Effect of Hydrogen Peroxide Application on Salt Stress Mitigation in Bell Pepper (Capsicum annuum L.)
Abstract
1. Introduction
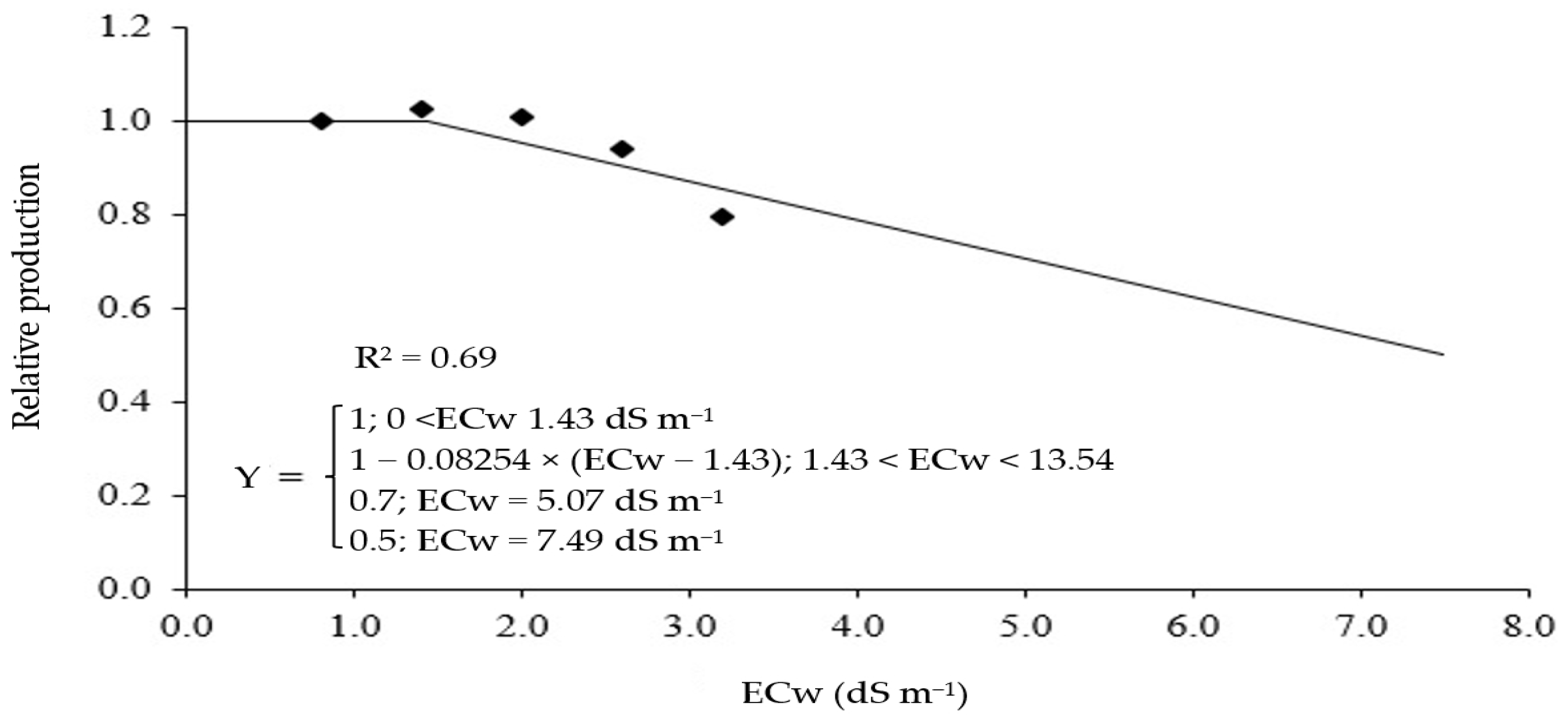
2. Results and Discussion
3. Materials and Methods
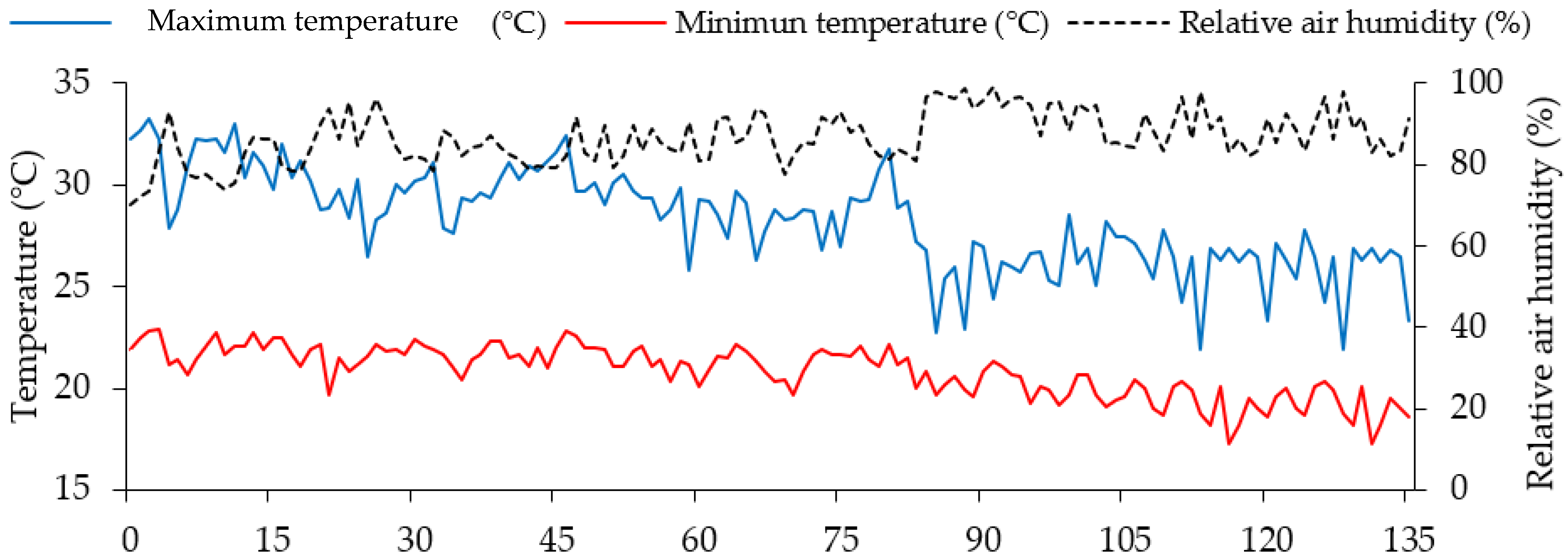
3.1. Location of the Experiment
3.2. Treatments and Experimental Design
3.3. Experiment Setup and Conduction
- Q = sum of cations (mmolc L−1); and
- ECw = electrical conductivity after discounting the ECw of water from the municipal supply system (dS m−1).
- VI = volume of water to be used in the next irrigation event (mL);
- Va = volume applied in the previous irrigation event (mL);
- Vd = volume drained (mL); and
- LF = leaching fraction of 0.10.
3.4. Variables Analyzed
- R/S—root/shoot ratio (g g−1);
- LDM—leaf dry mass (g per plant);
- STDM—stem dry mass (g per plant); and
- RDM—root dry mass (g per plant).
- WUE—water use efficiency (kg per m3);
- Production—Total production per plant (kg); and
- Water consumption—water consumption per plant (m3).
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data (accessed on 28 April 2023).
- Souza, I.L.; Tomazella, V.B.; Santos, A.J.N.; Moraes, T.; Silveira, L.C.P. Parasitoids diversity in organic Sweet Pepper (Capsicum annuum) associated with Basil (Ocimum basilicum) and Marigold (Tagetes erecta). Braz. J. Biol. 2019, 79, 603–611. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Veloso, L.L.d.S.A.; Souza, L.d.P.; de Fátima, R.T.; Silva, F.d.A.d.; Gheyi, H.R. Exogenous application of salicylic acid on the mitigation of salt stress in Capsicum annuum L. Ciênc. Rural 2023, 53, e20210447. [Google Scholar] [CrossRef]
- Francisco, V.d.S.S.; Geovani, S.d.L.; João, B.d.S.; Hans, R.G.; Lauriane, A.d.A.S.; Lourival, F.C.; Emanoela, P.d.P.; Leandro, d.P.S. Growth and physiological aspects of bell pepper (Capsicum annuum) under saline stress and exogenous application of proline. Afr. J. Biotechnol. 2016, 15, 1970–1976. [Google Scholar] [CrossRef]
- Fernandes, E.A.; Soares, L.A.d.A.; de Lima, G.S.; Silva Neta, A.M.S.; Roque, I.A.; da Silva, F.A.; Fernandes, P.D.; Lacerda, C.N. Cell damage, gas exchange, and growth of Annona squamosa L. under saline water irrigation and potassium fertilization. Semina Ciênc. Agrar. 2021, 42, 999–1018. [Google Scholar] [CrossRef]
- da Silva, S.S.; de Lima, G.S.; de Lima, V.L.A.; Gheyi, H.R.; Soares, L.A.d.A.; Lucena, R.C.M. Gas exchanges and production of watermelon plant under salinity management and nitrogen fertilization. Pesqui. Agropecu. Trop. 2019, 49. [Google Scholar] [CrossRef]
- Cavalcante, L.F.; Rebequi, A.M.; Sena, G.S.A.; Nunes, J.C. Irrigação com águas salinas e uso de biofertilizante bovino na formação de mudas de pinhão-manso. Irriga 2018, 16, 288–300. [Google Scholar] [CrossRef][Green Version]
- de Lima, G.S.; Dias, A.S.; Gheyi, H.R.; Soares, L.A.d.A.; Andrade, E.M.G. Saline water irrigation and nitrogen fertilization on the cultivation of colored fiber cotton. Rev. Caatinga 2018, 31, 151–160. [Google Scholar] [CrossRef]
- Kurunc, A.; Unlukara, A.; Cemek, B. Salinity and drought affect yield response of bell pepper similarly. Acta. Agricul. Scand Sect. B-Soil Plant Sci. 2011, 61, 514–522. [Google Scholar] [CrossRef]
- Piñero, M.C.; Houdusse, F.; García-Mina, J.; Garnica, M.; Del Amor, F. Regulation of hormonal responses of sweet pepper as affected by salinity and elevated CO2 concentration. Physiol. Plant. 2014, 151, 375–389. [Google Scholar] [CrossRef]
- Ahmed, B.A.E.; Moritani, I.S. Effect of saline water irrigation and manure application on the available water content, soil salinity, and growth of wheat. Agric. Water Manag. 2010, 97, 165–170. [Google Scholar] [CrossRef]
- da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Veloso, L.L.d.S.A.; Gheyi, H.R.; Soares, L.A.d.A. Salt stress and exogenous application of hydrogen peroxide on photosynthetic parameters of soursop. Rev. Bras. Eng. Agrí. Ambient. 2019, 23, 257–263. [Google Scholar] [CrossRef]
- Pinheiro, F.W.A.; de Lima, G.S.; Gheyi, H.R.; Soares, L.A.d.A.; de Oliveira, S.G.; da Silva, F.A. Gas exchange and yellow passion fruit production under irrigation strategies using brackish water and potassium. Rev. Ciênc. Agron. 2022, 53, e20217816. [Google Scholar] [CrossRef]
- Andrade, E.M.G.; de Lima, G.S.; de Lima, V.L.A.; da Silva, S.S.; Dias, A.S.; Gheyi, H.R. Hydrogen peroxide as attenuator of salt stress effects on the physiology and biomass of yellow passion fruit. Rev. Bras. Eng. Agrí. Ambient. 2022, 26, 571–578. [Google Scholar] [CrossRef]
- Veloso, L.L.d.S.A.; de Azevedo, C.A.V.; Nobre, R.G.; de Lima, G.S.; Capitulino, J.D.; Silva, F.d.A. H2O2 alleviates salt stress effects on photochemical efficiency and photosynthetic pigments of cotton genotypes. Rev. Bras. Eng. Agrí. Ambient. 2023, 27, 34–41. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C. Hydrogen peroxide alleviates salinity-induced damage through enhancing proline accumulation in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Veloso, L.L.d.S.A.; da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Gheyi, H.R.; Moreira, R.C.L. Growth and gas exchange of soursop under salt stress and hydrogen peroxide application. Rev. Bras. Eng. Agrí. Ambient. 2022, 26, 119–125. [Google Scholar] [CrossRef]
- Capitulino, J.D.; de Lima, G.S.; de Azevedo, C.A.V.; da Silva, A.A.R.; Arruda, T.F.L.; Soares, L.A.d.A.; Gheyi, H.R.; Fernandes, P.D.; Farias, M.S.S.; Silva, F.A. Influence of Foliar Application of Hydrogen Peroxide on Gas Exchange, Photochemical Efficiency, and Growth of Soursop under Salt Stress. Plants 2023, 12, e599. [Google Scholar] [CrossRef]
- Soares, L.A.d.A.; de Lima, G.S.; dos Santos, J.B.; Gheyi, H.R.; Nobre, R.G.; da Silva, S.S.; Dias, A.S.; Souza, L.d.P. Growth and physical characterization of fruits of bell pepper (Capsicum annuum L.) cv. ‘All Big’ subjected to saline stress and exogenous application of proline. Aust. J. Crop Sci. 2018, 12, 1528–1535. [Google Scholar] [CrossRef]
- Ramos, J.G.; de Lima, V.L.A.; de Lima, G.S.; Paiva, F.J.d.S.; Pereira, M.d.O.; Nunes, K.G. Hydrogen peroxide as salt stress attenuator in sour passion fruit. Rev. Caatinga 2022, 35, 412–422. [Google Scholar] [CrossRef]
- Dantas, M.V.; de Lima, G.S.; Gheyi, H.R.; Pinheiro, F.W.A.; Silva, L.A.; Fernandes, P.D. Summer squash morphophysiology under salt stress and exogenous application of H2O2 in hydroponic cultivation. Comun. Sci. 2021, 12, e3464. [Google Scholar]
- Dantas, M.V.; de Lima, G.S.; Gheyi, H.R.; Pinheiro, F.W.A.; Silva, P.C.C.; Soares, L.A.d.A. Gas exchange and hydroponic production of zucchini under salt stress and H2O2 application. Rev. Caatinga 2022, 35, 436–449. [Google Scholar] [CrossRef]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Silva, L.d.A.; Brito, M.E.; Sá, F.V.d.S.; Moreira, R.C.L.; Soares Filho, W.d.S.; Fernandes, P.D. Mecanismos fisiológicos em híbridos de citros sob estresse salino em cultivo hidropônico. Rev. Bras. Eng. Agrí. Ambient. 2014, 18, 1–7. [Google Scholar] [CrossRef][Green Version]
- Dias, A.S.; de Lima, G.S.; Gheyi, H.R.; Furtado, G.d.F.; Soares, L.A.d.A.; Nobre, R.G.; Fernandes, P.D. Chloroplast pigments and photochemical efficiency of West Indian cherry under salt stress and potassium-phosphorus fertilization. Semin. Cienc. Agrar. 2021, 42, 87–104. [Google Scholar] [CrossRef]
- Farouk, S.; Amira, M.S.A.Q. Enhancing seed quality and productivity as well as physio-anatomical responses of pea plants by folic acid and/or hydrogen peroxide application. Sci. Hortic. 2018, 240, 29–37. [Google Scholar] [CrossRef]
- Cintra, P.H.N.; de Melo, O.F.P.; de Menezes, J.O.S.; Padilha, R.C.; Rezende, A.G.; Matos, E.d.R. Análise de fluorescência da clorofila a em mudas de cafeeiro sob estresse hídrico. Braz. J. Dev. 2020, 6, 28006–28014. [Google Scholar] [CrossRef]
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2021, 401, e123365. [Google Scholar] [CrossRef]
- Peripolli, M.; Dornelles, S.H.; Lopes, S.J.; Tabaldi, L.A.; Trivisiol, V.S.; Rubert, J. Application of biostimulants in tomato subjected to water deficit: Physiological, enzymatic and production responses. Rev. Bras. Eng. Agrí. Ambient. 2021, 25, 90–95. [Google Scholar] [CrossRef]
- de Lima, G.S.; Dias, A.S.; Soares, L.A.d.A.; Gheyi, H.R.; Nobre, R.G.; da Silva, A.A.R. Phytochemical efficiency, photoassimilate partition and production of cotton under salt stress and nitrogen fertilization. Rev. Ciênc. Agrár. 2019, 42, 211–220. [Google Scholar] [CrossRef]
- Dias, A.S.; de Lima, G.S.; Pinheiro, F.W.A.; Gheyi, H.R.; Soares, L.A.d.A. Gas exchanges, quantum yield and photosynthetic pigments of West Indian Cherry under salt stress and potassium fertilization. Rev. Caatinga 2019, 32, 429–439. [Google Scholar] [CrossRef]
- Uzilday, B.; Ozgur, R.; Yalcinkaya, T.; Sonmez, M.C.; Turkan, I. Differential regulation of reactive oxygen species in dimorphic chloroplasts of single cell C4 plant Bienertia sinuspersici during drought and salt stress. Front. Plant Sci. 2023, 14, e1030413. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Y.; Testerink, C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2021, 45, 695–704. [Google Scholar] [CrossRef]
- Arif, M.R.; Islam, M.T.; Robin, A.H.K. Salinity Stress Alters Root Morphology and Root Hair Traits in Brassica napus. Plants 2019, 8, e192. [Google Scholar] [CrossRef]
- Jbir-Koubaa, R.; Charfeddine, S.; Bouaziz, D.; Ben Mansour, R.; Gargouri-Bouzid, R.; Nouri-Ellouz, O. Enhanced antioxidant enzyme activities and respective gene expressions in potato somatic hybrids under NaCl stress. Biol. Plant. 2019, 63, 633–642. [Google Scholar] [CrossRef]
- Bogoutdinova, L.R.; Lazareva, E.M.; Chaban, I.A.; Kononenko, N.V.; Dilovarova, T.; Khaliluev, M.R.; Baranova, E.N. Salt stress-induced structural changes are mitigated in transgenic tomato plants over-expressing superoxide dismutase. Biology 2020, 9, e297. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.A.R.; Sousa, P.F.N.; de Lima, G.S.; Soares, L.A.d.A.; Gheyi, H.R.; de Azevedo, C.A.V. Hydrogen peroxide reduces the effect of salt stress on growth and postharvest quality of hydroponic mini watermelon. Water Air Soil Poll. 2022, 233, e198. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Brestič, M.; Afrin, S.; Sakil, M.A.; Hossain, M.T.; Hossain, M.A.; Hossain, M.A. Exogenus salicylic acid and hydrogen peroxide attenuate drought stress in rice. Plant Soil Environ. 2020, 66, 7–13. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Feng, S.; Wang, J.; Huo, Z.; Ji, Q. Effects of irrigation water salinity on soil salt content distribution, soil physical properties and water use efficiency of maize for seed production in arid northwest China. Int. J. Agric. Eng. 2018, 11, 137–145. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Modesto, F.J.N.; dos Santos, M.Â.C.M.; Soares, T.M.; Santos, E.P.M. Crescimento, produção e consumo hídrico do quiabeiro submetido à salinidade em condições hidropônicas. Irriga 2019, 24, 86–97. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO, Food and agriculture organization of the United Nations: Rome, Italy, 1989; Volume 29, pp. 1–97. [Google Scholar]
- Sá, F.V.d.S.; Brito, M.E.B.; Silva, L.d.A.; Moreira, R.C.L.; Fernandes, P.D.; de Figueiredo, L.C. Physiology of perception of saline stress in ‘Common Sunki’ mandarin hybrids under saline hydroponic solution. Comun. Sci. 2015, 6, 463–470. [Google Scholar] [CrossRef]
- Veloso, L.L.d.S.A.; de Lima, G.S.; da Silva, A.A.R.; Souza, L.d.P.; de Lacerda, C.N.; da Silva, I.J.; Chaves, L.H.G.; Fernandes, P.D. Attenuation of salt stress on the physiology and production of bell peppers by treatment with salicylic acid. Semin. Cienc. Agrar. 2021, 42, 2751–2768. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. EROs as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Serenko, E.K.; Baranova, E.N.; Balakhnina, T.I.; Kurenina, L.V.; Gulevich, A.A.; Kosobruhov, A.A.; Polyakov, V.Y. Structural organization of chloroplast of tomato plants Solanum lycopersicum transformed by Fe-containing superoxide dismutase. Biochem. (Mosc.) Supl. Ser. A Membr. Cell Biol. 2011, 5, 177–184. [Google Scholar] [CrossRef]
- Carvalho, F.E.L.; Lobo, A.K.M.; Bonifácio, A.; Martins, M.O.; Lima Neto, M.C.; Silveira, J.A.G. Aclimatação ao estresse salino em plantas de arroz induzida pelo pré-tratamento com H2O2. Rev. Bras. Eng. Agrí. Ambient. 2011, 15, 416–423. [Google Scholar] [CrossRef]
- Antoniou, C.; Savvides, A.; Christou, A.; Fotopoulos, V. Unravelling chemical priming machinery in plants: The role of reactive oxygen–nitrogen–sulfur species in abiotic stress tolerance enhancement. Curr. Opin. Plant. 2016, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Kilic, S.; Kahraman, A. The Mitigation Effects of Exogenous Hydrogen Peroxide when Alleviating Seed Germination and Seedling Growth Inhibition on Salinity-Induced Stress in Barley. Pol. J. Environ. Stud. 2016, 25, 1053–1059. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa Solos: Brasília, Brazil, 2017; p. 573. [Google Scholar]
- Medeiros, J.F. Qualidade de Água de Irrigação e Evolução da Salinidade Nas Propriedades Assistidas Pelo GAT Nos Estados de RN, PB e CE. Dissertação de Mestrado; Universidade Federal da Paraíba: Campina Grande, Brazil, 1992; 196p. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; U.S. Department of Agriculture: Washington, DC, USA, 1954; 160p.
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio em ambiente controlado. In Métodos de Pesquisa em Fertilidade do Solo; Oliveira, A.J., Garrido, W.E., Araújo, J.D., Lourenço, S., Eds.; Embrapa SEA: Brasília, Brazil, 1991; pp. 189–253. [Google Scholar]
- Bione, M.A.A.; Soares, T.M.; Cova, A.M.W.; Silva Paz, V.P.; Gheyi, H.R.; Rafael, M.R.S.; Neves, B.S.L. Hydroponic production of ‘Biquinho’ pepper with brackish water. Agric. Water Manag. 2021, 245, e106607. [Google Scholar] [CrossRef]
- Govaerts, B.; Sayre, K.D.; Lichter, K.; Dendooven, L.; Deckers, J. Influence of permanent planting in high bed and residue management on physical and chemical soil quality in rainfed corn/wheat systems. Plant Soil. 2007, 291, 39–54. [Google Scholar] [CrossRef]
- Hotelling, H.; Eisenhart, C.; Hastay, M.W.; Wallis, W.A. Multivariate quality control. In Techniques of Statistical Analysis; John Wiley & Sons: New York, NY, USA, 1947; 73p. [Google Scholar]
- Hair, F.J.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tatham, R.L. Análise Multivariada de Dados, 6th ed.; Tradução Sant’Anna, A.S., Ed.; Bookman: Porto Alegre, Brazil, 2009; 688p. [Google Scholar]
- StatSoft, Inc. Programa Computacional Statistica, 7.0; Statsoft: Hamburg, Germany, 2004; Available online: https://statsoft-academic.com.br (accessed on 28 April 2023).
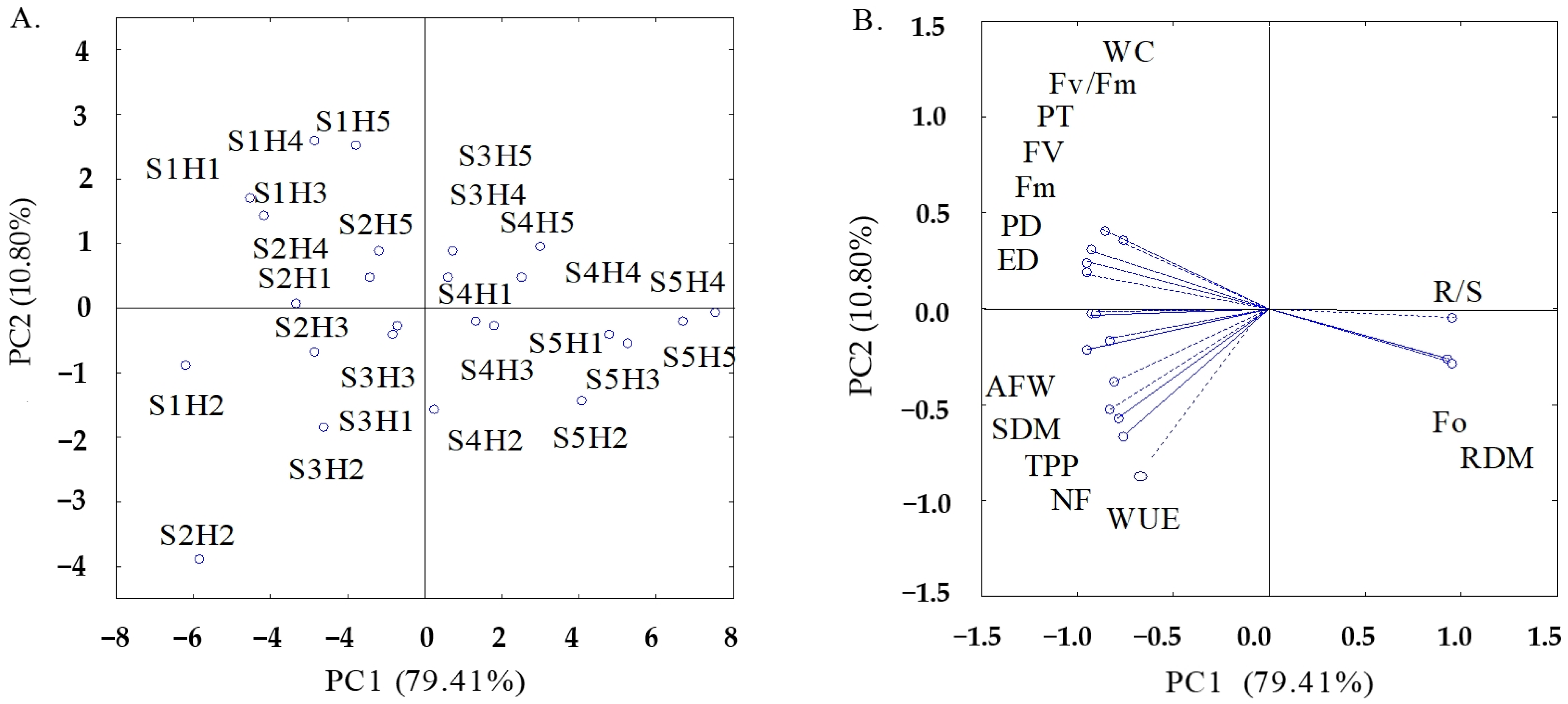

| Principal Components | ||
|---|---|---|
| PC1 | PC2 | |
| Eigenvalues (λ) | 14.29 | 1.94 |
| Percentage of total variance (S2%) | 79.41 | 10.80 |
| Hotelling test (T2) for electrical conductivity of irrigation water (ECw) | 0.01 | 0.01 |
| Hotelling test (T2) for hydrogen peroxide (H2O2) | 0.01 | 0.01 |
| Hotelling test (T2) for interaction (ECw × H2O2) | 0.01 | 0.03 |
| PCs | Correlation Coefficient (r) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F0 | Fm | Fv | Fv/Fm | SHDM | RDM | R/S | NF | AFW | TPP | PD | ED | PT | WC | WUE | |
| PC1 | 0.92 | −0.96 | −0.95 | −0.77 | −0.82 | 0.95 | 0.96 | −0.79 | −0.95 | −0.84 | −0.93 | −0.93 | −0.93 | −0.87 | −0.65 |
| PC2 | −0.26 | 0.18 | 0.25 | 0.35 | −0.39 | −0.28 | −0.05 | −0.57 | −0.21 | −0.53 | −0.03 | −0.03 | 0.30 | 0.41 | −0.75 |
| Mean Values | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F0 | Fm | Fv | Fv/Fm | SHDM | RDM | R/S | NF | AFW | TPP | PD | ED | PT | WC | WUE | |
| S1H1 | 420.0 | 2100.2 | 1642.7 | 0.782 | 14.6 | 4.5 | 0.31 | 6.7 | 85.3 | 569.7 | 74.9 | 89.9 | 5.38 | 264.5 | 12.7 |
| S1H2 | 422.0 | 2188.9 | 1692.7 | 0.773 | 17.9 | 4.7 | 0.26 | 10.7 | 90.5 | 965.3 | 71.5 | 85.8 | 5.37 | 264.5 | 21.5 |
| S1H3 | 428.0 | 2144.0 | 1665.0 | 0.777 | 14.3 | 5.1 | 0.36 | 8.0 | 75.9 | 607.6 | 71.3 | 85.6 | 5.46 | 264.5 | 13.5 |
| S1H4 | 424.3 | 2120.6 | 1651.3 | 0.779 | 13.3 | 5.3 | 0.40 | 5.7 | 73.4 | 417.6 | 66.5 | 79.8 | 5.37 | 264.5 | 9.3 |
| S1H5 | 424.0 | 2113.1 | 1588.0 | 0.752 | 12.5 | 5.4 | 0.43 | 5.0 | 70.8 | 353.7 | 67.1 | 80.5 | 5.42 | 264.5 | 7.9 |
| S2H1 | 428.4 | 2088.9 | 1593.3 | 0.763 | 14.1 | 5.5 | 0.39 | 7.3 | 87.3 | 638.9 | 64.7 | 77.7 | 5.11 | 234.2 | 15.9 |
| S2H2 | 430.4 | 2099.5 | 1574.7 | 0.750 | 15.9 | 6.0 | 0.38 | 12.8 | 95.9 | 1229.6 | 77.1 | 92.5 | 5.10 | 234.2 | 30.5 |
| S2H3 | 436.6 | 2078.8 | 1568.3 | 0.754 | 13.6 | 6.3 | 0.46 | 8.8 | 79.7 | 701.8 | 68.7 | 82.5 | 5.18 | 234.2 | 17.4 |
| S2H4 | 432.8 | 2068.6 | 1542.0 | 0.745 | 12.9 | 6.3 | 0.49 | 6.2 | 77.0 | 482.4 | 63.4 | 76.1 | 5.10 | 234.2 | 11.9 |
| S2H5 | 432.5 | 2067.5 | 1541.7 | 0.746 | 12.2 | 6.3 | 0.52 | 5.5 | 74.3 | 408.6 | 70.7 | 84.8 | 5.15 | 234.2 | 10.1 |
| S3H1 | 449.8 | 2063.8 | 1541.3 | 0.747 | 13.4 | 6.5 | 0.48 | 6.8 | 80.6 | 544.9 | 61.5 | 73.8 | 4.71 | 216.2 | 14.7 |
| S3H2 | 451.9 | 2075.4 | 1563.3 | 0.753 | 15.1 | 6.6 | 0.44 | 9.1 | 86.3 | 789.2 | 73.3 | 87.9 | 4.70 | 216.2 | 21.2 |
| S3H3 | 458.4 | 2042.7 | 1548.0 | 0.758 | 12.9 | 6.81 | 0.53 | 8.1 | 71.7 | 581.1 | 65.3 | 78.4 | 4.77 | 216.2 | 15.6 |
| S3H4 | 454.5 | 2037.9 | 1515.3 | 0.744 | 12.3 | 6.85 | 0.56 | 5.7 | 69.3 | 399.4 | 60.3 | 72.3 | 4.69 | 216.2 | 10.7 |
| S3H5 | 454.1 | 2024.0 | 1506.0 | 0.744 | 11.5 | 7.04 | 0.61 | 5.1 | 66.9 | 338.3 | 67.1 | 80.6 | 4.74 | 216.2 | 9.1 |
| S4H1 | 472.3 | 2020.9 | 1498.0 | 0.741 | 13.2 | 7.09 | 0.54 | 5.7 | 68.6 | 393.7 | 55.9 | 67.0 | 4.00 | 205.2 | 11.2 |
| S4H2 | 474.6 | 1996.5 | 1494.0 | 0.748 | 14.8 | 7.40 | 0.50 | 7.8 | 73.4 | 570.2 | 62.3 | 74.7 | 3.99 | 205.2 | 16.2 |
| S4H3 | 481.3 | 1991.4 | 1493.0 | 0.750 | 12.7 | 7.41 | 0.59 | 6.9 | 60.9 | 419.8 | 55.5 | 66.6 | 4.05 | 205.2 | 11.9 |
| S4H4 | 477.2 | 1984.6 | 1474.3 | 0.743 | 12.1 | 7.45 | 0.62 | 4.9 | 58.9 | 288.6 | 57.2 | 68.7 | 3.99 | 205.2 | 8.2 |
| S4H5 | 476.8 | 1959.4 | 1468.7 | 0.750 | 11.3 | 7.72 | 0.68 | 4.3 | 56.8 | 244.4 | 58.8 | 70.6 | 4.03 | 205.2 | 6.9 |
| S5H1 | 543.2 | 1947.5 | 1431.7 | 0.735 | 11.84 | 7.84 | 0.66 | 4.14 | 56.21 | 233.2 | 52.8 | 63.4 | 3.40 | 187.5 | 7.2 |
| S5H2 | 545.7 | 1920.1 | 1411.0 | 0.735 | 13.31 | 8.17 | 0.61 | 5.61 | 60.16 | 337.8 | 59.2 | 70.9 | 3.39 | 187.5 | 10.5 |
| S5H3 | 553.5 | 1901.3 | 1415.5 | 0.744 | 11.40 | 8.71 | 0.76 | 4.97 | 49.97 | 248.7 | 52.7 | 63.3 | 3.45 | 187.5 | 7.7 |
| S5H4 | 548.8 | 1893.1 | 1389.7 | 0.734 | 10.84 | 9.16 | 0.85 | 3.52 | 48.31 | 170.9 | 48.7 | 58.4 | 3.39 | 187.5 | 5.3 |
| S5H5 | 548.3 | 1867.3 | 1371.5 | 0.734 | 10.18 | 9.55 | 0.94 | 3.11 | 46.60 | 144.8 | 46.1 | 55.3 | 3.22 | 187.5 | 4.5 |
| Chemical Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| pH H2O | OM | P | K+ | Na+ | Ca2+ | Mg2+ | Al3+ | H+ |
| 1:2.5 | dag kg−1 | mg kg−1 | cmolc kg−1 | |||||
| 6.12 | 1.36 | 6.80 | 0.22 | 0.16 | 2.60 | 3.66 | 1.42 | 0.51 |
| Chemical characteristics | Physical characteristics | |||||||
| ECse | CEC | SARse | ESP | Particle size fraction (g kg−1) | Moisture (dag kg−1) | |||
| dS m−1 | cmolc kg−1 | (mmol L−1)0.5 | % | Sand | Silt | Clay | 33.42 kPa 1 | 1519.5 kPa 2 |
| 1.15 | 7.23 | 0.38 | 1.87 | 760.9 | 164.5 | 74.6 | 13.07 | 5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragão, J.; Lima, G.S.d.; Lima, V.L.A.d.; Silva, A.A.R.d.; Capitulino, J.D.; Caetano, E.J.M.; Silva, F.d.A.d.; Soares, L.A.d.A.; Fernandes, P.D.; Farias, M.S.S.d.; et al. Effect of Hydrogen Peroxide Application on Salt Stress Mitigation in Bell Pepper (Capsicum annuum L.). Plants 2023, 12, 2981. https://doi.org/10.3390/plants12162981
Aragão J, Lima GSd, Lima VLAd, Silva AARd, Capitulino JD, Caetano EJM, Silva FdAd, Soares LAdA, Fernandes PD, Farias MSSd, et al. Effect of Hydrogen Peroxide Application on Salt Stress Mitigation in Bell Pepper (Capsicum annuum L.). Plants. 2023; 12(16):2981. https://doi.org/10.3390/plants12162981
Chicago/Turabian StyleAragão, Jéssica, Geovani Soares de Lima, Vera Lúcia Antunes de Lima, André Alisson Rodrigues da Silva, Jessica Dayanne Capitulino, Edmilson Júnio Medeiros Caetano, Francisco de Assis da Silva, Lauriane Almeida dos Anjos Soares, Pedro Dantas Fernandes, Maria Sallydelândia Sobral de Farias, and et al. 2023. "Effect of Hydrogen Peroxide Application on Salt Stress Mitigation in Bell Pepper (Capsicum annuum L.)" Plants 12, no. 16: 2981. https://doi.org/10.3390/plants12162981
APA StyleAragão, J., Lima, G. S. d., Lima, V. L. A. d., Silva, A. A. R. d., Capitulino, J. D., Caetano, E. J. M., Silva, F. d. A. d., Soares, L. A. d. A., Fernandes, P. D., Farias, M. S. S. d., Gheyi, H. R., Borborema, L. D. A., Arruda, T. F. d. L., & Santos, L. F. S. (2023). Effect of Hydrogen Peroxide Application on Salt Stress Mitigation in Bell Pepper (Capsicum annuum L.). Plants, 12(16), 2981. https://doi.org/10.3390/plants12162981










