Transpiration Dynamics of Esparto Grass (Macrochloa tenacissima (L.) Kunth) in a Semi-Arid Mediterranean Climate: Unraveling the Impacts of Pine Competition
Abstract
1. Introduction
2. Results and Discussion
2.1. Seasonal Trends in Microclimatic Soil Variables and Transpiration Rates
2.2. Seasonal Variation in Microclimatic Soil Variables and Environmental Conditions in Function of Site
2.3. Correlations between Pine Competition, Soil Microclimatic Variables, Environmental Conditions, and Transpiration Rate of Green Leaves
2.4. Biometric Characteristics of Sampling Bushes: Plant Structure
2.5. Effects of Leaf Senescence on Transpiration Rate
2.6. Effects of Site and Maturity on Transpiration in Green Leaves
2.7. Seasonal Effects of Soil Water and Competition on Transpiration in Green and Senescent Leaves
3. Materials and Methods
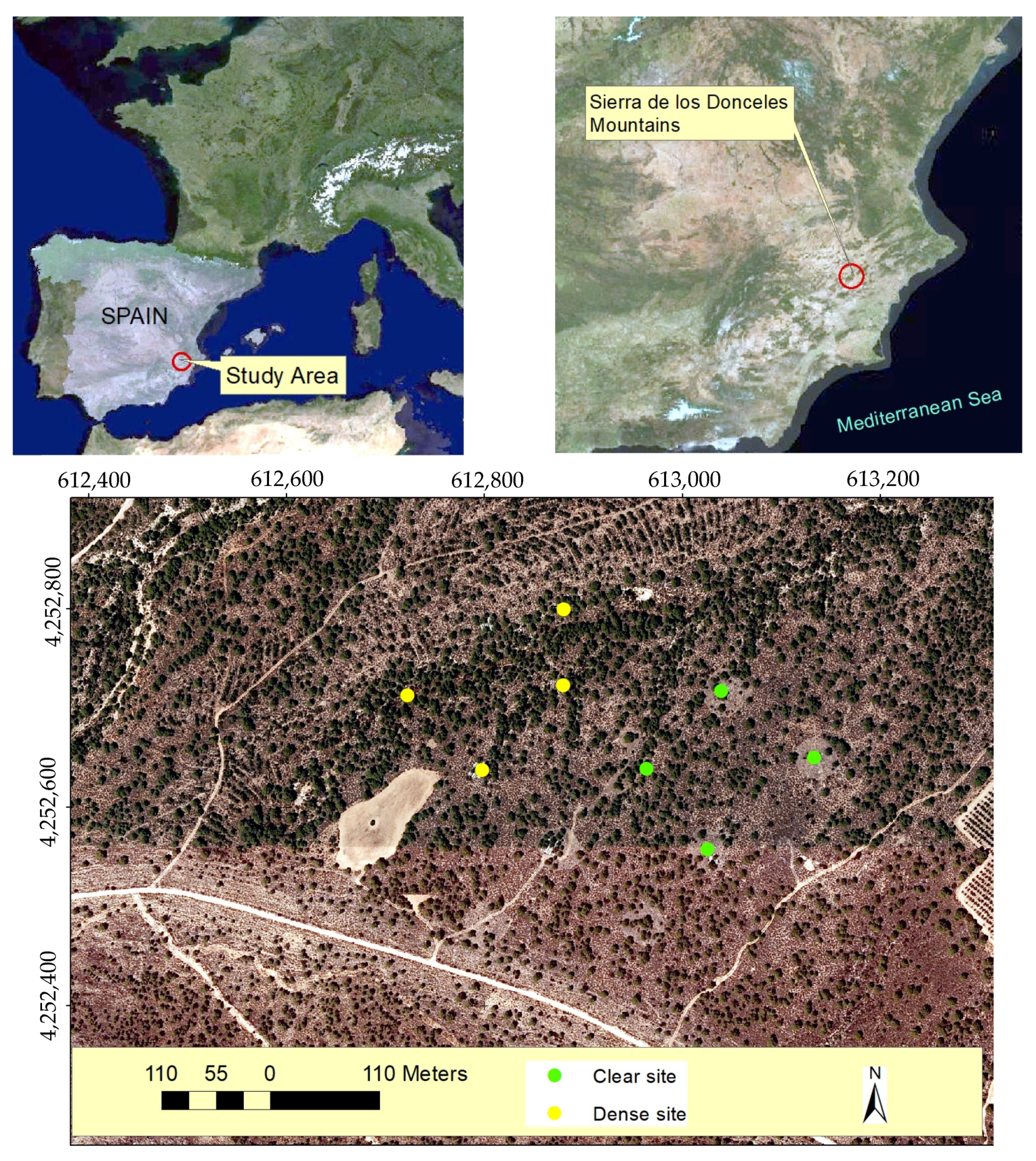
3.1. Study Area
| Characteristics | Closed Stands | Open Stands |
|---|---|---|
| Vegetation 1 | ||
| Tree density (pines ha−1) | 308 ± 40 | 88 ± 12 |
| Basal area (m2 ha−1) | 5.0 ± 0.7 | 3.1 ± 1.2 |
| Canopy cover (%) | 42.3 ± 12.1 | 18.3 ± 21.1 |
| Mean crown diameter | 4.54 ± 2.46 | 5.86 ± 2.09 |
| Volume growth (m3 ha−1 year−1) | 0.4 ± 0.1 | 0.2 ± 0.1 |
| Esparto cover (%) | 32 ± 23 | 65 ± 14 |
| CI (m m−1) | 0.22 ± 0.01 | <0.01 ± 0.001 |
| Soils 2 | ||
| Soil pH | 8.3 ± 0.1 | 8.4 ± 0.1 |
| Sand (%) | 46.3 ± 7.5 | 55.4 ± 4.6 |
| Clay (%) | 37.0 ± 7.9 | 31.4 ± 3.8 |
| Organic matter (%) | 4.2 ± 0.6 | 3.4 ± 0.7 |
| Total N (%) | 0.2 ± 0.01 | 0.1 ± 0.01 |
| Available P (ppm) | 5.0 ± 0.9 | 3.0 ± 1.2 |
| Water storage capacity (mm) | 54.4 ± 6.5 | 46.5 ± 4.4 |
3.2. Experimental Layout and Sampling of Bushes
3.3. Measurements of Transpiration at Leaf Level
3.4. Biometric Characterization of Sampled Bushes: Plant Structure
3.5. Microclimatic Soil Measurements and Environmental Conditions
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pugnaire, F.I.; Armas, C.; Maestre, F.T. Positive plant interactions in the Iberian Southeast: Mechanisms, environmental gradients, and ecosystem function. J. Arid. Environ. 2011, 75, 1310–1320. [Google Scholar] [CrossRef]
- Wu, W.; Li, H.; Feng, H.; Si, B.; Chen, G.; Meng, T.; Li, Y.; Siddique, K.H.M. Precipitation dominates the transpiration of both the economic forest (Malus pumila) and ecological forest (Robinia pseudoacacia) on the Loess Plateau after about 15 years of water depletion in deep soil. Agric. For. Meteorol. 2021, 297, 108244. [Google Scholar] [CrossRef]
- Sperlich, D.; Chang, C.T.; Peñuelas, J.; Gracia, C.; Sabaté, S. Seasonal variability of foliar photosynthetic and morphological traits and drought impacts in a Mediterranean mixed forest. Tree Physiol. 2015, 35, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, D.; Zhang, J.; Zhang, J.; Zhang, S.; Bai, Y.; Yang, S. Ecosystem Evapotranspiration Partitioning and Its Spatial–Temporal Variation Based on Eddy Covariance Observation and Machine Learning Method. Remote Sens. 2023, 15, 4831. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. (Eds.) Transpiration and Plant Water Balance. In Physiology of Woody Plants, 2nd ed.; Academic Press: San Diego, CA, USA, 1997; pp. 269–308. [Google Scholar]
- Buckley, T.N. Modeling Stomatal Conductance. Plant Physiol. 2017, 174, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.J.; Gower, S.T. 4—Forest Hydrology and Tree–Water Relations. In Applications of Physiological Ecology to Forest Management; Landsberg, J.J., Gower, S.T., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 89–124. [Google Scholar]
- Zhu, Y.; Cheng, Z.; Feng, K.; Chen, Z.; Cao, C.; Huang, J.; Ye, H.; Gao, Y. Influencing factors for transpiration rate: A numerical simulation of an individual leaf system. Therm. Sci. Eng. Prog. 2022, 27, 101110. [Google Scholar] [CrossRef]
- Li, X.; Zhai, J.; Sun, M.; Liu, K.; Zhao, Y.; Cao, Y.; Wang, Y. Characteristics of Changes in Sap Flow-Based Transpiration of Poplars, Locust Trees, and Willows and Their Response to Environmental Impact Factors. Forests 2024, 15, 90. [Google Scholar] [CrossRef]
- Smith, W.K.; Hinckley, T.M. Resource Physiology of Conifers: Acquisition, Allocation and Utilization; Academic Press: San Diego, CA, USA, 1995; 396p. [Google Scholar]
- Pallardy, S.G.; Čermák, J.; Ewers, F.M.; Kaufmann, M.R.; Parker, W.C.; Sperry, J.S. Water transport dynamics in trees and stands. In Resource Physiology of Conifers; Smith, W.K., Hinckley, T.M., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 381–389. [Google Scholar]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Vitale, M.; Anselmi, S.; Salvatori, E.; Manes, F. New approaches to study the relationship between stomatal conductance and environmental factors under Mediterranean climatic conditions. Atmos. Environ. 2007, 41, 5385–5397. [Google Scholar] [CrossRef]
- Han, T.; Feng, Q.; Yu, T.; Liu, W.; Ma, J.; Zhao, C.; Yang, L.; Zhang, J.; Li, H. Contrasting response of water use efficiency to soil moisture availability: From leaf to ecosystem in an arid oasis. Ecol. Indic. 2023, 147, 109964. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z. Effect of rainfall pulses on plant growth and transpiration of two xerophytic shrubs in a revegetated desert area: Tengger Desert, China. Catena 2016, 137, 269–276. [Google Scholar] [CrossRef]
- Ungar, E.D.; Rotenberg, E.; Raz-Yaseef, N.; Cohen, S.; Yakir, D.; Schiller, G. Transpiration and annual water balance of Aleppo pine in a semiarid region: Implications for forest management. For. Ecol. Manag. 2013, 298, 39–51. [Google Scholar] [CrossRef]
- Gasque, M.a.; García-Fayos, P. Interaction between Stipa tenacissima and Pinus halepensis: Consequences for reforestation and the dynamics of grass steppes in semi-arid Mediterranean areas. For. Ecol. Manag. 2004, 189, 251–261. [Google Scholar] [CrossRef]
- Sanchez, G.; Puigdefabregas, J. Interactions of plant growth and sediment movement on slopes in a semi-arid environment. Geomorphology 1994, 9, 243–260. [Google Scholar] [CrossRef]
- Belkhir, S.; Koubaa, A.; Khadhri, A.; Ksontini, M.; Smiti, S. Variations in the morphological characteristics of Stipa tenacissima fiber: The case of Tunisia. Ind. Crops Prod. 2012, 37, 200–206. [Google Scholar] [CrossRef]
- Redmann, R.E. Adaptation of Grasses to Water Stress-Leaf Rolling and Stomate Distribution. Ann. Mo. Bot. Gard. 1985, 72, 833–842. [Google Scholar] [CrossRef]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Valladares, F.; Blasco, A.; Bellot, J. Assessing transpiration in the tussock grass Stipa tenacissima L.: The crucial role of the interplay between morphology and physiology. Acta Oecol. 2006, 30, 386–398. [Google Scholar] [CrossRef]
- Valladares, F.; Pugnaire, F.I. Tradeoffs Between Irradiance Capture and Avoidance in Semi-arid Environments Assessed with a Crown Architecture Model. Ann. Bot. 1999, 83, 459–469. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Valladares, F.; Domingo, F.; Bellot, J. Seasonal water-use efficiency and chlorophyll fluorescence response in alpha grass (Stipa tenacissima L.) is affected by tussock size. Photosynthetica 2008, 46, 222–231. [Google Scholar] [CrossRef]
- Cerdà, A. The effect of patchy distribution of Stipa tenacissima L. on runoff and erosion. J. Arid. Environ. 1997, 36, 37–51. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, G.; Wang, Y.; Song, X. Sensitive Indicators of Zonal Stipa Species to Changing Temperature and Precipitation in Inner Mongolia Grassland, China. Front. Plant Sci. 2016, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Haase, P.; Pugnaire, F.I.; Clark, S.C.; Incoll, L.D. Environmental control of canopy dynamics and photosynthetic rate in the evergreen tussock grass Stipa tenacissima. Plant Ecol. 1999, 145, 327–339. [Google Scholar] [CrossRef]
- Krichen, K.; Vilagrosa, A.; Chaieb, M. Divergence of functional traits at early stages of development in Stipa tenacissima populations distributed along an environmental gradient of the Mediterranean. Plant Ecol. 2019, 220, 995–1008. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Haase, P. Comparative Physiology and Growth of Two Perennial Tussock Grass Species in a Semi-Arid Environment. Ann. Bot. 1996, 77, 81–86. [Google Scholar] [CrossRef]
- Ghiloufi, W.; Quero, J.L.; García-Gómez, M.; Chaieb, M. Potential impacts of aridity on structural and functional status of a southern Mediterranean Stipa tenacissima steppe. S. Afr. J. Bot. 2016, 103, 170–180. [Google Scholar] [CrossRef]
- Han, L.; Liu, L.; Peng, L.; Wang, N.; Zhou, P. Mixing of tree species with the same water use strategy might lead to deep soil water deficit. For. Ecol. Manag. 2023, 534, 120876. [Google Scholar] [CrossRef]
- Moreno-Gutiérrez, C.; Battipaglia, G.; Cherubini, P.; Saurer, M.; Nicolás, E.; Contreras, S.; Querejeta, J.I. Stand structure modulates the long-term vulnerability of Pinus halepensis to climatic drought in a semiarid Mediterranean ecosystem. Plant Cell Environ. 2012, 35, 1026–1039. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Valladares, F.; Blasco, A.; Bellot, J. Effects of tussock size and soil water content on whole plant gas exchange in Stipa tenacissima L.: Extrapolating from the leaf versus modelling crown architecture. Environ. Exp. Bot. 2008, 62, 376–388. [Google Scholar] [CrossRef]
- Maestre, F.T.; Cortina, J. Remnant shrubs in Mediterranean semi-arid steppes: Effects of shrub size, abiotic factors and species identity on understorey richness and occurrence. Acta Oecol. 2005, 27, 161–169. [Google Scholar] [CrossRef]
- Balaguer, L.; Pugnaire, F.I.; Martínez-Ferri, E.; Armas, C.; Valladares, F.; Manrique, E. Ecophysiological significance of chlorophyll loss and reduced photochemical efficiency under extreme aridity in Stipa tenacissima L. Plant Soil 2002, 240, 343–352. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Cosentino, S.L.; Brunetti, C.; De Carlo, A.; Avola, G.; Riggi, E.; Loreto, F.; Centritto, M. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environ. Exp. Bot. 2018, 147, 116–124. [Google Scholar] [CrossRef]
- Carignato, A.; Vázquez-Piqué, J.; Tapias, R.; Ruiz, F.; Fernández, M. Variability and Plasticity in Cuticular Transpiration and Leaf Permeability Allow Differentiation of Eucalyptus Clones at an Early Age. Forests 2019, 11, 9. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Z.; Li, Y.; Lv, Y.; Yang, D.; Yang, S.; Wu, J.; Li, X.; Gu, Z.; Sun, X.; et al. Uncovering the role of a positive selection site of wax ester synthase/diacylglycerol acyltransferase in two closely related Stipa species in wax ester synthesis under drought stress. J. Exp. Bot. 2020, 71, 4159–4170. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Q.; Mi, X.; Bai, Y.; Zhang, M.; Li, X. Grazing every month minimizes size but boosts photosynthesis in Stipa grandis in the steppe of Inner Mongolia, China. J. Arid Land 2018, 10, 601–611. [Google Scholar] [CrossRef]
- Zhou, A.; Liu, E.; Liu, J.; Feng, S.; Gong, S.; Wang, J. Characterization of increased cuticular wax mutant and analysis of genes involved in wax biosynthesis in Dianthus spiculifolius. Hortic. Res. 2018, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.C.; Burghardt, M.; Riederer, M. The ecophysiology of leaf cuticular transpiration: Are cuticular water permeabilities adapted to ecological conditions? J. Exp. Bot. 2017, 68, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.A.; Bellot, J.; Domingo, F.; Blasco, A. Can water responses in Stipa tenacissima L. during the summer season be promoted by non-rainfall water gains in soil? Plant Soil 2006, 291, 67–79. [Google Scholar] [CrossRef]
- Domingo, F.; Villagarcía, L.; Brenner, A.J.; Puigdefábregas, J. Evapotranspiration model for semi-arid shrub-lands tested against data from SE Spain. Agric. For. Meteorol. 1999, 95, 67–84. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Haase, P.; Incoll, L.D.; Clark, S.C. Response of the Tussock Grass Stipa tenacissima to Watering in a Semi-Arid Environment. Funct. Ecol. 1996, 10, 265–274. [Google Scholar] [CrossRef]
- El-Abbassi, F.E.; Assarar, M.; Ayad, R.; Bourmaud, A.; Baley, C. A review on alfa fibre (Stipa tenacissima L.): From the plant architecture to the reinforcement of polymer composites. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105677. [Google Scholar] [CrossRef]
- Raz-Yaseef, N.; Yakir, D.; Schiller, G.; Cohen, S. Dynamics of evapotranspiration partitioning in a semi-arid forest as affected by temporal rainfall patterns. Agric. For. Meteorol. 2012, 157, 77–85. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tanaka, T. Water flow and hydraulic characteristics of Japanese red pine and oak trees. Hydrol. Process. 2001, 15, 1731–1750. [Google Scholar] [CrossRef]
- García-Morote, F.; Martínez-García, E.; Andrés-Abellán, M.; Caballero, E.; Miettinen, H.; López-Serrano, F. Direct Seeding of Pinus halepensis Mill. for Recovery of Burned Semi-Arid Forests: Implications for Post-Fire Management for Improving Natural Regeneration. Forests 2017, 8, 353. [Google Scholar] [CrossRef]
- USDA—United States Department of Agriculture. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service: Washington, DC, USA, 1999; p. 869.
- Pérez-Anta, I. Efecto de Stipa tenacissima L. Sobre los Flujos de Agua del Sistema Suelo-Esparto en un Ecosistema Semiárido de Pinus Halepensis Mill. en el SE Peninsular: Implicaciones Para la Gestión Forestal. Ph.D. Thesis, University of Castilla-La Mancha, Albacete, Spain, 2021. [Google Scholar]
- Domingo Santos, J.M.; Fernández de Villarán San Juan, R.; Corral Pazos de Provens, E.; Rapp Arrarás, I. Estimation of water retention capacity in soil: Corrections to the CRA pedotransfer formula. For. Syst. 2006, 15, 14–23. [Google Scholar] [CrossRef]
- Leuning, R. A critical appraisal of a combined stomatal-photosynthesis model for C3 plants. Plant Cell Environ. 1995, 18, 339–355. [Google Scholar] [CrossRef]
- Hoshmand, H.R. Design of Experiments for Agriculture and the Natural Sciences, 2nd ed.; Champan & Hall/CRC: New York, NY, USA, 2006; p. 437. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-155. 2022. Available online: https://CRAN.R-project.org/package=nlme (accessed on 20 October 2023).
- Daniel, C.; Wood, F.S. Fitting Equations to Data: Computer Analysis of Multifactor Data; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1980. [Google Scholar]
- Neter, J.; Kutner, M.; Wasserman, W.; Nachtsheim, C. Applied Linear Statistical Models, 4th ed.; McGraw-Hill-Irwin: Chicago, IL, USA, 1996; p. xv, 720p. [Google Scholar]
- Belsley, D.A.; Kuh, E.; Welsch, R.E. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity; John Wiley & Sons, Ltd.: New York, NY, USA, 2013. [Google Scholar]
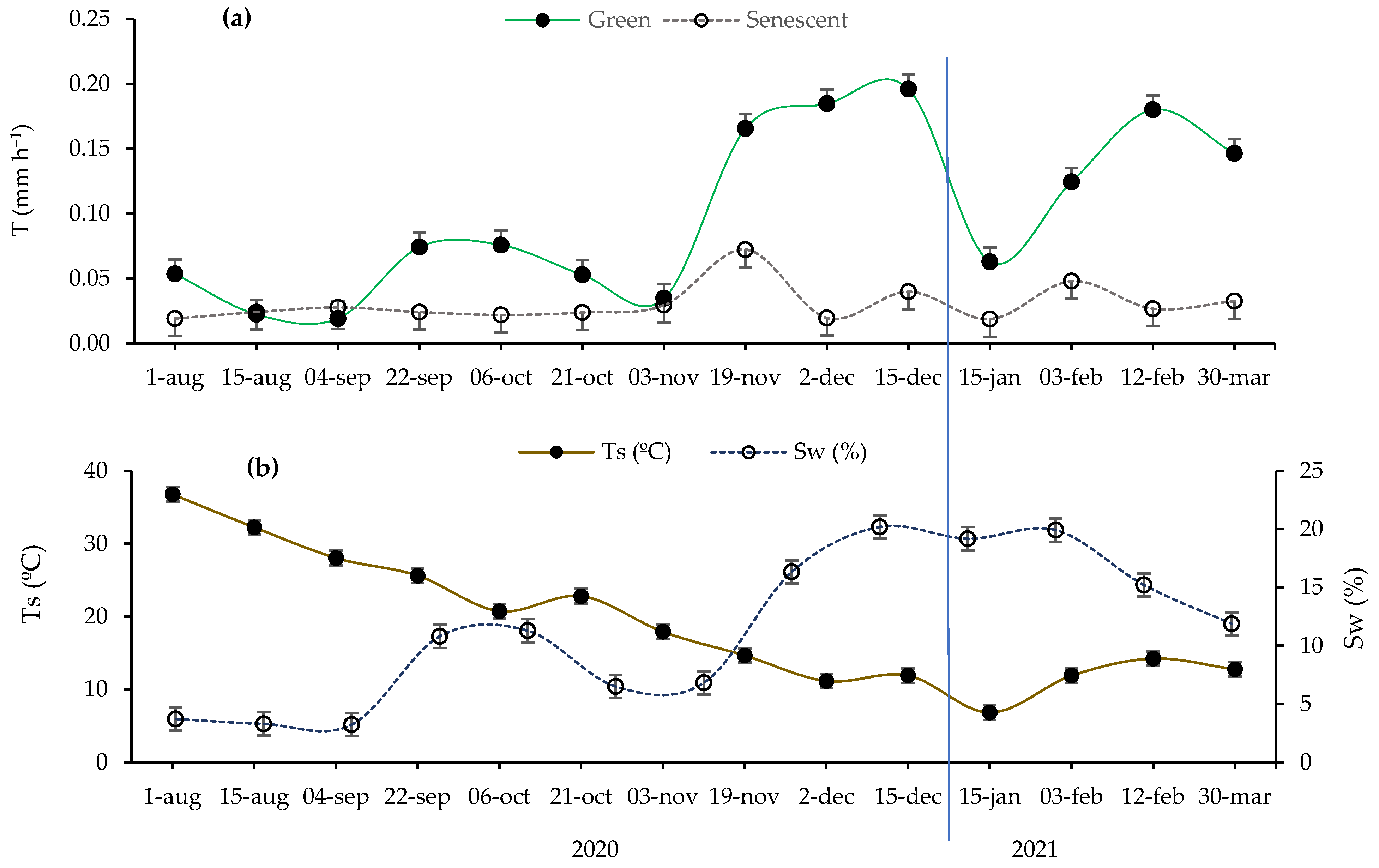


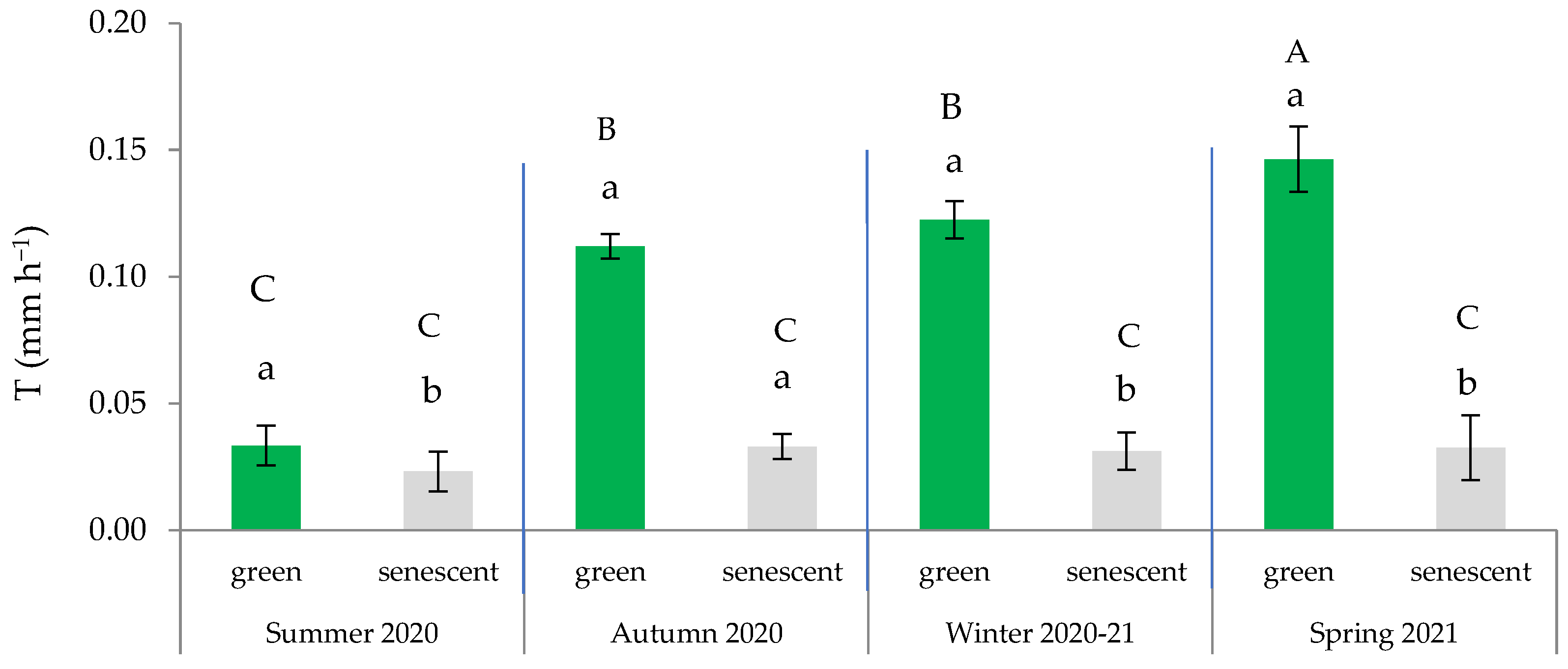
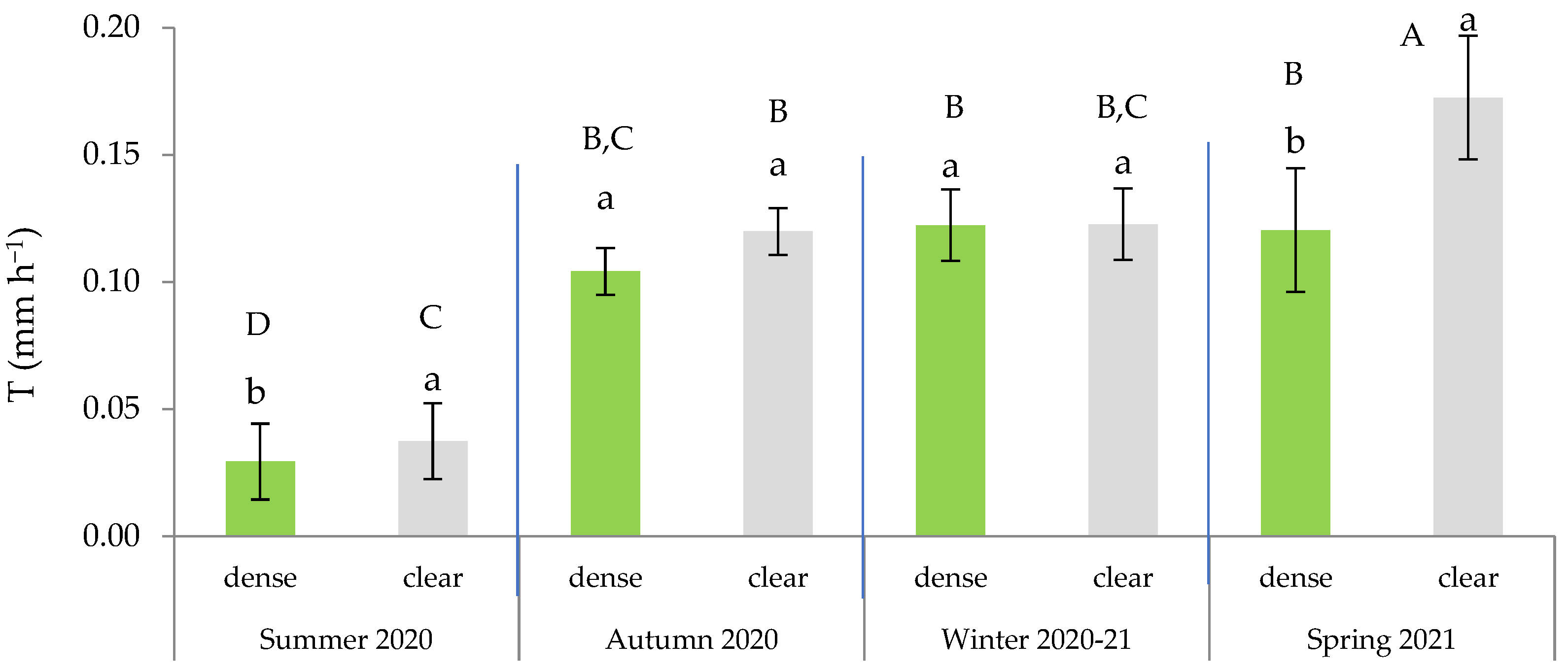
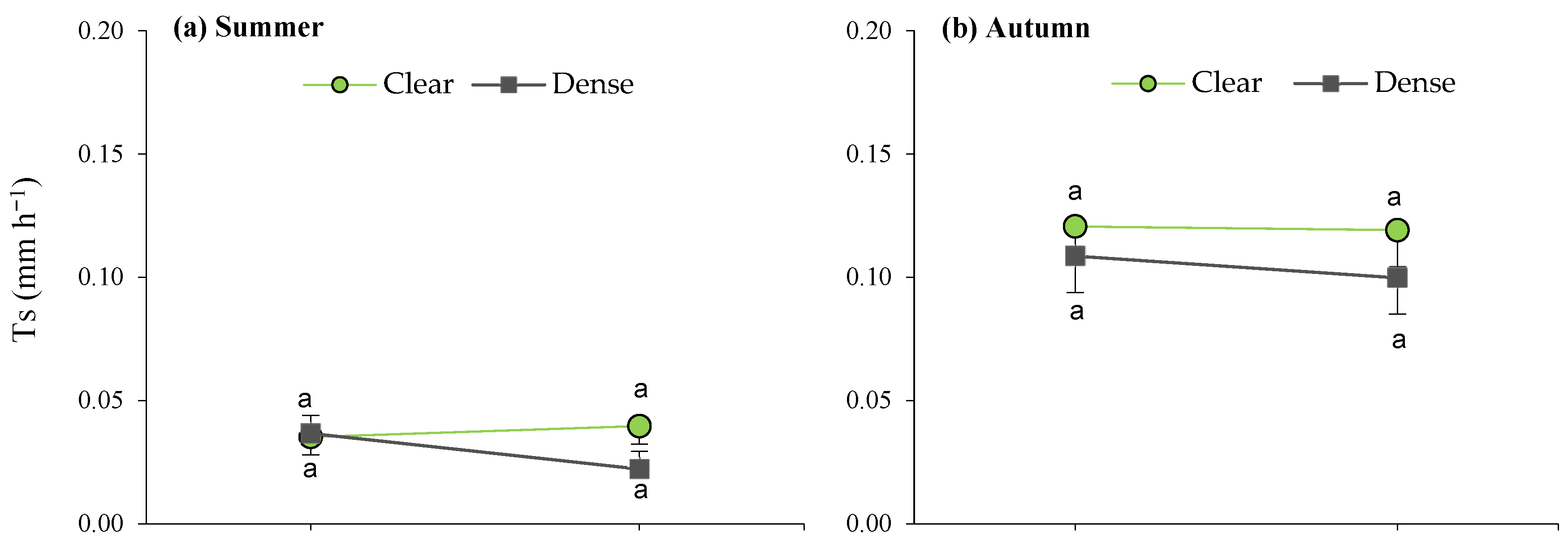



| Seasons | Variables | CI (m m−1) | Sw (%) | Ts (°C) | PAR (µmol m−2 s−1) | VPD (kPa) | T (mm h−1) |
|---|---|---|---|---|---|---|---|
| Summer 2020 | CI | −0.404 | −0.339 | −0.199 | −0.020 | −0.298 | |
| Sw | −0.404 | −0.396 | 0.194 | −0.322 | 0.536 | ||
| Ts | −0.339 | −0.396 | 0.454 | 0.432 | −0.471 | ||
| PAR | −0.199 | 0.194 | 0.454 | 0.321 | 0.517 | ||
| VPD | −0.020 | −0.322 | 0.432 | 0.321 | 0.234 | ||
| T | −0.298 | 0.536 | −0.471 | 0.517 | 0.234 | ||
| Autumn 2020 | CI | −0.093 | −0.154 | −0.032 | −0.123 | −0.088 | |
| Sw | −0.093 | −0.583 | 0.007 | −0.034 | 0.572 | ||
| Ts | −0.154 | −0.583 | 0.049 | −0.035 | −0.426 | ||
| PAR | −0.032 | 0.007 | 0.049 | 0.210 | 0.438 | ||
| VPD | −0.123 | −0.034 | −0.035 | 0.210 | −0.165 | ||
| T | −0.088 | 0.572 | −0.426 | 0.438 | −0.165 | ||
| Winter 2020–2021 | CI | −0.075 | −0.058 | 0.025 | −0.171 | 0.016 | |
| Sw | −0.075 | −0.407 | 0.134 | 0.035 | −0.302 | ||
| Ts | −0.058 | −0.407 | −0.229 | 0.144 | 0.579 | ||
| PAR | 0.025 | 0.134 | −0.229 | 0.556 | 0.399 | ||
| VPD | −0.171 | 0.035 | 0.144 | 0.556 | 0.345 | ||
| T | 0.016 | −0.300 | 0.579 | 0.399 | 0.345 | ||
| Spring 2021 | CI | −0.243 | −0.135 | −0.052 | −0.201 | −0.471 | |
| Sw | −0.243 | −0.423 | 0.187 | 0.154 | 0.360 | ||
| Ts | −0.135 | −0.423 | 0.135 | 0.567 | 0.218 | ||
| PAR | −0.052 | 0.187 | 0.135 | 0.204 | 0.632 | ||
| VPD | −0.201 | 0.154 | 0.567 | 0.204 | 0.134 | ||
| T | −0.470 | 0.360 | 0.218 | 0.632 | 0.134 |
| Seasons | |||||
|---|---|---|---|---|---|
| Main Effects | Spring | Summer | Autumn | Winter | Yearly |
| Leaf | <0.001 * | 0.533 | <0.000 * | 0.001 * | <0.001 * |
| Leaf × Season | - | - | - | - | <0.001 * |
| Leaf × Site | 0.858 | 0.687 | 0.571 | 0.873 | 0.317 |
| Leaf × Maturity | 0.322 | 0.223 | 0.447 | 0.784 | 0.295 |
| Leaf × Season × Site | - | - | - | - | 0.679 |
| Leaf × Season × Maturity | - | - | - | - | 0.724 |
| Seasons | |||||
|---|---|---|---|---|---|
| Main Effects | Spring | Summer | Autumn | Winter | Total Values |
| Season | - | - | - | - | <0.001 * |
| Site | 0.026 * | 0.003 * | 0.625 | 0.702 | 0.088 |
| Maturity | 0.512 | 0.098 | 0.775 | 0.240 | 0.305 |
| Season × Site | - | - | - | - | 0.126 |
| Season × Maturity | - | - | - | - | 0.164 |
| Site × Maturity | 0.086 | 0.021 * | 0.399 | 0.617 | 0.121 |
| Season × Site × Maturity | - | - | - | - | 0.607 |
| Seasons | Leaves | p | SEE | (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Summer 2020 | Greens | −8.62 ± 0.88 | 5.03 ± 1.05 | n.s. | n.s | 4.24 ± 0.81 | −4.18 ± 0.91 | 0.00 | 1.04 | 34.9 |
| Senescent | −3.91 ± 0.08 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| Autumn 2020 | Greens | −5.64 ± 0.27 | 0.19 ± 0.09 | n.s. | n.s. | 1.26 ± 0.11 | n.s. | 0.00 | 0.57 | 48.5 |
| Senescent | −3.97 ± 0.10 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| Winter 2020–2021 | Green | −1.67 ± 0.23 | n.s. | −0.048 ± 0.014 | n.s. | n.s. | n.s. | 0.00 | 0.39 | 23.8 |
| Senescent | −3.96 ± 0.14 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| Spring 2021 | Green | −2.14 ± 0.89 | n.s. | n.s. | 0.028 ± 0.010 | n.s. | n.s. | 0.00 | 0.31 | 23.6 |
| Senescent | −3.52 ± 0.09 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||
| Yearly (all data) | Greens | −5.82 ± 0.29 | 1.04 ± 0.35 | −0.053 ± 0.023 | n.s. | 1.60 ± 0.21 | −0.25 ± 0.15 | 0.00 | 0.90 | 46.0 |
| Senescent | −3.92 ± 0.06 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Seasons | Leaf Type–Site | T (mm h−1) | Sw (%) | Ts (°C) | PAR (µmol m−2 s−1) | VPD (kPa) |
|---|---|---|---|---|---|---|
| Summer 2020 | Green–dense | 3.4 ± 0.4 | 32.9 ± 0.5 | 959 ± 39 | 5.2 ± 2.9 | |
| Green–clear | ||||||
| Senescent | ||||||
| Autumn 2020 | Green–dense | 13.0 ± 0.3 | 17.8 ± 0.3 | 576 ± 24 | 2.7 ± 1.5 | |
| Green–clear | ||||||
| Senescent | ||||||
| Winter 2020–2021 | Green–dense | 16.3 ± 0.4 | 11.0 ± 0.5 | 458 ± 37 | 1.6 ± 0.6 | |
| Green–clear | ||||||
| Senescent | ||||||
| Spring 2021 | Green–dense | 11.9 ± 0.7 | 12.8 ± 0.8 | 198 ± 64 | 1.4 ± 0.3 | |
| Green–clear | ||||||
| Senescent | ||||||
| Yearly | Green–dense | 11.8 ± 6.5 | 18.9 ± 9.5 | 596 ± 490 | 2.8 ± 2.1 | |
| (all data) | Green–clear | |||||
| Senescent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Anta, I.; Rubio, E.; López-Serrano, F.R.; Garcés, D.; Andrés-Abellán, M.; Picazo, M.; Chebbi, W.; Arquero, R.; García-Morote, F.A. Transpiration Dynamics of Esparto Grass (Macrochloa tenacissima (L.) Kunth) in a Semi-Arid Mediterranean Climate: Unraveling the Impacts of Pine Competition. Plants 2024, 13, 661. https://doi.org/10.3390/plants13050661
Pérez-Anta I, Rubio E, López-Serrano FR, Garcés D, Andrés-Abellán M, Picazo M, Chebbi W, Arquero R, García-Morote FA. Transpiration Dynamics of Esparto Grass (Macrochloa tenacissima (L.) Kunth) in a Semi-Arid Mediterranean Climate: Unraveling the Impacts of Pine Competition. Plants. 2024; 13(5):661. https://doi.org/10.3390/plants13050661
Chicago/Turabian StylePérez-Anta, Iván, Eva Rubio, Francisco Ramón López-Serrano, Diego Garcés, Manuela Andrés-Abellán, Marta Picazo, Wafa Chebbi, Rocío Arquero, and Francisco Antonio García-Morote. 2024. "Transpiration Dynamics of Esparto Grass (Macrochloa tenacissima (L.) Kunth) in a Semi-Arid Mediterranean Climate: Unraveling the Impacts of Pine Competition" Plants 13, no. 5: 661. https://doi.org/10.3390/plants13050661
APA StylePérez-Anta, I., Rubio, E., López-Serrano, F. R., Garcés, D., Andrés-Abellán, M., Picazo, M., Chebbi, W., Arquero, R., & García-Morote, F. A. (2024). Transpiration Dynamics of Esparto Grass (Macrochloa tenacissima (L.) Kunth) in a Semi-Arid Mediterranean Climate: Unraveling the Impacts of Pine Competition. Plants, 13(5), 661. https://doi.org/10.3390/plants13050661






