Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil
Abstract
:1. Introduction
2. Results
2.1. Evaluation of Developed Nanoemulsion
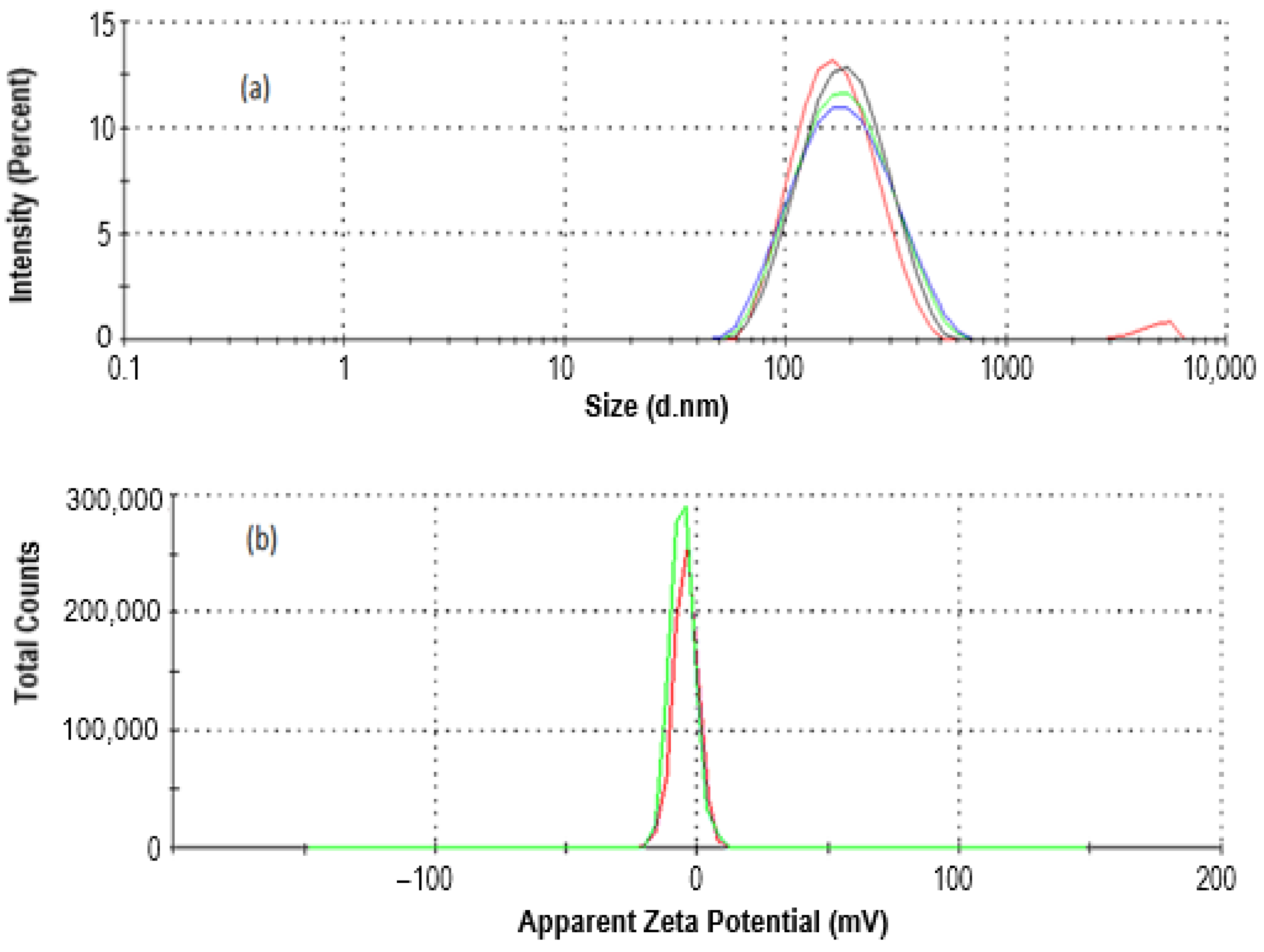
2.1.1. Size and Zeta Potential Analysis of CEO-Loaded Nanoemulsion via Dynamic Light Scattering (DLS) Study
2.1.2. Morphology Study
2.2. % Entrapment Efficiency (%EE)
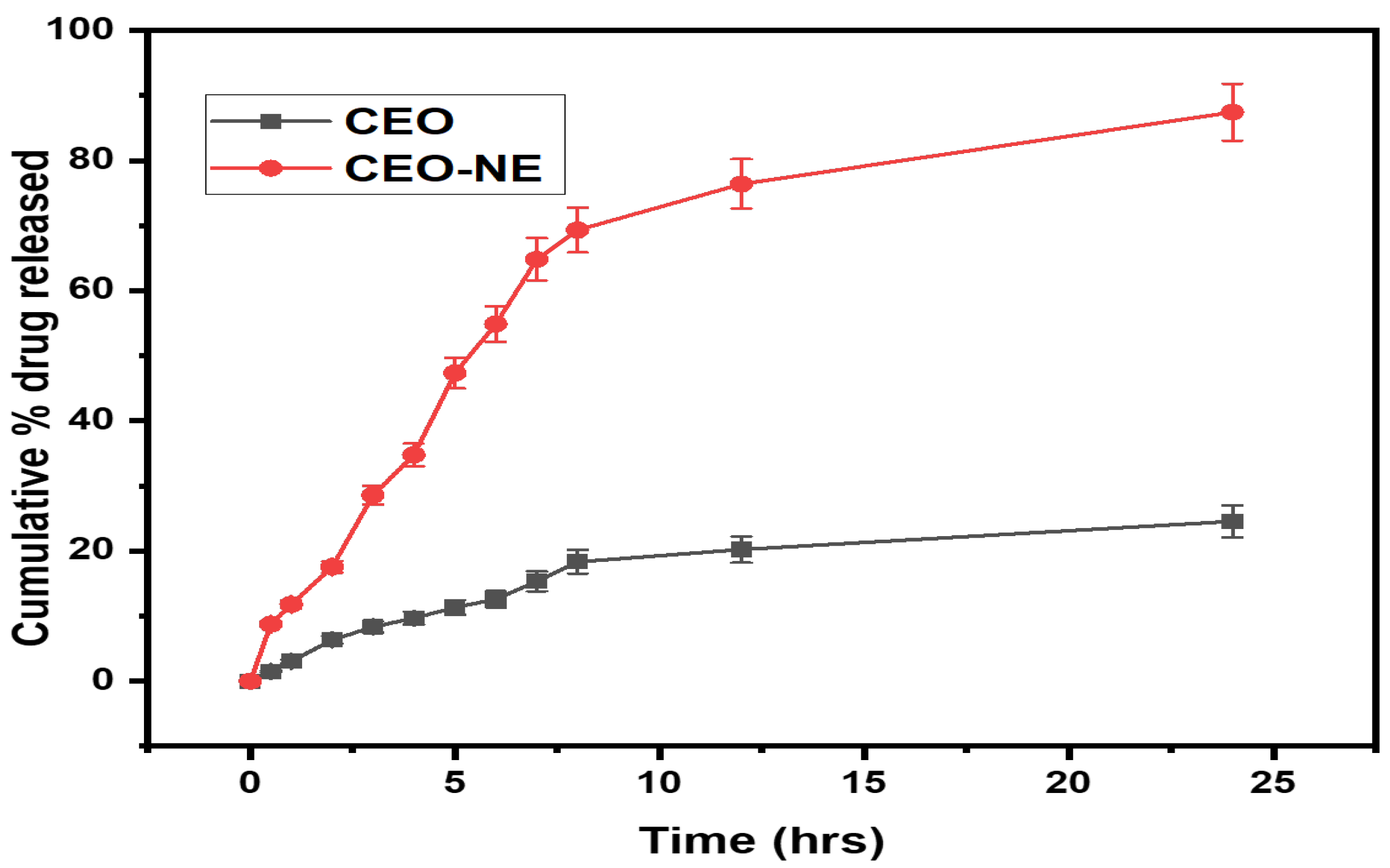
2.3. In Vitro Drug Release and Kinetic Study
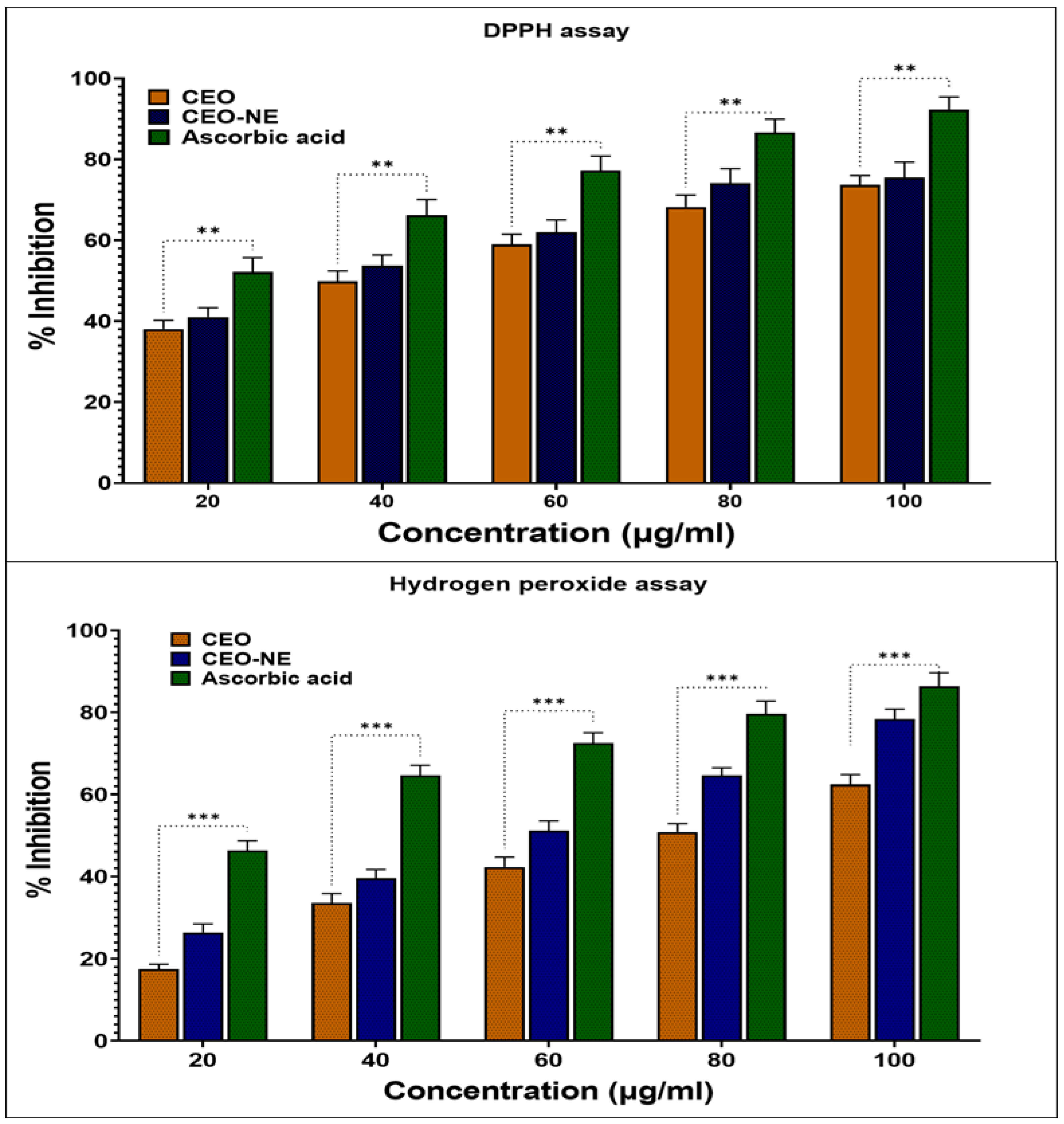
2.4. In-Vitro Anti-Oxidant Activity
2.5. In Vitro Antimicrobial Activity
2.6. Time-Kill Analysis
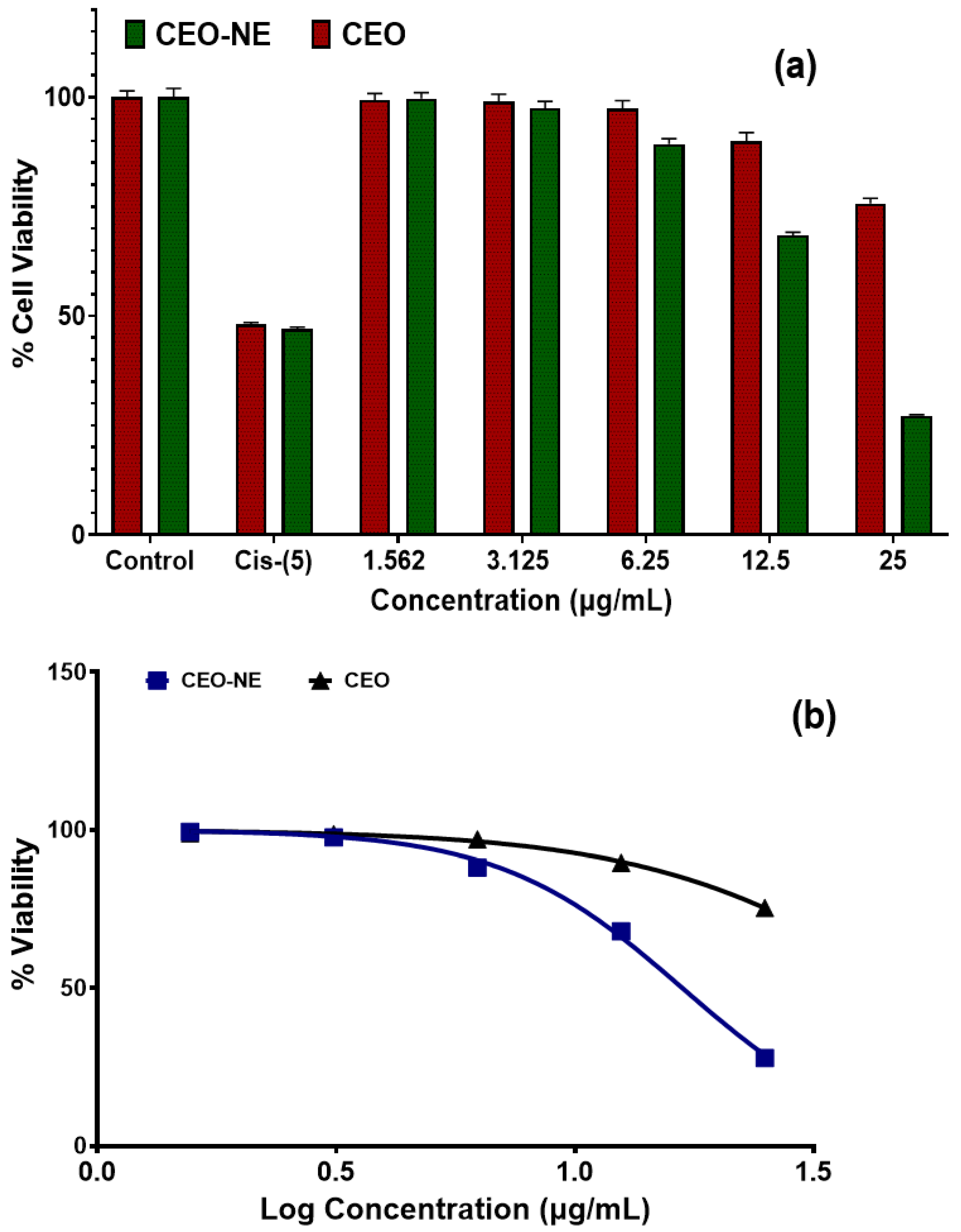
2.7. Anticancer Activity
2.8. Stability Study
3. Discussions
4. Materials and Methods
4.1. Nanoemulsion Preparation of Essential Oils
4.2. Study of Physical Appearance and Morphology
4.3. Size Distribution, Particle Size, and Zeta Potential
4.4. % Entrapment Efficiency (%EE) of CEO-NE
4.5. Drug Release and Kinetics Studies of CEO-NE
4.6. In Vitro Anti-Oxidant Activity
4.6.1. DPPH Radical Scavenging Activity
Preparation of sample/standard
Preparation of control
4.6.2. Hydrogen Peroxide Radical (H2O2) Scavenging Activity
4.7. Determination of Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.7.1. Preparation of Nutrient Media
4.7.2. Antibacterial Activity
4.8. Anticancer Activity
4.9. Short-Term Physical Stability
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linde, G.A.; Gazim, Z.C.; Cardoso, B.K.; Jorge, L.F.; Tešević, V.; Glamoćlija, J.; Soković, M.; Colauto, N.B. Antifungal and antibacterial activities of Petroselinum crispum essential oil. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Reis, D.; Jones, T. Aromatherapy: Using Essential Oils as a Supportive Therapy. Clin. J. Oncol. Nurs. 2017, 21, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Duarte, R.M.T.; Rodrigues, R.A.F.; Rodrigues, M.V.N. Essential Oils and Their Characteristics. Essent. Oils Food Process. Chem. Saf. Appl. 2017, 1–19. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Li, X.; Lu, H.Y.; Jiang, X.W.; Yang, Y.; Xing, B.; Yao, D.; Wu, Q.; Xu, Z.H.; Zhao, Q.C. Cinnamomum cassia extract promotes thermogenesis during exposure to cold via activation of brown adipose tissue. J. Ethnopharmacol. 2021, 266, 113413. [Google Scholar] [CrossRef]
- Chen, G.; Sun, F.; Wang, S.; Wang, W.; Dong, J.; Gao, F. Enhanced extraction of essential oil from Cinnamomum cassia bark by ultrasound assisted hydrodistillation. Chin. J. Chem. Eng. 2021, 36, 38–46. [Google Scholar] [CrossRef]
- Sadeghi, S.; Davoodvandi, A.; Pourhanifeh, M.H.; Sharifi, N.; ArefNezhad, R.; Sahebnasagh, R.; Moghadam, S.A.; Sahebkar, A.; Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019, 178, 131–140. [Google Scholar] [CrossRef]
- Yang, C.H.; Li, R.X.; Chuang, L.Y. Antioxidant activity of various parts of Cinnamomum cassia extracted with different extraction methods. Molecules 2012, 17, 7294–7304. [Google Scholar] [CrossRef]
- Kouame, K.; Peter, A.I.; Akang, E.N.; Moodley, R.; Naidu, E.C.; Azu, O.O. Histological and biochemical effects of Cinnamomum cassia nanoparticles in kidneys of diabetic Sprague-Dawley rats. Bosn. J. Basic Med. Sci. 2019, 19, 138–145. [Google Scholar] [CrossRef]
- Kacaniova, M.; Galovicova, L.; Valkova, V.; Tvrda, E.; Terentjeva, M.; Ziarovska, J.; Kunova, S.; Savitskaya, T.; Grinshpan, D.; Stefanikova, J.; et al. Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation. Open Chem. 2021, 19, 214–227. [Google Scholar] [CrossRef]
- Kaur, G.; Invally, M.; Khan, M.K.; Jadhav, P. A nutraceutical combination of Cinnamomum cassia & Nigella sativa for Type 1 diabetes mellitus. J. Ayurveda Integr. Med. 2018, 9, 27–37. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, L.; Fan, S.; Wang, J.; Luo, T.; Tang, Y.; Chen, Z.; Yu, L. Cinnamomum cassia Presl: A review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules 2019, 24, 3473. [Google Scholar] [CrossRef]
- Liang, D.; Feng, B.; Li, N.; Su, L.; Wang, Z.; Kong, F.; Bi, Y. Preparation, characterization, and biological activity of Cinnamomum cassia essential oil nano-emulsion. Ultrason. Sonochem. 2022, 86, 106009. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Liu, S.; Li, S. Cinnamon Cassia Oil Emulsions Stabilized by Chitin Nanofibrils: Physicochemical Properties and Antibacterial Activities. J. Agric. Food Chem. 2020, 68, 14620–14631. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef]
- Lai, W.; Liu, Y.; Kuang, Y.; Zhang, S.; Zhang, C.; Li, C.; Guo, B. Preparation and evaluation of microcapsules containing Rimulus Cinnamon and Angelica Sinenis essential oils. J. Dispers. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crops Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- Saleh, A.; Pirouzifard, M.K.; khaledabad, M.A.; Almasi, H. Optimization and Characterization of Lippia citriodora Essential Oil Loaded Niosomes: A Novel Plant-based Food Nano Preservative. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129480. [Google Scholar] [CrossRef]
- Gorjian, H.; Mihankhah, P.; Khaligh, N.G. Influence of tween nature and type on physicochemical properties and stability of spearmint essential oil (Mentha spicata L.) stabilized with basil seed mucilage nanoemulsion. J. Mol. Liq. 2022, 359, 119379. [Google Scholar] [CrossRef]
- Firooziyan, S.; Osanloo, M.; Basseri, H.R.; Moosa-Kazemi, S.H.; Hajipirloo, H.M.; Amani, A.; Sedaghat, M.M. Nanoemulsion of Myrtus communis essential oil and evaluation of its larvicidal activity against Anopheles stephensi. Arab. J. Chem. 2022, 15, 104064. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Rojekar, S.; Pai, R.; Abadi, L.F.; Mahajan, K.; Prajapati, M.K.; Kulkarni, S.; Vavia, P. Dual loaded nanostructured lipid carrier of nano-selenium and Etravirine as a potential anti-HIV therapy. Int. J. Pharm. 2021, 607, 120986. [Google Scholar] [CrossRef] [PubMed]
- Khine, Y.Y.; Callari, M.; Lu, H.; Stenzel, M.H. Direct Correlation Between Zeta Potential and Cellular Uptake of Poly(methacrylic acid) Post-Modified with Guanidinium Functionalities. Macromol. Chem. Phys. 2016, 217, 2302–2309. [Google Scholar] [CrossRef]
- Hagendorfer, H.; Kaegi, R.; Parlinska, M.; Sinnet, B.; Ludwig, C.; Ulrich, A. Characterization of silver nanoparticle products using asymmetric flow field flow fractionation with a multidetector approach—A comparison to transmission electron microscopy and batch dynamic light scattering. Anal. Chem. 2012, 84, 2678–2685. [Google Scholar] [CrossRef]
- Tuoriniemi, J.; Johnsson, A.C.J.H.; Holmberg, J.P.; Gustafsson, S.; Gallego-Urrea, J.A.; Olsson, E.; Pettersson, J.B.C.; Hassellöv, M. Intermethod comparison of the particle size distributions of colloidal silica nanoparticles. Sci. Technol. Adv. Mater. 2014, 15, 3. [Google Scholar] [CrossRef]
- Rehman, H.; Ali, W.; Khan, N.Z.; Aasim, M.; Khan, T. Delphinium uncinatum mediated biosynthesis of Zinc Oxide nanoparticles and in-vitro evaluation of their antioxidant, cytotoxic, antimicrobial, anti-diabetic, anti-inflammatory, and anti-aging activities. Saudi J. Biol. Sci. 2022, 30, 103485. [Google Scholar] [CrossRef]
- Ansarian, E.; Aminzare, M.; Azar, H.H.; Mehrasbi, M.R.; Bimakr, M. Nanoemulsion-based basil seed gum edible film containing resveratrol and clove essential oil: In vitro antioxidant properties and its effect on oxidative stability and sensory characteristic of camel meat during refrigeration storage. Meat Sci. 2022, 185, 108716. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Gharari, Z.; Hanachi, P.; Sadeghinia, H.; Walker, T.R. Cichorium intybus bio-callus synthesized silver nanoparticles: A promising antioxidant, antibacterial and anticancer compound. Int. J. Pharm. 2022, 625, 122062. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of size-dependent antimicrobial and cytotoxic properties of silver nanoparticles. Adv. Mater. Sci. Eng. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Topuz, O.K.; Özvural, E.B.; Zhao, Q.; Huang, Q.; Chikindas, M.; Gölükçü, M. Physical and antimicrobial properties of anise oil loaded nanoemulsions on the survival of foodborne pathogens. Food Chem. 2016, 203, 117–123. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Salkini, M.A.; Ross, S.A.; Yusufoglu, H.S. Phytochemical Screening, In Vitro and In Silico Studies of Volatile Compounds from Petroselinum crispum (Mill) Leaves Grown in Saudi Arabia. Molecules 2022, 27, 934. [Google Scholar] [CrossRef]
- Singh, S.; Numan, A.; Zhan, Y.; Singh, V.; Van Hung, T.; Nam, N.D. A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework. J. Hazard. Mater. 2020, 399, 123042. [Google Scholar] [CrossRef]
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Singh, S.; Alhakamy, N.A.; Bakhaidar, R.B.; Halwani, A.A.; Badr-Eldin, S.M. Gum Acacia Functionalized Colloidal Gold Nanoparticles of Letrozole as Biocompatible Drug Delivery Carrier for Treatment of Breast Cancer. Pharmaceutics 2021, 13, 1554. [Google Scholar] [CrossRef]
- Singh, S.; Aldawsari, H.M.; Alam, A.; Alqarni, M.H.S.; Ranjan, S.; Kesharwani, P. Synthesis and antimicrobial activity of vancomycin–conjugated zinc coordination polymer nanoparticles against methicillin-resistant staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2022, 70, 103255. [Google Scholar] [CrossRef]
- Athavale, A.; Jirankalgikar, N.; Nariya, P.; De, S. Evaluation of in-vitro antioxidant activity of Panchagavya: A traditional ayurvedic preparation. Int. J. Pharm. Sci. Res. 2012, 3, 2543–2549. [Google Scholar]
- Ruch, R.J.; Cheng, S.j.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Mohammadi-Sichani, M. Effect of different extracts of Stevia rebaudiana leaves on Streptococcus mutans growth. J. Med. Plants Res. 2012, 6, 4731–4734. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Mondal, L.; Mukherjee, B.; Dutta, L.; Ehsan, I.; Debnath, M.C.; Gaonkar, R.H.; Pal, M.M.; Majumdar, S. Apigenin loaded nanoparticle delayed development of hepatocellular carcinoma in rats. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1905–1917. [Google Scholar] [CrossRef] [PubMed]

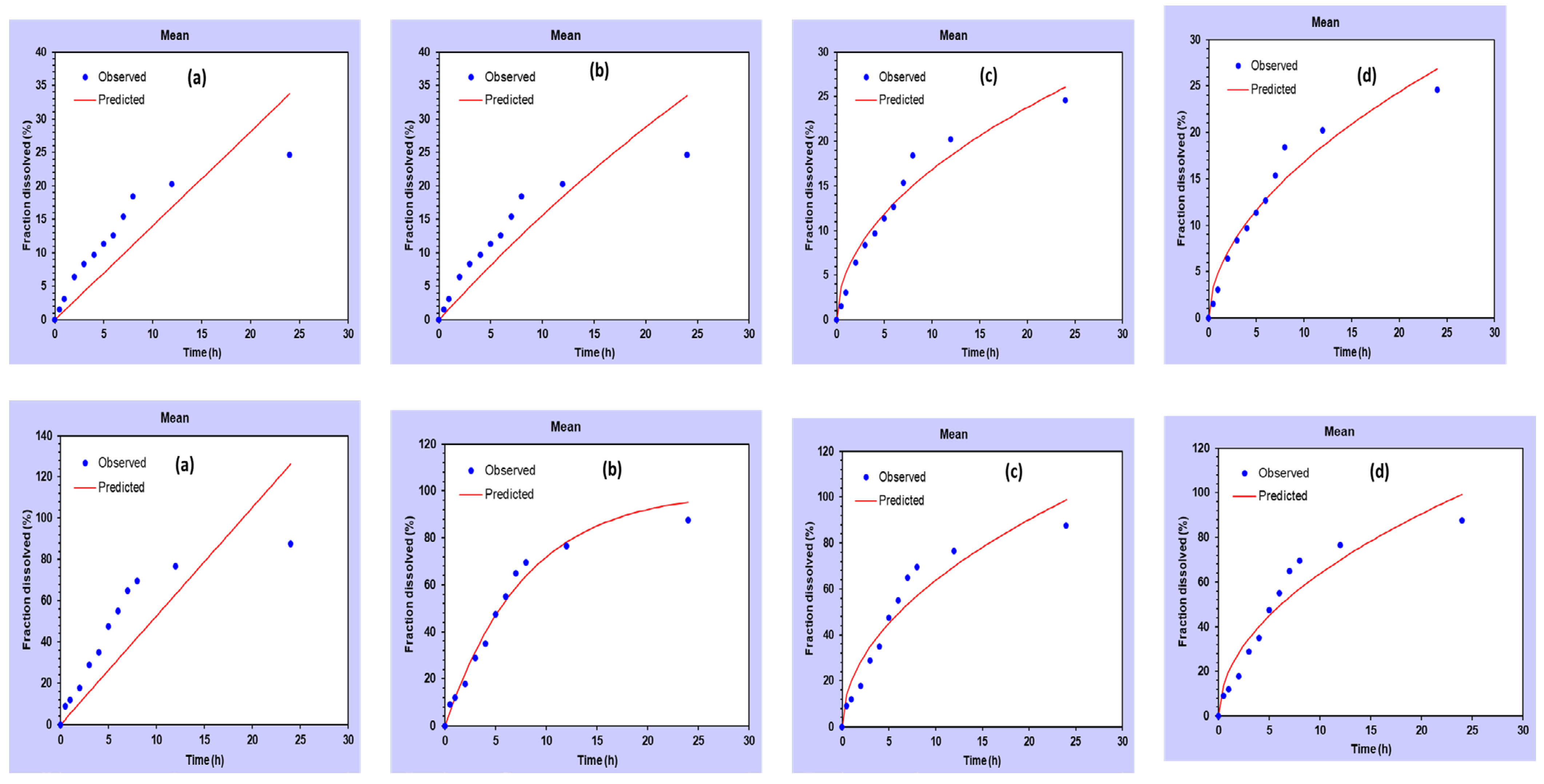

| Formulation | Zero Order | First Order | Higuchi | Kors–Peppas |
|---|---|---|---|---|
| CEO | 0.598 | 0.719 | 0.950 | 0.953 |
| CEO-NE | 0.498 | 0.978 | 0.916 | 0.907 |
| Treatment Given | IC50 (μg/mL) |
|---|---|
| DPPH radical scavenging assay of ascorbic acid | 10.42 ± 0.28 |
| DPPH radical scavenging assay of C.A. oil | 42.65 ± 1.7 |
| DPPH radical scavenging assay of C.A. nanoemulsion | 34.75 ± 1.54 |
| Hydrogen peroxide assay of ascorbic acid | 18.11 ± 0.7 |
| Hydrogen peroxide radical scavenging assay of C.A. oil | 83.21 ± 2.1 |
| Hydrogen peroxide radical scavenging assay of C.A. nanoemulsion | 70.89 ± 1.9 |
| Days | Temperature | %EE | Particle Size | PDI | ZP (mV) |
|---|---|---|---|---|---|
| 0th | 4 ℃ | 63.65 ± 3.182 | 221.8 ± 11.09 | 0.38 ± 0.019 | −5.6 ± 0.28 |
| Room temp. | 63.65 ± 3.182 | 221.7 ± 11.085 | 0.38 ± 0.019 | −5.6 ± 0.28 | |
| 15th | 4 ℃ | 64.75 ± 3.237 | 221.7 ± 11.085 | 0.38 ± 0.019 | −5.6 ± 0.28 |
| Room temp. | 63.45 ± 3.172 | 222.3 ± 11.115 | 0.45 ± 0.0225 | −5.9 ± 295 | |
| 30th | 4 ℃ | 66.23 ± 3.311 | 221.7 ± 11.085 | 0.38 ± 0.019 | −5.7 ± 0.285 |
| Room temp. | 59.87 ± 2.993 | 226.9 ± 11.345 | 0.51 ± 0.0255 | −6.8 ± 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.; Ansari, M.J.; Alqarni, M.H.; Salkini, M.A.; Raish, M. Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil. Plants 2023, 12, 834. https://doi.org/10.3390/plants12040834
Alam A, Ansari MJ, Alqarni MH, Salkini MA, Raish M. Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil. Plants. 2023; 12(4):834. https://doi.org/10.3390/plants12040834
Chicago/Turabian StyleAlam, Aftab, Mohammad Javed Ansari, Mohammed H. Alqarni, Mohammad Ayman Salkini, and Mohammad Raish. 2023. "Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil" Plants 12, no. 4: 834. https://doi.org/10.3390/plants12040834
APA StyleAlam, A., Ansari, M. J., Alqarni, M. H., Salkini, M. A., & Raish, M. (2023). Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum Cassia L. Essential Oil. Plants, 12(4), 834. https://doi.org/10.3390/plants12040834







