Inducing the Production of Secondary Metabolites by Foliar Application of Methyl Jasmonate in Peppermint
Abstract
:1. Introduction
2. Results
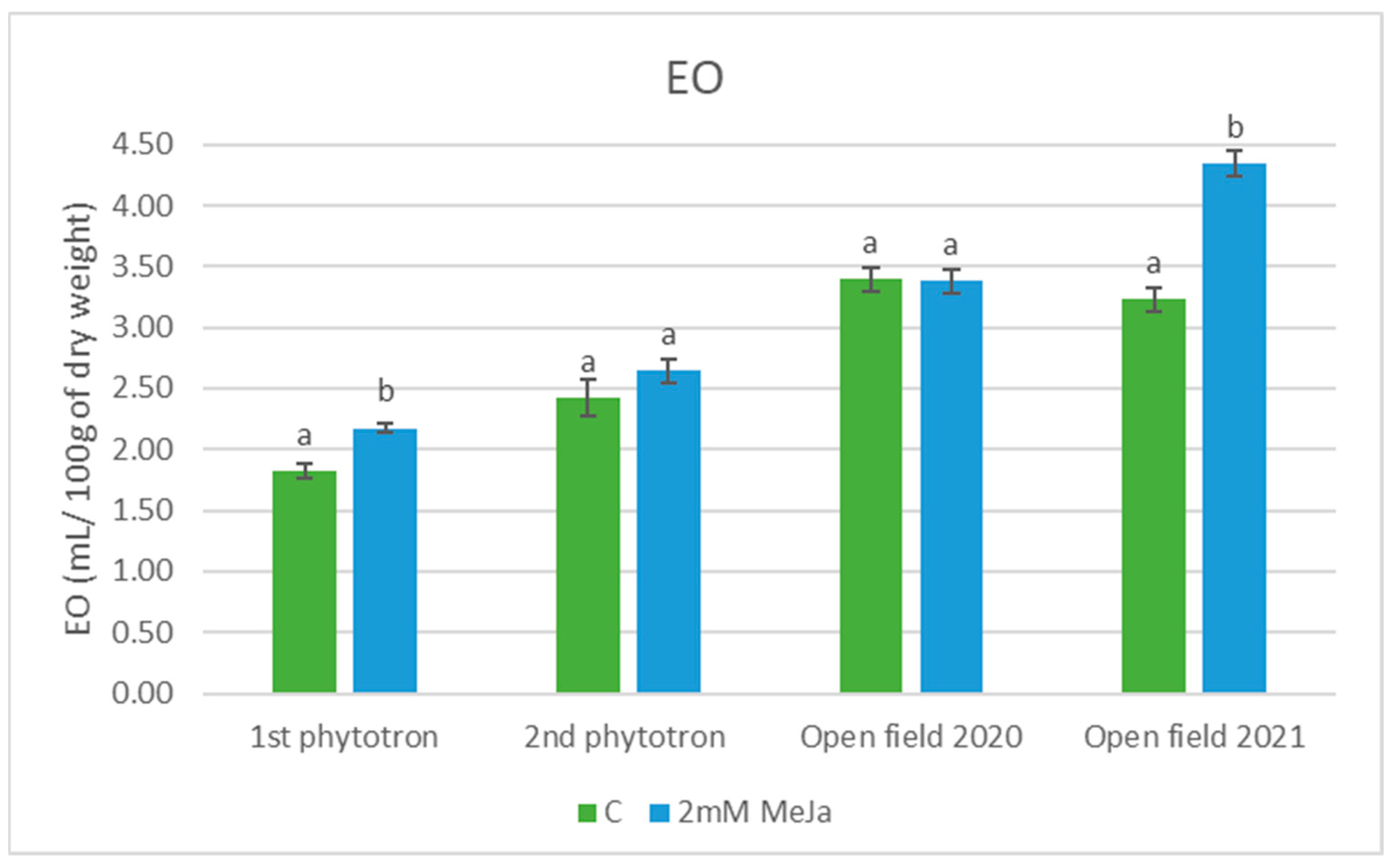
2.1. Essential Oil Content
2.2. Essential Oil Composition
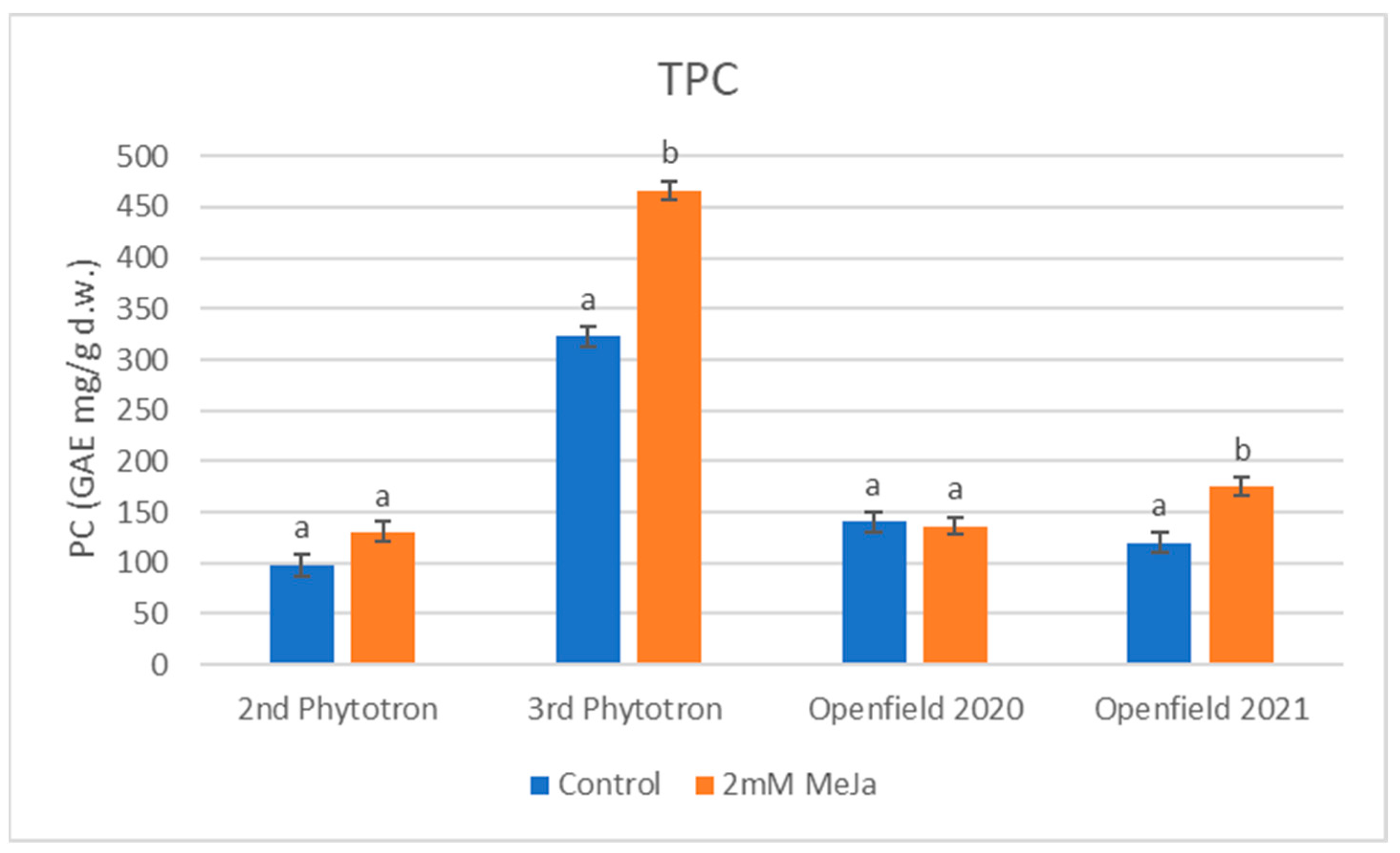
2.3. Total Phenolic Content
3. Discussion
4. Materials and Methods
4.1. Experimental Sites
4.2. Treatments
4.3. Harvesting
4.4. Essential Oil Extraction
4.5. Essential Oil Composition
4.6. Total Phenolic Content
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lawrence, B.M. Mint: The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–559. [Google Scholar] [CrossRef]
- Loolaie, M.; Moasefi, N.; Rasouli, H.; Adibi, H. Peppermint and Its Functionality: A Review. Arch. Clin. Microbiol. 2017, 8, 54. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product-Peppermint Tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, B.M. The Composition of Commercially Important Mints. In Mint: The Genus Mentha; CRC Press: Boca Raton, FL, USA, 2006; pp. 217–319. ISBN 9780849307980. [Google Scholar]
- Brown, N.; John, J.A.; Shahidi, F. Polyphenol Composition and Antioxidant Potential of Mint Leaves. Food Prod. Process. Nutr. 2019, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mahendran, G.; Rahman, L.U. Ethnomedicinal, Phytochemical and Pharmacological Updates on Peppermint (Mentha × piperita L.)—A Review. Phyther. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef] [PubMed]
- Németh-Zámbori, É. Natural Variability of Essential Oil Components. In Handbook of Essential Oils; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 85–124. ISBN 9781351246460. [Google Scholar]
- Murray, M.; Marble, P.; Lincoln, D.; Hefendehl, F. Peppermint Oil Quality Differences and the Reasons for Them. In Proceedings of the 10th International Congress of Essential Oils, Fragrances & Flavors, Washington, DC, USA, 16–20 November 1986; Lawrence, B.M., Mookherjee, B.D., Willis, B.J., Eds.; pp. 189–210. [Google Scholar]
- Mcconkey, M.E.; Gershenzon, J.; Croteau, R.B. Developmental Regulation of Monoterpene Biosynthesis in the Glandular Trichomes of Peppermint. Plant Physiol. 2000, 122, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Pirbalouti, A.; Gorgij, A.; Rahimmalek, M.; Hamedi, B. Phytochemical Response of Hyssop (Hyssopus officinalis L.) to Foliar Application of Jasmonic Acid. J. Herb. Drugs 2013, 4, 7–14. [Google Scholar]
- Jahani, F.; Tohidi-Moghadam, H.R.; Larijani, H.R.; Ghooshchi, F.; Oveysi, M. Influence of Zinc and Salicylic Acid Foliar Application on Total Chlorophyll, Phenolic Components, Yield and Essential Oil Composition of Peppermint (Mentha piperita L.) under Drought Stress Condition. Arab. J. Geosci. 2021, 14, 1–12. [Google Scholar] [CrossRef]
- Keshavarz Mirzamohammadi, H.; Modarres-Sanavy, S.A.M.; Sefidkon, F.; Mokhtassi-Bidgoli, A.; Mirjalili, M.H. Irrigation and Fertilizer Treatments Affecting Rosmarinic Acid Accumulation, Total Phenolic Content, Antioxidant Potential and Correlation between Them in Peppermint (Mentha piperita L.). Irrig. Sci. 2021, 39, 671–683. [Google Scholar] [CrossRef]
- Abdi, G.; Shokrpour, M.; Salami, S.A. Essential Oil Composition at Different Plant Growth Development of Peppermint (Mentha x piperita L.) Under Water Deficit Stress. J. Essent. Oil Bear. Plants 2019, 22, 431–440. [Google Scholar] [CrossRef]
- Maffei, M.; Scannerini, S. UV-B Effect on Photomorphogenesis and Essential Oil Composition in Peppermint (Mentha piperita L.). J. Essent. Oil Res. 2000, 12, 523–529. [Google Scholar] [CrossRef]
- Németh-Zámbori, É.; Szabó, K.; Rajhárt, P.; Inotai, K.; Seidler-Lozykowska, K.; Radácsi, P. Variability of phenolic compounds of four aromatic lamiaceae species in consequence of different water supply. Acta Sci. Pol. Hortorum Cultus 2017, 16, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Figueroa-Pérez, M.G.; Rocha-Guzmán, N.E.; Pérez-Ramírez, I.F.; Mercado-Silva, E.; Reynoso-Camacho, R. Metabolite Profile, Antioxidant Capacity, and Inhibition of Digestive Enzymes in Infusions of Peppermint (Mentha piperita) Grown under Drought Stress. J. Agric. Food Chem. 2014, 62, 12027–12033. [Google Scholar] [CrossRef] [PubMed]
- Goudarzian, A.; Pirbalouti, A.G.; Hossaynzadeh, M. Menthol, Balance of Menthol/Menthone, and Essential Oil Contents of Mentha × piperita L. under Foliar-Applied Chitosan and Inoculation of Arbuscular Mycorrhizal Fungi. J. Essent. Oil-Bear. Plants 2020, 23, 1012–1021. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandoudi, W.; Németh-Zámboriné, É. Stimulating Secondary Compound Accumulation by Elicitation: Is It a Realistic Tool in Medicinal Plants in Vivo? Phytochem. Rev. 2022, 21, 2007–2025. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate Action in Plant Growth and Development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, Metabolism, and Signaling by Proteins Activating and Repressing Transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Rohwer, C.L.; Erwin, J.E. Horticultural Applications of Jasmonates: A Review. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H.; Ali, G.S. Elicitation of Antioxidant Secondary Metabolites with Jasmonates and Gibberellic Acid in Cell Suspension Cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 2015, 120, 1099–1106. [Google Scholar] [CrossRef]
- Fard, F.R.; Omidbaigi, R.; Sharifi, M.; Sefidkon, F. Effect of Methyl Jasmonate on Essential Oil Content and Composition of Agastache foeniculum. J. Med. Plants Res. 2012, 6, 5701–5705. [Google Scholar] [CrossRef]
- Kandoudi, W.; Radácsi, P.; Gosztola, B.; Németh, É.Z. Elicitation of Medicinal Plants In Vivo-Is It a Realistic Tool? The Effect of Methyl Jasmonate and Salicylic Acid on Lamiaceae Species. Horticulturae 2022, 8, 5. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Jeliazkova, E. Effect of Foliar Application of Methyl Jasmonate and Extracts of Juniper and Sagebrush on Essential Oil Yield and Composition of “native” Spearmint. HortScience 2013, 48, 462–465. [Google Scholar] [CrossRef]
- Dastyar, Y.; Aelaei, M.; Kheiry, A. Effect of Salicylic Acid and Methyl Jasmonate on Morphological Traits, Enzymatic Activity and Essential Oil Percentage of Purple Coneflower Plant (Echinacea purpurea L.) in Zanjan Climate. Iran. J. Hortic. Sci. 2019, 50, 91–103. [Google Scholar] [CrossRef]
- Złotek, U.; Michalak-Majewska, M.; Szymanowska, U. Effect of Jasmonic Acid Elicitation on the Yield, Chemical Composition, and Antioxidant and Anti-Inflammatory Properties of Essential Oil of Lettuce Leaf Basil (Ocimum basilicum L.). Food Chem. 2016, 213, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving Production of Plant Secondary Metabolites through Biotic and Abiotic Elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Qian, Z.G.; Zhao, Z.J.; Xu, Y.; Qian, X.; Zhong, J.J. Novel Chemically Synthesized Hydroxyl-Containing Jasmonates as Powerful Inducing Signals for Plant Secondary Metabolism. Biotechnol. Bioeng. 2004, 86, 809–816. [Google Scholar] [CrossRef]
- Alavi-Samani, S.M.; Kachouei, M.A.; Pirbalouti, A.G. Growth, Yield, Chemical Composition, and Antioxidant Activity of Essential Oils from Two Thyme Species under Foliar Application of Jasmonic Acid and Water Deficit Conditions. Hortic. Environ. Biotechnol. 2015, 56, 411–420. [Google Scholar] [CrossRef]
- Kianersi, F.; Pour-aboughadareh, A.; Majdi, M.; Poczai, P. Effect of Methyl Jasmonate on Thymol, Carvacrol, Phytochemical Accumulation, and Expression of Key Genes Involved in Thymol/Carvacrol Biosynthetic Pathway in Some Iranian Thyme Species. Int. J. Mol. Sci. 2021, 22, 11124. [Google Scholar] [CrossRef]
- Soleymani, F.; Taheri, H.; Shafeinia, A.R. Relative Expression of Genes of Menthol Biosynthesis Pathway in Peppermint (Mentha piperita L.) after Chitosan, Gibberellic Acid and Methyl Jasmonate Treatments. Russ. J. Plant Physiol. 2017, 64, 59–66. [Google Scholar] [CrossRef]
- Turner, G.W.; Davis, E.M.; Croteau, R.B. Immunocytochemical Localization of Short-Chain Family Reductases Involved in Menthol Biosynthesis in Peppermint. Planta 2012, 235, 1185–1195. [Google Scholar] [CrossRef]
- Rios-Estepa, R.; Turner, G.W.; Lee, J.M.; Croteau, R.B.; Lange, B.M. A Systems Biology Approach Identifies the Biochemical Mechanisms Regulating Monoterpenoid Essential Oil Composition in Peppermint. Proc. Natl. Acad. Sci. USA 2008, 105, 2818–2823. [Google Scholar] [CrossRef] [Green Version]
- Commission, E. REGULATION (EC) No 1334/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC). Off. J. Eur. Union 2008, 50, 34–50. [Google Scholar]
- Sujana, P.; Sridhar, T.M.; Josthna, P.; Naidu, C.V. Antibacterial Activity and Phytochemical Analysis of Mentha piperita L. (Peppermint)—An Important Multipurpose Medicinal Plant. Am. J. Plant Sci. 2013, 2013, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of Methyl Jasmonate on Secondary Metabolites of Sweet Basil (Ocimum basilicum L.). J. Agric. Food Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Złotek, U.; Świeca, M.; Jakubczyk, A. Effect of Abiotic Elicitation on Main Health-Promoting Compounds, Antioxidant Activity and Commercial Quality of Butter Lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Improving Phenolic Total Content and Monoterpene in Mentha x piperita by Using Salicylic Acid or Methyl Jasmonate Combined with Rhizobacteria Inoculation. Int. J. Mol. Sci. 2019, 21, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenavaie Zare, A.; Ganjeali, A.; Vaezi Kakhki, M.R.; Cheniany, M.; Mashreghi, M. Plant Elicitation and TiO2 Nanoparticles Application as an Effective Strategy for Improving the Growth, Biochemical Properties, and Essential Oil of Peppermint. Physiol. Mol. Biol. Plants 2022, 28, 1391–1406. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [Green Version]
- Barrientos Carvacho, H.; Pérez, C.; Zúñiga, G.; Mahn, A. Effect of Methyl Jasmonate, Sodium Selenate and Chitosan as Exogenous Elicitors on the Phenolic Compounds Profile of Broccoli Sprouts. J. Sci. Food Agric. 2014, 94, 2555–2561. [Google Scholar] [CrossRef]
- Kandoudi, W.; Radácsi, P.; Zámboriné Németh, E. Regulation of Secondary Metabolites of Basil (Ocimum basilicum L.) by the Application of Elicitors in Vivo. Acta Hortic. 2023, 1358, 229–234. [Google Scholar] [CrossRef]
- Pharmacopoeia Hungarica, 7th ed.; Hungarian Pharmacopoeia Commission: Budapest, Hungary, 1986; Volume 1, pp. 395–398.
- Van Den Dool, H.; Dec Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456, ISBN 978-1932633214. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Tukey, J. Exploratory Data Analysis; Addison–Wesley: Boston, MA, USA, 1977. [Google Scholar]

| Components | RI 1 | Control | 2 mM MeJa |
|---|---|---|---|
| Limonene | 1029 | 2.27 a | 2.32 a |
| 1.8-cineole | 1034 | 2.30 a | 2.60 a |
| Menthone | 1158 | 37.30 a | 43.27 b |
| Menthofuran | 1167 | 18.21 b | 16.36 a |
| Menthol | 1171 | 14.04 a | 16.33 b |
| Pulegone | 1236 | 9.18 b | 5.43 a |
| Piperitone | 1249 | 1.00 b | 0.76 a |
| Isomenthyl acetate | 1291 | 9.54 b | 7.54 a |
| Total | 93.84 | 94.61 |
| Open Field 2020 | Open Field 2021 | ||||
|---|---|---|---|---|---|
| Components | RI 1 | Control | 2 mM MeJa | Control | 2 mM MeJa |
| Limonene | 1029 | 5.51 a | 5.23 a | 4.61 a | 6.38 b |
| 1.8-cineole | 1034 | 4.28 a | 3.94 a | 5.50 a | 7.22 b |
| Menthone | 1158 | 35.05 a | 35.15 a | 30.06 a | 34.59 b |
| Neo-menthol | 1159 | - | - | 6.86 a | 6.11 a |
| Menthofuran | 1167 | 7.70 a | 7.91 a | - | - |
| Menthol | 1171 | 27.79 a | 27.73 a | 35.10 b | 30.30 a |
| Pulegone | 1236 | 1.96 a | 2.06 a | 0.82 b | 0.43 a |
| Piperitone | 1249 | 1.57 a | 1.66 a | 2.05 b | 2.09 a |
| Isomenthyl acetate | 1291 | 4.57 a | 4.12 a | 7.45 b | 3.85 a |
| Total | 91.21 | 90.60 | 94.92 | 93.65 | |
| Propagation | 1st Treatment | 2nd Treatment | Harvest | |
|---|---|---|---|---|
| 1st phytotron experiment | 23 January 2020 | 25 February 2020 | 3 March 2020 | 11 March 2020 |
| 2nd phytotron experiment | 16 September 2020 | 5 November 2020 | 12 November 2020 | 19 November 2020 |
| 3rd phytotron experiment | 2 November 2021 | 21 January 2022 | 28 January 2022 | 3 February 2022 |
| 1st open field experiment | Perennial plantation | 26 June 2020 | 2 July 2020 | 10 July 2020 |
| 2nd open field experiment | 30 March 2021 | 28 June 2021 | 5 July 2021 | 12 July 2021 |
| Measured Parameter | pH H2O | Humus Content % | Lime Content% | Texture | NO2+NO3−N mg/kg | P2O5 mg/kg | K2O mg/kg | Zn mg/kg | Mg mg/kg | Mn mg/kg |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil in the experimental station 2020 | 7.82 | 2.84 | 0.34 | sandy loam | 6.93 | 412.89 | 245.54 | 4.09 | 131.78 | 25.64 |
| Soil in the experimental station 2021 | 7.47 | 1.62 | 4.20 | sandy | 16.70 | 398.67 | 826.33 | 8.08 | 174.19 | 138.99 |
| Soil in the phytotron pots | 5.49 | 8.16 | <0.20 | clay loam | 1401.50 | 875.50 | 3357.40 | 12.40 | 829.00 | 54.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandoudi, W.; Tavaszi-Sárosi, S.; Németh-Zámboriné, E. Inducing the Production of Secondary Metabolites by Foliar Application of Methyl Jasmonate in Peppermint. Plants 2023, 12, 2339. https://doi.org/10.3390/plants12122339
Kandoudi W, Tavaszi-Sárosi S, Németh-Zámboriné E. Inducing the Production of Secondary Metabolites by Foliar Application of Methyl Jasmonate in Peppermint. Plants. 2023; 12(12):2339. https://doi.org/10.3390/plants12122339
Chicago/Turabian StyleKandoudi, Wafae, Szilvia Tavaszi-Sárosi, and Eva Németh-Zámboriné. 2023. "Inducing the Production of Secondary Metabolites by Foliar Application of Methyl Jasmonate in Peppermint" Plants 12, no. 12: 2339. https://doi.org/10.3390/plants12122339
APA StyleKandoudi, W., Tavaszi-Sárosi, S., & Németh-Zámboriné, E. (2023). Inducing the Production of Secondary Metabolites by Foliar Application of Methyl Jasmonate in Peppermint. Plants, 12(12), 2339. https://doi.org/10.3390/plants12122339










