Growth and Performance of Guar (Cyamopsis tetragonoloba (L.) Taub.) Genotypes under Various Irrigation Regimes with and without Biogenic Silica Amendment in Arid Southwest US
Abstract
1. Introduction
2. Results
2.1. Growth and Biomass
2.2. Physiological Parameters
2.3. Water Use Efficiency
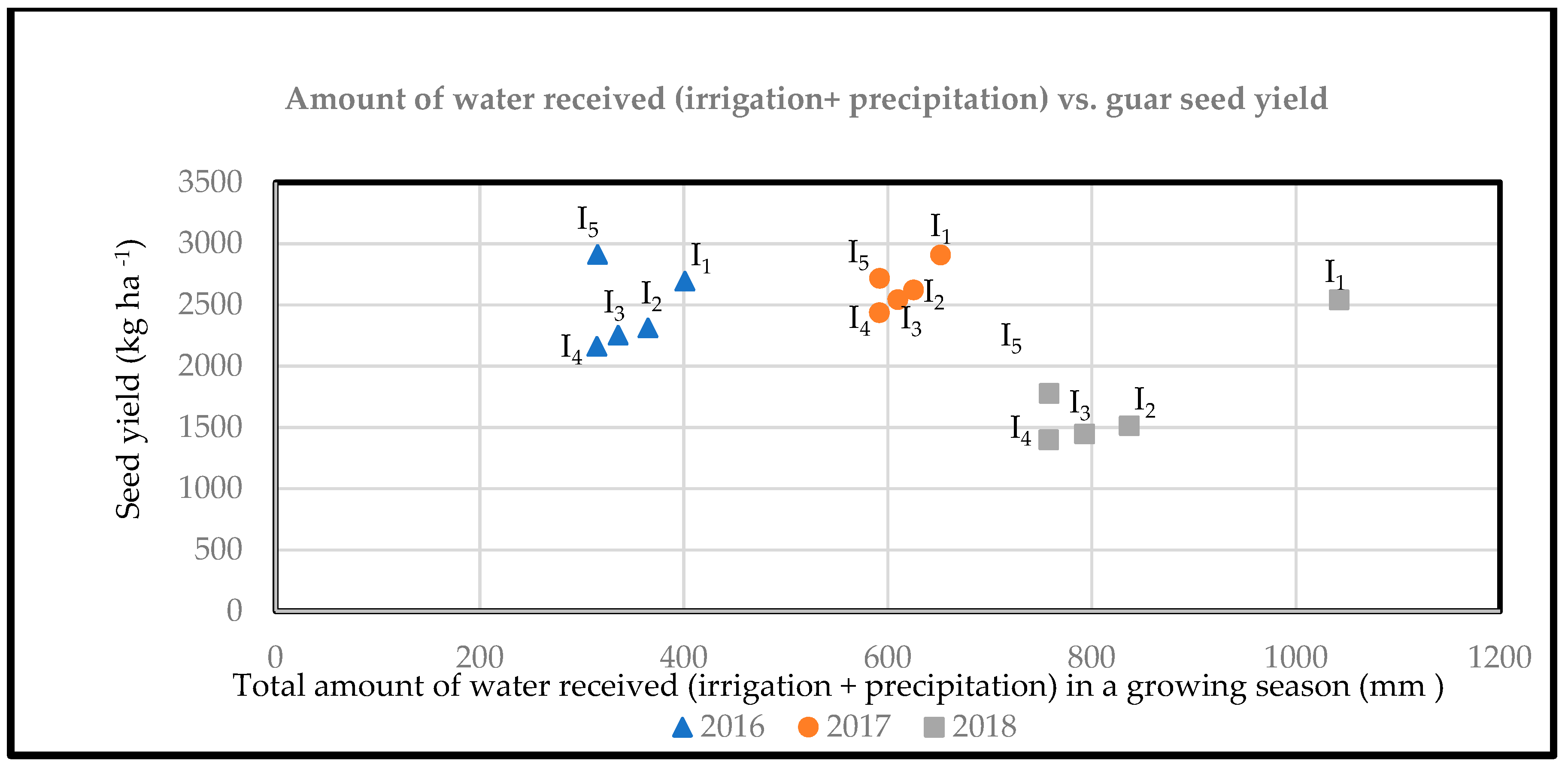
2.4. Yield Attributing Characteristics and Seed Yield
3. Materials and Methods
3.1. Experimental Site and Design Description
3.2. Field Preparation
3.3. Data Collection
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenny, J.F.; Barber, N.L.; Hutson, S.S.; Linsey, K.S.; Lovelace, J.K.; Maupin, M.A. Estimated Use of Water in the United States in 2005; United States Geological Survey: Reston, VA, USA, 2009. [CrossRef]
- Mapp, H.P. Irrigated Agriculture on the High Plains: An Uncertain Future. West. J. Agric. Econ. 1988, 13, 339–347. [Google Scholar]
- Scanlon, B.R.; Faunt, C.C.; Longuevergne, L.; Reedy, R.C.; Alley, W.M.; McGuire, V.L.; McMahon, P.B. Groundwater Depletion and Sustainability of Irrigation in the US High Plains and Central Valley. Proc. Natl. Acad. Sci. USA 2012, 109, 9320–9325. [Google Scholar] [CrossRef] [PubMed]
- Undersander, D.J.; Putnam, D.H.; Kaminski, A.R.; Kelling, K.A.; Doll, J.D.; Oplinger, E.S.; Gunsolus, J.L. Guar. In Alternative Field Crops Manual; Departments of Agronomy and Soil Science, College of Agricultural and Life Sciences and Cooperative Extension Service, University of Wisconsin: Madison, WI, USA; Department of Agronomy and Plant Genetics, University of Minnesota: St. Paul, MN, USA, 1991. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gresta, F.; Sortino, O.; Santonoceto, C.; Issi, L.; Formantici, C.; Galante, Y. Effects of sowing times on seed yield, protein, and galactomannans content of four varieties of guar (Cyamopsis tetragonoloba L.) in a Mediterranean environment. Ind. Crops Prod. 2013, 41, 46–52. [Google Scholar] [CrossRef]
- Singh, S.K. An Analysis of Performance of Guar Crop in INDIA; USDA Foreign Agricultural Service: Washington, DC, USA, 2014. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=An%20Analysis%20of%20Guar%20Crop%20in%20India_New%20Delhi_India_5-6-2014.pdf (accessed on 25 May 2023).
- Summers, H.M.; Sproul, E.; Seavert, C.; Angadi, S.; Robbs, J.; Khanal, S.; Gutierrez, P.; Teegerstrom, T.; Vazquez, D.A.Z.; Fan, N.; et al. Economic and environmental analyses of incorporating guar into the American southwest. Agric. Syst. 2021, 191, 103146. [Google Scholar] [CrossRef]
- Sandhu, D.; Pallete, A.; Pudussery, M.V.; Grover, K. Contrasting responses of guar genotypes shed light on multiple component traits of salinity tolerance mechanisms. Agronomy 2021, 11, 1068. [Google Scholar] [CrossRef]
- Acharya, B.R.; Sandhu, D.; Dueñas, C.; Ferreira, J.F.S.; Grover, K. Deciphering molecular mechanisms involved in salinity tolerance in Guar (Cyamopsis tetragonoloba (L.) Taub.) using transcriptome analyses. Plants 2022, 11, 291. [Google Scholar] [CrossRef]
- Sandhu, D.; Pallete, A.; William, M.; Ferreira, J.F.S.; Kaundal, A.; Grover, K. Salinity responses in 24 guar genotypes are linked to multigenic regulation explaining the complexity of tolerance mechanisms in planta. Crop Sci. 2022, 63, 585–597. [Google Scholar] [CrossRef]
- Singla, S.; Grover, K.; Angadi, S.; Schutte, B.; VanLeeuwen, D. Guar stand establishment, physiology and yield responses to planting dates in southern New Mexico. Agron. J. 2016, 108, 2289–2300. [Google Scholar] [CrossRef]
- Singla, S.; Grover, K.; Angadi, S.V.; Begna, S.H.; Schutte, B.; VanLeeuwen, D. Growth and yield of guar (Cyamopsis tetragonoloba L.) genotypes under different planting dates in the Semi-arid southern high plains. Am. J. Plant Sci. 2016, 7, 1246–1258. [Google Scholar] [CrossRef]
- Adams, C.B.; Boote, K.J.; Shrestha, R.; MacMillan, J.; Hinson, P.O.; Trostle, C. Growth stages and developmental patterns of guar. Agron. J. 2020, 112, 4990–5001. [Google Scholar] [CrossRef]
- Shrestha, R.; Adams, C.B.; Rajan, N. Does the drought tolerance of guar [Cyamopsis tetragonoloba (L.) Taub.] extend belowground to root nodules? J. Agron. Crop Sci. 2021, 208, 599–608. [Google Scholar] [CrossRef]
- Singh, J.; Guzman, I.; Begna, S.; Trostle, C.; Angadi, S. Germination and early growth response of guar cultivars to low temperatures. Ind. Crops Prod. 2021, 159, 113082. [Google Scholar] [CrossRef]
- Boote, K.J.; Hoogenboom, G.; Ale, S.; Adams, C.; Shreshta, R.; Mvuyekure, R.F.; Himanshu, S.K.; Grover, K.; Angadi, S. Adapting CROPGRO model to simulate growth and yield of guar, Cyamopsis tetragonoloba L, an industrial legume crop. Ind. Crops Prod. 2023, 197, 1–17. [Google Scholar] [CrossRef]
- Singh, J. Guar Growth and Development under Pre-Irrigation and in-Season Irrigation Management in the Southern High Plains. Master’s Thesis, New Mexico State University, Las Cruces, NM, USA, 2020. [Google Scholar]
- Pabuayon, I.L.B.; Singh, S.; Ritchie, G. Guar resilience in water-restricted production. Crop Sci. 2022, 62, 1937–1947. [Google Scholar] [CrossRef]
- Alexander, W.L.; Bucks, D.A.; Backhaus, R.A. Irrigation water management for guar seed production. Agron. J. 1988, 80, 447–453. [Google Scholar] [CrossRef]
- De Costa, W.A.J.M.; Dennett, M.D.; Ratnaweera, U.; Nyalemegbe, K. Effects of different water regimes on field-grown determinate and indeterminate faba bean (Vicia faba L.). II. Yield, yield components and harvest index. Field Crops Res. 1997, 52, 169–178. [Google Scholar] [CrossRef]
- Loggale, L.B. Responses of Guar to Supplemental Irrigation in Heavy Clay Soils of Abu Naama. J. Agric. Vet. Sci. 2018, 11, 12–16. [Google Scholar] [CrossRef]
- Robert, E.; Dennis, T.; Dennis, R.E.; Ray, D.T. Growing Guar in Arizona, Forage and Grain: A College of Agriculture Report; University of Arizona: Tucson, AZ, USA, 2016; pp. 28–31. [Google Scholar]
- Elsheikh, E.A.E.; Ibrahim, K.A. The effect of Bradyrhizobium inoculation on yield and seed quality of guar (Cyamopsis tetragonoloba L.). Food Chem. 1999, 65, 183–187. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. Available online: https://pubmed.ncbi.nlm.nih.gov/10585971/ (accessed on 17 June 2023). [CrossRef]
- Shakoor, S.A. Silicon to silica bodies and their potential roles: An overview. Int. J. Agric. Sci. 2014, 4, 111–120. [Google Scholar]
- Lewin, J.; Reimann, B.E.F. Silicon and Plant Growth. Annu. Rev. Plant Physiol. 1969, 20, 289–304. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2003, 50, 11–18. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lewin, J. Silicon as a nutrient element for Equisetum arvense. Can. J. Bot. 1969, 47, 125–131. [Google Scholar] [CrossRef]
- Sacala, E. Role of Silicon in Plant Resistance to Water Stress. J. Elem. 2009, 14, 619–636. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Putra, R.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Is it time to include legumes in plant silicon research? Funct. Ecol. 2020, 34, 1142–1157. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E.; Ryalls, J.M.W.; Frew, A.; DeGabriel, J.L.; Duncan, M.; Gherlenda, A.N. Silicon-induced root nodulation and synthesis of essential amino acids in a legume is associated with higher herbivore abundance. Funct. Ecol. 2017, 31, 1903–1909. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef]
- Köhler, I.H.; MacDonald, A.; Schnyder, H. Nutrient supply enhanced the increase in intrinsic water-use efficiency of a temperate seminatural grassland in the last century. Glob. Chang. Biol. 2012, 18, 3367–3376. [Google Scholar] [CrossRef]
- Fischer, R.A.; Turner, N.C. Plant Productivity in the Arid and Semiarid Zones. Annu. Rev. Plant Physiol. 2003, 29, 277–317. [Google Scholar] [CrossRef]
- Jákli, B.; Hauser, J.M.; Böttcher, F.; Meyer zur Müdehorst, J.; Senbayram, M.; Dittert, K. Leaf, canopy and agronomic water-use efficiency of field-grown sugar beet in response to potassium fertilization. J. Agron. Crop Sci. 2018, 204, 99–110. [Google Scholar] [CrossRef]
- Saxton, A. PDMIX800 Software Model; University of Tennessee: Knoxville, TN, USA, 2000. [Google Scholar]
- Ghorbani, M.; Ramazani, S.H.R.; Fallahi, H.; Koohi, S.M. Effect of drought stress and bio-fertilizer on yield and yield components of guar Cyamopsis tetragonoloba (L.) Taub. J. Med. Plants By-Prod. 2019, 1, 13–19. [Google Scholar]
- Ma, C.C.; Gao, Y.B.; Xin, T.R.; Ma, C.C.; Li, Q.F. Effects of silicon application on drought resistance of cucumber plants. Soil Sci. Plant Nutr. 2004, 50, 623–632. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Siti Nor Akmar, A.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Avola, G.; Riggi, E.; Trostle, C.; Sortino, O.; Gresta, F. Deficit irrigation on guar genotypes (Cyamopsis tetragonoloba (L.) Taub): Effects on seed yield and water use efficiency. Agronomy 2020, 10, 789. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hatamzadeh, A.; Sahraroo, A. Effect of irrigation period on some morphological traits of Guar (Cyamopsis Tetragonoloba) in karaj region. J. Biodivers. Environ. Sci. 2017, 11, 225–233. [Google Scholar]
- Gunes, A.; Kadioglu, Y.K.; Pilbeam, D.J.; Inal, A.; Coban, S.; Aksu, A. Influence of silicon on sunflower cultivars under drought stress, II: Essential and nonessential element uptake determined by polarized energy dispersive X-ray fluorescence. Commun. Soil Sci. Plant Anal. 2008, 39, 1904–1927. [Google Scholar] [CrossRef]
| SPAD Chlorophyll Content | Plant Height (cm) | AGDB (g/Plant) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50% Pod Formation Stage | 100% Pod Formation Stage | Maturity | Maturity | |||||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| Irrigation regime (I) | ||||||||||||
| I1 | 67.6 | 60.6 a | 66.1 bc | 68.8 b | 53.6 b | 63.6 a | 90.4 ab | 116.7 ab | 87.2 a | 157.6 ab | 153.9 a | 71.0 a |
| I2 | 69.7 | 59.3 ab | 65.5 c | 65.5 b | 55.8 b | 59.3 ab | 78.2 c | 125.3 a | 67.2 b | 110.7 b | 132.2 ab | 43.4 b |
| I3 | 68.4 | 55.7 c | 67.8 b | 68.4 b | 54.7 b | 56.6 b | 80.7 bc | 113.7 bc | 65.0 b | 127.0 b | 107.0 b | 36.0 b |
| I4 | 67.1 | 56.1 bc | 67.9 b | 69.5 b | 52.5 b | 57.3 b | 82.3 bc | 106.2 c | 62.7 b | 143.2 b | 112.1 b | 32.4 b |
| I5 | 67.3 | 57.8 abc | 70.4 a | 78.9 a | 62.0 a | 62.5 a | 97.6 a | 108.9 bc | 69.3 b | 198.4 a | 150.1 a | 51.7 ab |
| Standard Error | 1.1 | 1.4 | 0.9 | 3.9 | 1.5 | 2.2 | 4.2 | 3.9 | 4.8 | 16.2 | 10.1 | 10.0 |
| Genotype (G) | ||||||||||||
| Kinman | 64.3 c | 59.4 | 67.8 ab | 68.9 | 56.3 | 62.1 | 81.2 b | 112.0 | 71.0 | 127.6 b | 122.2 | 48.4 |
| Lewis | 69.0 ab | 56.9 | 69.6 a | 72.3 | 54.4 | 61.2 | 89.1 a | 117.6 | 72.1 | 153.1 ab | 141.3 | 48.1 |
| Matador | 71.0 a | 58.0 | 66.8 b | 69.8 | 55.0 | 59.2 | 85.5 ab | 113.0 | 69.0 | 135.9 b | 133.0 | 45.5 |
| NMSU-15-G1 | 67.9 b | 57.1 | 66.0 b | 69.8 | 57.0 | 57.0 | 87.6 a | 114.0 | 69.0 | 173.0 a | 127.7 | 45.7 |
| Standard Error | 1.0 | 1.4 | 0.9 | 3.7 | 1.2 | 2.1 | 3.3 | 3.2 | 2.5 | 12.7 | 10.7 | 8.8 |
| Interaction (I × G) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Photosynthetic Rate (µmol m−2 s−1) | Stomatal Conductance (mol m−2 s−1) | Transpiration Rate (mmol m−2 s−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| Irrigation regime (I) | |||||||||
| I1 | 25.6 | 26.9 | 25.7 a | 0.60 | 0.76 | 0.54 a | 6.2 | 9.4 ab | 9.4 a |
| I2 | 25.7 | 27.9 | 23.1 a | 0.57 | 0.88 | 0.42 ab | 6.0 | 10.2 a | 9.0 ab |
| I3 | 26.1 | 26.2 | 17.1 b | 0.59 | 0.82 | 0.21 c | 6.0 | 9.5 ab | 5.9 c |
| I4 | 24.9 | 26.3 | 18.2 b | 0.49 | 0.69 | 0.25 bc | 5.8 | 8.9 b | 6.6 bc |
| I5 | 26.0 | 26.6 | 21.8 ab | 0.66 | 0.61 | 0.33 bc | 6.3 | 8.6 b | 8.5 ab |
| Standard Error | 0.7 | 0.9 | 1.8 | 0.07 | 0.09 | 0.07 | 0.6 | 0.5 | 0.8 |
| Genotype (G) | |||||||||
| Kinman | 23.8 b | 26.0 | 20.7 | 0.48 | 0.74 | 0.33 | 5.6 b | 9.8 | 7.8 |
| Lewis | 26.6 a | 27.4 | 22.4 | 0.62 | 0.81 | 0.39 | 6.1 a | 9.0 | 8.3 |
| Matador | 26.4 a | 26.2 | 19.7 | 0.60 | 0.70 | 0.31 | 6.2 a | 9.1 | 7.4 |
| NMSU-15-G1 | 25.9 a | 27.5 | 21.9 | 0.62 | 0.75 | 0.38 | 6.4 a | 9.4 | 8.0 |
| Standard Error | 0.7 | 0.8 | 1.5 | 0.06 | 0.08 | 0.06 | 0.6 | 0.6 | 0.8 |
| Interaction (I × G) | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Intrinsic Water Use Efficiency (µmol m−2 s−1/mol m−2 s−1) | Instantaneous Water Use Efficiency (µmol m−2 s−1/mmol m−2 s−1) | Agronomic Water Use Efficiency (kg ha−1/mm−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 75% Pod Formation Stage | 75% Pod Formation Stage | Maturity | |||||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| Irrigation regime (I) | |||||||||
| I1 | 45.4 | 44.9 | 58.6 | 4.2 | 2.9 bc | 2.7 | 10.9 c | 5.6 | 2.9 ab |
| I2 | 47.6 | 34.9 | 66.2 | 4.4 | 2.8 c | 2.6 | 11.0 c | 5.3 | 2.2 c |
| I3 | 47.3 | 38.6 | 93.6 | 4.5 | 2.8 c | 2.9 | 12.4 bc | 5.3 | 2.3 bc |
| I4 | 53.4 | 46.1 | 87.4 | 4.5 | 3.0 ab | 2.8 | 13.5 b | 5.3 | 2.3 bc |
| I5 | 44.7 | 47.7 | 72.8 | 4.3 | 3.2 a | 2.6 | 18.1 a | 5.9 | 2.9 a |
| Standard Error | 3.3 | 5.7 | 10.3 | 0.4 | 0.2 | 0.1 | 0.6 | 0.3 | 0.2 |
| Genotype (G) | |||||||||
| Kinman | 50.9 | 40.9 | 74.1 | 4.5 | 2.7 | 2.7 | 13.6 a | 5.7 | 2.7 |
| Lewis | 47.2 | 38.2 | 73.8 | 4.5 | 3.1 | 2.7 | 13.3 a | 5.0 | 2.4 |
| Matador | 47.4 | 45.4 | 76.0 | 4.4 | 3.0 | 2.8 | 13.6 a | 5.6 | 2.7 |
| NMSU-15-G1 | 45.2 | 45.2 | 79.0 | 4.2 | 3.0 | 2.7 | 12.3 b | 5.4 | 2.2 |
| Standard Error | 3.1 | 5.0 | 7.4 | 0.4 | 0.2 | 0.1 | 0.5 | 0.3 | 0.2 |
| Interaction (I × G) | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Clusters per Plant | Pods per Plant | Seeds per Pod | Seeds per Plant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| Irrigation regime (I) | ||||||||||||
| I1 | 66.5 b | 47.6 | 30.9 | 279.6 ab | 226.7 a | 119.2 a | 7.1 | 8.5 | 7.6 | 2006 ab | 1918 a | 894 |
| I2 | 52.8 b | 40.5 | 23.2 | 208.9 b | 195.6 abc | 76.4 b | 7.1 | 8.2 | 7.1 | 1490 b | 1602 ab | 554 |
| I3 | 58.4 b | 36.7 | 22.0 | 231.5 b | 158.2 c | 66.6 b | 7.1 | 8.5 | 6.7 | 1639 b | 1346 b | 461 |
| I4 | 64.9 b | 41.9 | 17.8 | 258.5 b | 178.7 bc | 60.5 b | 7.2 | 8.4 | 7.0 | 1848 b | 1508 b | 432 |
| I5 | 84.5 a | 48.1 | 27.2 | 350.8 a | 223.1 ab | 95.8 ab | 7.3 | 8.6 | 7.0 | 2593 a | 1938 a | 661 |
| Standard Error | 5.4 | 4.4 | 4.8 | 27.9 | 18.2 | 18.8 | 0.1 | 0.1 | 0.2 | 206 | 137 | 153 |
| Genotype (G) | ||||||||||||
| Kinman | 74.2 a | 50.3 | 25.5 | 266.1 | 195.4 | 90.0 | 6.7 c | 8.1 b | 6.9 b | 1778 | 1585 | 639 |
| Lewis | 63.0 ab | 43.6 | 24.2 | 270.5 | 215.8 | 86.3 | 7.4 a | 8.4 a | 6.9 b | 2005 | 1833 | 589 |
| Matador | 53.9 b | 40.0 | 23.1 | 246.1 | 209.4 | 84.8 | 7.6 a | 8.6 a | 7.3 a | 1870 | 1794 | 631 |
| NMSU-15-G1 | 70.5 a | 37.9 | 24.1 | 280.7 | 165.4 | 73.7 | 7.1 b | 8.6 a | 7.2 ab | 2009 | 1437 | 543 |
| Standard Error | 4.2 | 4.3 | 4.4 | 22.8 | 17.3 | 15.9 | 0.1 | 0.1 | 0.2 | 170 | 133 | 125 |
| Interaction (I × G) | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 1000-Seed Weight (g) | Harvest Index | Seed Yield (kg ha−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |
| Irrigation regime (I) | |||||||||
| I1 | 36.3 | 34.6 ab | 36.7 | 0.43 a | 0.29 bc | 0.25 | 2696 a | 2908 | 2542 a |
| I2 | 36.4 | 32.3 c | 35.5 | 0.44 a | 0.28 c | 0.22 | 2314 b | 2622 | 1512 b |
| I3 | 35.7 | 33.3 c | 35.3 | 0.44 a | 0.31 ab | 0.21 | 2253 b | 2543 | 1447 b |
| I4 | 35.9 | 33.5 bc | 35.1 | 0.44 a | 0.32 a | 0.22 | 2163 b | 2436 | 1399 b |
| I5 | 35.2 | 35.1 a | 35.1 | 0.40 b | 0.32 a | 0.25 | 2913 a | 2717 | 1778 b |
| Standard Error | 0.5 | 0.4 | 0.6 | 0.01 | 0.01 | 0.02 | 107 | 157 | 145 |
| Genotype (G) | |||||||||
| Kinman | 35.1 bc | 32.7 b | 36.1 a | 0.43 | 0.33 a | 0.27 a | 2543 a | 2763 | 1845 |
| Lewis | 36.1 b | 33.5 b | 35.6 a | 0.43 | 0.29 b | 0.23 b | 2476 a | 2453 | 1668 |
| Matador | 34.8 c | 33.1 b | 33.7 b | 0.43 | 0.30 ab | 0.23 b | 2560 a | 2736 | 1876 |
| NMSU-15-G1 | 37.5 a | 35.8 a | 36.7 a | 0.43 | 0.29 b | 0.19 c | 2291 b | 2629 | 1554 |
| Standard Error | 0.4 | 0.5 | 0.6 | 0.01 | 0.01 | 0.01 | 86 | 140 | 130 |
| Interaction (I × G) | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.; Grover, K.; VanLeeuwen, D.; Stringam, B.; Schutte, B. Growth and Performance of Guar (Cyamopsis tetragonoloba (L.) Taub.) Genotypes under Various Irrigation Regimes with and without Biogenic Silica Amendment in Arid Southwest US. Plants 2023, 12, 2486. https://doi.org/10.3390/plants12132486
Garcia A, Grover K, VanLeeuwen D, Stringam B, Schutte B. Growth and Performance of Guar (Cyamopsis tetragonoloba (L.) Taub.) Genotypes under Various Irrigation Regimes with and without Biogenic Silica Amendment in Arid Southwest US. Plants. 2023; 12(13):2486. https://doi.org/10.3390/plants12132486
Chicago/Turabian StyleGarcia, Alonso, Kulbhushan Grover, Dawn VanLeeuwen, Blair Stringam, and Brian Schutte. 2023. "Growth and Performance of Guar (Cyamopsis tetragonoloba (L.) Taub.) Genotypes under Various Irrigation Regimes with and without Biogenic Silica Amendment in Arid Southwest US" Plants 12, no. 13: 2486. https://doi.org/10.3390/plants12132486
APA StyleGarcia, A., Grover, K., VanLeeuwen, D., Stringam, B., & Schutte, B. (2023). Growth and Performance of Guar (Cyamopsis tetragonoloba (L.) Taub.) Genotypes under Various Irrigation Regimes with and without Biogenic Silica Amendment in Arid Southwest US. Plants, 12(13), 2486. https://doi.org/10.3390/plants12132486







