Acclimation of the Grapevine Vitis vinifera L. cv. Assyrtiko to Water Deficit: Coordination of Structural and Functional Leaf Traits and the Dynamic of Calcium Oxalate Crystals
Abstract
:1. Introduction
2. Results
2.1. Leaf Functional Parameters
2.2. Leaf Structural Modifications
3. Discussion
4. Material and Methods
4.1. Research Sites and Plant Material
4.2. Morphological and Anatomical Estimations
4.3. Leaf Water Status
4.4. Chlorophyll Fluorescence
4.5. Leaf Gas Exchange Parameters
4.6. Properties of Calcium Oxalate Crystals
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, G.; Shevliakova, E.; Artaxo, P.; De Noblet-Ducoudré, N.; Houghton, R.; House, J.; Kitajima, K.; Lennard, C.; Popp, A.; Sirin, A.; et al. Land–climate interactions. In Special Report on Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019; pp. 133–206. Available online: https://www.ipcc.ch/srccl/chapter/chapter-2/ (accessed on 2 March 2021).
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperate drylands and intensifies drought in deep soils. Nat. Commun. 2017, 8, 14196. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, J.M. The Origins and Ancient History of Wine; Routledge: Oxfordshire, UK, 2003. [Google Scholar]
- Savo, V.; Kumbaric, A.; Caneva, G. Grapevine (Vitis vinifera L.) symbolism in the ancient Euro-Mediterranean cultures. Econ. Bot. 2016, 70, 190–197. [Google Scholar] [CrossRef]
- Moutinho-Pereira, J.; Gonçalves, B.; Bacelar, E.; Cunha, J.B.; Countinho, J.; Correira, C.M. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Physiological and yield attributes. Vitis-J. Grapevine Res. 2015, 48, 159–165. [Google Scholar]
- Leão, P.C.S. Genetic resources for table-grape breeding in Brazilian tropical semi-arid regions. Acta Hortic. 2018, 1248, 81–86. [Google Scholar] [CrossRef]
- de Oliveira, J.B.; Egipto, R.; Laureano, O.; de Castro, R.; Pereira, G.E.; Ricardo-da-Silva, J.M. Chemical characteristics of grapes cv. Syrah (Vitis vinifera L.) grown in the tropical semiarid region of Brazil (Pernambuco state): Influence of rootstock and harvest season. J. Sci. Food Agric. 2019, 99, 5050–5063. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef]
- Schultz, H.R.; Stoll, M. Some critical issues in environmental physiology of grapevines: Future challenges and current limitations. Aust. J. Grape Wine Res. 2010, 16, 4–24. [Google Scholar] [CrossRef]
- Shelden, M.C.; Vandeleur, R.; Kaiser, B.N.; Tyerman, S.D. A comparison of petiole hydraulics and aquaporin expression in an anisohydric and isohydric cultivar of grapevine in response to water-stress induced cavitation. Front. Plant Sci. 2017, 8, 1893. [Google Scholar] [CrossRef]
- Liakopoulos, G.; Nikolopoulos, D.; Karabourniotis, G. The first step from light to wine: Photosynthetic performance and photoprotection of grapevine (Vitis vinifera L.) leaves. Funct. Plant Sci. Biotechol. 2007, 1, 112–119. [Google Scholar]
- Lovisolo, C.; Lavoie-Lamoureux, A.; Tramontini, S.; Ferrandino, A. Grapevine adaptations to water stress: New perspectives about soil/plant interactions. Theor. Exp. Plant Physiol. 2016, 28, 53–66. [Google Scholar] [CrossRef]
- Marusig, D.; Tombesi, S. Abscisic acid mediates drought and salt stress responses in Vitis vinifera—A Review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, P.; Teixeira, G.; Lopes, C.M.; Monteiro, A. The role of grapevine leaf morphoanatomical traits in determining capacity for coping with abiotic stresses: A review. Ci. Técn. Vit. 2021, 36, 75–88. [Google Scholar] [CrossRef]
- Flexas, J.; Escalona, J.M.; Medrano, H. Down-regulation of photosynthesis by drought under field conditions in grapevine leaves. Aust. J. Plant Physiol. 1998, 25, 893–900. [Google Scholar] [CrossRef]
- Dayer, S.; Reingwirtz, I.; McElrone, A.J.; Gambetta, G.A. Response and recovery of grapevine to water deficit: From genes to physiology. In The Grape Genome; Cantu, D., Walker, M.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 223–245. [Google Scholar] [CrossRef]
- Cifre, J.; Bota, J.; Escalona, J.M.; Medrano, H.; Flexas, J. Physiological tools for irrigation scheduling in grapevine (Vitis vinifera L.): An open gate to improve water-use efficiency? Agric. Ecosyst. Environ. 2005, 106, 159–170. [Google Scholar] [CrossRef]
- Cancela, J.J.; Trigo-Córdoba, E.; Martínez, E.M.; Rey, B.J.; Bouzas-Cid, Y.; Fandiño, M.; Mirás-Avalos, J.M. Effects of climate variability on irrigation scheduling in white varieties of Vitis vinifera (L.) of NW Spain. Agric. Water Manag. 2016, 170, 99–109. [Google Scholar] [CrossRef]
- Canoura, C.; Kelly, M.T.; Ojeda, H. Effect of irrigation and timing and type of nitrogen application on the biochemical composition of Vitis vinifera L. cv. Chardonnay and Syrah grapeberries. Food Chem. 2018, 241, 171–181. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Fraga, H. Climate Change: A new challenge for the winemaking sector. Agronomy 2020, 10, 1465. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; DiBari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Arias, L.A.; Berli, F.; Fontana, A.; Bottini, R.; Piccoli, P. Climate change effects on grapevine physiology and biochemistry: Benefits and challenges of high altitude as an adaptation strategy. Front. Plant Sci. 2022, 13, 835425. [Google Scholar] [CrossRef] [PubMed]
- Mosedale, J.R.; Abernethy, K.E.; Smart, R.E.; Wilson, R.J.; Maclean, I.M.D. Climate change impact and adaptive strategies: Lessons from the grapevine. Glob. Chang. Biol. 2016, 22, 3814–3828. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, A.; Giannetto, C.; Zirilli, A.; Alibrandi, A.; Lanfranchi, M. How Mediterranean winegrowers perceive climate change. AIMS Agric. Food 2023, 8, 440–460. [Google Scholar] [CrossRef]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Constraints of photosynthetic performance and water status of four evergreen species co-occurring under field conditions. Bot. Stud. 2012, 53, 325–334. [Google Scholar]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Leaf functional traits of four evergreen species growing in Mediterranean environmental conditions. Acta Physiol. Plant. 2017, 39, 34. [Google Scholar] [CrossRef]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.; et al. “Alarm Photosynthesis”: Calcium oxalate crystals as an internal CO2 source in plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Karabourniotis, G.; Horner, H.T.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G. New insights on the functions of carbon-calcium-inclusions in plants. New Phytol. 2020, 228, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Reevaluation of the plant “gemstones”: Calcium oxalate crystals sustain photosynthesis under drought conditions. Plant Sign. Behav. 2016, 11, e1215793. [Google Scholar] [CrossRef]
- Tooulakou, G.; Nikolopoulos, D.; Dotsika, E.; Orkoula, M.G.; Kontoyiannis, C.G.; Liakopoulos, G.; Klapa, M.I.; Karabourniotis, G. Changes in size and composition of pigweed (Amaranthus hybridus L.) calcium oxalate crystals under CO2 starvation conditions. Physiol. Plant. 2018, 166, 862–872. [Google Scholar] [CrossRef]
- Fabbri, A.; Benelli, C.; di Collalto, G. Calcium oxalate crystals in vegetative and reproductive organs of the grapevine. Acta Hortic. 1992, 292, 107–112. [Google Scholar] [CrossRef]
- Arnott, H.J.; Webb, M.A. Twinned raphides of calcium oxalate in grape (Vitis): Implications for crystal stability and function. Int. J. Plant Sci. 2000, 161, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Jauregui-Zuniga, D.; Reyes-Grajeda, J.P.; Sepulveda-Sanchez, J.D.; Whitaker, J.R.; Moreno, A. Crystallochemical characterization of calcium oxalate crystals isolated from seed coats of Phaseolus vulgaris and leaves of Vitis vinifera. J. Plant Physiol. 2003, 160, 239–245. [Google Scholar] [CrossRef] [PubMed]
- De Bolt, S.; Hardie, J.; Tyerman, S.; Ford, C.M. Composition and synthesis of raphid crystals and druse crystals in berries of Vitis vinifera L. cv. Cabernet Sauvignon: Ascorbic acid as precursor for both oxalic and tartaric acids as revealed by radiolabelling studies. Austr. J. Grape Wine Res. 2004, 10, 134–142. [Google Scholar] [CrossRef]
- Webb, M.A. Analysis of the organic matrix associated with calcium oxalate crystals in Vitis mustangensis. In Mechanisms and Phylogeny of Mineralization in Biological Systems; Suga, S., Nakahara, H., Eds.; Springer: Tokyo, Japan, 1991; pp. 117–121. [Google Scholar]
- Webb, M.A.; Cavaletto, J.M.; Carpita, N.C.; Lopez, L.E.; Arnott, H.J. The intravacuolar organic matrix associated with calcium oxalate crystals in leaves of Vitis. Plant J. 1995, 7, 633–648. [Google Scholar] [CrossRef]
- Horner, H.T.; Wagner, B.L. Calcium oxalate formation in higher plants. In Calcium Oxalate in Biological Systems; Khan, S.R., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 53–72. [Google Scholar]
- Giannopoulos, A.; Bresta, P.; Nikolopoulos, D.; Liakopoulos, G.; Fasseas, C.; Karabourniotis, G. Changes in the properties of calcium-carbon inclusions during leaf development and their possible relationship with leaf functional maturation in three inclusion-bearing species. Protoplasma 2019, 256, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Karabourniotis, G.; Liakopoulos, G.; Bresta, P.; Nikolopoulos, D. The optical properties of leaf structural elements and their contribution to photosynthetic performance and photoprotection. Plants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Lukšić, K.; Mucalo, A.; Smolko, A.; Brkljačić, L.; Marinov, L.; Hančević, K.; Ozretić Zoković, M.; Bubola, M.; Maletić, E.; Karoglan Kontić, J.; et al. Biochemical Response and Gene Expression to Water Deficit of Croatian Grapevine Cultivars (Vitis vinifera L.) and a Specimen of Vitis sylvestris. Plants 2023, 12, 3420. [Google Scholar] [CrossRef]
- Marta, A.E.; Slabu, C.; Covasa, M.; Motrescu, I.; Lungoci, C.; Jitareanu, C.D. Influence of Environmental Factors on Some Biochemical and Physiological Indicators in Grapevine from Copou Vineyard, Iasi, Romania. Agronomy 2023, 13, 886. [Google Scholar] [CrossRef]
- Frioni, T.; Pastore, C.; Diago, M.P. Resilience of grapevine to climate change: From plant physiology to adaptation strategies-Volume II. Front. Plant Sci. 2023, 14, 1268158. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Cifre, J.; Bota, J.; Flexas, J. A ten-year study on the physiology of two Spanish grapevine cultivars under field conditions: Effects of water availability from leaf photosynthesis to grape yield and quality. Funct. Plant Biol. 2003, 30, 607–619. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Lopes, C.M.; Chaves, M.M. Grapevine varieties exhibiting differences in stomatal response to water deficit. Funct. Plant Biol. 2012, 39, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Fernández-Fernández, J.I.; Gil-Muñoz, R.; Botia, P. Vigour yield quality relationships in long-term deficit irrigated winegrapes grown under semiarid conditions. Theor. Exp. Plant Physiol. 2016, 28, 23–51. [Google Scholar] [CrossRef]
- Kolyva, F.; Rhizopoulou, S.; Meletiou-Christou, M.S.; Stratakis, E. Physiological characteristics of expanding and expanded leaves of Vitis vinifera L. cv. Assyrtiko in climate change conditions. Biol. Life Sci. Forum 2021, 4, 55. [Google Scholar] [CrossRef]
- Pagay, V.; Furlan, T.S.; Kidman, C.M.; Nagahatenna, D. Long-term drought adaptation of unirrigated grapevines (Vitis vinifera L.). Theor. Exp. Plant Physiol. 2022, 34, 215–225. [Google Scholar] [CrossRef]
- Pou, A.; Balda, P.; Cifre, J.; Ochogavia, J.M.; Ayestarán, B.; Guadalupe, Z.; Llompart, M.; Bota, J.; Martínez-Lapuente, L. Influence of non-irrigation and seasonality on wine colour, phenolic composition and sensory quality of a grapevine (Vitis vinifera cv. Callet) in a Mediterranean climate. OENO One 2023, 57, 217–233. [Google Scholar] [CrossRef]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Fyllas, N.M.; Theocharis, S.; Jones, G.V. Greek Wine Quality Assessment and Relationships with Climate: Trends, Future Projections and Uncertainties. Water 2022, 14, 573. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Gambetta, G.A.; Biniari, K. A comparative study on training systems and vine density in Santorini Island: Physiological, microclimate, yield and quality attributes. OENO One 2023, 57, 141–152. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M.; Ruiz, C.; Lissarrague, J.R. Effect of water stress on leaf area development, photosynthesis, and productivity in Chardonnay and Airén grapevines. Am. J. Enol. Vitic. 2002, 53, 138–143. [Google Scholar] [CrossRef]
- Briglia, N.; Montanaro, G.; Petrozza, A.; Summerer, S.; Cellini, F.; Nuzzo, V. Drought phenotyping in Vitis vinifera using RGB and NIR imaging. Sci. Hortic. 2019, 256, 108555. [Google Scholar] [CrossRef]
- Roig-Oliver, M.; Nadal, M.; Clemente-Moreno, M.J.; Bota, J.; Flexas, J. Cell wall components regulate photosynthesis and leaf water relations of Vitis vinifera cv. Grenache acclimated to contrasting environmental conditions. J. Plant Physiol. 2020, 244, 153084. [Google Scholar] [CrossRef]
- Zamorano, D.; Franck, N.; Pastenes, C.; Wallberg, B.; Garrido, M.; Silva, H. Improved physiological performance in grapevine (Vitis vinifera L.) cv. Cabernet Sauvignon facing recurrent drought stress. Aust. J. Grape Wine Res. 2021, 27, 258–268. [Google Scholar] [CrossRef]
- Pagay, V.; Zufferey, V.; Lakso, A.N. The influence of water stress on grapevine (Vitis vinifera L.) shoots in a cool, humid climate: Growth, gas exchange and hydraulics. Funct. Plant Biol. 2016, 43, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, A.J.; Davies, W.J. The coupled response of stomatal conductance to photosynthesis and transpiration. J. Exp. Bot. 1998, 49, 399–406. Available online: http://www.jstor.org/stable/23695973 (accessed on 10 September 2022). [CrossRef]
- Gómez-Espinoza, O.; González-Ramírez, D.; Méndez-Gómez, J.; Guillén-Watson, R.; Medaglia-Mata, A.; Bravo, L.A. Calcium oxalate crystals in leaves of the extremophile plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae). Plants 2021, 10, 1787. [Google Scholar] [CrossRef]
- Botelho, R.V.; Roberti, R.; Tessarin, P.; Garcia-Mina, J.M.; Rombolà, A.D. Physiological responses of grapevines to biodynamic management. Renew. Agric. Food Syst. 2016, 31, 402–413. [Google Scholar] [CrossRef]
- Düring, H. Stomatal responses to alterations of soil and air humidity in grapevines. Vitis 1987, 26, 9–18. [Google Scholar] [CrossRef]
- Merli, M.C.; Gatti, M.; Galbignani, M.; Bernizzoni, F.; Magnanini, E.; Poni, S. Water use efficiency in Sangiovese grapes (Vitis vinifera L.) subjected to water stress before veraison: Different levels of assessment lead to different conclusions. Funct. Plant Biol. 2014, 42, 198–208. [Google Scholar] [CrossRef]
- Merli, M.C.; Gatti, M.; Galbignani, M.; Bernizzoni, F.; Magnanini, E.; Poni, S. Comparison of whole-canopy water use efficiency and vine performance of cv. Sangiovese (Vitis vinifera L.) vines subjected to a post-veraison water deficit. Sci. Hortic. 2015, 185, 113–120. [Google Scholar] [CrossRef]
- Bertamini, M.; Zulini, L.; Zorer, R.; Muthuchelian, K.; Nedunchezhian, N. Photoinhibition of photosynthesis in water deficit leaves of grapevine (Vitis vinifera L.) plants. Photosynthetica 2007, 45, 426–432. [Google Scholar] [CrossRef]
- Flexas, J.; Baron, M.; Bota, J.; Ducruet, J.M.; Galle, A.; Galmes, J.; Medrano, H. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity and chlorophyll fluorescence of Vitis vinifera L. cv. Ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Kartal, C. Calcium oxalate crystals in some species of the tribe Cardueae (Asteraceae). Bot. Sci. 2016, 94, 107–119. [Google Scholar] [CrossRef]
- Gaberščik, A.; Grašič, M.; Vogel-Mikuš, K.; Germ, M.; Golob, A. Water shortage strongly alters formation of calcium oxalate druse crystals and leaf traits in Fagopyrum esculentum. Plants 2020, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N. Mechanisms linking drought, hydraulics, carbon metabolism and vegetation mortality. Plant Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 264–266. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, A.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, R.; Biehler, K.; Stuhlfauth, T.; Fock, H.P. Simultaneous gas exchange and fluorescence measurements indicate differences in the response of sunflower, bean and maize to water stress. Photosynth. Res. 1991, 27, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Biniari, K.; Xenaki, M.; Daskalakis, I.; Rusjan, D.; Bouza, D.; Stavrakaki, M. Polyphenolic compounds and antioxidants of skin and berry grapes of Greek Vitis vinifera cultivars in relation to climate conditions. Food Chem. 2020, 307, 125518. [Google Scholar] [CrossRef]
- Hellenic National Meteorological Service. Available online: http://www.emy.gr/emy (accessed on 10 September 2023).
- Carrara, M.; Catania, P.; Pipitone, F.; Vallone, M.; Piraino, S.; Salvia, M.; Paolino, C. Temperature and relative humidity distribution inside a greenhouse using wireless sensors. Acta Hortic. 2008, 801, 595–599. [Google Scholar] [CrossRef]
- Barraqué, B.; Karavitis, C.; Katsiardi, P. The range of existing circumstances in the WaterStrategyMan case studies. In Coping with Water Deficiency: From Research to Policymaking with Examples from Southern Europe, the Mediterranean and Developing Countries; Koundouri, E., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 45–112. [Google Scholar]
- Reid, K.; Oki, L. Irrigation and climate zone trials of perennial plants for sustainable landscapes. Acta Hortic. 2013, 980, 95–102. [Google Scholar] [CrossRef]
- Mapfumo, E.; Aspinall, D.; Hancock, T.W. Growth and development of roots of grapevine (Vitis vinifera L.) in relation to water uptake from soil. Ann. Bot. 1994, 74, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mishra, Ρ.Μ. Effect of urban air pollution on epidermal traits of road side tree species, Pongamia pinnata (L.) Merr. IOSR-JESTFT 2013, 2, 2319–2402. [Google Scholar] [CrossRef]
- Image-Pro Plus. Available online: http://www.mediacy.com/imageproplus (accessed on 12 December 2022).
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, E., Govindjee, G.C., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
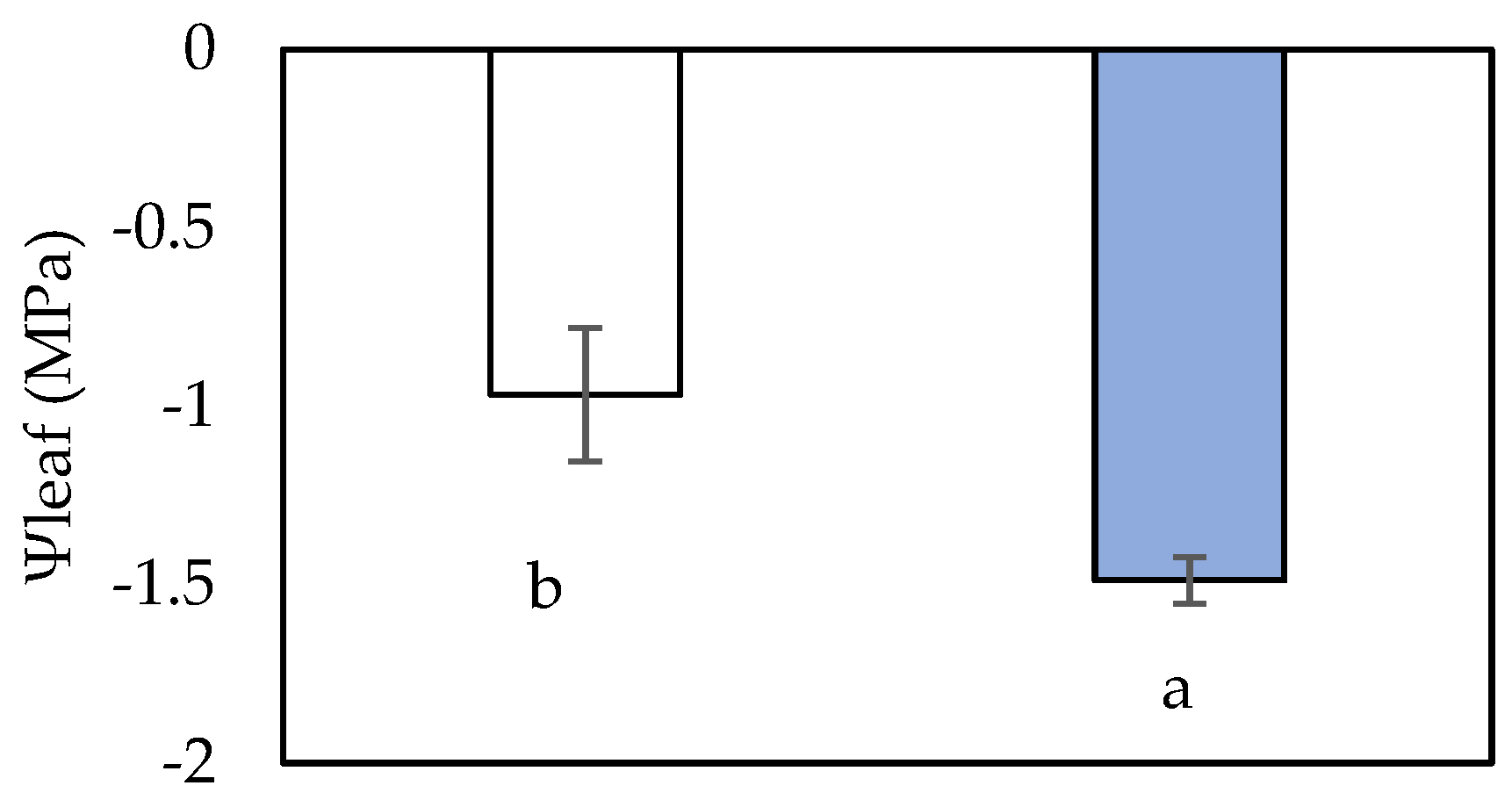
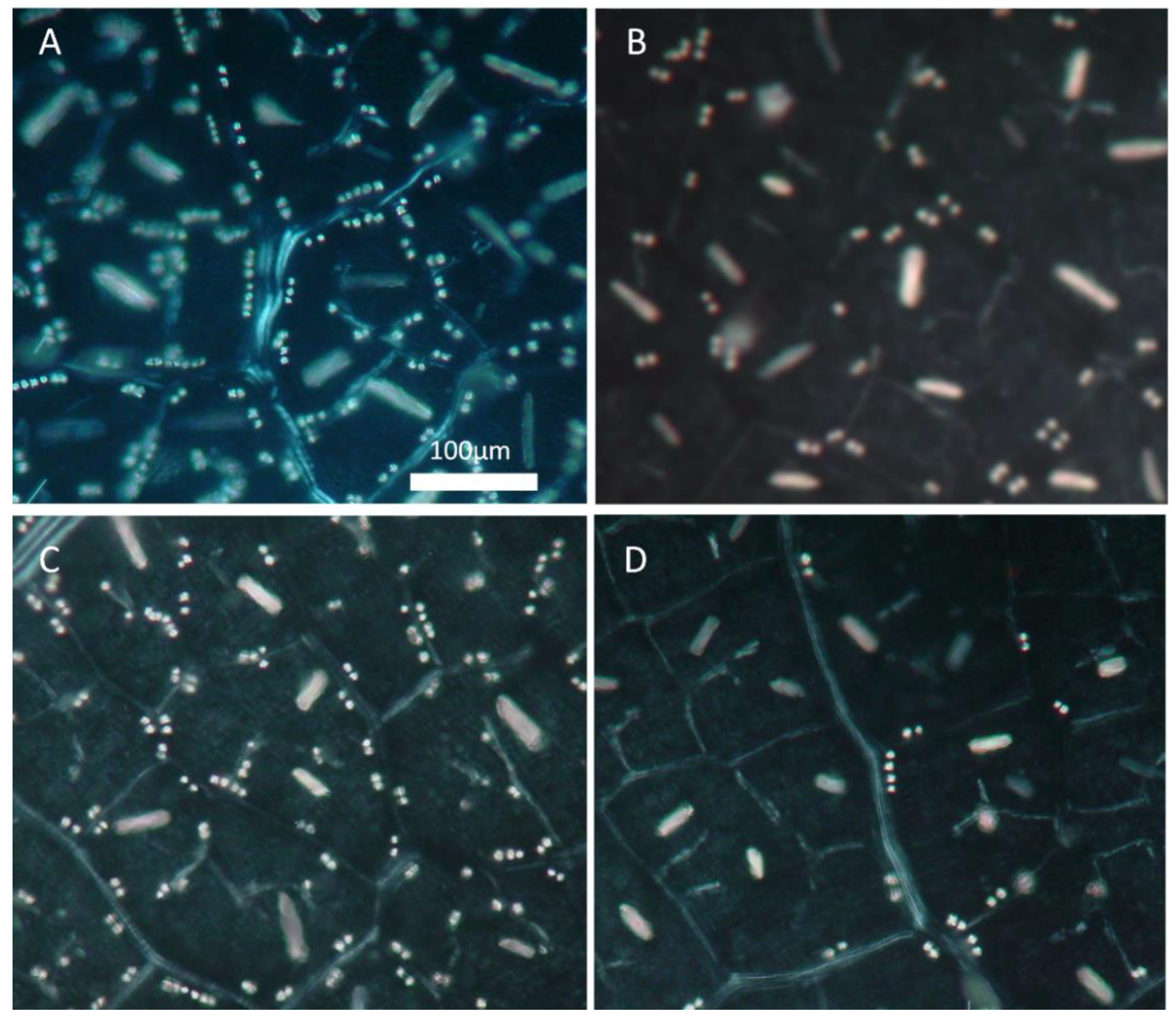



| Parameter (Units) | Well-Watered | Water-Deficit-Stressed |
|---|---|---|
| Stomatal density (mm−2) | 25.91 ± 2.80 a | 27.34 ± 3.20 a |
| Stomatal length (μm) | 55.10 ± 3.10 a | 42.40 ± 1.80 b |
| Leaf area (cm2) | 140.84 ± 14.53 a | 108.75 ± 8.90 b |
| LMA (g m−2) | 42.40 ± 1.66 a | 39.26 ± 2.52 b |
| Parameter (Units) | Well-Watered | Water-Deficit-Stressed |
|---|---|---|
| E (mmol H2O m−2 s−1) | 2.87 ± 0.08 a | 1.99 ± 0.09 b |
| gs (mol H2O m−2 s−1) | 0.15 ± 0.01 a | 0.09 ± 0.00 b |
| Amax (μmol CO2 m−2 s−1) | 15.12 ± 0.92 a | 4.82 ± 0.52 b |
| WUEi (μmol CO2 mmol−1 H2O) | 99.52 ± 4.33 a | 53.36 ± 6.26 b |
| WUE (μmol CO2 mol−1 H2O) | 5.23 ± 0.24 a | 2.44 ± 0.31 b |
| Fv/Fm | 0.80 ± 0.01 a | 0.79 ± 0.01 a |
| PItotal | 3.61 ± 0.54 a | 2.58 ± 0.19 b |
| Parameter (Units) | Well-Watered: Morning | Water-Deficit-Stressed: Morning | Well-Watered: Afternoon | Water-Deficit-Stressed: Afternoon |
|---|---|---|---|---|
| Raphides’ Length (μm) | 73.4 ± 4.91 ab | 84.0 ± 3.10 a | 69.0 ± 2.24 b | 66.0 ± 1.34 b |
| Raphides’ Width (μm) | 24.4 ± 0.20 a | 22.9 ± 0.59 a | 23.8 ± 0.63 a | 22.7 ± 0.36 a |
| Raphides’ Area (μm2) | 6449.4 ± 424.90 b | 7203.9 ± 312.74 a | 5836.2 ± 269.95 c | 5545.6 ± 163.62 c |
| Raphides’ Volume (μm3) | 34,304.05 a | 34,579.60 a | 30,681.22 b | 26,697.17 c |
| Raphides’ Density (mm–2) | 13.14 ± 0.57 a | 13.17 ± 0.70 a | 12.55 ± 1.24 a | 12.90 ± 0.60 a |
| Druses’ Density (mm–2) | 108.09 ± 10.92 a | 82.30 ± 10.37 c | 97.49 ± 11.77 b | 40.61 ± 5.96 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolyva, F.; Nikolopoulos, D.; Bresta, P.; Liakopoulos, G.; Karabourniotis, G.; Rhizopoulou, S. Acclimation of the Grapevine Vitis vinifera L. cv. Assyrtiko to Water Deficit: Coordination of Structural and Functional Leaf Traits and the Dynamic of Calcium Oxalate Crystals. Plants 2023, 12, 3992. https://doi.org/10.3390/plants12233992
Kolyva F, Nikolopoulos D, Bresta P, Liakopoulos G, Karabourniotis G, Rhizopoulou S. Acclimation of the Grapevine Vitis vinifera L. cv. Assyrtiko to Water Deficit: Coordination of Structural and Functional Leaf Traits and the Dynamic of Calcium Oxalate Crystals. Plants. 2023; 12(23):3992. https://doi.org/10.3390/plants12233992
Chicago/Turabian StyleKolyva, Foteini, Dimosthenis Nikolopoulos, Panagiota Bresta, Georgios Liakopoulos, George Karabourniotis, and Sophia Rhizopoulou. 2023. "Acclimation of the Grapevine Vitis vinifera L. cv. Assyrtiko to Water Deficit: Coordination of Structural and Functional Leaf Traits and the Dynamic of Calcium Oxalate Crystals" Plants 12, no. 23: 3992. https://doi.org/10.3390/plants12233992
APA StyleKolyva, F., Nikolopoulos, D., Bresta, P., Liakopoulos, G., Karabourniotis, G., & Rhizopoulou, S. (2023). Acclimation of the Grapevine Vitis vinifera L. cv. Assyrtiko to Water Deficit: Coordination of Structural and Functional Leaf Traits and the Dynamic of Calcium Oxalate Crystals. Plants, 12(23), 3992. https://doi.org/10.3390/plants12233992







