Screening and Evaluation of Excellent Blackberry Cultivars and Strains Based on Nutritional Quality, Antioxidant Properties, and Genetic Diversity
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Growth Statistics of Different Blackberry Cultivars and Strains
2.2. Comparison of Fruit Ripening Characteristics and Yield of Different Blackberry Cultivars and Strains
2.3. Comparison of Fruit Appearance Characteristics of Different Blackberry Cultivars and Strains
2.4. Analysis of Fruit Nutritional Characteristics of the 23 Blackberry Cultivars and Strains
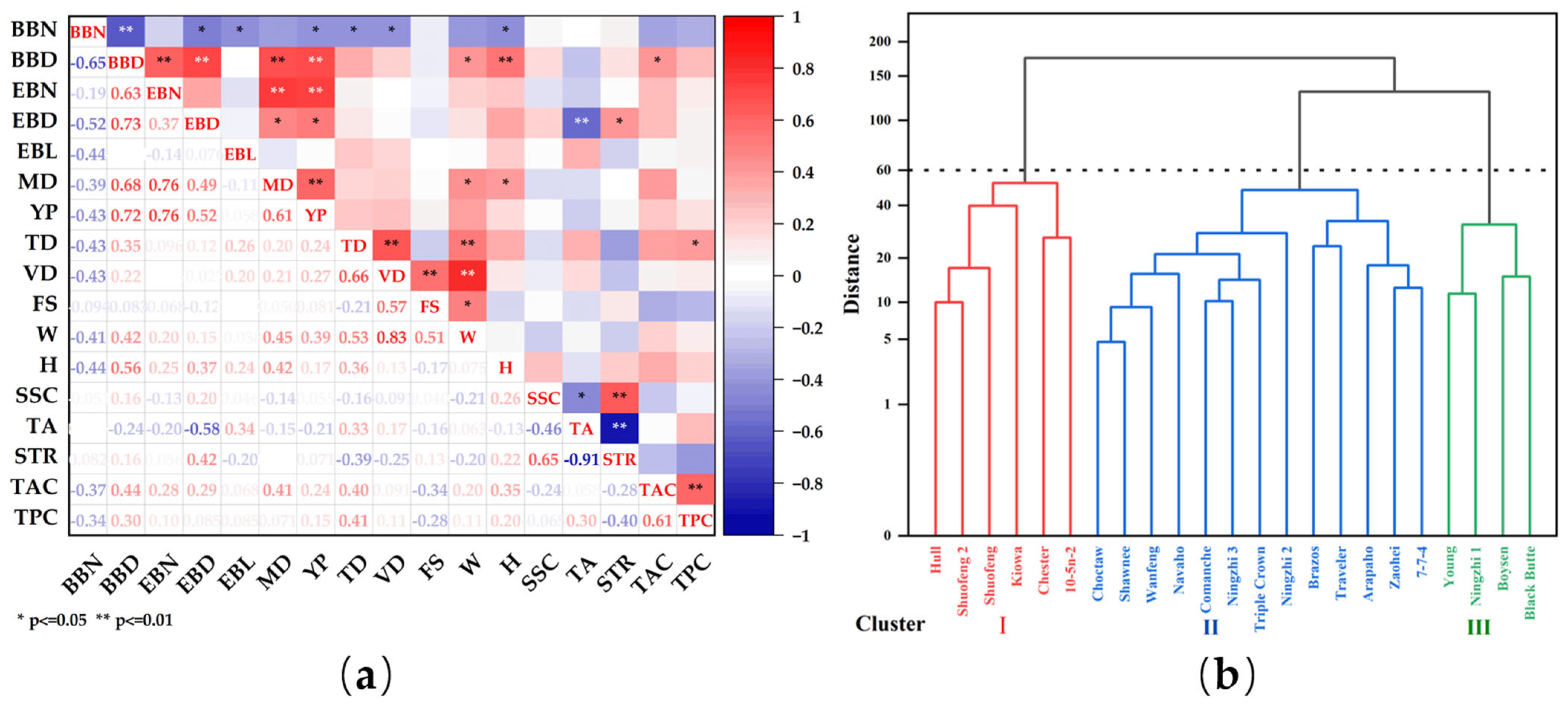
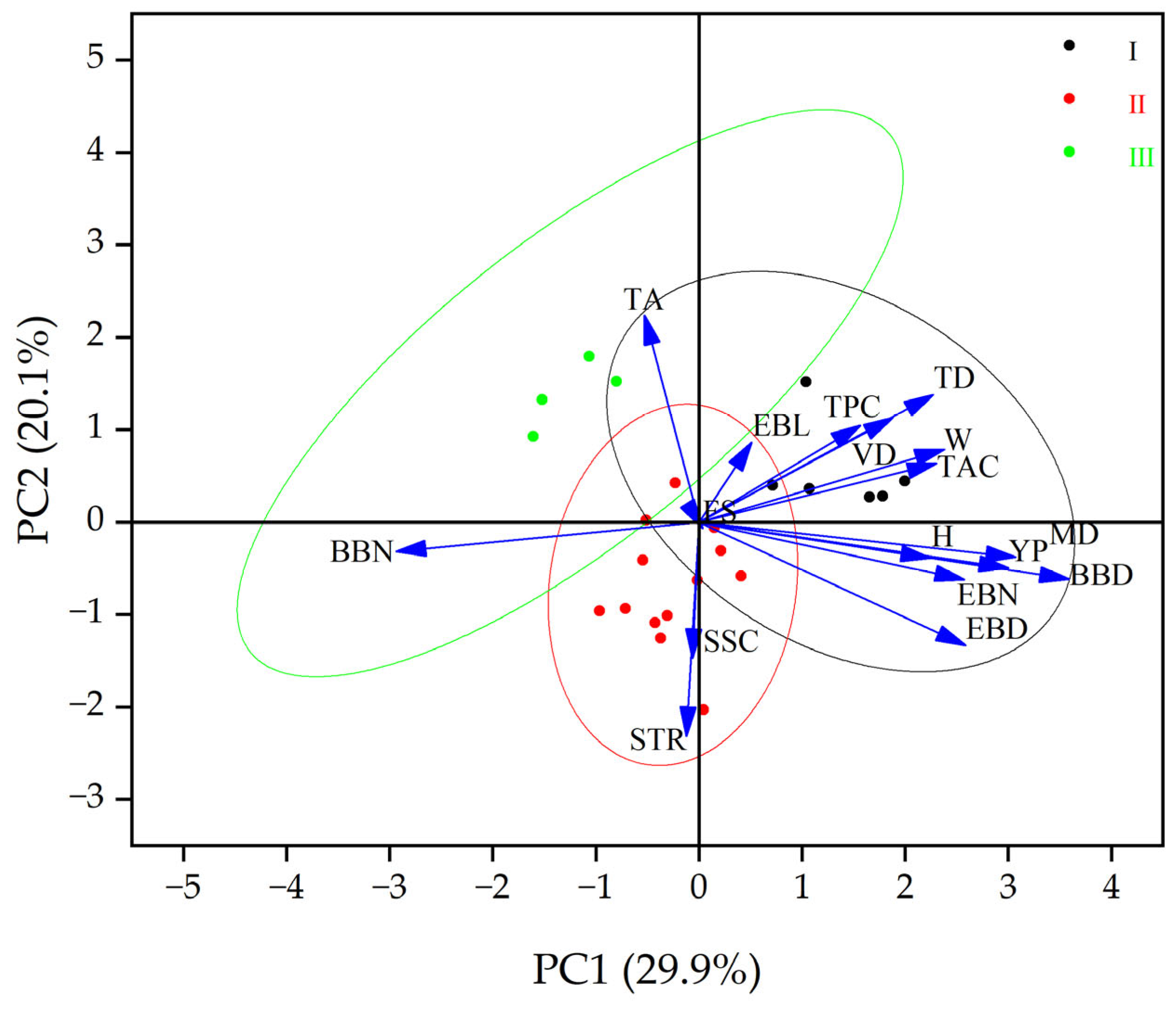
2.5. Correlation, Systematic Clustering and Principal Component Analysis among Various Indicators
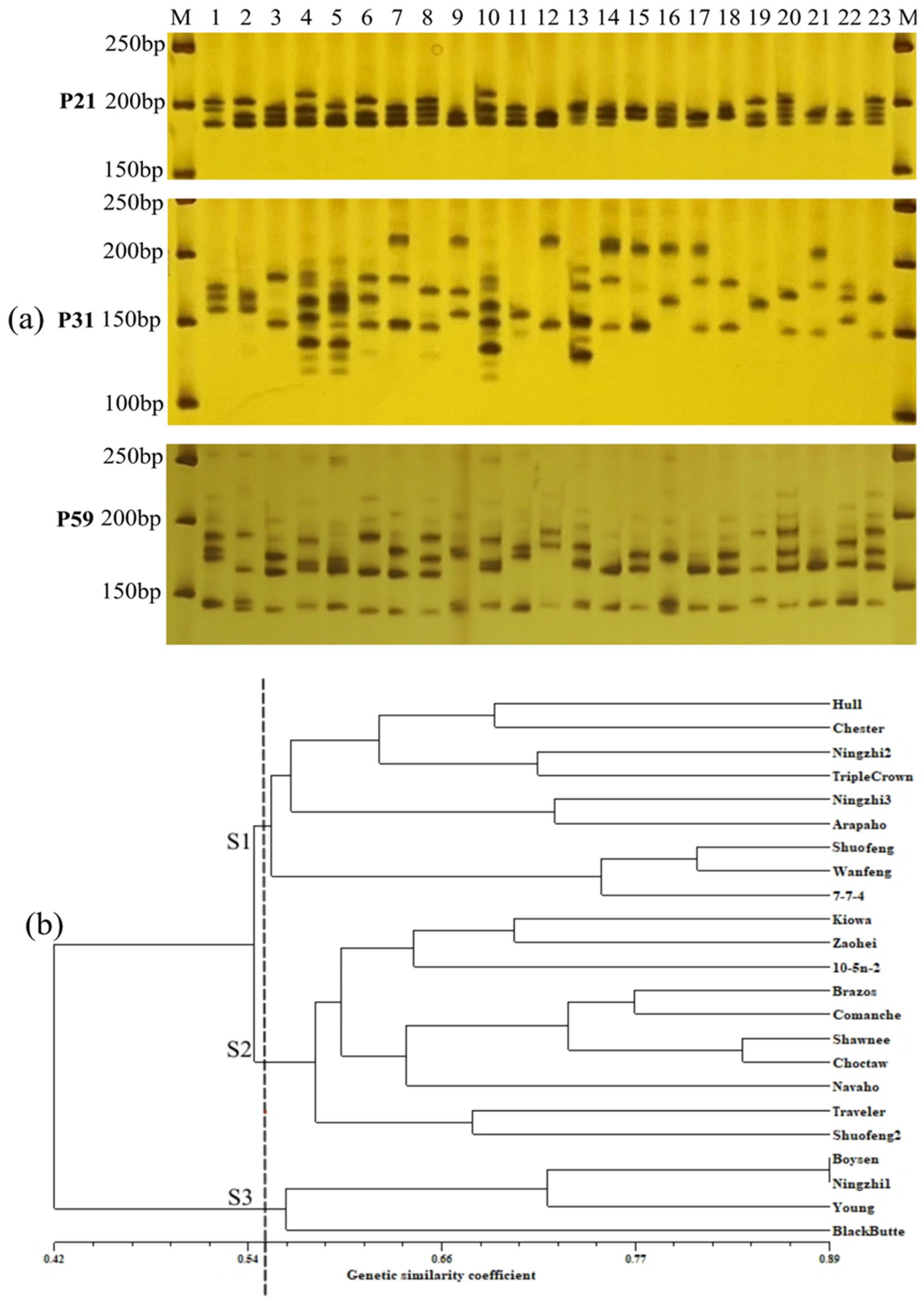
2.6. Genetic Diversity Analysis by SSR Markers
2.7. Comparison of Fruit Processing Characteristics of 10 Representative Blackberry Cultivars and Strains
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Growth Performance Survey Method
4.3. Method for Determination of Fruit Appearance Indexes
4.4. Methods for Determination of Fruit Nutritional Indexes
4.5. Genetic Diversity Analysis
4.5.1. DNA Extraction and Detection
4.5.2. SSR Marker Analysis
4.6. Methods for Determination of Fruit Processing Characteristics
4.7. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; Li, W.; Lyu, L.; Wang, X.; Zhao, H.; Fang, L.; Zhang, C.; Hu, M.; Zheng, H.; Zhang, M.; et al. Cultivation and Utilization of Blackberry in China; Jiangsu Science and Technology Press: Nanjing, China, 2010; pp. 17–18. [Google Scholar]
- Wu, W.; Chen, Y.; Lyu, L. Blackberry and raspberry introduction in Nanjing. J. Jiangsu For. Sci. Technol. 2006, 33, 13–15, 20. [Google Scholar]
- Wu, X.; Liu, S.; Chen, L. Blackberry introduction in Hunan and physiological characters of its fruit. J. Plant Resour. Environ. 1998, 8, 49–52. [Google Scholar]
- Toshima, S.; Hirano, T.; Kunitake, H. Comparison of anthocyanins, polyphenols, and antioxidant capacities among raspberry, blackberry, and Japanese wild Rubus species. Sci. Hortic. 2021, 285, 110204. [Google Scholar] [CrossRef]
- Dai, J.; Gupte, A.; Gates, L.; Mumper, R.J. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food Chem. Toxicol. 2009, 47, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Zhao, Y.; Hwang, J.; Cullen, A.; Deeb, C.; Akhavan, N.; Arjmandi, B.; Salazar, G. Gender differences in the effect of blackberry supplementation in vascular senescence and atherosclerosis in ApoE−/− mice. J. Nutr. Biochem. 2020, 80, 108375. [Google Scholar] [CrossRef]
- Dou, Z.M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef]
- Kishore, K. Phenological growth stages and heat unit requirement of Indian blackberry (Syzygium cumini L., Skeels). Sci. Hortic. 2019, 249, 455–460. [Google Scholar] [CrossRef]
- Joo, M.; Lewandowski, N.; Auras, R.; Harte, J.; Almenar, E. Comparative shelf life study of blackberry fruit in bio-based and petroleum-based containers under retail storage conditions. Food Chem. 2011, 126, 1734–1740. [Google Scholar] [CrossRef]
- Li, X.; Qiao, L.; Chen, B.; Zheng, Y.; Zhi, C.; Zhang, S.; Pan, Y.; Cheng, Z. SSR markers development and their application in genetic diversity evaluation of garlic (Allium sativum) germplasm. Plant Divers. 2022, 44, 481–491. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Lyu, L. Analysis on genetic diversity in different introduced Rubus spp. cultivars. Nonwood For. Res 2020, 38, 52–58. [Google Scholar] [CrossRef]
- Huang, Z.; Wen, Y.; Zhang, C. Comprehensive evaluation of five blackberry hybrid strains by analytic hierarchy process (AHP). J. Nanjing For. Univ. 2019, 43, 135–140. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, Y.; WenLong, W. DNA fingerprinting identification of blackberry hybrid strains with SSR molecular markers. J.Jiangsu For. Sci. Tech. 2015, 42, 19. [Google Scholar]
- Zhang, C.; WenLong, W.; Wang, X. Comparative observation of prickle between blackberry cv.‘Boysenberry’ and its bud mutation cv.‘Ningzhi 1’. Jiangsu Agric. Sci. 2013, 8, 26–29. [Google Scholar]
- Zhang, C.; Hu, S.; Lyu, L.; Wu, W.; Li, W. SSR identification of blackberry Ningzhi 2 and its parents. Jiangsu Agric. Sci. 2012, 40, 44–46. [Google Scholar] [CrossRef]
- Zhao, H.; Lyu, L.; Zhang, C. Rubus ‘Ningzhi 3’: A new blackberry cultivar. J. Nanjing For. Univ. 2020, 44, 231–232. [Google Scholar] [CrossRef]
- Wu, W.; Liu, H.; Lyu, L.; Li, W. ‘Ningzhi 3’, an early and sweet blackberry. Acta Hortic. 2020, 1277, 73–79. [Google Scholar] [CrossRef]
- Zhang, C.; Lyu, L.; Zhao, H. Rubus ‘Shuofeng’: A new blackberry cultivar. J. Nanjing For. Univ. 2020, 44, 245–246. [Google Scholar] [CrossRef]
- Lyu, L.; WenLong, W.; Li, W. ‘Zaohong’, a new blackberry (Rubus ursinus × idaeus) cultivar. J. Nanjing For. Univ. 2023. [Google Scholar]
- Van de Velde, F.; Méndez-Galarraga, M.P.; Pirovani, M.É. Effect of enriched O2 and CO2 atmospheres on the overall quality and the bioactive potential of fresh blackberries. Postharvest Biol. Tec. 2020, 164, 111166. [Google Scholar] [CrossRef]
- Mertoglu, K.; Eskimez, I.; Polat, M.; Okatan, V.; Korkmaz, N.; Gulbandilar, A.; Bulduk, I. Determination of anti-microbial and phyto-chemical characteristics of some blackberry cultivars. Fresen. Environ. Bull. 2021, 30, 1789–1795. [Google Scholar]
- Toshima, S.; Katsumi, I.; Kai, A.; Yahata, M.; Hirano, T.; Kunitake, H. Amphidiploid production of hybrid between black raspberry and Rubus parvifolius L., a wild Asian species, using colchicine treatment. Sci. Hortic. 2023, 312, 111863. [Google Scholar] [CrossRef]
- Abdi, N.; Moradi, H.; Hadadinejad, M. Evaluation of Genetic Diversity in Blackberry Germplasm in Iran by Using Inter Simple Sequence Repeats (ISSR) Markers. J. Agric. Sci. Technol. 2021, 23, 915–928. [Google Scholar]
- Moreno–Medina, B.L.; Casierra–Posada, F. Molecular characterization of a species in the genus Rubus in Boyacá, Colombia. Rev. Bras. Frutic. 2021, 43, 1–10. [Google Scholar] [CrossRef]
- Godwin, C.; Worthington, M.; Aryal, R.; Ashrafi, H.; Threlfall, R.; Clark, J. Genetic Control of Sweetness and Acidity in Blackberry. HortScience 2021, 56, S226–S227. [Google Scholar]
- Lebedev, V.G.; Subbotina, N.M.; Maluchenko, O.P.; Lebedeva, T.N.; Krutovsky, K.V.; Shestibratov, K.A. Transferability and Polymorphism of SSR Markers Located in Flavonoid Pathway Genes in Fragaria and Rubus Species. Genes 2019, 11, 11. [Google Scholar] [CrossRef]
- Perveen, N.; Cholin, S.S.; Hipparagi, K.; Prabhuling, G.; Murthy, B.N.S.; Peerjade, D. Molecular diversity assessment among the pomegranate genotypes belonging to diverse genetic background using microsatellite markers. Acta Physiol. Plant. 2023, 45, 92. [Google Scholar] [CrossRef]
- Jabari, M.; Golparvar, A.; Sorkhilalehloo, B.; Shams, M. Investigation of genetic diversity of Iranian wild relatives of bread wheat using ISSR and SSR markers. J. Genet. Eng. Biotechnol. 2023, 21, 73. [Google Scholar] [CrossRef]
- Finn, C.E.; Strik, B.C.; Yorgey, B.M.; Peterson, M.E.; Jones, P.A.; Lee, J.; Bassil, N.V.; Martin, R.R. ‘Twilight’ Thornless Semi-erect Blackberry. HortScience 2020, 55, 1148–1152. [Google Scholar] [CrossRef]
- Finn, C.E.; Strik, B.C.; Yorgey, B.M.; Peterson, M.E.; Jones, P.A.; Buller, G.; Serçe, S.; Lee, J.; Bassil, N.V.; Martin, R.R. ‘Eclipse’ Thornless Semi-erect Blackberry. HortScience 2020, 55, 749–754. [Google Scholar] [CrossRef]
- Mofokeng, M.A.; Mashilo, J.; Rantso, P.; Shimelis, H. Genetic variation and genetic advance in cowpea based on yield and yield-related traits. Acta. Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 381–391. [Google Scholar] [CrossRef]
- Agredano-De la Garza, C.S.; Balois-Morales, R.; Berumen-Varela, G.; León-Fernández, A.E.; Bautista-Rosales, P.U.; López-Guzmán, G.G.; Pérez-Ramírez, I.F. Physicochemical characterization and dietary fiber of 15 Nance (Byrsonima crassifolia L.) fruits selections from Nayarit. Sci. Hortic. 2021, 289, 110460. [Google Scholar] [CrossRef]
- Khadgi, A. Morphological Characterization of Prickled and Prickle-free Rubus Using Scanning Electron Microscopy. HortScience 2020, 55, 676–683. [Google Scholar] [CrossRef]
- Vance, A.J.; Jones, P.; Finn, C.E.; Strik, B.C. Fruit Development in Blackberry Types and Cultivars—Impact of Days and Temperature from Bloom to Stages of Ripening. J. Am. Pomol. Soc. 2019, 73, 227–239. [Google Scholar]
- Kaps, M.L. Blueberry and Blackberry Cultivar Evaluation in Missouri, 2011–2016. J. Am. Pomol. Soc. 2019, 73, 146–151. [Google Scholar]
- Garcia Munoz, M.C.; Cardona, W.A.; Calvo Salamanca, A.M.; Espitia Gonzalez, J.J.; Bolanos Benavides, M.M. Packaging design proposal motivated by the identification of damages in Andean blackberry (Rubus glaucus Benth). Heliyon 2020, 6, e05300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiong, Z.; Yang, H.; Wu, W. Changes in pericarp morphology, physiology and cell wall composition account for flesh firmness during the ripening of blackberry (Rubus spp.) fruit. Sci. Hortic. 2019, 250, 59–68. [Google Scholar] [CrossRef]
- Soto, M.; Pérez, A.M.; Cerdas, M.d.M.; Vaillant, F.; Acosta, Ó. Physicochemical characteristics and polyphenolic compounds of cultivated blackberries in Costa Rica. J. Berry Res. 2019, 9, 283–296. [Google Scholar] [CrossRef]
- Raseira, M.d.C.B.; Franzon, R.C.; Feldberg, N.P.; Antunes, L.E.C.; Scaranari, C. ‘BRS Cainguá’, a blackberry fresh-market cultivar. Crop Breed Appl. Biot. 2020, 20, 1–3. [Google Scholar] [CrossRef]
- D’Agostino, M.F.; Sicari, V.; Giuffrè, A.M.; Soria, A.C. Blackberries (Rubus ulmifolius Schott) from Calabria (Italy): A comprehensive characterisation. Eur. food Res. Technol. 2022, 248, 905–916. [Google Scholar] [CrossRef]
- Siriwoharn, T.; Wrolstad, R.E.; Finn, C.E.; Pereira, C.B. Influence of cultivar, maturity, and sampling on blackberry (Rubus L. Hybrids) anthocyanins, polyphenolics, and antioxidant properties. J. Agric. Food Chem. 2004, 52, 8021–8030. [Google Scholar] [CrossRef]
- Finn, C.E.; Strik, B.C.; Yorgey, B.M.; Peterson, M.E.; Jones, P.A.; Buller, G.; Lee, J.; Bassil, N.V.; Martin, R.R. ‘Galaxy’ Thornless Semierect Blackberry. HortScience 2020, 55, 967–971. [Google Scholar] [CrossRef]
- Segantini, D.M.; Leonel, S.; Clark, J.; Threlfall, R. Fruit Quality and Polyphenolic Compounds of ‘Tupy’ Blackberry Influenced by Pruning Time and Irrigation Management in a Subtropical Climate. HortScience 2015, 50, S339–S340. [Google Scholar]
- Huang, X.; Wu, Y.; Zhang, S.; Yang, H.; Wu, W.; Lyu, L.; Li, W. Variation in Bioactive Compounds and Antioxidant Activity of Rubus Fruits at Different Developmental Stages. Foods 2022, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Samaniego, I.; Brito, B.; Viera, W.; Cabrera, A.; Llerena, W.; Kannangara, T.; Vilcacundo, R.; Angos, I.; Carrillo, W. Influence of the Maturity Stage on the Phytochemical Composition and the Antioxidant Activity of Four Andean Blackberry Cultivars (Rubus glaucus Benth) from Ecuador. Plants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Putsakum, G.; Tzima, K.; Tiwari, B.K.; O’Donnell, C.P.; Rai, D.K. Effects of thermosonication on ascorbic acid, polyphenols and antioxidant activity in blackberry juice. Int. J. Food Sci. Technol. 2023, 58, 2304–2311. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Fu, H.Q.; Zhang, J.; Chen, T.; Sun, B.; Luo, Y.; Zhang, Y.; Tang, H.R.; Wang, X.R. Genome affinity in Rubus (Rosaceae) inferred from meiotic chromosome pairing of sixteen wild and cultivated bramble resources. Indian J. Genet. Pl. Br. 2018, 78, 496–506. [Google Scholar] [CrossRef]
- Lyu, L.; Huang, G.; Wu, W.; Li, W.; Wang, X. Growth Performance of Different Blackberry Cultivars in Nanjing. Nonwood For. Res. 2008, 26, 74–79. [Google Scholar] [CrossRef]
- Meyers, S.L.; Jennings, K.M.; Monks, D.W.; Mitchem, W.E. Effect of Weed-Free Strip Width on Newly Established ‘Navaho’ Blackberry Growth, Yield, and Fruit Quality. Weed Technol. 2014, 28, 426–431. [Google Scholar] [CrossRef]
- de Oliveira, J.T.; de Oliveira, R.A.; Silva, P.A.; Teodoro, P.E. Contribution to the selection of blackberry through fruit physical variables. HortScience 2021, 56, 1003–1004. [Google Scholar] [CrossRef]
- Nour, V. Quality characteristics, anthocyanin stability and antioxidant activity of apple (Malus domestica) and black chokeberry (Aronia melanocarpa) juice blends. Plants 2022, 11, 2027. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–159. [Google Scholar] [CrossRef]
- Graham, J.; Smith, K.; MacKenzie, K.; Jorgenson, L.; Hackett, C.; Powell, W. The construction of a genetic linkage map of red raspberry (Rubus idaeus subsp idaeus) based on AFLPs, genomic-SSR and EST-SSR markers. Theor. Appl. Genet. 2004, 109, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.R.F.; Reed, B.M.; Graham, J.; Fernandez-Fernandez, F.; Bassil, N.V. Microsatellite Markers for Raspberry and Blackberry. J. Am. Soc. Hortic. Sci. 2010, 135, 271–278. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Wang, K.; Sun, S.; Tian, W.; Li, Z.; Wang, G.; Lu, X.; Liu, Z.; Li, Q.; et al. Genetic Structure and Molecular Identities of 46 Apple Landraces (Malus Mill.) in China. Agronomy 2023, 13, 1262. [Google Scholar] [CrossRef]
- Guo, R.; Xia, X.; Chen, J.; An, Y.; Mi, X.; Li, R.; Zhang, C.; Chen, M.; Wei, C.; Liu, S. Genetic relationship analysis and molecular fingerprint identification of the tea germplasms from Guangxi Province, China. Breed. Sci. 2021, 71, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Xu, S.Y.; Jin, M.K. Effects of different maceration enzymes on yield, clarity and anthocyanin and other polyphenol contents in blackberry juice. Int. J. Food Sci. Technol. 2009, 44, 2342–2349. [Google Scholar] [CrossRef]
| Cultivar/Strain | Thorns | Basal Branch Number | Basal Branch Diameter/cm | Effective Branch Number | Effective Branch Diameter/cm | Effective Branch Length/cm | Yield per Plant/kg | Ripening Time | Ripening Type | Maturity Duration/d |
|---|---|---|---|---|---|---|---|---|---|---|
| Ningzhi 1 | none | 3.0 ± 0.17 | 1.40 ± 0.08 | 16 ± 1.6 | 0.62 ± 0.05 | 133 ± 12.74 | 2.8 ± 0.17 | Late May to mid-June | Early ripe | 25 |
| Ningzhi 3 | none | 2.8 ± 0.26 | 1.70 ± 0.09 | 20 ± 1.92 | 0.72 ± 0.04 | 91 ± 7.14 | 4.8 ± 0.41 | Early June to late June | Early ripe | 25 |
| Zaohei | none | 2.4 ± 0.15 | 1.90 ± 0.16 | 17 ± 1.50 | 0.74 ± 0.04 | 88 ± 7.64 | 3.6 ± 0.26 | Late May to mid-June | Early ripe | 20 |
| Young | none | 2.2 ± 0.21 | 1.35 ± 0.13 | 11 ± 0.63 | 0.62 ± 0.03 | 132 ± 10.79 | 2.2 ± 0.15 | Late May to early June | Early ripe | 20 |
| Arapaho | none | 3.6 ± 0.35 | 1.60 ± 0.10 | 18 ± 1.76 | 0.71 ± 0.04 | 56 ± 4.19 | 2.4 ± 0.22 | Late May to mid-June | Early ripe | 30 |
| Traveler | none | 2.7 ± 0.15 | 1.80 ± 0.15 | 16 ± 1.11 | 0.82 ± 0.07 | 76 ± 4.35 | 3.8 ± 0.35 | Late May to mid-June | Early ripe | 25 |
| Boysen | yes | 3.2 ± 0.29 | 1.50 ± 0.08 | 16 ± 1.45 | 0.63 ± 0.05 | 128 ± 9.91 | 3.8 ± 0.33 | Late May to mid-June | Early ripe | 25 |
| Brazos | yes | 2.8 ± 0.19 | 1.60 ± 0.13 | 28 ± 1.51 | 0.70 ± 0.04 | 98 ± 7.97 | 5.3 ± 0.37 | Late May to late June | Early ripe | 30 |
| Black Butte | yes | 2.2 ± 0.12 | 1.20 ± 0.06 | 10 ± 0.99 | 0.60 ± 0.05 | 120 ± 6.06 | 1.3 ± 0.09 | Late May to mid-June | Early ripe | 20 |
| Choctaw | yes | 2.3 ± 0.23 | 1.80 ± 0.16 | 21 ± 2.06 | 0.77 ± 0.04 | 116 ± 6.22 | 4.8 ± 0.34 | Early June to late June | Early ripe | 30 |
| Shawnee | yes | 2.5 ± 0.14 | 1.85 ± 0.10 | 25 ± 1.36 | 0.71 ± 0.06 | 118 ± 10.03 | 4.5 ± 0.25 | Early June to early July | Early ripe | 30 |
| Hull | none | 2.2 ± 0.20 | 1.85 ± 0.18 | 24 ± 2.18 | 0.78 ± 0.04 | 126 ± 10.24 | 5.6 ± 0.45 | Mid-June to early July | Medium ripe | 30 |
| Shuofeng | none | 1.4 ± 0.09 | 2.30 ± 0.20 | 18 ± 1.78 | 0.81 ± 0.05 | 135 ± 9.20 | 6.5 ± 0.62 | Mid-June to mid-July | Medium ripe | 30 |
| Ningzhi 2 | none | 2.1 ± 0.14 | 2.10 ± 0.19 | 22 ± 1.27 | 0.78 ± 0.07 | 128 ± 6.61 | 3.6 ± 0.34 | Late June to late July | Medium ripe | 30 |
| Triple Crown | none | 1.8 ± 0.13 | 1.90 ± 0.10 | 15 ± 1.35 | 0.88 ± 0.08 | 132 ± 9.01 | 4.2 ± 0.26 | Mid-June to early July | Medium ripe | 30 |
| Navaho | none | 2.6 ± 0.16 | 1.75 ± 0.15 | 18 ± 1.41 | 0.76 ± 0.05 | 105 ± 9.68 | 3.8 ± 0.22 | Mid-June to mid-July | Medium ripe | 30 |
| Comanche | yes | 2.2 ± 0.15 | 1.80 ± 0.16 | 20 ± 1.76 | 0.8 ± 0.06 | 112 ± 5.72 | 4.6 ± 0.38 | Mid-June to early July | Medium ripe | 25 |
| Chester | none | 1.8 ± 0.16 | 2.41 ± 0.15 | 30 ± 2.01 | 0.82 ± 0.06 | 119 ± 6.09 | 6.5 ± 0.35 | Mid July to early August | Late ripe | 40 |
| Wanfeng | none | 2.2 ± 0.13 | 1.60 ± 0.12 | 20 ± 1.63 | 0.77 ± 0.07 | 124 ± 10.02 | 3.5 ± 0.28 | Late June to late July | Late ripe | 35 |
| Shuofeng 2 | none | 2.1 ± 0.15 | 2.10 ± 0.15 | 22 ± 2.05 | 0.76 ± 0.04 | 119 ± 6.23 | 6.0 ± 0.48 | Late June to late July | Late ripe | 30 |
| 10-5n-2 | none | 1.9 ± 0.13 | 2.10 ± 0.13 | 18 ± 1.41 | 0.79 ± 0.05 | 113 ± 9.26 | 2.3 ± 0.19 | Late June to late July | Late ripe | 30 |
| 7-7-4 | none | 2.6 ± 0.19 | 1.70 ± 0.09 | 19 ± 1.53 | 0.76 ± 0.04 | 73 ± 4.72 | 3.9 ± 0.33 | Early July to late July | Late ripe | 30 |
| Kiowa | yes | 1.6 ± 0.11 | 2.40 ± 0.21 | 28 ± 1.88 | 0.76 ± 0.08 | 83 ± 5.57 | 6.3 ± 0.45 | Late June to late July | Late ripe | 45 |
| Mean | 2.4 ± 0.52 | 1.80 ± 0.32 | 20 ± 5.02 | 0.74 ± 0.07 | 110 ± 22.1 | 4.2 ± 1.44 | - | - | 28.9 ± 5.83 | |
| Min | 1.4 | 1.2 | 10 | 0.6 | 56 | 1.3 | - | - | 20 | |
| Max | 3.6 | 2.41 | 30 | 0.88 | 135 | 6.5 | - | - | 45 | |
| CV/% | 22 | 17.5 | 25.6 | 9.67 | 20.2 | 34.4 | - | - | 20.2 |
| Cultivar/Strain | Transverse Diameter/mm | Vertical Diameter/mm | Fruit Shape Index | Fruit Weight/g | Hardness/kg/cm2 |
|---|---|---|---|---|---|
| Ningzhi 1 | 17.95 ± 1.56 | 20.15 ± 1.44 | 1.12 ± 0.16 | 4.54 ± 0.53 | 1.24 ± 0.50 |
| Ningzhi 3 | 21.25 ± 1.78 | 24.61 ± 1.32 | 1.16 ± 0.12 | 5.87 ± 0.89 | 2.13 ± 0.17 |
| Zaohei | 20.57 ± 1.44 | 23.11 ± 2.28 | 1.12 ± 0.12 | 4.89 ± 1.16 | 1.72 ± 0.38 |
| Young | 18.82 ± 1.39 | 23.07 ± 2.40 | 1.23 ± 0.08 | 4.05 ± 0.75 | 2.01 ± 0.75 |
| Arapaho | 19.26 ± 1.61 | 23.11 ± 1.91 | 1.20 ± 0.11 | 5.08 ± 0.91 | 2.11 ± 0.33 |
| Traveler | 16.22 ± 1.33 | 23.72 ± 2.66 | 1.46 ± 0.13 | 6.86 ± 1.34 | 1.41 ± 0.28 |
| Boysen | 21.79 ± 2.43 | 28.65 ± 0.94 | 1.31 ± 0.09 | 7.03 ± 0.79 | 1.75 ± 0.21 |
| Brazos | 18.63 ± 1.79 | 24.20 ± 2.02 | 1.31 ± 0.13 | 5.73 ± 0.86 | 1.13 ± 0.20 |
| Black Butte | 20.82 ± 1.80 | 30.68 ± 1.93 | 1.47 ± 0.11 | 7.26 ± 1.07 | 1.73 ± 0.96 |
| Choctaw | 18.06 ± 1.76 | 24.69 ± 2.86 | 1.37 ± 0.04 | 5.80 ± 1.21 | 2.08 ± 0.44 |
| Shawnee | 18.39 ± 2.15 | 22.10 ± 2.05 | 1.20 ± 0.11 | 5.40 ± 0.86 | 2.01 ± 0.25 |
| Hull | 23.77 ± 0.95 | 28.10 ± 1.76 | 1.24 ± 0.08 | 6.44 ± 0.78 | 2.00 ± 0.55 |
| Shuofeng | 22.82 ± 1.10 | 31.02 ± 1.81 | 1.36 ± 0.13 | 7.43 ± 1.07 | 2.07 ± 0.38 |
| Ningzhi 2 | 17.49 ± 1.08 | 23.96 ± 2.16 | 1.37 ± 0.11 | 4.76 ± 0.78 | 2.73 ± 0.80 |
| Triple Crown | 21.34 ± 1.54 | 25.22 ± 1.51 | 1.18 ± 0.10 | 6.41 ± 0.68 | 1.99 ± 0.37 |
| Navaho | 15.83 ± 0.81 | 19.72 ± 1.43 | 1.25 ± 0.02 | 5.21 ± 0.55 | 2.06 ± 0.61 |
| Comanche | 18.63 ± 1.00 | 21.57 ± 1.75 | 1.16 ± 0.11 | 4.76 ± 0.51 | 1.56 ± 0.61 |
| Chester | 20.94 ± 0.86 | 22.82 ± 1.38 | 1.10 ± 0.06 | 5.48 ± 0.68 | 2.78 ± 0.51 |
| Wanfeng | 20.37 ± 2.00 | 25.94 ± 4.07 | 1.28 ± 0.12 | 5.48 ± 1.27 | 2.20 ± 0.61 |
| Shuofeng 2 | 22.13 ± 1.87 | 26.92 ± 0.92 | 1.22 ± 0.05 | 7.05 ± 1.20 | 2.39 ± 0.65 |
| 10-5n-2 | 24.83 ± 1.84 | 24.62 ± 2.89 | 0.99 ± 0.11 | 6.69 ± 1.16 | 2.52 ± 0.22 |
| 7-7-4 | 17.98 ± 1.34 | 21.27 ± 0.99 | 1.11 ± 0.09 | 4.27 ± 1.10 | 1.56 ± 0.82 |
| Kiowa | 21.94 ± 1.56 | 31.62 ± 1.15 | 1.44 ± 0.12 | 10.43 ± 0.95 | 1.98 ± 0.21 |
| Mean | 19.99 ± 2.34 | 24.82 ± 3.33 | 1.25 ± 0.13 | 5.95 ± 1.39 | 1.96 ± 0.42 |
| Min | 15.83 | 19.72 | 0.99 | 4.05 | 1.13 |
| Max | 24.83 | 31.62 | 1.47 | 10.43 | 2.78 |
| CV/% | 11.72 | 13.42 | 10.12 | 23.39 | 21.46 |
| Cultivar/Strain | Soluble Solid Content/% | Total Acid Content/% | Solid-Acid Ratio | Total Anthocyanin Content (mg/100 g FW) | Total Polyphenol Content (mg/g FW) |
|---|---|---|---|---|---|
| Ningzhi 1 | 10.8 ± 0.28 | 1.77 ± 0.01 | 6.12 ± 0.13 | 102.6 ± 2.40 | 2.25 ± 0.10 |
| Ningzhi 3 | 11.8 ± 0.17 | 0.54 ± 0.01 | 21.88 ± 0.44 | 84.6 ± 7.94 | 2.26 ± 0.13 |
| Zaohei | 12.6 ± 0.07 | 1.23 ± 0.01 | 10.20 ± 0.25 | 103.4 ± 5.62 | 2.83 ± 0.09 |
| Young | 10.5 ± 0.14 | 1.86 ± 0.01 | 5.63 ± 0.03 | 54.0 ± 3.60 | 2.54 ± 0.18 |
| Arapaho | 11.7 ± 0.14 | 0.68 ± 0.01 | 17.25 ± 0.35 | 102.5 ± 7.00 | 2.33 ± 0.13 |
| Traveler | 10.1 ± 0.17 | 0.63 ± 0.02 | 16.03 ± 0.11 | 68.4 ± 6.19 | 2.09 ± 0.09 |
| Boysen | 10.3 ± 0.28 | 1.78 ± 0.08 | 5.79 ± 0.22 | 88.7 ± 2.82 | 2.07 ± 0.13 |
| Brazos | 9.2 ± 0.14 | 1.07 ± 0.03 | 8.60 ± 0.12 | 128.0 ± 11.10 | 2.65 ± 0.08 |
| Black Butte | 10.6 ± 0.21 | 1.42 ± 0.02 | 7.42 ± 0.07 | 119.7 ± 6.70 | 2.51 ± 0.05 |
| Choctaw | 11.2 ± 0.17 | 0.86 ± 0.02 | 13.04 ± 0.23 | 138.2 ± 5.02 | 2.66 ± 0.13 |
| Shawnee | 11.1 ± 0.15 | 0.70 ± 0.03 | 15.86 ± 0.06 | 128.9 ± 3.39 | 2.49 ± 0.10 |
| Hull | 11.9 ± 0.28 | 1.21 ± 0.04 | 9.83 ± 0.09 | 94.2 ± 5.44 | 2.67 ± 0.16 |
| Shuofeng | 12.4 ± 0.28 | 0.88 ± 0.03 | 14.06 ± 0.45 | 171.4 ± 2.05 | 3.11 ± 0.14 |
| Ningzhi 2 | 13.2 ± 0.17 | 0.53 ± 0.02 | 25.05 ± 0.87 | 71.3 ± 3.60 | 1.82 ± 0.12 |
| Triple Crown | 11.6 ± 0.35 | 0.71 ± 0.02 | 16.38 ± 0.26 | 99.1 ± 3.99 | 2.22 ± 0.09 |
| Navaho | 12.9 ± 0.17 | 0.70 ± 0.02 | 18.49 ± 0.72 | 145.7 ± 13.67 | 2.72 ± 0.03 |
| Comanche | 11.0 ± 0.28 | 0.62 ± 0.04 | 17.89 ± 0.12 | 98.3 ± 9.58 | 1.93 ± 0.08 |
| Chester | 10.1 ± 0.21 | 1.48 ± 0.05 | 6.79 ± 0.08 | 166.5 ± 13.03 | 2.90 ± 0.10 |
| Wanfeng | 10.5 ± 0.35 | 0.85 ± 0.02 | 12.37 ± 0.31 | 143.9 ± 8.30 | 2.05 ± 0.14 |
| Shuofeng 2 | 10.8 ± 0.27 | 1.08 ± 0.03 | 10.00 ± 0.26 | 187.7 ± 3.95 | 2.33 ± 0.04 |
| 10-5n-2 | 9.5 ± 0.36 | 1.42 ± 0.04 | 6.71 ± 0.17 | 225.4 ± 15.41 | 3.24 ± 0.12 |
| 7-7-4 | 10.9 ± 0.37 | 1.02 ± 0.01 | 10.64 ± 0.18 | 153.1 ± 7.84 | 2.29 ± 0.06 |
| Kiowa | 10.5 ± 0.28 | 1.22 ± 0.02 | 8.64 ± 0.05 | 114.7 ± 7.63 | 2.30 ± 0.14 |
| Mean | 11.07 ± 1.03 | 1.05 ± 0.41 | 12.38 ± 5.64 | 121.3 ± 41.22 | 2.44 ± 0.37 |
| Min | 9.2 | 0.53 | 5.63 | 54.0 | 1.82 |
| Max | 13.2 | 1.86 | 25.05 | 225.4 | 3.24 |
| CV/% | 9.29 | 39.31 | 43.96 | 33.98 | 14.96 |
| Primer Name | Repeat Motif | Forward Primer (5′—3′) | Allele Size/bp | Allele Number | PIC Value |
|---|---|---|---|---|---|
| P4 | (CT)16(CA)32 | TGCATGTGACTTTGCATCTCT/GCACTGAAAAATCATGCATCTG | 115~200 | 6 | 0.677 |
| P5 | (CT)7(AT)6(GT)10 | ATTCCCCGCCTCAGAATAAT/AAGGTTTGTGACGGGAACAG | 123~240 | 6 | 0.707 |
| P6 | (AT)8(GT)11 | TGTTGTACGTGTTGGGCTTT/GGGTGTTTGCCAGTTTCAGT | 130~177 | 6 | 0.785 |
| P15 | (T)10-(A)11-(GA)15 | GAGGGGCAATTAAAGGGTTT/TGTTGTAATTTGGTTTATCCTTGG | 130~275 | 8 | 0.770 |
| P21 | (CT)5-(CT)4 | CTCACCCGAAATGTTCAACC/GGCTAGGCCGAATGACTACA | 185~210 | 5 | 0.711 |
| P31 | (AG)8 | CAGCAGCTAGCATTTTACTGGA/GCACTCTCCACCCATTTCAT | 120~210 | 9 | 0.844 |
| P40 | (TC)9 | AACCCTAAGCCAAGGACCAT/CACCACCCATGACAGTCAGA | 150~200 | 5 | 0.755 |
| P44 | (GA)41 | TGGACAGCTTTGTGCAGAGT/GCTTGCTTGTATCTCCATTGC | 98~155 | 7 | 0.816 |
| P46 | (TTTTC)3 | CATGCTTGCATGATCACCAC/TGAGCCATAAATTTAGAGGGATT | 140~150 | 2 | 0.374 |
| P48 | (GA)10 | GCATCAGCCATTGAATTTCC/CCCACCTCCATTACCAACTC | 140~187 | 6 | 0.756 |
| P55 | (AG)15 | TGCATGAAGGCGATATAAAGG/TCCGCAAGGGTTGTATCCTA | 200~250 | 7 | 0.785 |
| P59 | (GA)10 | GCATCAGCCATTGAATTTCC/CCCACCTCCATTACCAACTC | 140~185 | 7 | 0.810 |
| P60 | (AG)27 | CACAACCAGTCCCGAGAAAT/CATTTCATCCAAATGCAACC | 107~150 | 7 | 0.809 |
| P61 | (A)11(AG)8 | GCCCCATCCTGTACAAAGAA/TTGCAACAAAGGTACGTAATGG | 185~265 | 5 | 0.722 |
| Rh37 | (AG)10 | TTTGGCCCATGTTTGCTCTC/CACTACGCCAAATCAGCTCC | 285~320 | 5 | 0.763 |
| Rh72 | (AAC)8 | TTCCGAATCAAGCTCAAAGT/AAACAATAGGTACACGGCTT | 325~360 | 5 | 0.759 |
| Rh100 | (GAGT)5 | CCTCACCATCCCACAATTAA/TTTGCTCACCGAATCTGTAT | 205~240 | 4 | 0.629 |
| Rh114 | (TAAT)5 | TCGTTCTACACTGTGTTTGT/CGCTGATATCGACTCTGAAT | 245~270 | 4 | 0.670 |
| Rh118 | (TTGGA)5 | AGTTTTCCACATGCGTAGAT/TGTACTGCATATTCGAGGAC | 140~180 | 7 | 0.814 |
| Rh121 | (TTGCTC)5bv | AAAAGTCTGTTGGTAGGCAA/TGACTGATGCAAATCTCACA | 300~400 | 8 | 0.825 |
| Cultivar/Strain | Yield of Juice/% | pH | Small Fruit Number | Small Fruit Ratio/% | Dry Matter Content/% |
|---|---|---|---|---|---|
| Hull | 71.0 ± 2.93 | 2.8 ± 0.05 | 61.7 ± 5.13 | 91.5 ± 6.34 | 14.3 ± 0.41 |
| Chester | 72.0 ± 7.06 | 2.9 ± 0.19 | 52.3 ± 5.86 | 93.1 ± 8.68 | 12.3 ± 1.18 |
| Kiowa | 70.6 ± 3.61 | 2.8 ± 0.05 | 106.8 ± 11.03 | 92.4 ± 7.95 | 14.6 ± 0.04 |
| Shuofeng | 71.0 ± 1.47 | 3.0 ± 0.02 | 68.0 ± 11.53 | 92.1 ± 13.02 | 15.8 ± 0.95 |
| Shuofeng2 | 70.0 ± 3.82 | 3.0 ± 0.08 | 37.0 ± 3.23 | 92.4 ± 6.72 | 16.1 ± 0.79 |
| 10-5n-2 | 68.7 ± 6.98 | 2.9 ± 0.14 | 51.0 ± 6.93 | 94.7 ± 10.72 | 11.0 ± 1.06 |
| Wanfeng | 69.0 ± 4.64 | 2.9 ± 0.08 | 75.0 ± 2.00 | 94.5 ± 2.10 | 14.9 ± 0.21 |
| Ningzhi 3 | 66.3 ± 1.31 | 3.0 ± 0.07 | 58.3 ± 8.06 | 87.4 ± 10.07 | 13.1 ± 1.24 |
| Zaohei | 64.9 ± 3.45 | 2.9 ± 0.02 | 68.8 ± 8.02 | 82.6 ± 8.03 | 17.2 ± 1.04 |
| Arapaho | 67.9 ± 2.41 | 3.0 ± 0.13 | 76.0 ± 5.68 | 85.4 ± 5.32 | 16.4 ± 1.53 |
| Mean | 69.1 ± 2.25 | 2.9 ± 0.09 | 65.5 ± 18.82 | 90.6 ± 4.06 | 14.6 ± 1.95 |
| Min | 64.9 | 2.8 | 37.0 | 82.6 | 11.0 |
| Max | 72.0 | 3.0 | 106.8 | 94.7 | 17.2 |
| CV/% | 3.26 | 3.11 | 28.7 | 4.48 | 13.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Wu, Y.; Wu, W.; Li, W.; Jin, Y. Screening and Evaluation of Excellent Blackberry Cultivars and Strains Based on Nutritional Quality, Antioxidant Properties, and Genetic Diversity. Plants 2023, 12, 2982. https://doi.org/10.3390/plants12162982
Zhao H, Wu Y, Wu W, Li W, Jin Y. Screening and Evaluation of Excellent Blackberry Cultivars and Strains Based on Nutritional Quality, Antioxidant Properties, and Genetic Diversity. Plants. 2023; 12(16):2982. https://doi.org/10.3390/plants12162982
Chicago/Turabian StyleZhao, Huifang, Yaqiong Wu, Wenlong Wu, Weilin Li, and Yongcan Jin. 2023. "Screening and Evaluation of Excellent Blackberry Cultivars and Strains Based on Nutritional Quality, Antioxidant Properties, and Genetic Diversity" Plants 12, no. 16: 2982. https://doi.org/10.3390/plants12162982
APA StyleZhao, H., Wu, Y., Wu, W., Li, W., & Jin, Y. (2023). Screening and Evaluation of Excellent Blackberry Cultivars and Strains Based on Nutritional Quality, Antioxidant Properties, and Genetic Diversity. Plants, 12(16), 2982. https://doi.org/10.3390/plants12162982





