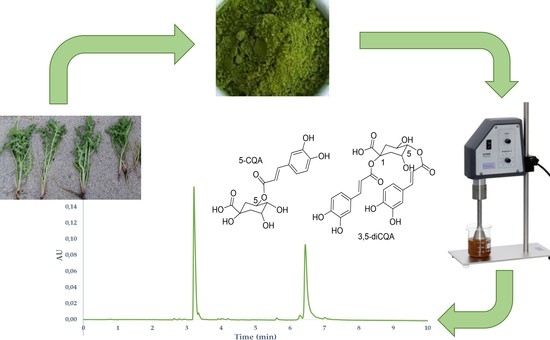
Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solvent Composition Optimization
2.2. Temperature Stability Study
2.3. UAE Method Optimization
2.4. Optimal Extraction Time
2.5. Repeatability and Intermediate Precision of the Developed Method
2.6. Application to Real Samples
2.7. Antioxidant Capacity of the Real Samples
3. Materials and Methods
3.1. Biological Samples
3.2. Solvents and Reagents
3.3. Ultrasound Assisted Extraction
3.3.1. Validation of the Extraction Method
3.3.2. Box–Behnken Experiment Design
3.4. Identification of CQAs by UHPLC-QToF-MS
3.5. Detecting CQAs by Ultra High-Performance Liquid Chromatography (UHPLC)
3.6. Antioxidant Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vázquez, F.M. The Genus Scolymus Tourn. Ex L. (Asteraceae): Taxonomy and Distribution. In Anales del Jardín Botánico de Madrid; Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2000; Volume 58, pp. 83–100. [Google Scholar] [CrossRef] [Green Version]
- Polo, S.; Tardío, J.; Vélez-del-Burgo, A.; Molina, M.; Pardo-de-Santayana, M. Knowledge, Use and Ecology of Golden Thistle (Scolymus hispanicus L.) in Central Spain. J. Ethnobiol. Ethnomed. 2009, 5, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Disciglio, G.; Tarantino, A.; Frabboni, L.; Gagliardi, A.; Giuliani, M.M.; Tarantino, E.; Gatta, G. Qualitative Characterization of Cultivated and Wild Edible Plants: Mineral Elements, Phenols Content and Antioxidant Capacity. Ital. J. Agron. 2017, 11, 383–394. [Google Scholar] [CrossRef] [Green Version]
- De Cortes Sánchez-Mata, M.; Tardío, J. Mediterranean Wild Edible Plants; De Cortes Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; ISBN 978-1-4939-3327-3. [Google Scholar]
- Marmouzi, I.; El Karbane, M.; El Hamdani, M.; Kharbach, M.; Naceiri Mrabti, H.; Alami, R.; Dahraoui, S.; El Jemli, M.; Ouzzif, Z.; Cherrah, Y.; et al. Phytochemical and Pharmacological Variability in Golden Thistle Functional Parts: Comparative Study of Roots, Stems, Leaves and Flowers. Nat. Prod. Res. 2017, 31, 2669–2674. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Wild Food Plants Used in Traditional Vegetable Mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Abu-Lafi, S.; Rayan, M.; Masalha, M.; Abu-Farich, B.; Al-Jaas, H.; Abu-Lafi, M.; Rayan, A. Phytochemical Composition and Biological Activities of Wild Scolymus maculatus L. Medicines 2019, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Pieroni, A.; Janiak, V.; Dürr, C.M.; Lüdeke, S.; Trachsel, E.; Heinrich, M. In Vitro Antioxidant Activity of Non-Cultivated Vegetables of Ethnic Albanians in Southern Italy. Phytother. Res. 2002, 16, 467–473. [Google Scholar] [CrossRef]
- Ozkol, H.; Tuluce, Y.; Dilsiz, N.; Koyuncu, I. Therapeutic Potential of Some Plant Extracts Used in Turkish Traditional Medicine on Streptozocin-Induced Type 1 Diabetes Mellitus in Rats. J. Membr. Biol. 2013, 246, 47–55. [Google Scholar] [CrossRef]
- Semaoui, M.; Mesli, F.; Dib, M.E.A.; Tabti, B.; Achiri, R.; Costa, J.; Muselli, A. Statistical Analysis/Theoretical Investigations of Novel Vascular Endothelial Growth Factor of Davanoide from Scolymus Grandifloras Desf as Potent Anti-Angiogenic Drug Properties. J. Biomol. Struct. Dyn. 2022, 40, 3850–3870. [Google Scholar] [CrossRef]
- Kirimer, N.; Tunalier, Z.; Başer, K.H.C.; Cingi, I. Antispasmodic and Spasmogenic Effects of Scolymus hispanicus and Taraxasteryl Acetate on Isolated Ileum Preparations. Planta Med. 1997, 63, 556–558. [Google Scholar] [CrossRef]
- Rubio, B.; Diaz, A.M.; Velazquez, M.P.; Villaescusa, L. Caffeoyl and Flavonoid Compounds in Scolymus hispanicus. Planta Med. 1991, 57, A130. [Google Scholar] [CrossRef]
- Sanz, M.J.; Terencio, M.C.; Mañez, S.; Rios, J.L.; Soriano, C. A New Quercetin-Acylglucuronide from Scolymus hispanicus. J. Nat. Prod. 1993, 56, 1995–1998. [Google Scholar] [CrossRef]
- Rubio, B.; Villaescusa, L.; Diaz, A.; Fernandez, L.; Martin, T. Flavonol Glycosides from Scolymus hispanicus and Jasonia glutinosa. Planta Med. 1995, 61, 583. [Google Scholar] [CrossRef] [PubMed]
- Rubio, B.; Díaz, A.M. Flavonoid Glycosides in Leaves and Flowers from Scolymus hispanicus. Pharmazie 1995, 50, 629–631. [Google Scholar]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, T. Safety of Quercetin for Clinical Application (Review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Cheminat, A.; Zawatzky, R.; Becker, H.; Brouillard, R. Caffeoyl Conjugates from Echinacea Species: Structures and Biological Activity. Phytochemistry 1988, 27, 2787–2794. [Google Scholar] [CrossRef]
- Ma, C.M.; Kawahata, T.; Hattori, M.; Otake, T.; Wang, L.; Daneshtalab, M. Synthesis, Anti-HIV and Anti-Oxidant Activities of Caffeoyl 5,6-Anhydroquinic Acid Derivatives. Bioorg. Med. Chem. 2010, 18, 863–869. [Google Scholar] [CrossRef]
- Romussi, G.; Ciarallo, G. Flavonoid Compounds from Scolymus hispanicus L. Pharmazie 1978, 33, 685–686. [Google Scholar]
- Trichopoulou, A.; Vasilopoulou, E.; Hollman, P.; Chamalides, C.; Foufa, E.; Kaloudis, T.; Kromhout, D.; Miskaki, P.; Petrochilou, I.; Poulima, E.; et al. Nutritional composition and flavonoid content of edible wild greens and green pies: A potential rich source of antioxidant nutrients in the Mediterranean diet. Food Chem. 2000, 70, 319–323. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Andrade, P.B.; Valentão, P.; Romano, A. Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. Eur. Food Res. Technol. 2019, 245, 753–762. [Google Scholar] [CrossRef]
- Rees, S.; Harborne, J. Flavonoids and Other Phenolics of Cichorium and Related Members of the Lactuceae (Compositae). Bot. J. Linn. Soc. 1984, 89, 313–319. [Google Scholar] [CrossRef]
- Rubio, B.; Elias, R.; Faure, R.; Diaz, A.M.; Debrauwer, L.; Balansard, G. Flavonol Glucuronosides from Scolymus hispanicus Leaves. Pharm. Pharmacol. Lett. 1994, 3, 207–208. [Google Scholar]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef] [PubMed]
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of Temperature and Photoperiod on Yield and Chemical Composition of Northern and Southern Clones of Bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [Green Version]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Veremeichik, G.N. Anticancer Polyphenols from Cultured Plant Cells: Production and New Bioengineering Strategies. Curr. Med. Chem. 2018, 25, 4671–4692. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS Profiling of Artemisia Herba-Alba and Evaluation of Its Bioactive Properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some pro-Oxidant Induced Oxidative Stress in Rats’ Brain-in Vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and Neuroprotective Effects of Chlorogenic Acid. Nutr. Neurosci. 2016, 20, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic Acid Isomers Directly Interact with Keap 1-Nrf2 Signaling in Caco-2 Cells. Mol. Cell Biochem. 2019, 457, 105–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.-W.; Bai, J.-P.; Zhang, Q.; Hu, X.-L.; Tian, X.; Zhu, J.; Liu, J.; Meng, W.-H.; Zhao, Q.-C. Caffeoylquinic Acid Derivatives from the Roots of Arctium lappa L. (Burdock) and Their Structure–Activity Relationships (SARs) of Free Radical Scavenging Activities. Phytochem. Lett. 2016, 15, 159–163. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and Cytoprotective Effects of the Di-O-Caffeoylquinic Acid Family: The Mechanism, Structure–Activity Relationship, and Conformational Effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef] [Green Version]
- Indy Tamayose, C.; dos Santos, E.A.; Roque, N.; Costa-Lotufo, L.V.; Pena Ferreira, M.J. Caffeoylquinic Acids: Separation Method, Antiradical Properties and Cytotoxicity. Chem. Biodivers. 2019, 16, e1900093. [Google Scholar] [CrossRef] [PubMed]
- González-de-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Barbero, G.F.; Palma, M. Extraction of Antioxidant Compounds from Onion Bulb (Allium cepa L.) Using Individual and Simultaneous Microwave-Assisted Extraction Methods. Antioxidants 2022, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Su, J.Y.; Yang, C.Y. Ultrasound-Assisted Aqueous Extraction of Chlorogenic Acid and Cynarin with the Impact of Inulin from Burdock (Arctium lappa L.) Roots. Antioxidants 2022, 11, 1219. [Google Scholar] [CrossRef]
- Nicolescu, A.; Babotă, M.; Zhang, L.; Bunea, C.I.; Gavrilaș, L.; Vodnar, D.C.; Mocan, A.; Crișan, G.; Rocchetti, G. Optimized Ultrasound-Assisted Enzymatic Extraction of Phenolic Compounds from Rosa canina L. Pseudo-Fruits (Rosehip) and Their Biological Activity. Antioxidants 2022, 11, 1123. [Google Scholar] [CrossRef] [PubMed]
- Gkioni, M.D.; Andriopoulos, V.; Koutra, E.; Hatziantoniou, S.; Kornaros, M.; Lamari, F.N. Ultrasound-Assisted Extraction of Nannochloropsis oculata with Ethanol and Betaine: 1,2-Propanediol Eutectic Solvent for Antioxidant Pigment-Rich Extracts Retaining Nutritious the Residual Biomass. Antioxidants 2022, 11, 1103. [Google Scholar] [CrossRef]
- Silva, A.M.; Pinto, D.; Moreira, M.M.; Costa, P.C.; Delerue-Matos, C.; Rodrigues, F. Valorization of Kiwiberry Leaves Recovered by Ultrasound-Assisted Extraction for Skin Application: A Response Surface Methodology Approach. Antioxidants 2022, 11, 763. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Typek, R. The influence of pH on the thermal stability of 5-O-caffeoylquinic acids in aqueous solutions. Eur. Food Res. Technol. 2011, 233, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Liu, J.; Qiu, S.; Wang, J.; Song, G.; Chu, B.; Li, L.; Xiao, G.; Gong, J.; Zheng, F. Ultrasonic degradation kinetics and isomerization of 3-and 4-O-caffeoylquinic acid at various pH: The protective effects of ascorbic acid and epigallocatechin gallate on their stability. Ultrason. Sonochem. 2021, 80, 105812. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barrero, G.F.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Yerena-Prieto, B.J.; Gonzalez-Gonzalez, M.; Vázquez-Espinosa, M.; González-De-Peredo, A.V.; García-Alvarado, M.A.; Palma, M.; Rodríguez-Jimenes, G.; Barbero, G.F. Optimization of an Ultrasound-Assisted Extraction Method Applied to the Extraction of Flavonoids from Moringa Leaves (Moringa oleífera Lam.). Agronomy 2022, 12, 261. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferrerio-González, M.; Carrera, C.; Palma, M.; Barroso, C.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Pandino, G.; Bonomo, A.; Scavo, A.; Mauromicale, G.; Lombardo, S. Caffeoylquinic acids and flavones profile in Cynara cardunculus L. seedlings under controlled conditions as affected by light and water-supply treatments. Sci. Hortic. 2022, 302, 111180. [Google Scholar] [CrossRef]
- Rejeb, I.B.; Dhen, N.; Gargouri, M.; Boulila, A. Chemical Composition, Antioxidant Potential and Enzymes Inhibitory Properties of Globe Artichoke By-Products. Chem. Biodivers. 2020, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Dagron, S. Die International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). In Handbuch Ethik und Recht der Forschung am Menschen; Springer: Berlin/Heidelberg, Germany, 2014; pp. 541–545. [Google Scholar]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]






| Factor | Low Level (−1) | Intermediate Level (0) | High Level (+1) |
|---|---|---|---|
| % MeOH | 50 | 75 | 100 |
| T (°C) | 10 | 35 | 60 |
| Ratio (g) | 0.1 | 0.2 | 0.3 |
| pH | 2 | 5 | 8 |
| Amplitude (%) | 20 | 40 | 60 |
| Cycle (s) | 0.2 | 0.6 | 1.0 |
| Variable | p-Value | Estimated Coefficient | Variable | p-Value | Estimated Coefficient |
|---|---|---|---|---|---|
| A: % MeOH | 0.0001 | −1.27214 × 106 | BD | 0.3236 | −34,003.5 |
| B: T (°C) | 0.7148 | 44,703.2 | BE | 0.6983 | 1086.55 |
| C: Ratio (g) | 0.0000 | 4256.03 | BF | 0.7553 | −29.9245 |
| D: pH | 0.0013 | 2.27012 × 106 | CC | 0.5985 | 2267.5 |
| E: Amplitude (%) | 0.9437 | −254,745 | CD | 0.2824 | 3.04617 × 106 |
| F: Cycle (s) | 0.9709 | 14,799.6 | CE | 0.7223 | −355,591 |
| AA | 0.0006 | −248,702 | CF | 0.8609 | 11,635.9 |
| AB | 0.8393 | −357.327 | DD | 0.3466 | 270,292 |
| AC | 0.2821 | −17.6942 | DE | 0.6229 | 13,694.2 |
| AD | 0.1999 | 28,468.3 | DF | 0.6062 | −806.016 |
| AE | 0.5861 | 1205.1 | EE | 0.2549 | 56,353.1 |
| AF | 0.7640 | 71.4463 | EF | 0.8892 | −166.323 |
| BB | 0.7465 | 2621.08 | FF | 0.6473 | −151,854 |
| BC | 0.1275 | −20.7393 | Constant | - | −1.27214 × 106 |
| Factor | Optimal Levels |
|---|---|
| % MeOH | 81 |
| T (°C) | 40 |
| Ratio (g) | 0.3 |
| pH | 3 |
| Amplitude (%) | 52 |
| Cycle (s) | 0.6 |
| Repeatability | Intermediate Precision | |
|---|---|---|
| Media (mg/g) | 5.79 | 5.67 |
| Standard deviation (mg/g) | 0.19 | 0.26 |
| Variance coefficient (%) | 3.36 | 4.61 |
| Zone | Name | Latitude | Longitude | Altitude | Soil Moisture |
|---|---|---|---|---|---|
| 1 | El Salao | 36°31′45″ N | 5°54′03″ W | 57 m | + |
| 2 | Las Piletas | 36°31′43″ N | 5°57′05″ W | 93 m | ++ |
| 3 | Pozo la Lapa | 36°32′18″ N | 5°53′14″ W | 70 m | ++ |
| 4 | La Peña | 36°30′46″ N | 5°49′29″ W | 100 m | + |
| 5 | La Joya | 36°29′57″ N | 5°46′10″ W | 81 m | +++ |
| 6 | Magaña | 36°30′45″ N | 5°43′49″ W | 108 m | ++ |
| Zone 1: El Salao | Zone 2: Las Piletas | Zone 3: Pozo la Lapa | Zone 4: La Peña | Zone 5: La Joya | Zone 6: Magaña | |
|---|---|---|---|---|---|---|
| mg trolox equivalent/ g sample | 5.39 ± 0.11 | 11.66 ± 0.21 | 9.79 ± 0.31 | 4.54 ± 0.12 | 17.41 ± 0.21 | 9.17 ± 0.22 |
| mg 5-CQA/g sample | 1.36 ± 0.07 | 4.30 ± 0.05 | 3.31 ± 0.20 | 1.24 ± 0.09 | 4.15 ± 0.10 | 2.82 ± 0.12 |
| mg 3,5-diCQA/g sample | 0.43 ± 0.04 | 1.08 ± 0.08 | 1.54 ± 0.03 | 0.42 ± 0.05 | 2.59 ± 0.06 | 1.56 ± 0.06 |
| mg total CQA/g sample | 1.79 ± 0.06 | 5.38 ± 0.07 | 4.85 ± 0.12 | 1.66 ± 0.07 | 6.74 ± 0.08 | 4.38 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruano-González, A.; Pinto, A.A.; Chinchilla, N.; Palma, M.; Barbero, G.F.; Carrera, C.; Vázquez-Espinosa, M. Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction. Plants 2023, 12, 2340. https://doi.org/10.3390/plants12122340
Ruano-González A, Pinto AA, Chinchilla N, Palma M, Barbero GF, Carrera C, Vázquez-Espinosa M. Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction. Plants. 2023; 12(12):2340. https://doi.org/10.3390/plants12122340
Chicago/Turabian StyleRuano-González, Antonio, Ana A. Pinto, Nuria Chinchilla, Miguel Palma, Gerardo F. Barbero, Ceferino Carrera, and Mercedes Vázquez-Espinosa. 2023. "Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction" Plants 12, no. 12: 2340. https://doi.org/10.3390/plants12122340
APA StyleRuano-González, A., Pinto, A. A., Chinchilla, N., Palma, M., Barbero, G. F., Carrera, C., & Vázquez-Espinosa, M. (2023). Determination of Caffeoylquinic Acids Content by UHPLC in Scolymus hispanicus Extracts Obtained through Ultrasound-Assisted Extraction. Plants, 12(12), 2340. https://doi.org/10.3390/plants12122340