Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco
Abstract
:1. Introduction
2. Results
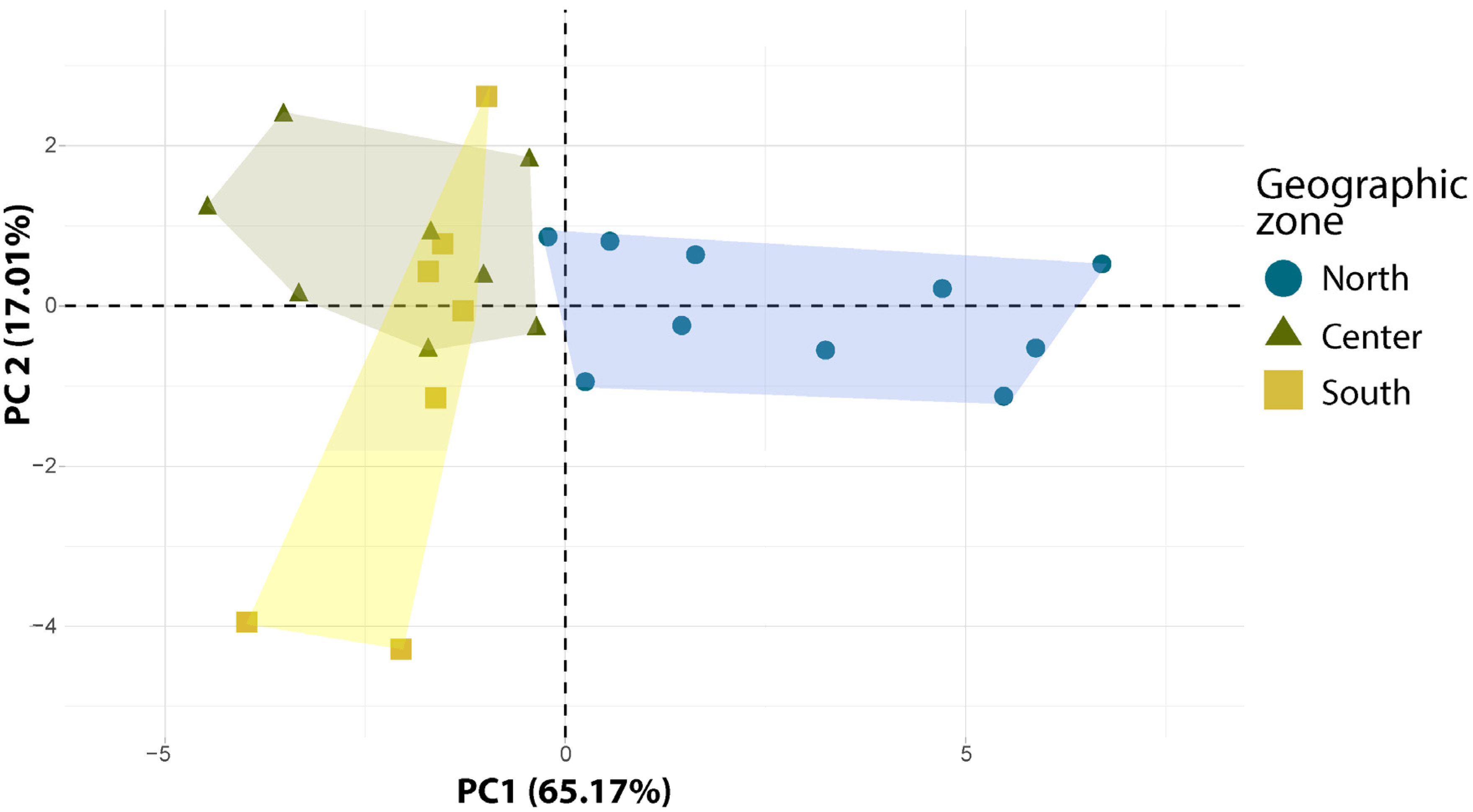
2.1. Variation in Carob Pod and Seed Traits between Populations
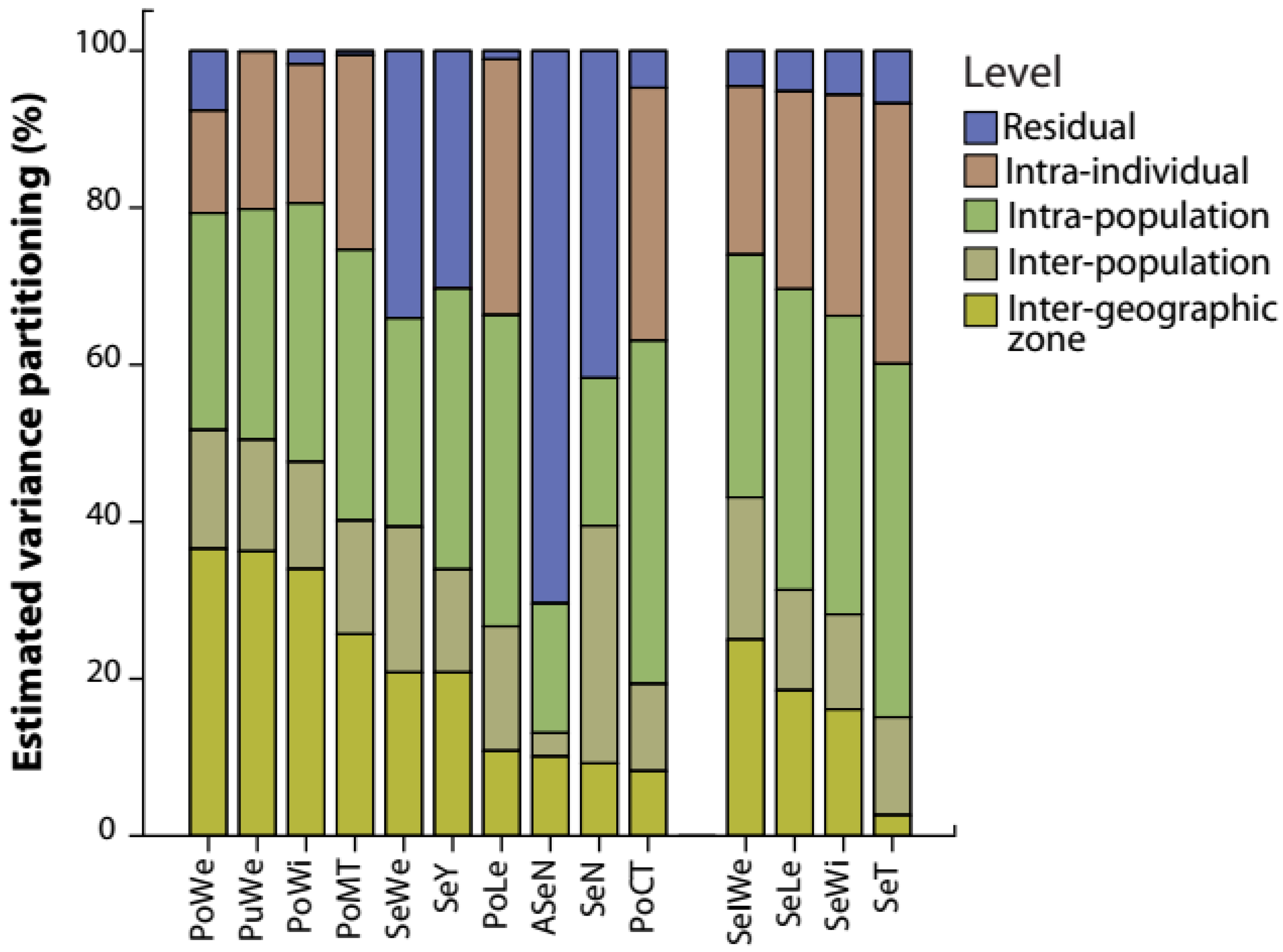
2.2. Structure and Amount of Intraspecific Variation in Carob Traits
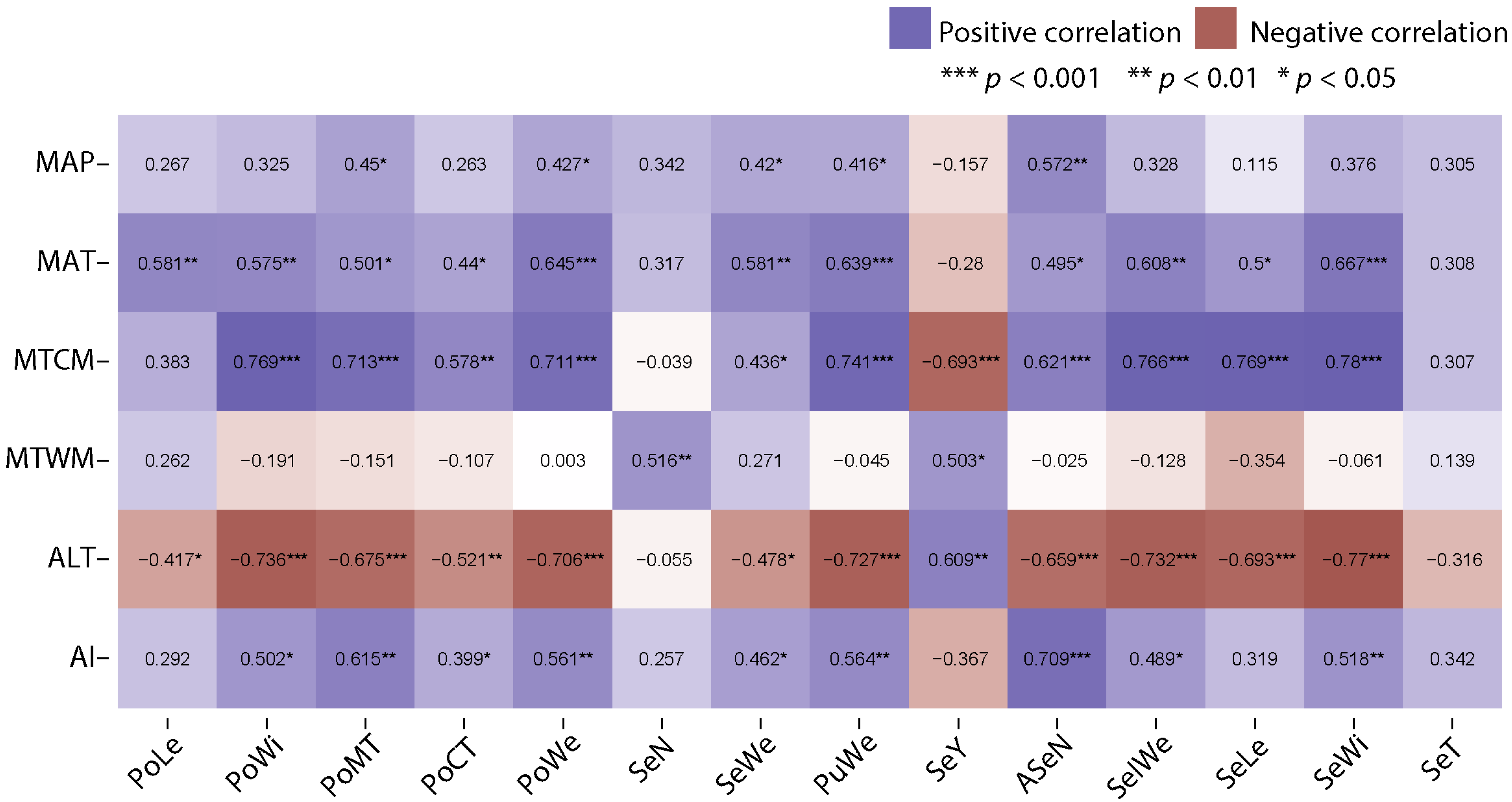
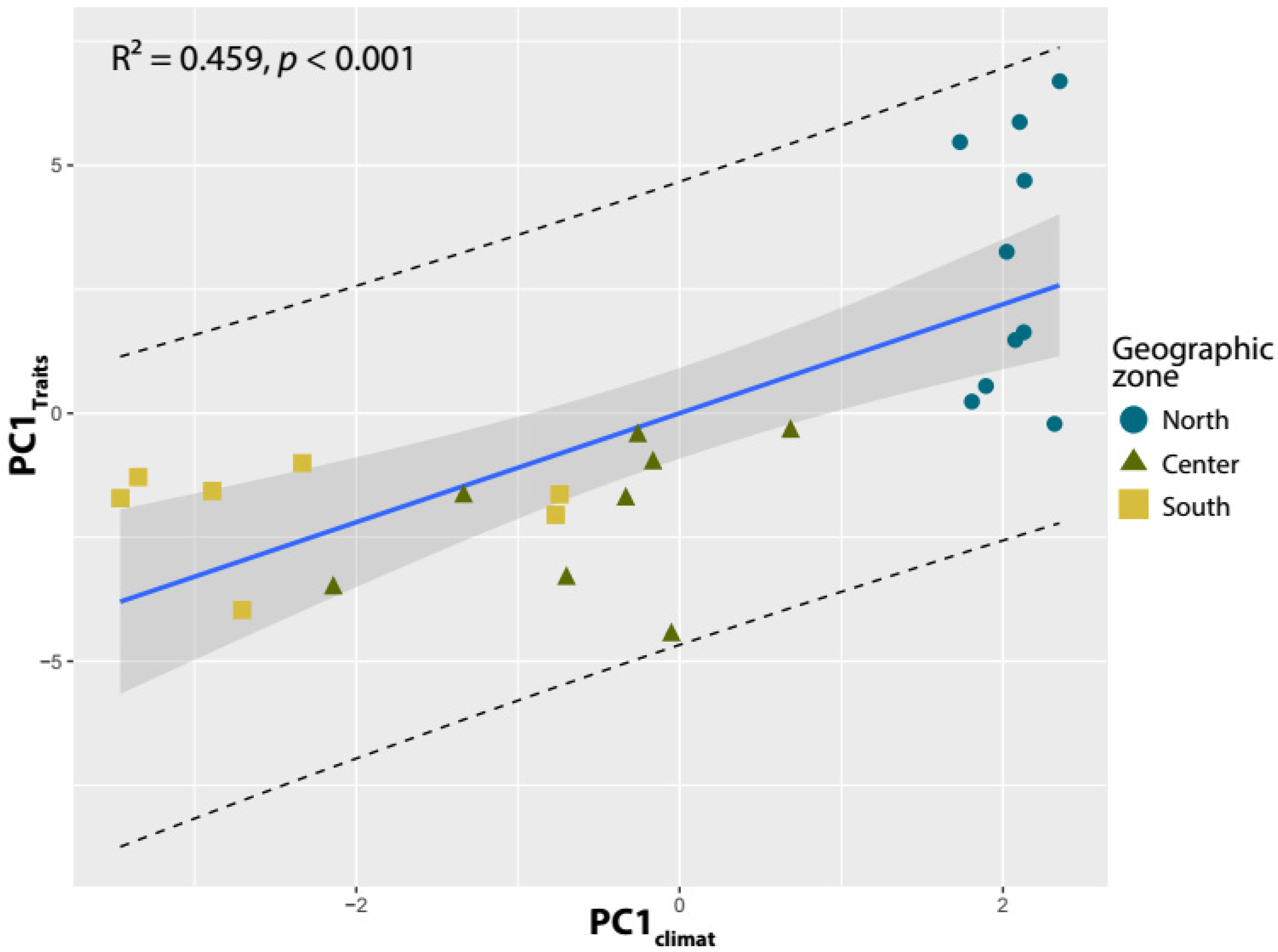
2.3. Carob Traits Variation According to Environmental Variables
3. Discussion
3.1. Variability in Pod and Seed Traits across the Studied Populations
3.2. Impact of the Geographic Origin on the Expression of Carob Pod and Seed Traits
3.3. Structure and Extent of Intraspecific Trait Variability in Carob Tree
3.4. Carob Pod and Seed Trait Variations and Environmental Variables
4. Materials and Methods
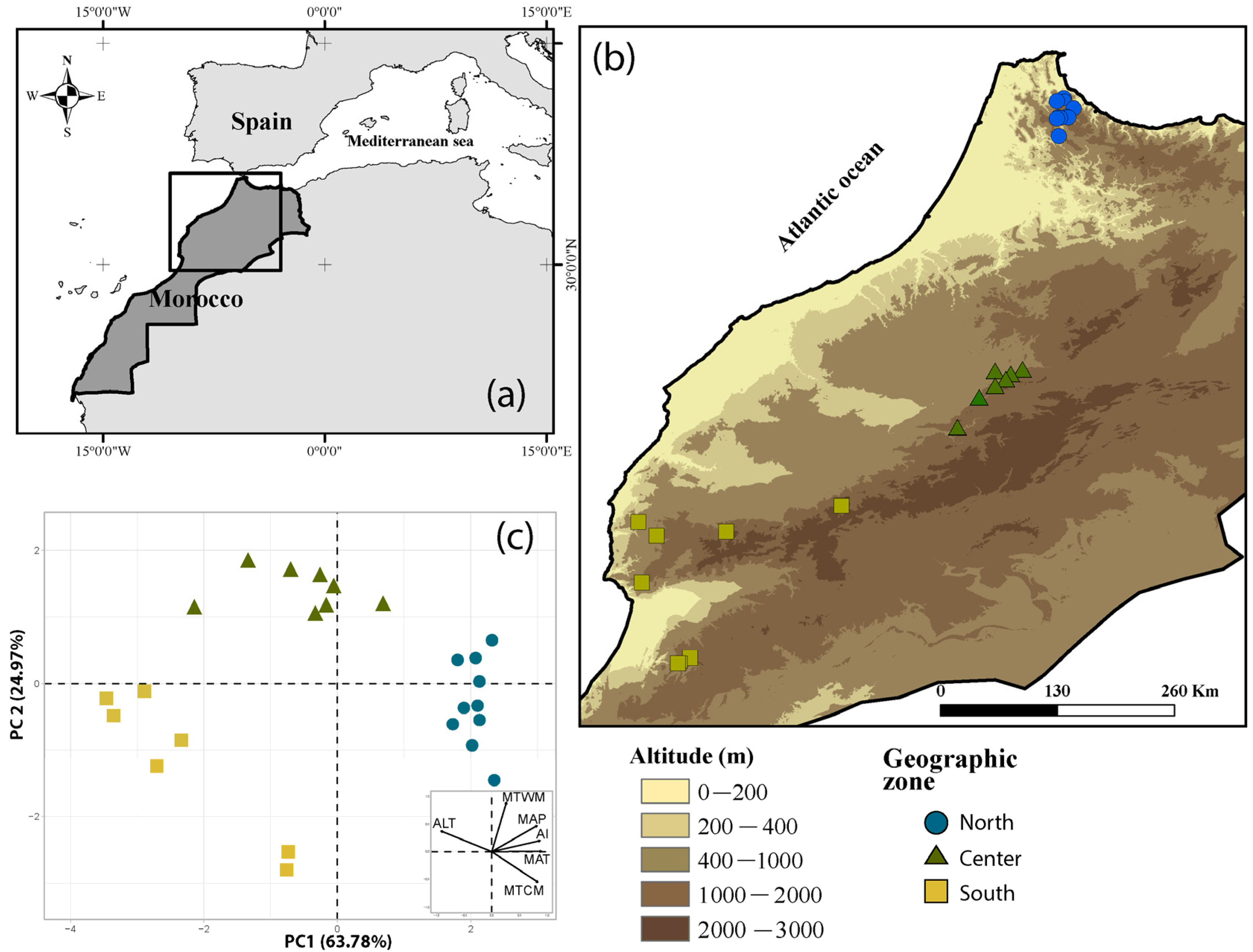
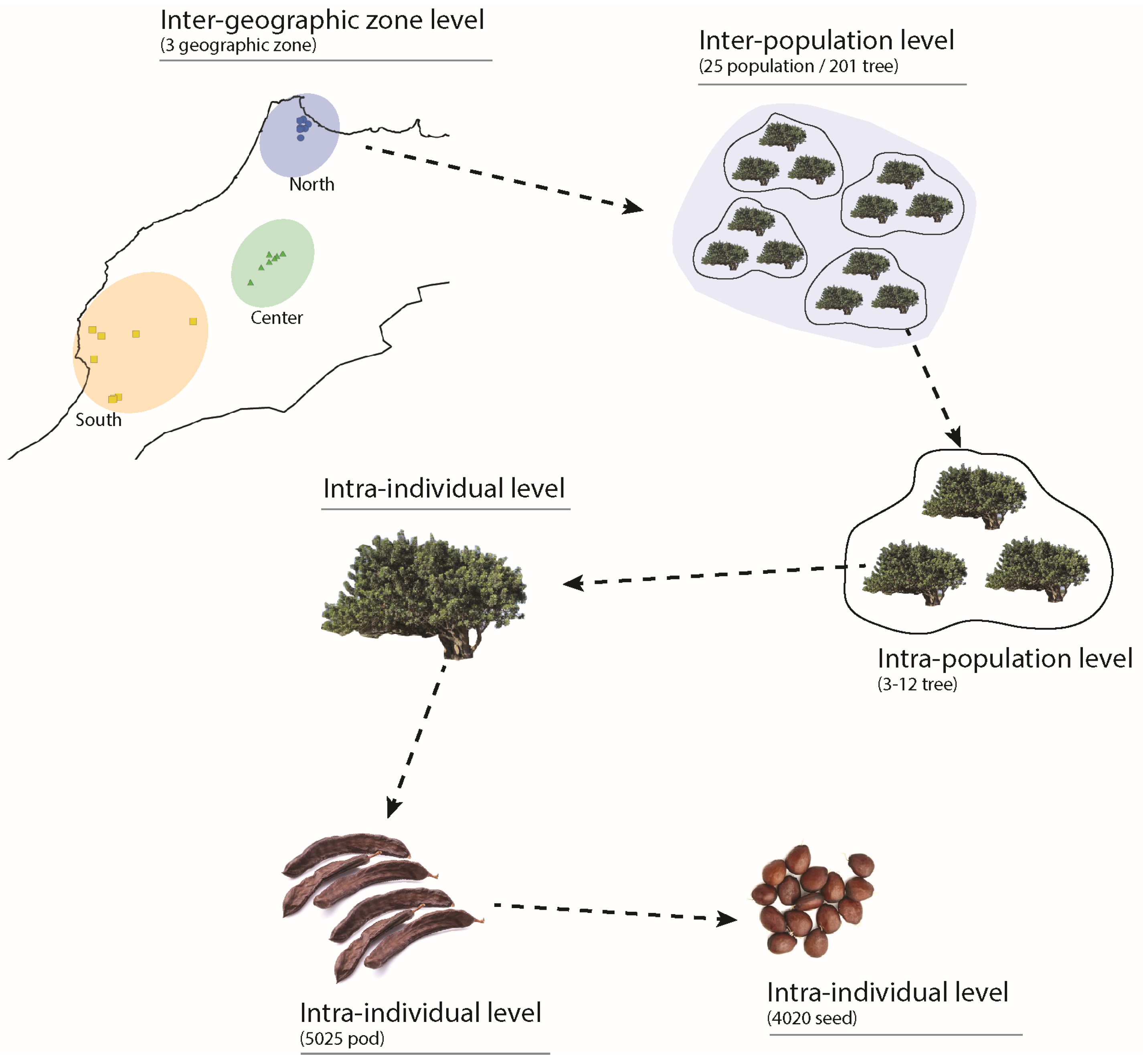
4.1. Study Area and Field Sampling
4.2. Morphological Traits Measurements
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghalambor, C.K.; McKAY, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus Non-Adaptive Phenotypic Plasticity and the Potential for Contemporary Adaptation in New Environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using Plant Functional Traits to Understand Ecological Processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Change 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Lloret, F.; de la Riva, E.G.; Pérez-Ramos, I.M.; Marañón, T.; Saura-Mas, S.; Díaz-Delgado, R.; Villar, R. Climatic Events Inducing Die-off in Mediterranean Shrublands: Are Species’ Responses Related to Their Functional Traits? Oecologia 2016, 180, 961–973. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E.; Valladares, F. Global Change and the Evolution of Phenotypic Plasticity in Plants. Ann. N. Y. Acad. Sci. 2010, 1206, 35–55. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Terral, J.-F.; Badal, E.; Heinz, C.; Roiron, P.; Thiebault, S.; Figueiral, I. A Hydraulic Conductivity Model Points to Post-neogene Survival of the Mediterranean Olive. Ecology 2004, 85, 3158–3165. [Google Scholar] [CrossRef]
- Kassout, J.; Ater, M.; Ivorra, S.; Barbara, H.; Limier, B.; Ros, J.; Girard, V.; Paradis, L.; Terral, J.-F. Resisting Aridification: Adaptation of Sap Conduction Performance in Moroccan Wild Olive Subspecies Distributed Over an Aridity Gradient. Front. Plant Sci. 2021, 12, 663721. [Google Scholar] [CrossRef]
- Stotz, G.C.; Salgado-Luarte, C.; Escobedo, V.M.; Valladares, F.; Gianoli, E. Global Trends in Phenotypic Plasticity of Plants. Ecol. Lett. 2021, 24, 2267–2281. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic Plasticity for Plant Development, Function and Life History. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Kassout, J.; Terral, J.-F.; Hodgson, J.G.; Ater, M. Trait-Based Plant Ecology a Flawed Tool in Climate Studies? The Leaf Traits of Wild Olive That Pattern with Climate Are Not Those Routinely Measured. PLoS ONE 2019, 14, e0219908. [Google Scholar] [CrossRef]
- Gratani, L.; Covone, F.; Larcher, W. Leaf Plasticity in Response to Light of Three Evergreen Species of the Mediterranean Maquis. Trees 2006, 20, 549–558. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Batlle, I. The Carob Tree: Botany, Horticulture, and Genetic Resources. Hortic. Rev. 2013, 41, 385–456. [Google Scholar]
- Palamarev, E. Paleobotanical Evidences of the Tertiary History and Origin of the Mediterranean Sclerophyll Dendroflora. Pl Syst Evol. 1989, 162, 93–107. [Google Scholar] [CrossRef]
- Zohary, D. Domestication of the Carob (Ceratonia siliqua L.). Isr. J. Plant Sci. 2002, 50, 141–145. [Google Scholar] [CrossRef]
- Ramón-Laca, L.; Mabberley, D.J. The Ecological Status of the Carob-Tree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Bot. J. Linn. Soc. 2004, 144, 431–436. [Google Scholar] [CrossRef]
- Battle, I.; Tous, J. Carob Tree (Ceratonia siliqua L.). Promoting the Conservation and Use of Underutilized and Neglected Crops. Bioversity Int. 1997, 17, 1–92. [Google Scholar]
- Meyer, R.S.; DuVal, A.E.; Jensen, H.R. Patterns and Processes in Crop Domestication: An Historical Review and Quantitative Analysis of 203 Global Food Crops. New Phytol. 2012, 196, 29–48. [Google Scholar] [CrossRef]
- Baumel, A.; Nieto Feliner, G.; Médail, F.; La Malfa, S.; Di Guardo, M.; Bou Dagher Kharrat, M.; Lakhal-Mirleau, F.; Frelon, V.; Ouahmane, L.; Diadema, K. Genome-wide Footprints in the Carob Tree (Ceratonia siliqua) Unveil a New Domestication Pattern of a Fruit Tree in the Mediterranean. Mol. Ecol. 2022, 31, 4095–4111. [Google Scholar] [CrossRef]
- Viruel, J.; Le Galliot, N.; Pironon, S.; Nieto Feliner, G.; Suc, J.-P.; Lakhal-Mirleau, F.; Juin, M.; Selva, M.; Bou Dagher Kharrat, M.; Ouahmane, L. A Strong East–West Mediterranean Divergence Supports a New Phylogeographic History of the Carob Tree (Ceratonia siliqua, Leguminosae) and Multiple Domestications from Native Populations. J. Biogeogr. 2020, 47, 460–471. [Google Scholar] [CrossRef]
- Baumel, A.; Mirleau, P.; Viruel, J.; Bou Dagher Kharrat, M.; La Malfa, S.; Ouahmane, L.; Diadema, K.; Moakhar, M.; Sanguin, H.; Médail, F. Assessment of Plant Species Diversity Associated with the Carob Tree (Ceratonia siliqua, Fabaceae) at the Mediterranean Scale. Plant Ecol. Evol. 2018, 151, 185–193. [Google Scholar] [CrossRef]
- Kassout, J.; Hmimsa, Y.; El Fatehi, S.; El Ouahrani, A.; Kadaoui, K.; Chakkour, S.; Ariza-Mateos, D.; Palacios-Rodríguez, G.; Navarro-Cerrillo, R.; Ater, M. Image Analysis of Moroccan Carob Seeds (Ceratonia siliqua L.) Revealed Substantial Intraspecific Variations Depending on Climate and Geographic Origin. Ecol. Process. 2022, 11, 34. [Google Scholar] [CrossRef]
- Correia, P.J.; Gama, F.; Pestana, M.; Martins-Loução, M.A. Tolerance of Young (Ceratonia siliqua L.) Carob Rootstock to NaCl. Agric. Water Manag. 2010, 97, 910–916. [Google Scholar] [CrossRef]
- Ozturk, M.; Dogan, Y.; Sakcali, M.S.; Doulis, A.; Karam, F. Ecophysiological Responses of Some Maquis (Ceratonia siliqua L., Olea oleaster Hoffm. & Link, Pistacia lentiscus and Quercus coccifera L.) Plant Species to Drought in the East Mediterranean Ecosystem. J. Environ. Biol. 2010, 31, 233. [Google Scholar]
- Barracosa, P.; Osorio, J.; Cravador, A. Evaluation of Fruit and Seed Diversity and Characterization of Carob (Ceratonia siliqua L.) Cultivars in Algarve Region. Sci. Hortic. 2007, 114, 250–257. [Google Scholar] [CrossRef]
- Naghmouchi, S.; Khouja, M.L.; Romero, A.; Tous, J.; Boussaid, M. Tunisian Carob (Ceratonia siliqua L.) Populations: Morphological Variability of Pods and Kernel. Sci. Hortic. 2009, 121, 125–130. [Google Scholar] [CrossRef]
- Sidina, M.M.; El Hansali, M.; Wahid, N.; Ouatmane, A.; Boulli, A.; Haddioui, A. Fruit and Seed Diversity of Domesticated Carob (Ceratonia siliqua L.) in Morocco. Sci. Hortic. 2009, 123, 110–116. [Google Scholar] [CrossRef]
- Chami, M.; Hajj, A.; Kahwaji, J.; Youssef, H.; Ghaith, S.; Fakih, L.; Smaha, M.; Nabbout, R.; El Raichy, M.; As-Sadi, F. Assessment of Ancient Carob Germplasm of Lebanon by Morphological Traits. J. Am. Pomol. Soc. 2018, 72, 260–278. [Google Scholar]
- Haddarah, A.; Bassal, A.; Ismail, A.; Gaiani, C.; Ioannou, I.; Charbonnel, C.; Hamieh, T.; Ghoul, M. The Structural Characteristics and Rheological Properties of Lebanese Locust Bean Gum. J. Food Eng. 2014, 120, 204–214. [Google Scholar] [CrossRef]
- El Bouzdoudi, B.; El Ansari, Z.N.; Mangalagiu, I.; Mantu, D.; Badoc, A.; Lamarti, A. Determination of Polyphenols Content in Carob Pulp from Wild and Domesticated Moroccan Trees. Am. J. Plant Sci. 2016, 7, 1937–1951. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]
- Boublenza, I.; Ghezlaoui, S.; Mahdad, M.; Vasaï, F.; Chemat, F. Algerian Carob (Ceratonia siliqua L.) Populations. Morphological and Chemical Variability of Their Fruits and Seeds. Sci. Hortic. 2019, 256, 108537. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Taibi, M.; Ouassou, H.; Ouahhoud, S.; Ou-Yahia, D.; Loukili, E.H.; Aherkou, M.; Mansouri, F.; Bencheikh, N.; Laaraj, S. Exploring the Multi-Faceted Potential of Carob (Ceratonia siliqua var. Rahma) Leaves from Morocco: A Comprehensive Analysis of Polyphenols Profile, Antimicrobial Activity, Cytotoxicity against Breast Cancer Cell Lines, and Genotoxicity. Pharmaceuticals 2023, 16, 840. [Google Scholar] [CrossRef]
- Fadel, F.; El Mehrach, K.; Chebli, B.; Fahmi, F.; El Hafa, M.; Amri, O.; Ait Bihi, M.; Hatimi, A.; Tahrouch, S. Morphometric and Physicochemical Characteristics of Carob Pods in Three Geographical Regions of Morocco. SN Appl. Sci. 2020, 2, 2173. [Google Scholar] [CrossRef]
- Kyratzis, A.C.; Antoniou, C.; Papayiannis, L.C.; Graziani, G.; Rouphael, Y.; Kyriacou, M.C. Pod Morphology, Primary and Secondary Metabolite Profiles in Non-Grafted and Grafted Carob Germplasm Are Configured by Agro-Environmental Zone, Genotype, and Growing Season. Front. Plant Sci. 2021, 11, 612376. [Google Scholar] [CrossRef]
- Brassesco, M.E.; Brandão, T.R.; Silva, C.L.; Pintado, M. Carob Bean (Ceratonia siliqua L.): A New Perspective for Functional Food. Trends Food Sci. Technol. 2021, 114, 310–322. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Romano, A.; Moreno-Rojas, J.M. Carob Pulp: A Nutritional and Functional by-Product Worldwide Spread in the Formulation of Different Food Products and Beverages. A Review. Processes 2021, 9, 1146. [Google Scholar] [CrossRef]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.; Cross, A.T. A Research Agenda for Seed-trait Functional Ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific Functional Variability: Extent, Structure and Sources of Variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Kuppler, J.; Albert, C.H.; Ames, G.M.; Armbruster, W.S.; Boenisch, G.; Boucher, F.C.; Campbell, D.R.; Carneiro, L.T.; Chacón-Madrigal, E.; Enquist, B.J.; et al. Global Gradients in Intraspecific Variation in Vegetative and Floral Traits Are Partially Associated with Climate and Species Richness. Glob. Ecol. Biogeogr. 2020, 29, 992–1007. [Google Scholar] [CrossRef]
- Siefert, A.; Violle, C.; Chalmandrier, L.; Albert, C.H.; Taudiere, A.; Fajardo, A.; Aarssen, L.W.; Baraloto, C.; Carlucci, M.B.; Cianciaruso, M.V. A Global Meta-analysis of the Relative Extent of Intraspecific Trait Variation in Plant Communities. Ecol. Lett. 2015, 18, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Des Roches, S.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The Ecological Importance of Intraspecific Variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Westerband, A.C.; Funk, J.L.; Barton, K.E. Intraspecific Trait Variation in Plants: A Renewed Focus on Its Role in Ecological Processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 64, 715–716. [Google Scholar] [CrossRef]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.I.N.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The Return of the Variance: Intraspecific Variability in Community Ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Shipley, B.; De Bello, F.; Cornelissen, J.H.C.; Laliberté, E.; Laughlin, D.C.; Reich, P.B. Reinforcing Loose Foundation Stones in Trait-Based Plant Ecology. Oecologia 2016, 180, 923–931. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The Worldwide Leaf Economics Spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The Global Spectrum of Plant Form and Function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative Estimation of Phenotypic Plasticity: Bridging the Gap between the Evolutionary Concept and Its Ecological Applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A Multi-Trait Approach Reveals the Structure and the Relative Importance of Intra- vs. Interspecific Variability in Plant Traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Schupp, E.W.; Zwolak, R.; Jones, L.R.; Snell, R.S.; Beckman, N.G.; Aslan, C.; Cavazos, B.R.; Effiom, E.; Fricke, E.C.; Montaño-Centellas, F. Intrinsic and Extrinsic Drivers of Intraspecific Variation in Seed Dispersal Are Diverse and Pervasive. AoB Plants 2019, 11, plz067. [Google Scholar] [CrossRef] [PubMed]
- Geber, M.A.; Griffen, L.R. Inheritance and Natural Selection on Functional Traits. Int. J. Plant Sci. 2003, 164, S21–S42. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A.; Hermoso, J.F.; Ninot, A.; Plana, J.; Batlle, I. Agronomic and Commercial Performance of Four Spanish Carob Cultivars. HortTechnology 2009, 19, 465–470. [Google Scholar] [CrossRef]
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: A Sustainable Opportunity for Metabolic Health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef]
- Bahuguna, R.N.; Jagadish, K.S. Temperature Regulation of Plant Phenological Development. Environ. Exp. Bot. 2015, 111, 83–90. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Gianoli, E.; Morris, W.F.; Bozinovic, F. Ecological and Evolutionary Impacts of Changing Climatic Variability. Biol. Rev. 2017, 92, 22–42. [Google Scholar] [CrossRef] [PubMed]
- Albanell, E.; Caja, G.; Plaixats, J. Characterization of Carob Fruits (Ceratonia siliqua L.), Cultivated in Spain for Agroindustrial Use. Int. Tree Crops J. 1996, 9, 1–9. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Lechowicz, M.J. How Do Traits Vary across Ecological Scales? A Case for Trait-Based Ecology. Ecol. Lett. 2010, 13, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Laforest-Lapointe, I.; Martínez-Vilalta, J.; Retana, J. Intraspecific Variability in Functional Traits Matters: Case Study of Scots Pine. Oecologia 2014, 175, 1337–1348. [Google Scholar] [CrossRef]
- Evangelista, C.; Olden, J.D.; Lecerf, A.; Cucherousset, J. Scale-Dependent Patterns of Intraspecific Trait Variations in Two Globally Invasive Species. Oecologia 2019, 189, 1083–1094. [Google Scholar] [CrossRef]
- Fajardo, A.; Piper, F.I. Intraspecific Trait Variation and Covariation in a Widespread Tree Species (Nothofagus pumilio) in Southern Chile. New Phytol. 2011, 189, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Messier, J.; McGill, B.J.; Enquist, B.J.; Lechowicz, M.J. Trait Variation and Integration across Scales: Is the Leaf Economic Spectrum Present at Local Scales? Ecography 2017, 40, 685–697. [Google Scholar] [CrossRef]
- Qi, W.; Guo, S.; Chen, X.; Cornelissen, J.H.; Bu, H.; Du, G.; Cui, X.; Li, W.; Liu, K. Disentangling Ecological, Allometric and Evolutionary Determinants of the Relationship between Seed Mass and Elevation: Insights from Multiple Analyses of 1355 Angiosperm Species on the Eastern Tibetan Plateau. Oikos 2014, 123, 23–32. [Google Scholar] [CrossRef]
- Llanderal-Mendoza, J.; Gugger, P.F.; Oyama, K.; Uribe-Salas, D.; González-Rodríguez, A. Climatic Determinants of Acorn Size and Germination Percentage of Quercus rugosa (Fagaceae) along a Latitudinal Gradient in Mexico. Bot. Sci. 2017, 95, 37–45. [Google Scholar] [CrossRef]
- Mojzes, A.; Ónodi, G.; Lhotsky, B.; Kalapos, T.; Csontos, P.; Kröel-Dulay, G. Within-Generation and Transgenerational Plasticity in Growth and Regeneration of a Subordinate Annual Grass in a Rainfall Experiment. Oecologia 2018, 188, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Baraloto, C.; Forget, P.-M. Seed Size, Seedling Morphology, and Response to Deep Shade and Damage in Neotropical Rain Forest Trees. Am. J. Bot. 2007, 94, 901–911. [Google Scholar] [CrossRef]
- Souza, A.F.; de Matos, D.U.; Forgiarini, C.; Martinez, J. Seed Crop Size Variation in the Dominant South American Conifer Araucaria Angustifolia. Acta Oecologica 2010, 36, 126–134. [Google Scholar] [CrossRef]
- Benabid, A. Etude Phytoécologique des Peuplements Forestiers et Préforestiers du Rif Centro-Occidental (Maroc); Institut Scientifique, Université Mohammed V: Rabat, Morocco, 1984. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR Consortium for Spatial Information. 2017. Available online: http://www.csi.cgiar.org (accessed on 10 July 2023).
- R Development Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 July 2023).
- Sokal, R.R.; Braumann, C.A. Significance Tests for Coefficients of Variation and Variability Profiles. Syst. Biol. 1980, 29, 50–66. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- de Mendiburu, F.; Yaseen, M. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.4.02020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 July 2023).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial; R Package Version 1.7; Helsinki University Press: Helsinki, Finland, 2013. [Google Scholar]
- Grishin, V.N.; Grishin, N.V. Euclidian Space and Grouping of Biological Objects. Bioinformatics 2002, 18, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Statistics and Computing: Modern Applied Statistics with S; Springer: New York, NY, USA, 2013. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models, R package version 31-141. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 9 August 2023).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
| Trait | Abbreviation | Units | Mean | S.D | Min | Max | CV (%) | ANOVA F |
|---|---|---|---|---|---|---|---|---|
| Pod traits | ||||||||
| Pod length | PoLe | cm | 13.00 | 2.56 | 3.8 | 22.7 | 19.70 | 85.58 *** |
| Pod width | PoWi | mm | 17.99 | 2.74 | 9.15 | 28.89 | 15.23 | 175.79 *** |
| Pod marginal thickness | PoMT | mm | 6.55 | 1.27 | 3.44 | 12.98 | 19.43 | 132.22 *** |
| Pod central thickness | PoCT | mm | 4.53 | 1.16 | 1.52 | 9.76 | 25.60 | 56.55 *** |
| Pod weight | PoWe | g | 8.68 | 3.91 | 1.5 | 33.53 | 45.03 | 211.55 *** |
| Seed number | SeN | number/pod | 10.17 | 2.68 | 1 | 18 | 26.36 | 77.45 *** |
| Seed weight | SeWe | g | 1.81 | 0.63 | 0.11 | 4.18 | 35.11 | 110.82 *** |
| Pulp weight | PuWe | g | 6.87 | 3.47 | 1.21 | 30.42 | 50.51 | 215.70 *** |
| Seed yield | SeY | % | 22.40 | 6.77 | 3.15 | 51.70 | 30.25 | 99.26 *** |
| Aborted seed number | ASeN | number/pod | 0.80 | 1.13 | 0 | 9 | 141.22 | 29.69 *** |
| Seed traits | ||||||||
| Seed individual weight | SeIW | g | 0.18 | 0.038 | 0.07 | 0.4 | 20.25 | 127.30 *** |
| Seed length | SeLe | mm | 9.07 | 0.959 | 5.68 | 12.08 | 10.57 | 76.65 *** |
| Seed width | SeWi | mm | 6.80 | 0.730 | 3.71 | 9.83 | 10.74 | 72.05 *** |
| Seed thickness | SeT | mm | 4.28 | 0.493 | 2.12 | 6.92 | 11.51 | 38.26 *** |
| Population Name (Tree Number Per Pop.) a | Geo. Zone | Latitude (°) | Longitude (°) | ALT (m) | MAP (mm) | MAT (°C) | MTCM (°C) | MTWM (°C) | AI |
|---|---|---|---|---|---|---|---|---|---|
| Iaasfa (7) | North | 35.456 | −5.288 | 418 | 727 | 17.1 | 5.9 | 30.5 | 0.601 |
| Toughza (10) | North | 35.440 | −5.280 | 264 | 682 | 17.7 | 6.7 | 30.8 | 0.561 |
| Amtel (10) | North | 35.425 | −5.357 | 389 | 789 | 17.3 | 5.8 | 31.0 | 0.65 |
| Afertane (10) | North | 35.353 | −5.188 | 31 | 588 | 18.5 | 7.8 | 31.2 | 0.499 |
| Zaouia (10) | North | 35.283 | −5.347 | 546 | 868 | 16.6 | 4.4 | 31.7 | 0.694 |
| Ikhoujja (10) | North | 35.269 | −5.258 | 223 | 704 | 17.8 | 6.1 | 32.0 | 0.573 |
| Taghzoute (10) | North | 35.262 | −5.244 | 326 | 732 | 17.4 | 5.6 | 31.8 | 0.586 |
| Kebbache (10) | North | 35.256 | −5.328 | 380 | 820 | 17.3 | 5.2 | 32.0 | 0.651 |
| Taghbaloute (19) | North | 35.249 | −5.356 | 480 | 876 | 17.0 | 4.6 | 32.1 | 0.695 |
| Bni Ouella (10) | North | 35.076 | −5.338 | 446 | 914 | 17.4 | 4.6 | 33.2 | 0.682 |
| Tizim Tazart (10) | Center | 32.737 | −5.701 | 886 | 649 | 16.4 | 1.6 | 35.4 | 0.435 |
| Sidi Said (6) | Center | 32.720 | −5.975 | 686 | 648 | 17.8 | 2.8 | 37.3 | 0.409 |
| Ait Taleb (4) | Center | 32.691 | −5.819 | 925 | 705 | 16.3 | 1.4 | 35.8 | 0.465 |
| Komch (10) | Center | 32.638 | −5.867 | 1116 | 702 | 15.5 | 0.3 | 35.4 | 0.471 |
| Imheouach (10) | Center | 32.573 | −5.975 | 1288 | 677 | 14.8 | −0.5 | 35.1 | 0.46 |
| Tongha (5) | Center | 32.455 | −6.134 | 1000 | 659 | 16.6 | 1.2 | 36.8 | 0.422 |
| Ouaouizeght (4) | Center | 32.154 | −6.352 | 877 | 519 | 17.3 | 2.1 | 37.0 | 0.324 |
| Arbaa Tighdouin (10) | Center | 31.368 | −7.512 | 1278 | 447 | 15.4 | −0.6 | 34.7 | 0.293 |
| Imi N’Tlit (3) | South | 31.203 | −9.546 | 527 | 326 | 15.8 | 5.7 | 24.8 | 0.303 |
| Tadart (10) | South | 31.109 | −8.667 | 1282 | 445 | 13.3 | −0.6 | 28.5 | 0.347 |
| Ait Daoued (3) | South | 31.066 | −9.362 | 1173 | 414 | 13.0 | 1.2 | 25.2 | 0.367 |
| Immouzzer Ida Outanane (6) | South | 30.598 | −9.509 | 517 | 289 | 16.6 | 5.3 | 26.4 | 0.249 |
| Amskassen (6) | South | 29.841 | −9.026 | 1478 | 317 | 13.8 | −0.4 | 29.1 | 0.248 |
| Ougougane (5) | South | 29.800 | −9.117 | 1133 | 265 | 15.6 | 1.8 | 29.8 | 0.213 |
| Talant (12) | South | 29.786 | −9.148 | 1409 | 302 | 14.0 | 0.1 | 28.8 | 0.223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassout, J.; Hmimsa, Y.; Fatehi, S.E.; Kadaoui, K.; Houssni, M.; Chakkour, S.; Sahli, A.; El Chami, M.A.; Ariza-Mateos, D.; Palacios-Rodríguez, G.; et al. Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco. Plants 2023, 12, 3447. https://doi.org/10.3390/plants12193447
Kassout J, Hmimsa Y, Fatehi SE, Kadaoui K, Houssni M, Chakkour S, Sahli A, El Chami MA, Ariza-Mateos D, Palacios-Rodríguez G, et al. Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco. Plants. 2023; 12(19):3447. https://doi.org/10.3390/plants12193447
Chicago/Turabian StyleKassout, Jalal, Younes Hmimsa, Salama El Fatehi, Khalil Kadaoui, Mhammad Houssni, Soufian Chakkour, Abdelouahab Sahli, Mohamad Ali El Chami, David Ariza-Mateos, Guillermo Palacios-Rodríguez, and et al. 2023. "Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco" Plants 12, no. 19: 3447. https://doi.org/10.3390/plants12193447
APA StyleKassout, J., Hmimsa, Y., Fatehi, S. E., Kadaoui, K., Houssni, M., Chakkour, S., Sahli, A., El Chami, M. A., Ariza-Mateos, D., Palacios-Rodríguez, G., Navarro-Cerrillo, R. M., & Ater, M. (2023). Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco. Plants, 12(19), 3447. https://doi.org/10.3390/plants12193447








