21.2 ± 2.6 (18.3–23.6) |
| b | 3.2 ± 0.1 (2.9–3.3) | 3.6 ± 0.1 (3.3–3.7) | 2.9, 2.3 | 3.3 ± 0.5 (2.8–4.0) |
| c | 14.3 ± 1.2 (13.0–16.3) | 12.4 ± 0.9 (11.2–13.8) | 12.5, 11.5 | 13.8 ± 1.0 (13.0–15.2) |
| c′ | 2.6 ± 0.3 (2.2–3.0) | 2.9 ± 0.2 (2.6–3.3) | 2.5, 2.4 | 2.3 |
Habitat and Locality
Molecular Characterization
Diagnosis (Based on Specimens Examined)
Remarks
2.3. Molecular Characterization and Morphological Illustrations of Paratylenchus spp. Populations from Localities Other than Florida
2.3.1. Paratylenchus hamatus Thorne & Allen, 1950 [18]
Description
Habitat and Locality
Molecular Characterization
2.3.2. Paratylenchus hamicaudatus (Cid del Prado Vera & Maggenti, 1988) Brzeski, 1998 [19,20]

Description
Habitat and Locality
Molecular Characterization
2.3.3. Paratylenchus holdemani Raski, 1975 [21]
Description
Habitat and Locality
Molecular Characterization
2.3.4. Paratylenchus pedrami Clavero-Camacho, Cantalapiedra-Navarrete, Archidona-Yuste, Castillo & Palomares-Rius, 2021 [25]
Description
Habitat and Locality
Molecular Characterization
2.3.5. Paratylenchus projectus Jenkins, 1956 [26]
Description
Habitat and Locality
Molecular Characterization
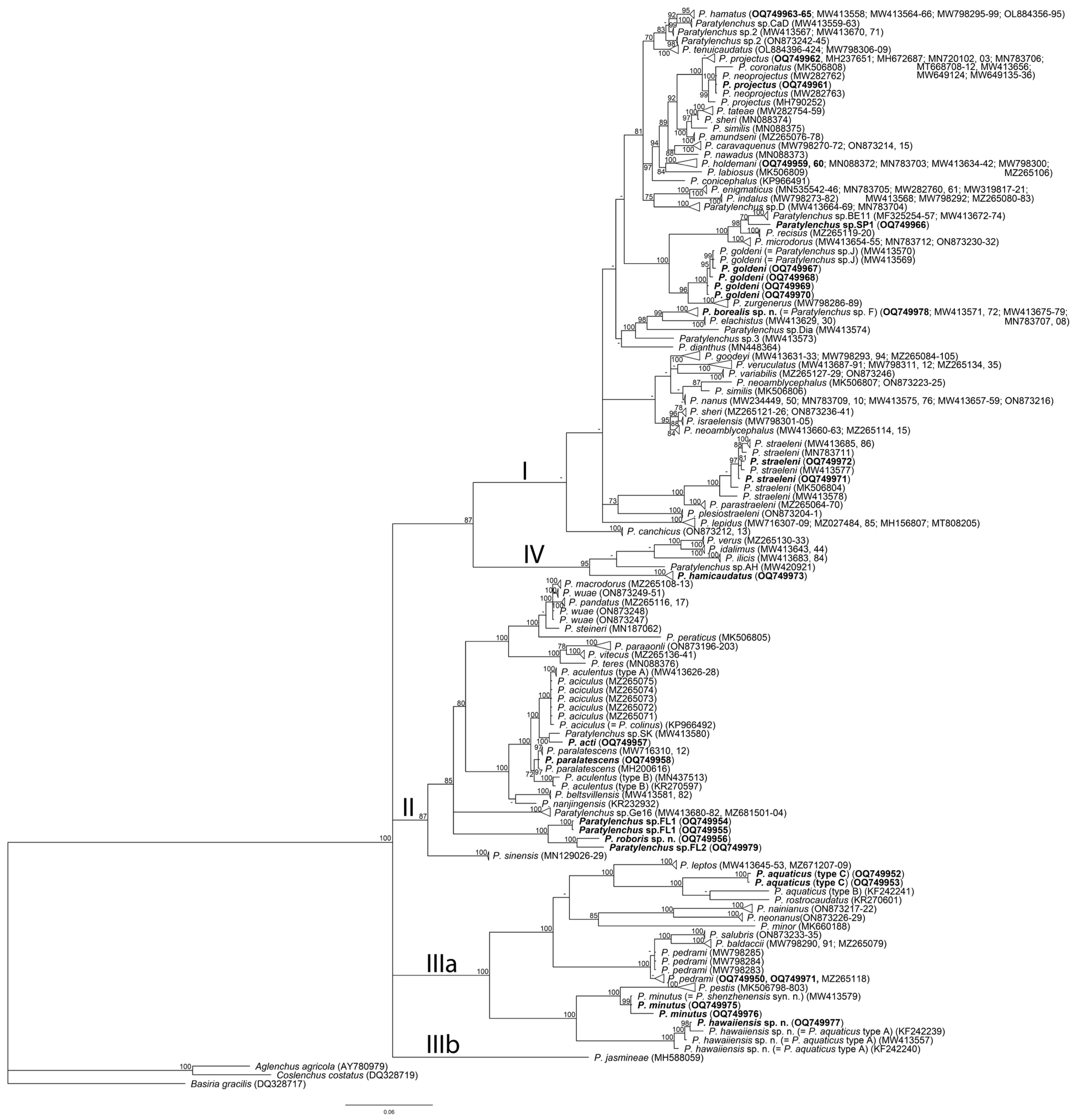
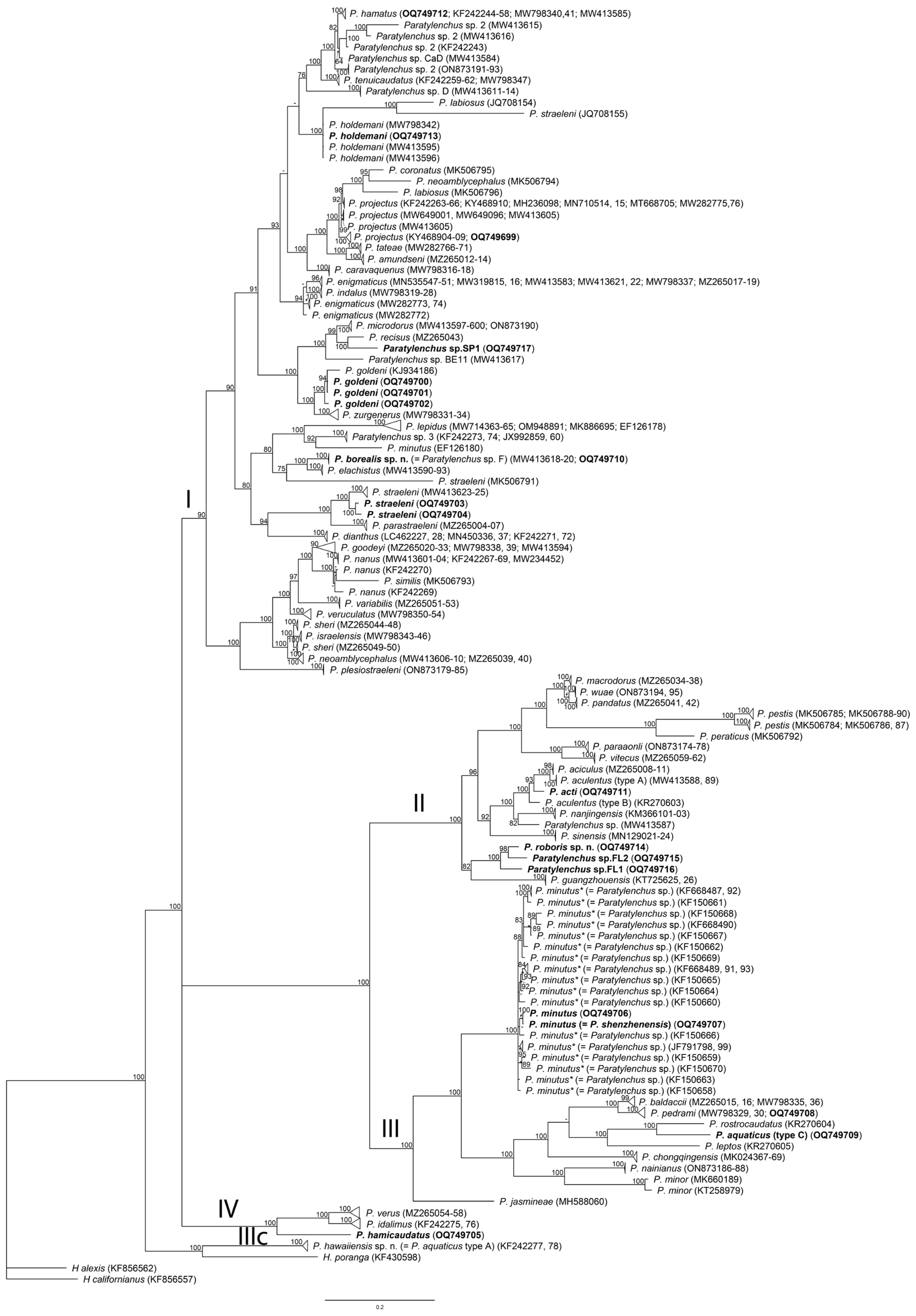
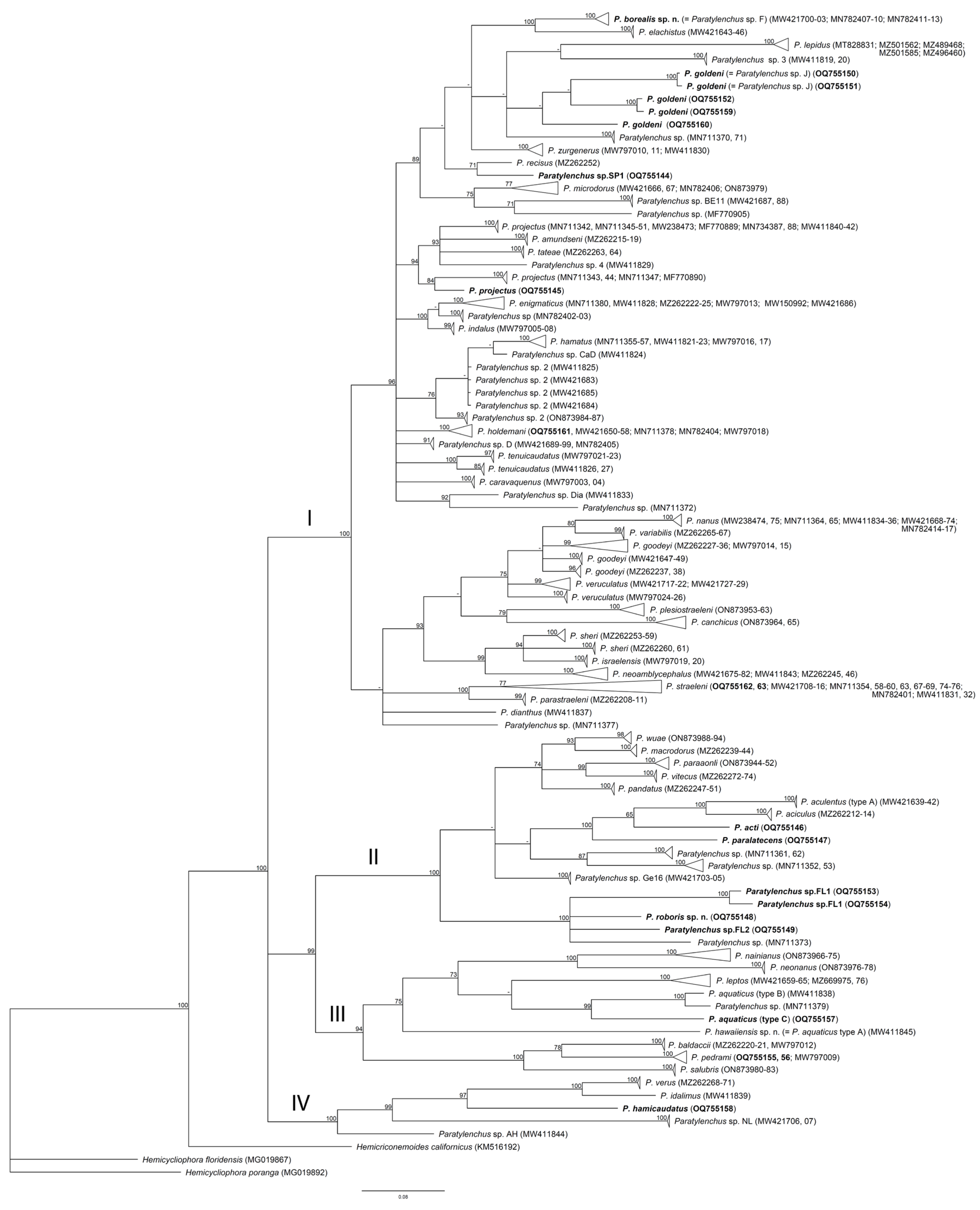
2.4. Phylogenetic Relationships of Paratylenchus Species
3. Discussion
4. Materials and Methods
4.1. Nematode Populations
4.2. Light Microscopic Study and Morphological Identification
4.3. DNA Extraction, PCR, and Sequencing
4.4. Phylogenetic and Sequence Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crow, W.; Duncan, L. Management of Plant Parasitic Nematode Pests in Florida. In Plant Parasitic Nematodes in Sustainable Agriculture of North America; Subbotin, S.A., Chitambar, J., Eds.; Sustainability in Plant and Crop Protection; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Christie, J.R. Plant Nematodes: Their Bionomics and Control; Agricultural Experiment Stations, University of Florida: Gainesville, FL, USA, 1959; 256p. [Google Scholar]
- Esser, R.P. A diagnostic compendium to species included in Paratylenchinae Thorne, 1949 and Tylenchocriconematinae Raski & Siddiqi, 1975 (Nematoda: Criconematoidea). Nematologica 1992, 38, 146–163. [Google Scholar] [CrossRef]
- Lehman, P.S. Phytoparasitic Nematodes Reported from Florida; Nematology Booklet; Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Bureau of Entomology, Nematology and Plant Pathology, Nematology Section: Gainesville, FL, USA, 2002; 18p. [Google Scholar]
- Raski, D.J. Revision of the genus Paratylenchus Micoletzky, 1922 and descriptions of new species. Part III of three parts. J. Nematol. 1976, 8, 97–115. [Google Scholar] [PubMed]
- Siddiqi, M.R. Tylenchida: Parasites of Plants and Insects; Commonwealth Institute of Parasitology: Slough, UK, 1986; 645p. [Google Scholar]
- Tarjan, A.C. A review of the genus Paratylenchus Micoletzky, 1922 (Paratylenchinae: Nematoda) with a description of two new species. Ann. N. Y. Acad. Sci. 1960, 84, 329–390. [Google Scholar] [CrossRef]
- Troccoli, A.; Vovlas, N.; Inserra, R.N. Parasitism of timber bamboo roots by Gracilacus latescens Raski, 1976 and morpho-biological notes on mature and immature life stages. Nematropica 2002, 32, 87–102. [Google Scholar]
- Inserra, R.N.; Achor, D.; Duncan, L.W.; Troccoli, A. Ultrastructure of the attachment and feeding sites of Gracilacus latescens Raski, 1976 in timber bamboo roots and selected anatomical details of the female stylet. Nematology 2003, 5, 307–312. [Google Scholar] [CrossRef]
- Steiner, G. Plant nematodes the grower should know. Proc. Soil Sci. Soc. Flo. 1949, IV, 37–39. [Google Scholar]
- Raski, D.J. Paratylenchidae n. fam, with descriptions of five new species of Gracilacus n. g. and an ememdation of Cacopaurus Thorne, 1943, Paratylenchus Mikoletzky, 1922 and Criconematidae Thorne, 1943. Proc. Helminthol. Soc. Wash. 1962, 29, 189–207. [Google Scholar]
- Siddiqi, M.R.; Goodey, J.B. The status of the genera and subfamilies of the Criconematidae (Nematoda); with a comment on the position of Fergusobia. Nematologica 1964, 9, 363–377. [Google Scholar] [CrossRef]
- Wang, K.; Xie, H.; Li, Y.; Xu, C.L.; Yu, L.; Wang, D.W. Paratylenchus shenzhenensis n. sp. (Nematoda: Paratylenchinae) from the rhizosphere soil of Anthurium andraeanum in China. Zootaxa 2013, 3750, 167–175. [Google Scholar] [CrossRef]
- Singh, P.R.; Karssen, G.; Couvreur, M.; Subbotin, S.A.; Bert, W. Integrative taxonomy and molecular phylogeny of the plant-parasitic nematode genus Paratylenchus (Nematoda: Paratylenchinae): Linking species with molecular barcodes. Plants 2021, 10, 408. [Google Scholar] [CrossRef]
- Eroshenko, A.S. Pathogenic nematodes of pine forests in the south of Sakhalin Island. Fitogel’mintologicheskie Issled. 1978, 32, 33–37. [Google Scholar]
- Merny, G. Nématodes d’Afrique tropicale: Un Nouveau Paratylenchus (Criconematidae), deux nouveaux Longidorus et observations sur Longidorus laevicapitatus Williams, 1959 (Dorylaimidae). Nematologica 1966, 12, 385–395. [Google Scholar] [CrossRef]
- Raski, D.J. Revision of the genus Paratylenchus Micoletzky, 1922 and descriptions of new species. Part I of three parts. J. Nematol. 1975, 7, 15–34. [Google Scholar] [PubMed]
- Thorne, G.; Allen, M.W. Paratylenchus hamatus n. sp. and Xiphinema index n. sp.; two new nematodes associated with fig roots, with a note on Paratylenchus anceps Cobb. Proc. Helminthol. Soc. Wash. 1950, 17, 27–35. [Google Scholar]
- Cid del Prado Vera, I.; Maggenti, A.R. Description of Gracilacus hamicaudata sp. n. (Nemata: Criconematidae) with biological and histopathological observations. Rev. Nématologie 1988, 11, 29–33. [Google Scholar]
- Brzeski, M.W. Nematodes of Tylenchina in Poland and Temperate Europe; Muzeum i Instytut Zoologii Polska Akademia Nauk: Warsaw, Poland, 1998; 397p. [Google Scholar]
- Raski, D.J. Revision of the genus Paratylenchus Micoletzky, 1922 and descriptions of new species. Part II of three parts. J. Nematol. 1975, 7, 275–295. [Google Scholar]
- Linford, M.B.; Oliveira, J.M.; Ishii, M. Paratylenchus minutus n. sp. a nematode parasitic on roots. Pac. Sci. 1949, 3, 111–119. [Google Scholar]
- Munawar, M.; Cai, R.; Ye, W.; Powers, T.O.; Zheng, J. Description of Gracilacus paralatescens n. sp. (Nematoda: Paratylenchinae) found from the rhizosphere of Bamboo in Zhejiang, China. J. Nematol. 2018, 50, 611–622. [Google Scholar] [CrossRef]
- Munawar, M.; Miao, W.; Castillo, P.; Zheng, J.-W. A new pin nematode, Paratylenchus sinensis n. sp. (Nematoda: Paratylenchinae) in the rhizosphere of white mulberry from Zhejiang Province, China. Eur. J. Plant Pathol. 2020, 156, 1023–1039. [Google Scholar] [CrossRef]
- Clavero-Camacho, I.; Cantalapiedra-Navarrete, C.; Archidona-Yuste, A.; Castillo, P.; Palomares-Rius, J.E. Remarkable cryptic diversity of Paratylenchus spp. (Nematoda: Tylenchulidae) in Spain. Animals 2021, 11, 1161. [Google Scholar] [CrossRef]
- Jenkins, W.R. Paratylenchus projectus, new species (Nematoda: Criconematidae) with a key to the species of Paratylenchus. J. Wash. Acad. Sci. 1956, 46, 296–298. [Google Scholar]
- De Conink, L.A.P. Sur trois espècies nouvelles de nematodes libres trouvés en Belgium. Bull. Musée R. D’histoire Nat. Belg. 1931, 7, 1–15. [Google Scholar]
- Oostenbrink, M. The family Criconematidae. In Nematology; Sasser, J.N., Jenkins, W.R., Eds.; University of North Carolina Press: Chapel Hill, NC, USA, 1960; pp. 196–205. [Google Scholar]
- Etongwe, C.M.; Singh, P.R.; Bert, W.; Subbotin, S.A. Molecular characterisation of some plant-parasitic nematodes (Nematoda: Tylenchida) from Belgium. Russ. J. Nematol. 2020, 28, 1–28. [Google Scholar]
- Van den Berg, E.; Tiedt, L.R.; Subbotin, S.A. Morphological and molecular characterisation of several Paratylenchus Micoletzky, 1922 (Tylenchida: Paratylenchidae) species from South Africa and USA, together with some taxonomic notes. Nematology 2014, 16, 323–358. [Google Scholar] [CrossRef]
- Ghaderi, R.; Kashi, L.; Karegar, A. Contribution to the study of the genus Paratylenchus Micoletzky, 1922 sensu lato (Nematoda: Tylenchulidae). Zootaxa 2014, 3841, 151–187. [Google Scholar] [CrossRef]
- Cobb, N.A. Notes on Paratylenchus, a genus of nemas. J. Wash. Acad. Sci. 1923, 13, 254–257. [Google Scholar]
- Subbotin, S.A.; Yan, G.; Kantor, M.; Handoo, Z. On the molecular identity of Paratylenchus nanus Cobb, 1923 (Nematoda: Tylenchida). J. Nematol. 2020, 52, 1–7. [Google Scholar] [CrossRef]
- Wouts, W.M. Paratylenchus halophilus (Nematoda: Criconematidae), a new species from New Zealand. N. Z. J. Sci. 1966, 9, 281–286. [Google Scholar]
- Geraert, E. The genus Paratylenchus. Nematologica 1965, 11, 301–334. [Google Scholar] [CrossRef]
- Bajaj, H.K. On the species of Paratylenchus Micoletzky (Nematoda: Criconematina) from Haryana, India. Indian J. Nematol. 1987, 17, 318–326. [Google Scholar]
- Andrássy, I. Neue und wenig bekannte nematoden aus Jugoslavien. Ann. Hist. Natur. Mus. Nat. Hungar. 1959, 51, 259–275. [Google Scholar]
- Raski, D.J. Paratylenchoides gen. n. and two new species (Nematoda: Paratylenchidae). Proc. Helminthol. Soc. Wash. 1973, 40, 230–233. [Google Scholar]
- Wu, Y.L. Paratylenchus veruculatus n. sp. (Criconematidae: Nematoda) from Scotland. Can. J. Zool. 1962, 40, 773–775. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Baldwin, J.G.; Choi, Y.E. New records of Paratylenchus Micoletzky, 1922 (Nematoda: Paratylenchinae) from Vietnam with description of Paratylenchus laocaiensis sp. n. J. Nematode Morphol. Syst. 2004, 7, 51–76. [Google Scholar]
- Golden, A.M. Paratylenchus steineri (Criconematidae) a new species of plant nematode. Proc. Helminthol. Soc. Wash. 1961, 28, 9–11. [Google Scholar]
- Pramodini, M.; Mohilal, N.; Dhanachand, C. Gracilacus vitecus sp. n. and record of G. raskii Phukan & Sanwal from Manipur, India. Indian J. Nematol. 2006, 36, 272–276. [Google Scholar]
- Brzeski, M.W. Paratylenchus macrodorus n. sp. (Nematoda: Paratylenchidae), a new plant parasitic nematode from Poland. Bull. I’Académie Pol. Sci. 1963, 11, 277–280. [Google Scholar]
- Misra, S.L.; Edward, J.C. Two new species of the genus Paratylenchus with description of their larval stages and a note on P. nawadus a synonym of P. nainianus. Allahabad Farmer 1971, 45, 345–350. [Google Scholar]
- Edward, J.C.; Misra, S.L.; Singh, G.R. Paratylenchus micoletzkyi n. sp. with the description of the allotype of P. nainianus Edward & Misra, 1963. Nematologica 1967, 13, 347–352. [Google Scholar] [CrossRef]
- Colbran, R.C. Plant and soil nematodes. Qld. J. Agric. Anim. Sci. 1969, 26, 181–192. [Google Scholar]
- Phukan, P.N.; Sanwal, K.C. Taxonomic studies on nematodes from Assam, India, (Paratylenchidae: Tylenchida). Indian J. Nematol. 1979, 9, 20–26. [Google Scholar]
- Inserra, R.N.; Vovlas, N.; O’Bannon, J.H.; Esser, R.P. Tylenchulus graminis n. sp. and T. palustris n. sp. from native flora in Florida, with notes on T. semipenetrans and T. furcus. J. Nematol. 1988, 20, 266–287. [Google Scholar] [PubMed]
- Brown, G.L. Three new species of the genus Paratylenchus from Canada (Nematoda: Criconematidae). Proc. Helminthol. Soc. Wash. 1959, 26, 1–8. [Google Scholar]
- Huang, C.S.; Raski, D.J. Four new species of Gracilacus Raski, 1962 (Criconematoidea: Nemata). Rev. Nématologie 1986, 9, 347–356. [Google Scholar]
- Inserra, R.N.; Troccoli, A.; Gozel, U.; Bernard, E.C.; Dunn, D.; Duncan, L. Pratylenchus hippeastri n. sp. (Nematoda Pratylenchidae) from amaryllis in Florida with notes on P. scribneri and P. hexincisus. Nematology 2007, 9, 25–42. [Google Scholar] [CrossRef]
- Huang, C.S.; Raski, D.J. New records of Paratylenchus Micoletzky, 1922 from Brazil with descriptions of two new species (Tylenchulidae: Nemata). J. Nematol. 1987, 19, 69–76. [Google Scholar]
- Brzeski, M.W. Paratylenchinae: Morphology of some known species and description of Gracilacus bilineata and G. vera sp. n. (Nematoda: Tylenchulidae). Nematologica 1995, 41, 535–565. [Google Scholar] [CrossRef]
- Zeng, Y.; Ye, W.; Tredway, L.; Martin, S.; Martin, M. Taxonomy and morphology of plant-parasitic nematodes associated with turfgrasses in North and South Carolina, USA. Zootaxa 2012, 3452, 1–46. [Google Scholar] [CrossRef]
- Zeng, Y.; Ye, W.; Kerns, J.; Tredway, L.; Martin, S.; Martin, M. Molecular characterization and phylogenetic relationships of plant-parasitic nematodes associated with turfgrasses in North Carolina and South Carolina, United States. Plant Dis. 2015, 99, 982–993. [Google Scholar] [CrossRef]
- Thorne, G. Cacopaurus pestis nov. gen. nov. spec. (Nematoda: Criconematinae), a destructive parasite of the walnut, Juglans regia Linn. Proc. Helminthol. Soc. Wash. 1943, 10, 78–83. [Google Scholar]
- Goodey, T. Soil and Freshwater Nematodes; Methuen & Co. Ltd.: London, UK, 1963; 544p. [Google Scholar]
- Micoletzky, H. Die freilebenden Erd-Nematoden mit besonderer Berücksichtigung der Steiermark und der Bukowina, zugleich met einer Revision sämtlicher nicht mariner, freilebender Nematoden in Form von Genus-Beschreibungen und Bestimmungsschlüsseln. Arch. Für Nat. A 1922, 87, 1–650. [Google Scholar]
- Ghaderi, R.; Geraert, E.; Karegar, A. The Tylenchulidae of the World, Identification of the Family Tylenchulidae (Nematoda: Tylenchida); Academy Press: Gent, Belgium, 2016; 453p. [Google Scholar]
- Brzeski, M.W.; Háněl, L. Paratylenchinae: Postembryonic developmental stages of Paratylenchus straeleni (De Conink, 1931) and P. steineri Golden, 1961 (Nematoda: Tylenchulidae). Nematology 1999, 1, 673–680. [Google Scholar] [CrossRef]
- Van den Berg, E.; Tiedt, L.R. New records of Criconematoidea (Nematoda) from South Africa, with the description of Criconema zantene n. sp. Nematology 2001, 3, 797–815. [Google Scholar] [CrossRef]
- Wu, Y.L. Paratylenchus tenuicaudatus n. sp. (Nematoda: Criconematidae). Can. J. Zool. 1961, 39, 163–165. [Google Scholar] [CrossRef]
- Singh, P.R.; Lokker, B.; Couvreur, M.; Bert, W.; Karssen, G. Paratylenchus ilicis n. sp. (Nematoda: Paratylenchinae) associated with holly from the Netherlands and new taxonomical and phylogenetic support for the synonymization of Cacopaurus with Paratylenchus. J. Nematol. 2022, 54, 20220037. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.V.; Kimpinski, J. Paratylenchus labiosus n. sp. (Nematoda: Paratylenchidae) from Canada. Can. J. Zool. 1977, 55, 1992–1996. [Google Scholar] [CrossRef]
- Wu, L.Y.; Hawn, E.J. Paratylenchus neoprojectus n. sp. (Paratylenchinae: Nematoda) from alfalfa fields in Alberta, Canada. Can. J. Zool. 1975, 53, 1841–1843. [Google Scholar] [CrossRef]
- Clavero-Camacho, I.; Palomares-Rius, J.E.; Cantalapiedra-Navarrete, C.; León-Ropero, G.; Martín-Barbarroja, J.; Archidona-Yuste, A.; Castillo, P. Taxonomy reveals hidden cryptic diversity within pin nematodes of the genus Paratylenchus (Nematoda: Tylenchulidae). Plants 2021, 10, 1454. [Google Scholar] [CrossRef]
- Munawar, M.; Yevtushenko, D.P.; Palomares-Rius, J.E.; Castillo, P. Species diversity of pin nematodes (Paratylenchus spp.) from potato growing regions of southern Alberta, Canada. Plants 2021, 10, 188. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Archidona-Yuste, A.; Clavero-Camacho, I.; de la Fuente, J.A.C.; Rey, A.; Viñegla, B.; Liébanas, G.; Cantalapiedra-Navarrete, C.; Castillo, P. DNA barcoding and morphometry reveal further cryptic biodiversity of pin nematodes of the genus Paratylenchus (Nematoda: Tylenchulidae). Plants 2022, 11, 3385. [Google Scholar] [CrossRef]
- Phani, V.; Somvanshi, V.S.; Rao, U.; Khan, M.R. Paratylenchus jasmineae sp. n. (Nematoda: Paratylenchinae) from rhizosphere of Jasminum sambac in India. Nematology 2019, 21, 469–478. [Google Scholar] [CrossRef]
- Mokaram Hesar, A.; Karegar, A.; Ghaderi, R. Phylogenetic relationships of Cacopaurus pestis Thorne, 1943 within representatives of the Tylenchulidae Skarbilovich, 1947 as inferred from ITS and D2-D3 expansion segments of 28S-rRNA sequences. Nematology 2019, 21, 971–994. [Google Scholar] [CrossRef]
- Doucet, M.E. New data on Gracilacus colina Huang; Raski, 1986 (Nemata: Criconematoidea). Fundam. Appl. Nematol. 1994, 17, 117–121. [Google Scholar]
- Zhuo, K.; Liu, X.T.; Tao, Y.; Wang, H.H.; Lin, B.R.; Liao, J.L. Morphological and molecular characterisation of three species of Paratylenchus Micoletzky, 1922 (Tylenchida: Paratylenchidae) from China, with a first description of the male P. rostrocaudatus. Nematology 2018, 20, 837–850. [Google Scholar] [CrossRef]
- Siddiqi, M.R. Tylenchida: Parasites of Plants and Insects, 2nd ed.; CABI Publishing: Wallingford, UK, 2000; 833p. [Google Scholar]
- Raski, D.J.; Siddiqui, I.A. Tylenchocriconema alleni n. gen. n. sp. from Guatemala (Tylenchocriconematidae n. fam.; Tylenchocriconematoidea n. sperfam.; Nematoda). J. Nematol. 1975, 7, 247–251. [Google Scholar]
- Siddiqi, M.R. Nematodes of tropical rainforests 3. Three new genera and five new species of tylenchs. Afro-Asian J. Nematol. 1994, 4, 22–31. [Google Scholar]
- Poghossian, E.E. New genus and species of nematode of the family Heteroderidae from Armenian SSR (Nematoda). DAN ArmSSR 1966, 42, 117–123. [Google Scholar]
- Raski, D.J.; Sher, S.A. Sphaeronema californicum nov. gen. nov. spec. (Criconematidae: Sphaeronematinae nov. subfam.) an endoparasite of the roots of certain plants. Proc. Helminthol. Soc. Wash. 1952, 19, 77–80. [Google Scholar]
- Raski, D.J. Trophotylenchulus and Trophonema two new genera of Tylenchulidae n. fam. (Nematoda). Nematologica 1957, 2, 85–90. [Google Scholar] [CrossRef]
- Cobb, N.A. Notes on Mononchus and Tylenchulus. J. Wash. Acad. Sci. 1913, 3, 287–288. [Google Scholar]
- Inserra, R.N.; Vovlas, N. Parasitic habits of Gracilacus peratica on olive feeder roots. Nematol. Medit. 1977, 5, 345–348. [Google Scholar]
- Inserra, R.N.; Vovlas, N. Parasitism of walnut, Juglans regia, by Cacopaurus pestis. J. Nematol. 1981, 13, 546–548. [Google Scholar]
- Palomares-Rius, J.E.; León-Ropero, G.; Clavero-Camacho, I.; Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Castillo, P. New interactive web-based polytomous key for species identification of pin nematodes of the genus Paratylenchus Micoletzky, 1922 (Nematoda: Paratylenchinae) with the use of ribosomal and mitochondrial genes. J. Zool. Syst. Evol. Res. 2022, 6216266. [Google Scholar] [CrossRef]
- Jenkins, W.R. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Report. 1964, 48, 692. [Google Scholar]
- Young, T.W. An incubation method for collecting migratory endo-parasitic nematodes. Plant Dis. Report. 1954, 38, 794–795. [Google Scholar]
- Esser, R.P. A water agar en face technique. Proc. Helminthol. Soc. Wash. 1986, 53, 254–255. [Google Scholar]
- Southey, J.F. (Ed.) Laboratory Methods for Work with Plant and Soil Nematodes; Technical Bulletin 2 HMSO; Ministry of Agriculture, Fisheries and Food: London, UK, 1970; 148p. [Google Scholar]
- Subbotin, S.A. Molecular identification of nematodes using polymerase chain reaction (PCR). In Techniques for Work with Plant and Soil Nematodes; Perry, R.N., Hunt, D.J., Subbotin, S.A., Eds.; CAB International: Wallingford, UK, 2021; pp. 218–239. [Google Scholar]
- Subbotin, S.A.; Sturhan, D.; Chizhov, V.N.; Vovlas, N.; Baldwin, J.G. Phylogenetic analysis of Tylenchida Thorne, 1949 as inferred from D2 and D3 expansion fragments of the 28S rRNA gene sequences. Nematology 2006, 8, 455–474. [Google Scholar] [CrossRef]
- Derycke, S.; Remerie, T.; Vierstraete, A.; Backeljau, T.; Vanfleteren, J.; Vincx, M.; Moens, T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 2005, 300, 91–103. [Google Scholar] [CrossRef]
- Powers, T.O.; Bernard, E.C.; Harris, T.; Higgins, R.; Olson, M.; Lodema, M.; Mullin, P.; Sutton, L.; Powers, K.S. COI haplotype groups in Mesocriconema (Nematoda: Criconematidae) and their morphospecies associations. Zootaxa 2014, 3827, 101–146. [Google Scholar] [CrossRef]
- Chenna, R.; Sugawara, H.; Koike, T.; Lopez, R.; Gibson, T.J.; Higgins, D.G.; Thompson, J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef]
- Chen, D.Y.; Ni, H.F.; Yen, J.H.; Tsay, T.T. Identification of a new recorded pin nematode Paratylenchus minutus (Nematoda: Criconematoidea, Tylenchulidae) in Taiwan. Plant Pathol. Bull. 2009, 18, 167–174. [Google Scholar]
- Wang, K.; Li, Y.; Xie, H.; Xu, C.-L.; Wu, W.-J. Morphology and molecular analysis of Paratylenchus guangzhouensis n. sp. (Nematoda: Paratylenchinae) from the soil associated with Bambusa multiplex in China. Eur. J. Plant Pathol. 2016, 145, 255–264. [Google Scholar] [CrossRef]
- Wang, K.; Xie, H.; Li, Y.; Wu, W.-J.; Xu, C.-L. Morphology and molecular analysis of Paratylenchus nanjingensis n. sp. (Nematoda: Paratylenchinae) from the rhizosphere soil of Pinus massoniana in China. J. Helminthol. 2016, 90, 166–173. [Google Scholar] [CrossRef]
- Yu, Q.; Ye, W.; Powers, T. Morphological and Molecular Characterization of Gracilacus wuae n. sp. (Nematoda: Criconematoidea) Associated with Cow Parsnip (Heracleum maximum) in Ontario, Canada. J. Nematol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Ki, Y.; Wang, K.; Xie, H.; Xu, C.-L. Morphology and molecular analysis of Paratylenchus chongqingensis n. sp. (Nematoda: Paratylenchinae) from soil associated with Ophiopogon japonicus in China. Eur. J. Plant Pathol. 2019, 154, 597–605. [Google Scholar] [CrossRef]
- Kantor, M.R.; Handoo, Z.A.; Subbotin, S.A.; Bauchan, G.R.; Mowery, J.D. Morphological and molecular characterization of Paratylenchus beltsvillensis n. sp. from the rhizosphere of pine tree (Pinus virginiana Mill) in Maryland, USA. J. Nematol. 2021, 53, e2021-79. [Google Scholar] [CrossRef]
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP∗: Phylogenetic Analysis Using Parsimony (∗and Other Methods), version 4.0b 10; Sinauer Associates: Sunderland, MA, USA, 2003. [Google Scholar]



| Species | Location | Host | Sample Code | GenBank Accession Number | Source | ||
|---|---|---|---|---|---|---|---|
| D2–D3 of 28S rRNA | ITS rRNA | COI | |||||
| Paratylenchus acti | Avon Park, Highlands County, FL, USA | Andropogon virginicus | CD3566, N21-01034-2 | OQ749957 | OQ749711 | OQ755146 | R.N. Inserra & J. D. Stanley |
| P. aquaticus type C | Okeechobee, Okeechobee County, FL, USA | Stenotaphrum secundatum | CD3480, N21-00445 | OQ749952 | OQ749709 | OQ755157 | R.N. Inserra |
| P. aquaticus type C | Immokalee, Collier County, FL, USA | Zoysia sp. | CD3707, N22-00239 | OQ749953 | - | - | R.N. Inserra |
| P. borealis sp. n. | Anchorage, AK, USA | Unknown plants | CD3781 | OQ749978 | OQ749710 | - | S.A. Subbotin |
| P. goldeni | Madison, Madison County, FL, USA | Zoysia sp. | CD3651, N21-01426-4 | OQ749968 | OQ749702 | OQ755160 | J. D. Stanley |
| P. goldeni | Morriston, Levy County, FL, USA | Ilex sp. | CD3443, N21-00015-4 | OQ749969 | OQ749700 | OQ755152 | R.N. Inserra |
| P. goldeni | Milton, Santa Rosa County, FL, USA | Enydra sp. | CD3453, N21-00238 | OQ749970 | OQ749701 | OQ755159 | R.N. Inserra |
| P. goldeni | Lewis Creek Trail, Madera County, CA, USA | Unknown plants | CD3470 | OQ749967 | - | - | S.A. Subbotin |
| P. goldeni | Skokomish, Mason County, WA, USA | Unknown plant | CD3216 | MW413570 | - | OQ755151 | S.A. Subbotin |
| P. goldeni | Oakland, Douglas County, OR, USA | Unknown plant | CD3220 | MW413569 | - | OQ755150 | S.A. Subbotin |
| P. hamatus | Kern County, CA, USA | Peach | CD3584 | OQ749965 | - | - | S.A. Subbotin |
| P. hamatus | Kern County, CA, USA | Grapevine | CD3625 | OQ749963 | - | - | S.A. Subbotin |
| P. hamatus | San Jose, Santa Clara County, CA, USA | Ornamental pear | CD3628 | OQ749964 | OQ749712 | - | S.A. Subbotin |
| P. hamicaudatus | Lagunitas Lake (type locality), San Rafael, Marin County, CA, USA | Sequoia sempervirens | CD3447a,b | OQ749973, OQ749974 | OQ749705 | OQ755158 | S.A. Subbotin |
| P. hawaiiensis sp. n. | Princeton, Miami-Dade County, FL, USA | Bromeliad (Aechmea sp.) | CD3688 | OQ749977 | - | - | S.A. Subbotin |
| P. holdemani | Marin County, CA, USA | Unknown plants | CD3469 | OQ749960 | - | - | S.A. Subbotin |
| P. holdemani | Área Recreativa “El Sotillo”, Villaviciosa de Odón, Madrid, Spain | Quercus ilex | CD3527, CD3528 | OQ749959 | OQ749713 | OQ755161 | S. Álvarez-Ortega |
| P. minutus | Kalaheo, Kauai Island, HI, USA | Coffea sp. | CD3538, N21-01001 | OQ749976 | OQ749706 | - | K.H. Wang |
| P. minutus | Jasper, Hamilton County, FL, USA | Hemerocallis sp. | CD3465, N21-00389-1 | OQ749975 | OQ749707 | - | R.N. Inserra |
| P. paralatescens | Gainesville, Alachua County, FL, USA | Phyllostachys nigra | CD3396, N20-01262-1 | OQ749958 | - | OQ755147 | R.N. Inserra |
| P. pedrami | Yolo County, CA, USA | Grapevine | CD3609 | OQ749971 | OQ749708 | OQ755156 | S.A. Subbotin |
| P. pedrami | San Jose, Santa Clara County, CA, USA | Unknown plants | CD3461 | OQ749950 | - | OQ755155 | S.A. Subbotin |
| P. projectus | Canada | Fragaria x ananassa | CD3623, N21-01275-1 | OQ749962 | - | - | R.N. Inserra |
| P. projectus | Villamanta, Madrid, Spain | Grape field | PP329 | OQ749961 | OQ749699 | OQ755145 | S. Álvarez-Ortega |
| P. roboris sp. n. | High Springs, Alachua County, FL, USA | Q. virginiana | CD3450, N21-00211 | OQ749956 | OQ749714 | OQ755148 | R.N. Inserra |
| P. straeleni | Ocala, Marion County, FL, USA | Q. virginiana | CD3633, N21-01260-3 | OQ749971 | OQ749704 | OQ755162 | R.N. Inserra |
| P. straeleni | Turnbull Hammock, Volusia County, FL, USA | Rapidhophyllum hystrix | CD3708, N22-00084 | OQ749972 | OQ749703 | OQ755163 | R.N. Inserra |
| Paratylenchus sp.FL1 | Fort Lauderdale, Broward County, FL, USA | Cynodon dactylon | CD3637, N21-01410 | OQ749954 | OQ749716 | OQ755153 | R.N. Inserra |
| Paratylenchus sp.FL1 | Punta Gorda, Charlotte County, FL, USA | Unidentified plant from family Palmae | CD3706, N22-00102 | OQ749955 | - | OQ755154 | R.N. Inserra |
| Paratylenchus sp.FL2 | Oviedo, Seminole County, FL, USA | Unidentified plant from family Palmae | CD3750, N22-00888 | OQ749979 | OQ749715 | OQ755149 | R.N. Inserra |
| Paratylenchus sp.SP1 | Villanueva de Perales, Madrid, Spain | Salix sp. | PP317A | OQ749966 | OQ749717 | OQ755144 | S. Álvarez-Ortega |
| Reference | Van den Berg et al. [30] | This Study | Total Range | |
|---|---|---|---|---|
| Population | Hawaii, USA (CD619) | Florida, USA (CD3688) | ||
| Character | ♀ (Fixed) | ♀ (Fixed) | ♀ | |
| Holotype | Paratypes | |||
| n | 5 | 1 | 4 | 10 |
| L | 366 ± 27.1 (342–409) | 362 | 368.6 ± 20.0 (339.0–383.0) | 339–409 |
| Stylet length (St) | 21 ± 1 (20–23) | 20.5 | 22.8 ± 2.0 (20.0–24.7) | 20–25 |
| Conus length | 13 ± 0.8 (12–14) | 12.5 | 14.0 ± 1.1 (12.5–14.8) | 12–15 |
| Stylet shaft + knob height | 8 ± 0.4 (8–9) | 8.0 | 8.8 ± 1.0 (7.5–9.9) | 7.5–10.0 |
| Knob width | 3.0 | 3.0 | 4.1 ± 0.1 (4.0–4.2) | 3.0–4.0 |
| Knob height | 2 ± 0.4 (1.5–2.0) | 2.0 | 2.6 ± 0.3 (2.3–2.9) | 1.5–3.0 |
| DGO | - | 4.0 | 4.0 ± 0.1 (3.8–4.0) | 4.0 |
| Median bulb valve length | - | 5.0 | 5.0 ± 0.0 (5.0–5.0) | 5.0 |
| Median bulb width | - | 7.0 | 8.3 ± 0.8 (7.5–9.4) | 7.0–9.5 |
| Isthmus length | - | 22.0 | 18.7 ± 1.1 (17.8–20.0) | 18–22 |
| Pharynx length | 90 ± 1.9 (89–92) | 87 | 91.5 ± 5.4 (84.1–97.0) | 84–97 |
| Anterior end to excretory pore (Ep) | 68 ± 3.1 (63.5–72) | 68 | 75.4 ± 4.5 (70.3–81.2) | 63–81 |
| Max. body width | 12 ± 1.1 (11–14) | 12.0 | 13.6 ± 1.3 (12.0–15.0) | 11–15 |
| Body width at vulva | - | 10.5 | 11.0 ± 1.4 (9.5–12.3) | 9.5–12.5 |
| Body width at anus | - | 7.5 | 8.8 ± 0.7 (8.1–9.4) | 7.5–9.5 |
| Anterior end to median bulb base | - | 51 | 56.1 ± 3.5 (53.4–61.3) | 51–61 |
| Lateral field width | 1.8 ± 0.3 (1.5–2.0) | 2.0 | 2.1 ± 0.1 (2.0–2.3) | 1.5–2.5 |
| Anterior end-vulva distance | - | 298 | 300.7 ± 13.5 (281.0–311.8) | 281–312 |
| Vulva-tail terminus distance | - | 64 | 67.9 ± 6.6 (58.0–71.3) | 58–71 |
| Genital tract length | - | 115 | 118.6 ± 26.0 (85.0–143.5) | 85–144 |
| Vulva-anus distance | 42 ± 6 (35–51) | 35.5 | 39.8 ± 4.4 (33.5–43.5) | 33–51 |
| Tail length | 27 ± 2.2 (25.5–31) | 28.0 | 28.1 ± 2.7 (24.5–30.5) | 24–31 |
| PERCENTAGES | ||||
| V | 81 ± 1.2 (79–82) | 82 | 81.4 ± 0.5 (81.0–82.0) | 79–82 |
| G or T | - | 32 | 32.3 ± 7.9 (22.6–39.5) | 23–40 |
| St/L | - | 5.7 | 6.2 ± 0.8 (5.3–7.3) | 5.5–7.5 |
| Ep/L | 18.6 ± 0.8 (17.6–19.9) | 18.8 | 20.5 ± 1.5 (18.6–22.1) | 18–22 |
| RATIOS | ||||
| a | 30.6 ± 3.8 (24.8–34.8) | 30.0 | 27.4 ± 3.8 (22.6–31.2) | 23–35 |
| b | 4.2 ± 0.2 (4.0–4.4) | 4.2 | 4.0 ± 0.3 (3.7–4.5) | 3.7–4.5 |
| c | 13.4 ± 0.7 (12.3–14.1) | 12.5 | 13.1 ± 0.8 (12.2–13.8) | 12–14 |
| c′ | 3.6 ± 0.4 (3.1–4.1) | 4.1 | 3.2 ± 0.5 (2.6–3.7) | 2.6–4.1 |
| Population | Avon Park, Highlands County (CD3566—N21-01034) | Paratypes of P. acti from Sakhalin Island Eroshenko [15] | ||
|---|---|---|---|---|
| Character | Vermiform ♀ (Live, Molted Cuticle Attached) | Vermiform ♂ (Live, Molted Cuticle Attached) | ♀ | ♂ |
| n | 8 | 4 | 11 | 2 |
| L | 289.9 ± 13.8 (277.0–311.0) | 299.0 ± 6.2 (291.0–305.9) | 240–280 | 250 |
| Stylet length (St) | 60.8 ± 2.5 (55.5–64.3) | - | 56–62 | - |
| Conus length | 55.0 ± 2.2 (50.0–57.4) | - | - | - |
| Stylet shaft + knob height | 5.8 ± 0.8 (4.9–7.0) | - | - | - |
| Knob width | 2.9 ± 0.9 (2.5–3.3) | - | - | - |
| Knob height | 1.5 ± 0.1 (1.3–1.7) | - | - | - |
| DGO | 5.5 ± 0.8 (5.0–7.0) | - | - | - |
| Median bulb valve length | 9.7 ± 0.3 (9.4–10.2) | - | - | - |
| Median bulb width | 8.7 ± 0.8 (7.5–9.8) | - | - | - |
| Isthmus length | 15.0 ± 1.7 (12.0–17.5) | - | - | - |
| Pharynx length | 109.5 ± 4.4 (104.0–114.8) | 88.1 ± 5.1 (82.1–96.0) | - | - |
| Anterior end to excretory pore (Ep) | 69.7 ± 5.1 (60.5–77.2) | 77.8 ± 2.9 (74.2–82.1) | - | - |
| Max body width | 12.8 ± 0.3 (12.3–13.3) | 12.6 ± 0.4 (12.0–13.0) | - | - |
| Body width at vulva | 11.3 ± 0.8 (10.0–12.1) | - | - | - |
| Body width at anus | 6.9 ± 0.3 (6.4–7.4) | 10.0 ± 0.5 (9.4–10.8) | - | - |
| Anterior end to median bulb base | 83.6 ± 2.8 (80.0–87.2) | - | - | - |
| Lateral field width | - | - | - | - |
| Anterior end-vulva distance | 212.0 ± 9.0 (202.0–228.7) | - | - | - |
| Vulva-tail terminus distance | 79.0 ± 6.3 (71.0–92.4) | - | - | - |
| Genital tract length | 36.6 ± 7.3 (29.0–45.5) | 105.9 ± 9.8 (96.0–118.8) | - | - |
| Vulva-anus distance | 56.9 ± 6.3 (50.0–71.2) | - | - | - |
| Tail length | 21.1 ± 1.1 (21.8–24.8) | 27.2 ± 1.1 (25.7–28.7) | 25 | - |
| Stylet base (Stb) to median bulb valve base (v) | 20.4 ± 6.0 (13.0–33.0) | - | - | - |
| Spicule length | - | 17.6 ± 0.2 (17.4–17.8) | - | 16–17 |
| Gubernaculum length | - | 4.1 ± 0.1 (4.0–4.2) | - | - |
| PERCENTAGES | ||||
| V | 72.8 ± 1.3 (70.0–74.4) | - | 69–73 | - |
| G or T | 12.5 ± 2.2 (10.0–16.0) | 35.4 ± 3.5 (31.5–40.2) | - | 34–35 |
| Stb-v/St | 33.6 ± 9.5 (21.2–51.3) | - | - | - |
| St/L | 20.6 ± 1.3 (19.0–22.4) | - | 22–24 | - |
| Ep/L | 23.6 ± 1.1 (21.8–24.8) | 26.0 ± 0.6 (25.4–27.0) | - | - |
| RATIOS | ||||
| a | 22.5 ± 1.1 (21.0–24.6) | 23.7 ± 1.1 (22.3–25.4) | 19–23 | 23 |
| b | 2.6 ± 0.1 (2.4–2.9) | 3.4 ± 0.2 (3.1–3.5) | 2.5–2.6 | 3.5 |
| c | 13.8 ± 0.8 (12.2–14.7) | 11.0 ± 0.3 (10.6–11.3) | 10–11 | 10 |
| c′ | 3.0 ± 0.2 (2.6–3.4) | 2.7 ± 0.2 (2.4–3.0) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Ortega, S.; Subbotin, S.A.; Wang, K.-H.; Stanley, J.D.; Vau, S.; Crow, W.; Inserra, R.N. Morphological and Molecular Diversity among Pin Nematodes of the Genus Paratylenchus (Nematoda: Paratylenchidae) from Florida and Other Localities and Molecular Phylogeny of the Genus. Plants 2023, 12, 2770. https://doi.org/10.3390/plants12152770
Álvarez-Ortega S, Subbotin SA, Wang K-H, Stanley JD, Vau S, Crow W, Inserra RN. Morphological and Molecular Diversity among Pin Nematodes of the Genus Paratylenchus (Nematoda: Paratylenchidae) from Florida and Other Localities and Molecular Phylogeny of the Genus. Plants. 2023; 12(15):2770. https://doi.org/10.3390/plants12152770
Chicago/Turabian StyleÁlvarez-Ortega, Sergio, Sergei A. Subbotin, Koon-Hui Wang, Jason D. Stanley, Silvia Vau, William Crow, and Renato N. Inserra. 2023. "Morphological and Molecular Diversity among Pin Nematodes of the Genus Paratylenchus (Nematoda: Paratylenchidae) from Florida and Other Localities and Molecular Phylogeny of the Genus" Plants 12, no. 15: 2770. https://doi.org/10.3390/plants12152770
APA StyleÁlvarez-Ortega, S., Subbotin, S. A., Wang, K.-H., Stanley, J. D., Vau, S., Crow, W., & Inserra, R. N. (2023). Morphological and Molecular Diversity among Pin Nematodes of the Genus Paratylenchus (Nematoda: Paratylenchidae) from Florida and Other Localities and Molecular Phylogeny of the Genus. Plants, 12(15), 2770. https://doi.org/10.3390/plants12152770









