Atrazine Toxicity: The Possible Role of Natural Products for Effective Treatment
Abstract
1. Introduction
1.1. Atrazine Toxicity
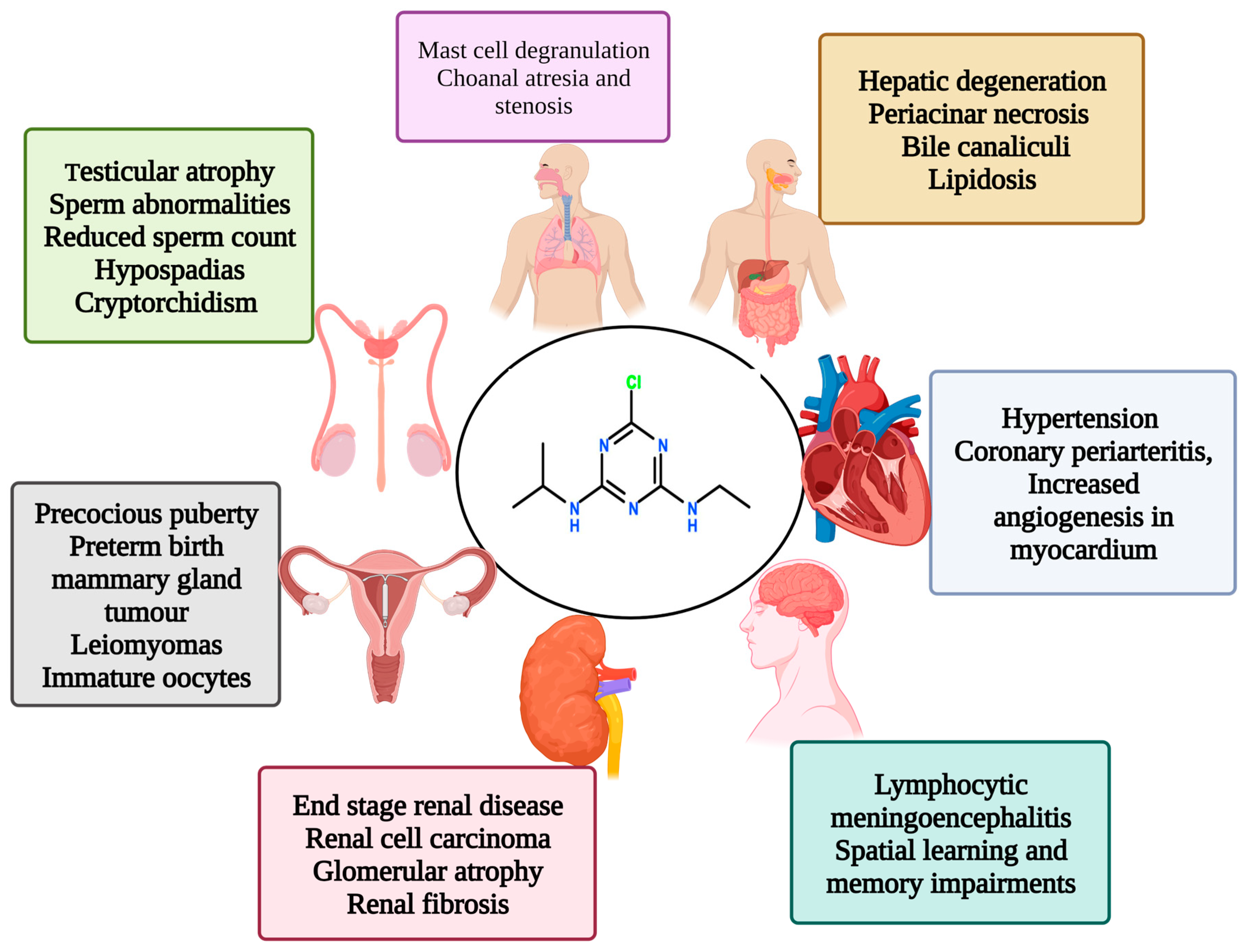
1.2. Effects of Atrazine on Various Systems of the Body
2. Mechanism of Action of Atrazine
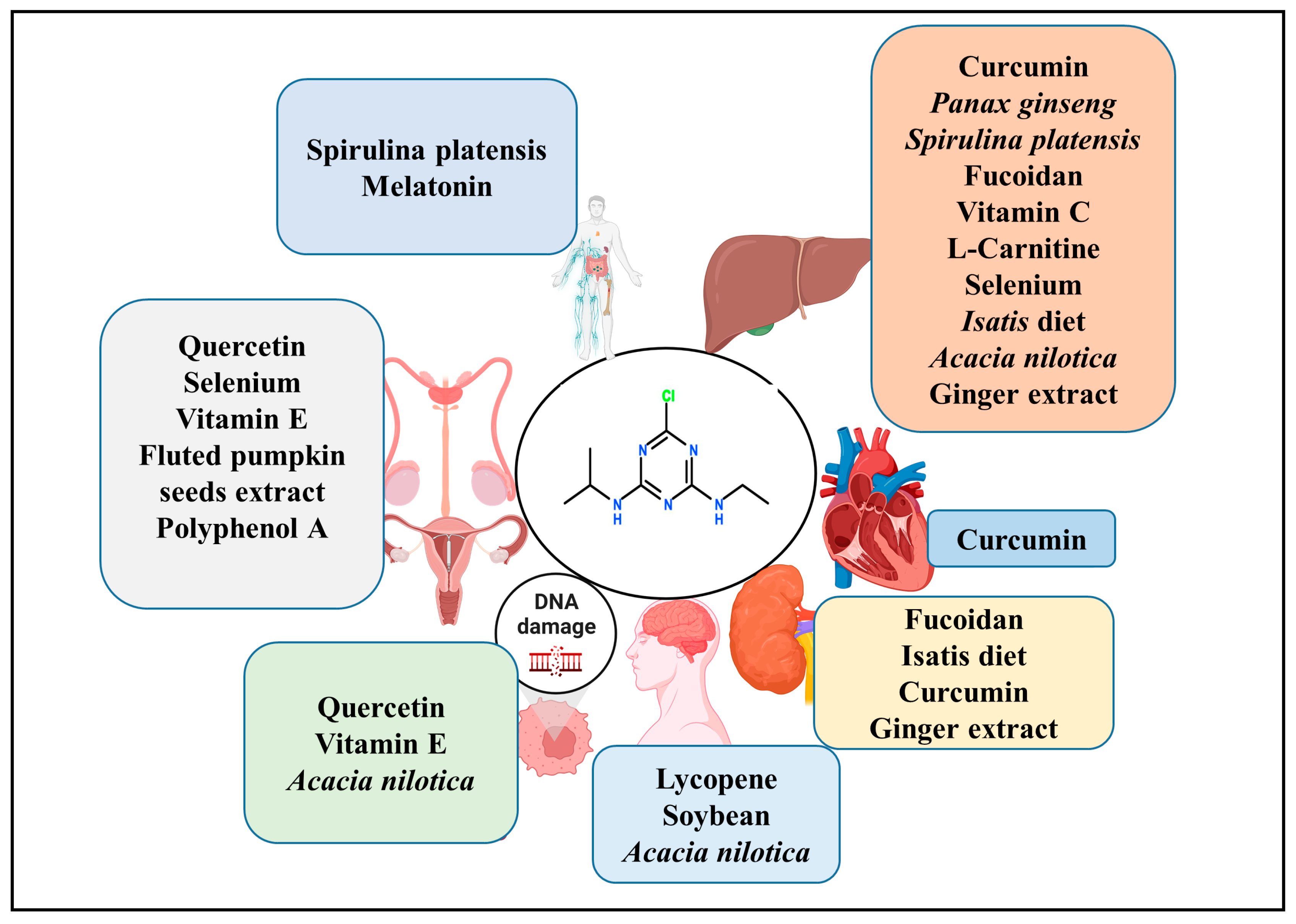
3. Approaches to Counteract Atrazine Toxicity
4. Natural Products and Compounds as Possible Agents to Counteract Atrazine Toxicity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nwani, C.D.; Lakra, W.S.; Nagpure, N.S.; Kumar, R.; Kushwaha, B.; Srivastava, S.K. Toxicity of the herbicide atrazine: Effects on lipid peroxidation and activities of antioxidant enzymes in the freshwater fish Channa punctatus (Bloch). Int. J. Environ. Res. Public Health 2010, 7, 3298–3312. [Google Scholar] [CrossRef] [PubMed]
- IPCS International Programme on Chemical Safety. Atrazine: Health and Safety Guide; WHO: Geneva, Switzerland, 1990; Available online: https://apps.who.int/iris/bitstream/handle/10665/39868/9241510471_eng.pdf?sequence=1&isAllowed=y (accessed on 27 April 2023).
- Pathak, R.K.; Dikshit, A.K. Atrazine and human health. Int. J. Ecosyst. 2012, 1, 14–23. [Google Scholar] [CrossRef]
- Rohr, J.R.; Crumrine, P.W. Effects of an herbicide and an insecticide on pond community structure and processes. Ecol. Appl. 2005, 15, 1135–1147. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P.; Rosenfeld, P.F. Handbook of Pollution Prevention and Cleaner Production Vol. 3: Best Practices in the Agrochemical Industry; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- de Campos Ventura, B.; de Angelis, D.D.F.; Marin-Morales, M.A. Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic. Biochem. Physiol. 2008, 90, 42–51. [Google Scholar] [CrossRef]
- Geng, Y.; Ma, J.; Jia, R.; Xue, L.Q.; Tao, C.J.; Li, C.J.; Ma, X.D.; Lin, Y. Impact of long-term atrazine use on groundwater safety in Jilin Province, China. J. Integr. Agric. 2013, 12, 305–313. [Google Scholar] [CrossRef]
- Beaulieu, M.; Cabana, H.; Taranu, Z.; Huot, Y. Predicting atrazine concentrations in waterbodies across the contiguous United States: The importance of land use, hydrology, and water physicochemistry. Limnol. Oceanogr. 2020, 65, 2966–2983. [Google Scholar] [CrossRef]
- Basave, B.L.; Noreña, H.A.S.; Aguilar, M.R.; Sanchez, J.V.; Tovar, M.A.M. Occurrence and Risk Assessment of Atrazine and Diuron in Well and Surface Water of a Cornfield Rural Region. Water 2022, 14, 3790. [Google Scholar] [CrossRef]
- Lakra, W.S.; Nagpure, N.S. Genotoxicological studies in fishes: A review. Indian J. Anim. Sci. 2009, 79, 93–97. Available online: https://epubs.icar.org.in/index.php/IJAnS/article/view/5210 (accessed on 27 April 2023).
- Achuba, F.; Osakwe, S. Petroleum—Induced free radical toxicity in African catfish (Clarias gariepinus). Fish. Physiol. Biochem. 2003, 29, 97–103. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Liu, J.; Huang, D. The role of reactive oxygen species in the herbicide acetochlor-induced DNA damage on Bufo raddei tadpole liver. Aquat. Toxicol. 2006, 78, 21–26. [Google Scholar] [CrossRef]
- Dorsey, A. Toxicological Profile for Atrazine; Agency for Toxic Substances and Disease Registry: Atlanta, Ga, USA, 2003. Available online: https://core.ac.uk/display/61992847 (accessed on 27 April 2023).
- Rusiecki, J.A.; De Roos, A.; Lee, W.J.; Dosemeci, M.; Lubin, J.H.; Hoppin, J.A.; Blair, A.; Alavanja, M.C. Cancer incidence among pesticide applicators exposed to atrazine in the Agricultural Health Study. Natl. Cancer Inst. 2004, 96, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.E.; Rusiecki, J.A.; Hoppin, J.A.; Lubin, J.H.; Koutros, S.; Andreotti, G.; Zahm, S.H.; Hines, C.J.; Coble, J.B.; Barone-Adesi, F.; et al. Atrazine and cancer incidence among pesticide applicators in the agricultural health study (1994–2007). Environ. Health Perspect. 2011, 119, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- De Roos, A.J.; Zahm, S.H.; Cantor, K.P.; Weisenburger, D.D.; Holmes, F.F.; Burmeister, L.F.; Blair, A. Integrative assessment of multiple pesticides as risk factors for non-Hodgkin’s lymphoma among men. Occup. Environ. Med. 2003, 60, E11. [Google Scholar] [CrossRef] [PubMed]
- Hoar, S.K.; Blair, A.; Holmes, F.F.; Boysen, C.D.; Robel, R.J.; Hoover, R.; Fraumeni, J.F., Jr. Agricultural herbicide use and risk of lymphoma and soft-tissue sarcoma. JAMA 1986, 256, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Ahn, S.Y.; Song, I.C.; Chung, M.H.; Jang, H.C.; Park, K.S.; Lee, K.U.; Pak, Y.K.; Lee, H.K. Chronic exposure to the herbicide, atrazine, causes mitochondrial dysfunction and insulin resistance. PLoS ONE 2009, 4, e5186. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Maske, P.; Mote, C.; Dighe, V. Exposure to Atrazine through gestation and lactation period led to impaired sexual maturation and subfertility in F1 male rats with congenital deformities in F2 progeny. Food. Chem. Toxicol. 2021, 157, 112586. [Google Scholar] [CrossRef]
- Durand, P.; Blondet, A.; Martin, G.; Carette, D.; Pointis, G.; Perrard, M.H. Effects of a mixture of low doses of atrazine and benzo [a] pyrene on the rat seminiferous epithelium either during or after the establishment of the blood-testis barrier in the rat seminiferous tubule culture model. Toxicol. In Vitro 2020, 62, 104699. [Google Scholar] [CrossRef]
- Martins-Santos, E.; Pimenta, C.G.; Campos, P.R.N.; Franco, M.B.; Gomes, D.A.; Mahecha, G.A.B.; Oliveira, C.A. Persistent testicular structural and functional alterations after exposure of adult rats to atrazine. Reprod. Toxicol. 2017, 73, 201–213. [Google Scholar] [CrossRef]
- Harper, A.P.; Finger, B.J.; Green, M.P. Chronic Atrazine Exposure Beginning Prenatally Impacts Liver Function and Sperm Concentration With Multi-Generational Consequences in Mice. Front. Endocrinol. 2020, 11, 580124. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Akiri, O.F.; Durojaiye, M.A.; Adenike, A. Combined effects of repeated administration of Bretmont Wipeout (glyphosate) and Ultrazin (atrazine) on testosterone, oxidative stress and sperm quality of Wistar rats. Toxicol. Mech. Methods 2015, 25, 70–80. [Google Scholar] [CrossRef]
- Cook, L.E.; Finger, B.J.; Green, M.P.; Pask, A.J. Exposure to atrazine during puberty reduces sperm viability, increases weight gain and alters the expression of key metabolic genes in the liver of male mice. Reprod. Fertil. Devel. 2019, 31, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Adesiyan, A.C.; Oyejola, T.O.; Abarikwu, S.O.; Oyeyemi, M.O.; Farombi, E.O. Selenium provides protection to the liver but not the reproductive organs in an atrazine-model of experimental toxicity. Exp. Toxicol. Pathol. 2011, 63, 201–207. [Google Scholar] [CrossRef] [PubMed]
- DeSesso, J.M.; Scialli, A.R.; White, T.E.; Breckenridge, C.B. Multigeneration reproduction and male developmental toxicity studies on atrazine in rats. Birth Defects. Res. B Dev. Reprod. Toxicol. 2014, 101, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wu, G.; Wang, S.; Lawless, J.; Sinn, A.; Chen, D.; Zheng, Z. Prenatal exposure to atrazine induces cryptorchidism and hypospadias in F1 male mouse offspring. Birth. Defects Res. 2021, 113, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, E.T.; Ore, A.; Adewole, K.E.; Oyerinde, O. Evaluation of the toxicological effects of atrazine-metolachlor in male rats: In vivo and in silico studies. Environ. Anal. Health Toxicol. 2022, 37, e2022021. [Google Scholar] [CrossRef]
- Gao, J.G.; He, Y.; Hua, Y.L.; Chen, S.X.; Jiang, Y.; Zheng, J.T.; Wang, P. Atrazine changes meiosis and reduces spermatogenesis in male mice. Zhonghua Nan Ke Xue 2020, 26, 963–968. [Google Scholar]
- Govers, L.C.; Harper, A.P.; Finger, B.J.; Mattiske, D.M.; Pask, A.J.; Green, M.P. Atrazine induces penis abnormalities including hypospadias in mice. J. Dev. Orig. Health Dis. 2020, 11, 246–249. [Google Scholar] [CrossRef]
- Quignot, N.; Arnaud, M.; Robidel, F.; Lecomte, A.; Tournier, M.; Cren-Olivé, C.; Barouki, R.; Lemazurier, E. Characterization of endocrine-disrupting chemicals based on hormonal balance disruption in male and female adult rats. Reprod. Toxicol. 2012, 33, 339–352. [Google Scholar] [CrossRef]
- Foradori, C.D.; Hinds, L.R.; Hanneman, W.H.; Handa, R.J. Effects of atrazine and its withdrawal on gonadotropin-releasing hormone neuroendocrine function in the adult female Wistar rat. Biol. Reprod. 2009, 81, 1099–1105. [Google Scholar] [CrossRef]
- Breckenridge, C.B.; Foradori, C.D.; Sawhney Coder, P.; Simpkins, J.W.; Sielken, R.L.; Handa, R.J., Jr. Changes in Sensitivity to the Effects of Atrazine on the Luteinizing Hormone Surge in Female Sprague-Dawley Rats after Repeated Daily Doses: Correlation with Liver Enzyme Expression. Birth Defects Res. 2018, 110, 246–258. [Google Scholar] [CrossRef]
- McBirney, M.; King, S.E.; Pappalardo, M.; Houser, E.; Unkefer, M.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Winchester, P.; Skinner, M.K. Atrazine induced epigenetic transgenerational inheritance of disease, lean phenotype and sperm epimutation pathology biomarkers. PLoS ONE 2017, 12, e0184306. [Google Scholar] [CrossRef] [PubMed]
- Laws, S.C.; Ferrell, J.M.; Stoker, T.E.; Schmid, J.; Cooper, R.L. The effects of atrazine on female wistar rats: An evaluation of the protocol for assessing pubertal development and thyroid function. Toxicol. Sci. 2000, 58, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Lee, S.; So, C.; Manhas, R.; Kim, C.; Wibowo, T.; Hori, M.; Hunter, N. Oocyte Development and Quality in Young and Old Mice following Exposure to Atrazine. Environ. Health Perspect. 2022, 130, 117007. [Google Scholar] [CrossRef] [PubMed]
- Gely-Pernot, A.; Saci, S.; Kernanec, P.Y.; Hao, C.; Giton, F.; Kervarrec, C.; Tevosian, S.; Mazaud-Guittot, S.; Smagulova, F. Embryonic exposure to the widely-used herbicide atrazine disrupts meiosis and normal follicle formation in female mice. Sci. Rep. 2017, 7, 3526. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Winship, A.L.; Stringer, J.M.; Swindells, E.O.; Harper, A.P.; Finger, B.J.; Hutt, K.J.; Green, M.P. Prolonged atrazine exposure beginning in utero and adult uterine morphology in mice. J. Dev. Orig. Health Dis. 2022, 13, 39–48. [Google Scholar] [CrossRef]
- Kolaitis, N.D.; Finger, B.J.; Merriner, D.J.; Nguyen, J.; Houston, B.J.; O’Bryan, M.K.; Stringer, J.M.; Zerafa, N.; Nguyen, N.; Hutt, K.J.; et al. Impact of Chronic Multi-Generational Exposure to an Environmentally Relevant Atrazine Concentration on Testicular Development and Function in Mice. Cells 2023, 12, 648. [Google Scholar] [CrossRef]
- Rinsky, J.L.; Hopenhayn, C.; Golla, V.; Browning, S.; Bush, H.M. Atrazine exposure in public drinking water and preterm birth. Public Health Rep. 2012, 127, 72–80. [Google Scholar] [CrossRef]
- Pogrmic-Majkic, K.; Samardzija, D.; Stojkov-Mimic, N.; Vukosavljevic, J.; Trninic-Pjevic, A.; Kopitovic, V.; Andric, N. Atrazine suppresses FSH-induced steroidogenesis and LH-dependent expression of ovulatory genes through PDE-cAMP signaling pathway in human cumulus granulosa cells. Mol. Cell. Endocrinol. 2018, 461, 79–88. [Google Scholar] [CrossRef]
- Villanueva, C.M.; Durand, G.; Coutté, M.B.; Chevrier, C.; Cordier, S. Atrazine in municipal drinking water and risk of low birth weight, preterm delivery, and small-for-gestational-age status. Occup. Environ. Med. 2005, 62, 400–405. [Google Scholar] [CrossRef]
- Ochoa-Acuña, H.; Frankenberger, J.; Hahn, L.; Carbajo, C. Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ. Health Perspect. 2009, 117, 1619–1624. [Google Scholar] [CrossRef]
- Munger, R.; Isacson, P.; Hu, S.; Burns, T.; Hanson, J.; Lynch, C.F.; Cherryholmes, K.; Van Dorpe, P.; Hausler, W.J., Jr. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environ. Health Perspect. 1997, 105, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.H.; AbuBakr, H.O.; Ahmad, I.M.; Ahmed, Z.S.O. Histopathological, Immunohistochemical, and Molecular Alterations in Brain Tissue and Submandibular Salivary Gland of Atrazine-Induced Toxicity in Male Rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 30697–30711. [Google Scholar] [CrossRef] [PubMed]
- Riera, J.; Matus, E.; Matus, L.; Molino, J. Toxicity of commercial atrazine in rattus novergicus organs as a function of concentration: Histopathological, ultrastructural and hematological evaluation. An. Acad. Bras. Cienc. 2022, 94, e20201125. [Google Scholar] [CrossRef] [PubMed]
- Campos-Pereira, F.D.; Oliveira, C.A.; Pigoso, A.A.; Silva-Zacarin, E.C.; Barbieri, R.; Spatti, E.F.; Marin-Morales, M.A.; Severi-Aguiar, G.D. Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: A morphological, immunohistochemical, biochemical, and molecular study. Ecotoxicol. Environ. Saf. 2012, 78, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhao, H.; Ye, H.; Cui, J.; Fang, X.; Zhang, Y.; Ye, L. Toxic Effects of Atrazine on Liver and Underlying Mechanism: A Review. Expo. Health 2023. [Google Scholar] [CrossRef]
- Zimmerman, A.D.; Breckenridge, C.B.; Yi, K.D.; Sawhney Coder, P.; Wanders, D.; Judd, R.L.; Foradori, C.D. Changes in hepatic phase I and phase II biotransformation enzyme expression and glutathione levels following atrazine exposure in female rats. Xenobiotica 2018, 48, 867–881. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, H.S.; Xiang, L.R.; Xia, J.; Wang, L.L.; Li, X.N.; Li, J.L.; Zhang, Y. Lycopene protects against atrazine-induced hepatic ionic homeostasis disturbance by modulating ion-transporting ATPases. J. Nutr. Biochem. 2016, 27, 249–256. [Google Scholar] [CrossRef]
- Santa Maria, C.; Moreno, J.; Lopez-Campos, J.L. Hepatotoxicity induced by the herbicide atrazine in the rat. J. Appl. Toxicol. 1987, 7, 373–378. [Google Scholar] [CrossRef]
- Rajkovic, V.; Djolai, M.; Matavulj, M. Alterations in jejunal morphology and serotonin-containing enteroendocrine cells in peripubertal male rats associated with subchronic atrazine exposure. Ecotoxicol. Environ. Saf. 2011, 74, 2304–2309. [Google Scholar] [CrossRef]
- Greenman, S.B.; Rutten, M.J.; Fowler, W.M.; Scheffler, L.; Shortridge, L.A.; Brown, B.; Sheppard, B.C.; Deveney, K.E.; Deveney, C.W.; Trunkey, D.D. Herbicide/pesticide effects on intestinal epithelial growth. Environ. Res. 1997, 75, 85–93. [Google Scholar] [CrossRef]
- Genovese, T.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Morabito, R.; Cuzzocrea, S.; et al. Atrazine Inhalation Causes Neuroinflammation, Apoptosis and Accelerating Brain Aging. Int. J. Mol. Sci. 2021, 22, 7938. [Google Scholar] [CrossRef] [PubMed]
- Agopian, A.J.; Cai, Y.; Langlois, P.H.; Canfield, M.A.; Lupo, P.J. Maternal residential atrazine exposure and risk for choanal atresia and stenosis in offspring. J. Pediatr. 2013, 162, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.S.O.; Tahon, M.A.; Hasan, R.S.; El-Sayed, H.G.M.; AbuBaker, H.O.; Ahmed, I.M.; Ahmed, Y.H. Histopathological, immunohistochemical, and molecular investigation of atrazine toxic effect on some organs of adult male albino rats with a screening of Acacia nilotica as a protective trial. Environ. Sci. Pollut. Res. Int. 2022, 29, 83797–83809. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Lin, J.; Zhu, S.Y.; Guo, J.Y.; Cui, J.G.; Li, J.L. Atrazine-induced oxidative damage via modulating xenobiotic-sensing nuclear receptors and cytochrome P450 systems in cerebrum and antagonism of lycopene. Food. Chem. Toxicol. 2022, 170, 113462. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, B.; Wu, Y.; Li, B. The Effects of Maternal Atrazine Exposure and Swimming Training on Spatial Learning Memory and Hippocampal Morphology in Offspring Male Rats via PSD95/NR2B Signaling Pathway. Cell. Mol. Neurobiol. 2019, 39, 1003–1015. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Bi, H.; Ma, K.; Li, B. Developmental Exposure to Atrazine Impairs Spatial Memory and Downregulates the Hippocampal D1 Dopamine Receptor and cAMP-Dependent Signaling Pathway in Rats. Int. J. Mol. Sci. 2018, 19, 2241. [Google Scholar] [CrossRef]
- Walters, J.L.; Lansdell, T.A.; Lookingland, K.J.; Baker, L.E. The effects of gestational and chronic atrazine exposure on motor behaviors and striatal dopamine in male Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2015, 289, 185–192. [Google Scholar] [CrossRef]
- Lin, Z.; Dodd, C.A.; Xiao, S.; Krishna, S.; Ye, X.; Filipov, N.M. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits and selectively alters monoaminergic systems in C57BL/6 mouse dams, juvenile and adult offspring. Toxicol. Sci. 2014, 141, 90–102. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.S.; Yang, J.W.; Yu, J.; Wu, Y.P.; Li, B.X. Exposure to atrazine during gestation and lactation periods: Toxicity effects on dopaminergic neurons in offspring by downregulation of Nurr1 and VMAT2. Int. J. Mol. Sci. 2014, 15, 2811–2825. [Google Scholar] [CrossRef]
- Chávez-Pichardo, M.E.; Reyes-Bravo, D.Y.; Mendoza-Trejo, M.S.; Marín-López, A.G.; Giordano, M.; Hernández-Chan, N.; Domínguez-Marchan, K.; Ortega-Rosales, L.C.; Rodríguez, V.M. Brain alterations in GABA, glutamate and glutamine markers after chronic atrazine exposure in the male albino rat. Arch. Toxicol. 2020, 94, 3217–3230. [Google Scholar] [CrossRef]
- Reyes-Bravo, D.Y.; Villalobos-Aguilera, P.; Almonte-Zepeda, J.T.; Mendoza-Trejo, M.S.; Giordano, M.; Orozco, A.; Rodríguez, V.M. Chronic atrazine exposure increases the expression of genes associated with GABAergic and glutamatergic systems in the brain of male albino rat. Front. Toxicol. 2022, 4, 933300. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.M.; Mendoza-Trejo, M.S.; Hernandez-Plata, I.; Giordano, M. Behavioral effects and neuroanatomical targets of acute atrazine exposure in the male Sprague-Dawley rat. Neurotoxicology 2017, 58, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Dodd, C.A.; Filipov, N.M. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol. Teratol. 2013, 39, 26–35. [Google Scholar] [CrossRef]
- Shan, W.; Hu, W.; Wen, Y.; Ding, X.; Ma, X.; Yan, W.; Xia, Y. Evaluation of atrazine neurodevelopment toxicity in vitro-application of hESC-based neural differentiation model. Reprod. Toxicol. 2021, 103, 149–158. [Google Scholar] [CrossRef]
- Rogers, J.M.; Ellis-Hutchings, R.G.; Grey, B.E.; Zucker, R.M.; Norwood, J., Jr.; Grace, C.E.; Gordon, C.J.; Lau, C. Elevated blood pressure in offspring of rats exposed to diverse chemicals during pregnancy. Toxicol. Sci. 2014, 137, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.C.; Chang, S.C.; Hsuan, S.L.; Chien, M.S.; Lee, W.C.; Kang, J.J.; Wang, S.C.; Liao, J.W. Cardiovascular effects of herbicides and formulated adjuvants on isolated rat aorta and heart. Toxicol. In Vitro 2007, 21, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, V.; Kovac, R.; Koledin, I.; Matavulj, M. Atrazine-induced changes in the myocardial structure of peripubertal rats. Toxicol. Ind. Health 2014, 30, 250–258. [Google Scholar] [CrossRef]
- Lebov, J.F.; Engel, L.S.; Richardson, D.; Hogan, S.L.; Hoppin, J.A.; Sandler, D.P. Pesticide use and risk of end-stage renal disease among licensed pesticide applicators in the Agricultural Health Study. Occup. Environ. Med. 2016, 73, 3–12. [Google Scholar] [CrossRef]
- Andreotti, G.; Beane Freeman, L.E.; Shearer, J.J.; Lerro, C.C.; Koutros, S.; Parks, C.G.; Blair, A.; Lynch, C.F.; Lubin, J.H.; Sandler, D.P.; et al. Occupational Pesticide Use and Risk of Renal Cell Carcinoma in the Agricultural Health Study. Environ. Health Perspect. 2020, 128, 67011. [Google Scholar] [CrossRef]
- Jestadi, D.B.; Phaniendra, A.; Babji, U.; Srinu, T.; Shanmuganathan, B.; Periyasamy, L. Effects of short term exposure of atrazine on the liver and kidney of normal and diabetic rats. J. Toxicol. 2014, 2014, 536759. [Google Scholar] [CrossRef]
- Liu, W.; Du, Y.; Liu, J.; Wang, H.; Sun, D.; Liang, D.; Zhao, L.; Shang, J. Effects of atrazine on the oxidative damage of kidney in Wister rats. Int. J. Clin. Exp. Med. 2014, 7, 3235–3243. [Google Scholar] [PubMed]
- Xia, J.; Lin, J.; Li, X.N.; Zhang, C.; Li, N.; Du, Z.H.; Li, Y.H.; Li, J.L. Atrazine-induced environmental nephrosis was mitigated by lycopene via modulating nuclear xenobiotic receptors-mediated response. J. Nutr. Biochem. 2018, 51, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Lin, J.; Huang, Y.Q.; Talukder, M.; Yu, L.; Li, J.L. AQP2 as a target of lycopene protects against atrazine-induced renal ionic homeostasis disturbance. Food. Funct. 2021, 12, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Duru, Q.C.; Njoku, R.C.; Amadi, B.A.; Tamunoibuomie, A.; Keboh, E. Effects of co-exposure to atrazine and ethanol on the oxidative damage of kidney and liver in Wistar rats. Ren. Fail. 2017, 39, 588–596. [Google Scholar] [CrossRef]
- Wang, T.; Huang, X.; Liu, J.; Liu, W.; Yang, Z.; He, K.; Chen, J.; Zhao, L. Prolonged exposure to the herbicide atrazine promotes kidney fibrosis by activating Wnt/β-catenin signaling in rats. Environ. Toxicol. 2023, 38, 1143–1152. [Google Scholar] [CrossRef]
- Shearer, J.J.; Sandler, D.P.; Andreotti, G.; Murata, K.; Shrestha, S.; Parks, C.G.; Liu, D.; Alavanja, M.C.; Landgren, O.; Beane Freeman, L.E.; et al. Pesticide use and kidney function among farmers in the Biomarkers of Exposure and Effect in Agriculture study. Environ. Res. 2021, 199, 111276. [Google Scholar] [CrossRef]
- Ademola, J.I.; Sedik, L.E.; Wester, R.C.; Maibach, H.I. In vitro percutaneous absorption and metabolism in man of 2-chloro-4-ethylamino-6-isopropylamine-s-triazine (atrazine). Arch. Toxicol. 1993, 67, 85–91. [Google Scholar] [CrossRef]
- Ikonen, R.; Kangas, J.; Savolainen, H. Urinary atrazine metabolites as indicators for rat and human exposure to atrazine. Toxicol. Lett. 1988, 44, 109–112. [Google Scholar] [CrossRef]
- Shah, P.V.; Fisher, H.L.; Sumler, M.R.; Monroe, R.J.; Chernoff, N.; Hall, L.L. Comparison of the penetration of 14 pesticides through the skin of young and adult rats. J. Toxicol. Environ. Health 1987, 21, 353–366. [Google Scholar] [CrossRef]
- Chang, J.; Liang, C.; Wang, W.; Yong, L.; Mao, W.; Yang, H.; Jia, X.; Liu, Z.; Song, Y. Toxic effects of atrazine on immune function in BALB/c mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 37978–37994. [Google Scholar] [CrossRef]
- Cimino-Reale, G.; Ferrario, D.; Casati, B.; Brustio, R.; Diodovich, C.; Collotta, A.; Vahter, M.; Gribaldo, L. Combined in utero and juvenile exposure of mice to arsenate and atrazine in drinking water modulates gene expression and clonogenicity of myeloid progenitors. Toxicol. Lett. 2008, 180, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Fiaz Khan, M.; Tabassum, S.; Zahran, E. Adverse effects of atrazine on blood parameters, biochemical profile and genotoxicity of snow trout (Schizothorax plagiostomus). Saudi J. Biol. Sci. 2021, 28, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Grattan, D.R. 60 Years of neuroendocrinology: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.L.; Stoker, T.E.; Tyrey, L.; Goldman, J.M.; McElroy, W.K. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol. Sci. 2000, 53, 297–307. [Google Scholar] [CrossRef]
- Wirbisky, S.E.; Freeman, J.L. Atrazine Exposure and Reproductive Dysfunction through the Hypothalamus-Pituitary-Gonadal (HPG) Axis. Toxics 2015, 3, 414–450. [Google Scholar] [CrossRef]
- Victor-Costa, A.B.; Bandeira, S.M.; Oliveira, A.G.; Mahecha, G.A.; Oliveira, C.A. Changes in testicular morphology and steroidogenesis in adult rats exposed to Atrazine. Reprod. Toxicol. 2010, 29, 323–331. [Google Scholar] [CrossRef]
- Fa, S.; Pogrmic-Majkic, K.; Samardzija, D.; Glisic, B.; Kaisarevic, S.; Kovacevic, R.; Andric, N. Involvement of ERK1/2 signaling pathway in atrazine action on FSH-stimulated LHR and CYP19A1 expression in rat granulosa cells. Toxicol. Appl. Pharmacol. 2013, 270, 1–8. [Google Scholar] [CrossRef]
- Pogrmic-Majkic, K.; Samardzija, D.; Fa, S.; Hrubik, J.; Glisic, B.; Kaisarevic, S.; Andric, N. Atrazine enhances progesterone production through activation of multiple signaling pathways in FSH-stimulated rat granulosa cells: Evidence for premature luteinization. Biol. Reprod. 2014, 91, 124. [Google Scholar] [CrossRef]
- Roberge, M.; Hakk, H.; Larsen, G. Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol. Lett. 2004, 154, 61–68. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Seinen, W.; Giesy, J.P.; van den Berg, M. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: A novel mechanism for estrogenicity? Toxicol. Sci. 2000, 54, 121–127. [Google Scholar] [CrossRef]
- Kucka, M.; Pogrmic-Majkic, K.; Fa, S.; Stojilkovic, S.S.; Kovacevic, R. Atrazine acts as an endocrine disrupter by inhibiting cAMP-specific phosphodiesterase-4. Toxicol. Appl. Pharmacol. 2012, 265, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Holloway, A.C.; Anger, D.A.; Crankshaw, D.J.; Wu, M.; Foster, W.G. Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J. Appl. Toxicol. 2008, 28, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.M.; Davis, L.K.; Murr, A.S.; Cooper, R.L. Atrazine-induced elevation or attenuation of the LH surge in the ovariectomized, estrogen-primed female rat: Role of adrenal progesterone. Reproduction 2013, 146, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Karn, E.; Kells, B.; Orlowski, J. Assessment of the Benefits of Atrazine and the Impacts of Potential Mitigation for Field Corn, Sweet Corn, Sorghum, and Sugarcane; United States Environmental Protection Agency: Washington, DC, USA, 2020. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2013-0266-1624 (accessed on 27 April 2023).
- Amaral, P.; Partlan, E.; Li, M.; Lapolli, F.; Mefford, O.T.; Karanfil, T.; Ladner, D.A. Superfine powdered activated carbon (S-PAC) coatings on microfiltration membranes: Effects of milling time on contaminant removal and flux. Water. Res. 2016, 100, 429–438. [Google Scholar] [CrossRef]
- Sánchez, V.; López-Bellido, F.J.; Cañizares, P.; Rodríguez, L. Assessing the phytoremediation potential of crop and grass plants for atrazine-spiked soils. Chemosphere 2017, 185, 119–126. [Google Scholar] [CrossRef]
- Liebman, M.; Davis, A.S. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–48. [Google Scholar] [CrossRef]
- Leblanc, M.L.; Cloutier, D.C.; Stewart, K.A. Rotary hoe cultivation in sweet corn. HortTechnology 2006, 16, 583–589. [Google Scholar] [CrossRef]
- Council, N.R. Professional Societies and Ecologically Based Pest Management: Proceedings of a Workshop; National Academies Press: Washington, DC, USA, 2000; Available online: https://nap.nationalacademies.org/read/9888/chapter/1 (accessed on 27 April 2023).
- Liebman, M.; Gibson, L.R.; Sundberg, D.N.; Heggenstaller, A.H.; Westerman, P.R.; Chase, C.A.; Hartzler, R.G.; Menalled, F.D.; Davis, A.S.; Dixon, P.M. Agronomic and economic performance characteristics of conventional and low-external-input cropping systems in the central Corn Belt. Agron. J. 2008, 100, 600–610. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Ghadage, S.R.; Mane, K.A.; Agrawal, R.S.; Pawar, V.N. Tomato lycopene: Potential health benefits. Pharma Innov. J. 2019, 8, 1245–1248. [Google Scholar]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P.R.R. Can lycopene be considered an effective protection against cardiovascular disease? Food. Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Althagafy, H.S.; Abd-Alhameed, E.K.; Al-Thubiani, W.S.; Hassanein, E.H.M. Promising hepatoprotective effects of lycopene in different liver diseases. Life Sci. 2022, 310, 121131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.Y.; Li, C.X.; Tong, Y.X.; Xu, Y.R.; Wang, Z.Y.; Li, J.L. IL-6/STAT3/Foxo1 axis as a target of lycopene ameliorates the atrazine-induced thymic mitophagy and pyroptosis cross-talk. Food Funct. 2022, 13, 8871–8879. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.A.D.; Indrayanto, G. Chapter Three-Curcumin. Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 113–204. [Google Scholar]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Chen, G.; Wang, D.; Tang, S.; Deng, H.; Wang, J.; Li, S.; Lan, J.; Tong, J.; et al. Curcumin Attenuates Asthmatic Airway Inflammation and Mucus Hypersecretion Involving a PPARγ-Dependent NF-κB Signaling Pathway In Vivo and In Vitro. Mediat. Inflamm. 2019, 2019, 4927430. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef]
- Altinay, S.; Cabalar, M.; Isler, C.; Yildirim, F.; Celik, D.S.; Zengi, O.; Tas, A.; Gulcubuk, A. Is Chronic Curcumin Supplementation Neuroprotective Against Ischemia for Antioxidant Activity, Neurological Deficit, or Neuronal Apoptosis in an Experimental Stroke Model? Turk. Neurosurg. 2017, 27, 537–545. [Google Scholar] [CrossRef]
- Mohebbati, R.; Anaeigoudari, A.; Khazdair, M.R. The effects of Curcuma longa and curcumin on reproductive systems. Endocr. Regul. 2017, 51, 220–228. [Google Scholar] [CrossRef]
- Keshk, W.A.; Soliman, N.A.; Abo El-Noor, M.M.; Wahdan, A.A.; Shareef, M.M. Modulatory effects of curcumin on redox status, mitochondrial function, and caspace-3 expression during atrazin-induced toxicity. J. Biochem. Mol. Toxicol. 2014, 28, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Noor, M.; Wahdan, A.; Shareef, M.; Soliman, N.; Keshk, W. The Possible Ameliorative Effect of Curcumin on Atrazine-Induced Oxidative Stress, DNA Damage, Mitochondrial Dysfunction, and Apoptosis in the Kidney of Adult Male Albino Rats. Ain Shams J. Forensic Med. Clin. Toxicol. 2014, 23, 61–70. [Google Scholar] [CrossRef]
- Ndufeiya-Kumasi, L.C.; Abarikwu, S.O.; Ohanador, R.; Omoregie, E.S. Curcumin improves the protective effects of quercetin against atrazine-induced testicular injury in adult Wistar rats. Andrologia 2002, 54, e14445. [Google Scholar] [CrossRef]
- Yuan, C.S.; Wang, C.Z.; Wicks, S.M.; Qi, L.W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J. Ginseng. Res. 2010, 34, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Mehrim, A.I.; Refaey, M.M.; Hassan, M.A.E.; Zaki, M.A.; Zenhom, O.A. Ginseng® as a reproductive enhancer agent for African catfish, Clarias gariepinus (Burchell, 1822). Fish Physiol. Biochem. 2022, 48, 15–32. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Mohammed, A.T.; Farag, M.R.; Hassan, M.A.; Mawed, S.A.; Alagawany, M.; Zizzadoro, C. Dietary Supplementation of Nile Tilapia (Oreochromis niloticus) With Panax ginseng Essential Oil: Positive Impact on Animal Health and Productive Performance, and Mitigating Effects on Atrazine-Induced Toxicity. Front. Mar. Sci. 2022, 9, 920057. [Google Scholar] [CrossRef]
- van den Driessche, J.J.; Plat, J.; Konings, M.C.J.M.; Mensink, R.P. Effects of spirulina and wakame consumption on intestinal cholesterol absorption and serum lipid concentrations in non-hypercholesterolemic adult men and women. Eur. J. Nutr. 2020, 59, 2229–2236. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential application of microalga Spirulina platensis as a protein source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef]
- Dasgupta, T.; Banejee, S.; Yadav, P.K.; Rao, A.R. Chemomodulation of carcinogen metabolising enzymes, antioxidant profiles and skin and forestomach papillomagenesis by Spirulina platensis. Mol. Cell. Biochem. 2001, 226, 27–38. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxid. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef]
- Chen, J.C.; Liu, K.S.; Yang, T.J.; Hwang, J.H.; Chan, Y.C.; Lee, I.T. Spirulina and C-phycocyanin reduce cytotoxicity and inflammation-related genes expression of microglial cells. Nutr. Neurosci. 2012, 15, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.A.V.; Joventino, I.P.; Joventino, F.P.; de Almeida, A.C.; Neves, K.R.T.; do Carmo, M.R.; Leal, L.K.A.M.; de Andrade, G.M.; de Barros Viana, G.S. Neuroprotective Activities of Spirulina platensis in the 6-OHDA Model of Parkinson’s Disease Are Related to Its Anti-Inflammatory Effects. Neurochem. Res. 2017, 42, 3390–3400. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Shahzad, S.; Batool, Z.; Sadir, S.; Liaquat, L.; Tabassum, S.; Perveen, T. Spirulina platensis reduces the schizophrenic-like symptoms in rat model by restoring altered APO-E and RTN-4 protein expression in prefrontal cortex. Life Sci. 2021, 277, 119417. [Google Scholar] [CrossRef]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef] [PubMed]
- Toughan, H.; Khalil, S.R.; El-Ghoneimy, A.A.; Awad, A.; Seddek, A.S. Effect of dietary supplementation with Spirulina platensis on Atrazine-induced oxidative stress- mediated hepatic damage and inflammation in the common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2018, 149, 135–142. [Google Scholar] [CrossRef]
- Khalil, S.R.; Reda, R.M.; Awad, A. Efficacy of Spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine. Fish. Shellfish. Immunol. 2017, 67, 119–128. [Google Scholar] [CrossRef]
- Hedayatirad, M.; Mirvaghefi, A.; Nematollahi, M.A.; Forsatkar, M.N.; Brown, C. Transgenerational disrupting impacts of atrazine in zebrafish: Beneficial effects of dietary spirulina. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 230, 108685. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Kim, S.K. Anticancer effects of fucoidan. Adv. Food. Nutr. Res. 2014, 72, 195–213. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Sousa, R.B.; Franco, Á.X.; Costa, J.V.; Neves, L.M.; Ribeiro, R.A.; Sutton, R.; Criddle, D.N.; Soares, P.M.; de Souza, M.H. Protective effects of fucoidan, a P- and L-selectin inhibitor, in murine acute pancreatitis. Pancreas 2014, 43, 82–87. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Q.; Chen, L.; Ren, S.; Xu, P.; Tang, Y.; Luo, D. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thromb. Res. 2010, 125, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lean, Q.Y.; Eri, R.D.; Fitton, J.H.; Patel, R.P.; Gueven, N. Fucoidan Extracts Ameliorate Acute Colitis. PLoS ONE 2015, 10, e0128453. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yim, E.K.F. Fucoidan for cardiovascular application and the factors mediating its activities. Carbohydr. Polym. 2021, 270, 118347. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Han, X.; Ma, Y.; Zhang, Z.; Zhao, L.; Guan, F.; Ma, S. Fucoidan: A promising agent for brain injury and neurodegenerative disease intervention. Food. Funct. 2021, 12, 3820–3830. [Google Scholar] [CrossRef] [PubMed]
- McBean, S.E.; Church, J.E.; Thompson, B.K.; Taylor, C.J.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y.; van der Poel, C. Oral fucoidan improves muscle size and strength in mice. Physiol. Rep. 2021, 9, e14730. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef]
- Abdel-Warith, A.A.; Younis, E.M.; Al-Asgah, N.A.; Gewaily, M.S.; El-Tonoby, S.M.; Dawood, M.A.O. Role of Fucoidan on the Growth Behavior and Blood Metabolites and Toxic Effects of Atrazine in Nile Tilapia Oreochromis niloticus (Linnaeus, 1758). Animals 2021, 11, 1448. [Google Scholar] [CrossRef]
- Ariharan, V.N.; Kalirajan, K.; Devi, V.N.; Prasad, P. An exotic fruit which forms the new natural source for vitamin-C. Rasayan. J. Chem. 2012, 5, 356–359. [Google Scholar]
- Gull, J.; Sultana, B.; Anwar, F.; Naseer, R.; Ashraf, M.; Ashrafuzzaman, M. Variation in antioxidant attributes at three ripening stages of guava (Psidium guajava L.) fruit from different geographical regions of Pakistan. Molecules 2012, 17, 3165–3180. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Öberg, E.; Johansson, E.; Andersson, S.C.; Rumpunen, K. Phenols and ascorbic acid in black currants (Ribes nigrum L.): Variation due to genotype, location, and year. J. Agric. Food Chem. 2013, 61, 9298–9306. [Google Scholar] [CrossRef]
- Dhariwal, K.R.; Hartzell, W.O.; Levine, M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 1991, 54, 712–716. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. Adv. Food. Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian. J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Sirasanagandla, S.R.; Rooben, R.K.; Rajkumar; Narayanan, S.N.; Jetti, R. Ascorbic Acid ameliorates nicotine exposure induced impaired spatial memory performances in rats. West Indian Med. J. 2014, 63, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef]
- Guarnieri, S.; Loft, S.; Riso, P.; Porrini, M.; Risom, L.; Poulsen, H.E.; Dragsted, L.O.; Møller, P. DNA repair phenotype and dietary antioxidant supplementation. Br. J. Nutr. 2008, 99, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.B.; Zhang, Y.P.; Zhang, J.; Zhang, Y.B. Evaluation of Vitamin C Supplementation on Kidney Function and Vascular Reactivity Following Renal Ischemic Injury in Mice. Kidney Blood Press. Res. 2016, 41, 460–470. [Google Scholar] [CrossRef]
- Weber, C.; Erl, W.; Weber, K.; Weber, P.C. Increased adhesiveness of isolated monocytes to endothelium is prevented by vitamin C intake in smokers. Circulation 1986, 93, 1488–1492. [Google Scholar] [CrossRef]
- Gomes, J.L.C.; do Amaral, A.M.B.; Storck, T.R.; Moraes, B.S.; Loro, V.L.; Clasen, B. Can Vitamin C Supplementation Improve the Antioxidant Capacity of Rhamdia quelen Fish Exposed to Atrazine? Arch. Environ. Contam. Toxicol. 2022, 82, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic acid induced atrazine degradation. J. Hazard. Mater. 2017, 327, 71–78. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Miadoková, E. Isoflavonoids—An overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- Liao, W.; Jin, G.; Zhao, M.; Yang, H. The effect of genistein on the content and activity of α- and β-secretase and protein kinase C in Aβ-injured hippocampal neurons. Basic. Clin. Pharmacol. Toxicol. 2013, 112, 182–185. [Google Scholar] [CrossRef]
- Grossini, E.; Farruggio, S.; Raina, G.; Mary, D.; Deiro, G.; Gentilli, S. Effects of Genistein on Differentiation and Viability of Human Visceral Adipocytes. Nutrients 2018, 10, 978. [Google Scholar] [CrossRef]
- Qin, W.; Du, N.; Zhang, L.; Wu, X.; Hu, Y.; Li, X.; Shen, N.; Li, Y.; Yang, B.; Xu, C.; et al. Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. Br. J. Pharmacol. 2015, 172, 5559–5572. [Google Scholar] [CrossRef] [PubMed]
- Yoo, N.Y.; Jeon, S.; Nam, Y.; Park, Y.J.; Won, S.B.; Kwon, Y.H. Dietary Supplementation of Genistein Alleviates Liver Inflammation and Fibrosis Mediated by a Methionine-Choline-Deficient Diet in db/db Mice. J. Agric. Food Chem. 2015, 63, 4305–4311. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Yang, K.; Zhu, P.; Zhao, H.Q.; Song, Y.H.; Liu, K.C.; Huang, W.F. Genistein Ameliorates Ischemia/Reperfusion-Induced Renal Injury in a SIRT1-Dependent Manner. Nutrients 2017, 9, 403. [Google Scholar] [CrossRef]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods 2021, 10, 774. [Google Scholar] [CrossRef]
- Li, P.; Yao, L.Y.; Jiang, Y.J.; Wang, D.D.; Wang, T.; Wu, Y.P.; Li, B.X.; Li, X.T. Soybean isoflavones protect SH-SY5Y neurons from atrazine-induced toxicity by activating mitophagy through stimulation of the BEX2/BNIP3/NIX pathway. Ecotoxicol. Environ. Saf. 2021, 227, 112886. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.; Yao, L.; Wu, Y.; Li, B. Soybean isoflavones prevent atrazine-induced neurodegenerative damage by inducing autophagy. Ecotoxicol. Environ. Saf. 2020, 190, 110065. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food. Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Oh, W.Y.; Ambigaipalan, P.; Shahidi, F. Preparation of Quercetin Esters and Their Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 10653–10659. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.C.; Tripathi, A.N.; Kumar, P. Tripathi and P. Kumar. Influence of different solvents on antibacterial potential of three species of himalayan oaks. Int. J. Pharm. Sci. Res. 2022, 13, 197–205. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Davis, B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1071–R1077. [Google Scholar] [CrossRef]
- Hee Lee, J.; Huh, C.H.; Yoon, H.J.; Cho, K.H.; Chung, J.H. Photo-epilation results of axillary hair in dark-skinned patients by intense pulsed light: Comparison between different wavelengths and pulse width. Dermatol. Surg. 2006, 32, 234–240. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Loboda, A.; Wagner, A.E.; Stachurska, A.; Jozkowicz, A.; Dulak, J.; Döring, F.; Wolffram, S.; Rimbach, G. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: Role of miR-155. J. Nutr. Biochem. 2011, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Ringseis, R.; Keller, J.; Eder, K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: Evidence from experimental and clinical studies. Eur. J. Nutr. 2013, 52, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.B.; Constantin-Teodosiu, D.; Greenhaff, P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007, 58, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, X.; Cao, L.; Zhao, S.; Zhao, C.; Yin, S.; Hu, H. A Multi-Ingredient Formula Ameliorates Exercise-Induced Fatigue by Changing Metabolic Pathways and Increasing Antioxidant Capacity in Mice. Foods 2021, 10, 3120. [Google Scholar] [CrossRef] [PubMed]
- Abdel Aziz, R.L.; Abdel-Wahab, A.; Abo El-Ela, F.I.; Hassan, N.E.Y.; El-Nahass, E.S.; Ibrahim, M.A.; Khalil, A.A.Y. Dose- dependent ameliorative effects of quercetin and l-Carnitine against atrazine- induced reproductive toxicity in adult male Albino rats. Biomed. Pharmacother. 2018, 102, 855–864. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Farombi, E.O. Quercetin ameliorates atrazine-induced changes in the testicular function of rats. Toxicol. Ind. Health 2016, 32, 1278–1285. [Google Scholar] [CrossRef]
- Farombi, E.O.; Abarikwu, S.O.; Adesiyan, A.C.; Oyejola, T.O. Quercetin exacerbates the effects of subacute treatment of atrazine on reproductive tissue antioxidant defence system, lipid peroxidation and sperm quality in rats. Andrologia 2013, 45, 256–265. [Google Scholar] [CrossRef]
- Njoku, R.C.; Abarikwu, S.O. Antifertility and profertility effects of the leaves and seeds of fluted pumpkin: Sperm quality, hormonal effects and histomorphological changes in the testes of experimental animal models. J. Integr. Med. 2021, 19, 104–110. [Google Scholar] [CrossRef]
- Osukoya, O.A.; Adegbenro, D.; Onikanni, S.A.; Ojo, O.A.; Onasanya, A. Antinociceptive and Antioxidant Activities of the Methanolic Extract of Telfairia occidentalis Seeds. Anc. Sci. Life 2016, 36, 98–103. [Google Scholar] [CrossRef]
- Njoku, R.C.; Abarikwu, S.O.; Uwakwe, A.A.; Mgbudom-Okah, C.J. Telfairia occidentalis-supplemented diet induces changes in sperm parameters and testosterone level in rats. Andrologia 2018, 50, e13044. [Google Scholar] [CrossRef] [PubMed]
- Ezim, O.E.; Abarikwu, S.O. Fluted pumpkin seeds protect the spermatogenesis score index and testicular histology of caffeine treated rats. Andrologia 2022, 54, e14578. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oyeniran, O.H.; Jimoh, T.O.; Oboh, G.; Boligon, A.A. Fluted pumpkin (Telfairia occidentalis) seed modulates some markers of erectile function in isolated rat’s corpus cavernosum: Influence of polyphenol and amino acid constituents. J. Food Biochem. 2019, 43, e13037. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Mgbudom-Okah, C.J.; Onuah, C.L.; Ogunlaja, A. Fluted pumpkin seeds protect against busulfan-induced oxidative stress and testicular injuries in adult mice. Drug Chem. Toxicol. 2022, 45, 22–32. [Google Scholar] [CrossRef]
- Ezirim, C.Y.; Abarikwu, S.O.; Uwakwe, A.A.; Mgbudom-Okah, C.J. Protective effects of Anthocleista djalonensis A. Chev root extracts against induced testicular inflammation and impaired spermatogenesis in adult rats. Mol. Biol. Rep. 2019, 46, 5983–5994. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Oleribe, A.L.; Mgbudom-Okah, C.J.; Onuah, C.L.; Chikwendu, C.S.; Onyeike, E.N. The protective effect of fluted pumpkin seeds against atrazine-induced testicular injury. Drug Chem. Toxicol. 2022, 45, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Saremi, A.; Arora, R. Vitamin E and cardiovascular disease. Am. J. Ther. 2010, 17, e56–e65. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaffarin, A.S.; Ng, S.F.; Ng, M.H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int. J. Nanomed. 2020, 15, 9961–9974. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef]
- Zingg, J.M. Vitamin E: A Role in Signal Transduction. Annu. Rev. Nutr. 2015, 35, 135–173. [Google Scholar] [CrossRef]
- Icer, M.A.; Arslan, N.; Gezmen-Karadag, M. Effects of vitamin E on neurodegenerative diseases: An update. Acta Neurobiol. Exp. 2021, 81, 21–33. [Google Scholar] [CrossRef]
- Yuan, X.; Duan, Y.; Xiao, Y.; Sun, K.; Qi, Y.; Zhang, Y.; Ahmed, Z.; Moiani, D.; Yao, J.; Li, H.; et al. Vitamin E Enhances Cancer Immunotherapy by Reinvigorating Dendritic Cells via Targeting Checkpoint SHP1. Cancer Discov. 2022, 12, 1742–1759. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, P.; Sandhir, R.; Kiran, R. Protective effects of vitamin E against atrazine-induced genotoxicity in rats. Mutation. Res. 2008, 654, 145–149. [Google Scholar] [CrossRef]
- Singh, M.; Sandhir, R.; Kiran, R. Effects on antioxidant status of liver following atrazine exposure and its attenuation by vitamin E. Exp. Toxicol. Pathol. 2011, 63, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Rezaie Agdam, H.; Razi, M.; Amniattalab, A.; Malekinejad, H.; Molavi, M. Co-Administration of Vitamin E and Testosterone Attenuates The Atrazine-Induced Toxic Effects on Sperm Quality and Testes in Rats. Cell J. 2017, 19, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Griboff, J.; Morales, D.; Bertrand, L.; Bonansea, R.I.; Monferrán, M.V.; Asis, R.; Wunderlin, D.A.; Amé, M.V. Oxidative stress response induced by atrazine in palaemonetes argentinus: The protective effect of vitamin E. Ecotoxicol. Environ. Saf. 2014, 108, 1–8. [Google Scholar] [CrossRef]
- Sharma, R.K.; Fulia, A.; Chauhan, P.K. Antioxidant attenuation of atrazine induced histopathological changes in testicular tissue of goat in vitro. Toxicol. Int. 2012, 19, 260–266. [Google Scholar] [CrossRef]
- Dogara, A.M.; Hamad, S.W.; Hama, H.A.; Bradosty, S.W.; Kayfi, S.; Al-Rawi, S.S.; Lema, A.A. Biological evaluation of garcinia kola heckel. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 3837965. [Google Scholar] [CrossRef] [PubMed]
- Nkono Ya Nkono, B.L.; Rouamba, A.; Kinyok, M.J.; Omokolo, J.G.S.; Tcheudi, B.T.; Tigui, B.A.; Dzeufiet, P.D.D.; Sokeng, S.D.; Kamtchouing, P. Antidiabetic and antiradical effects of garcinia kola seeds in dexamethasone-induced hyperglycemic rats. Int. J. Appl. Basic. Med. Res. 2022, 12, 203–210. [Google Scholar] [CrossRef]
- Yakubu, M.T.; Quadri, A.L. Garcinia kola seeds: Is the aqueous extract a true aphrodisiac in male wistar rats? Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 530–535. [Google Scholar] [CrossRef]
- Ilechie, A.A.; Jeduah, M.M.; Abraham, C.H.; Ocansey, S.; Abu, E.; Okyere, T.; Ngosaro, O. Oral consumption of garcinia kola(bitter kola) lowers intraocular pressure. Acta Ophthalmol. 2020, 98, e1028–e1033. [Google Scholar] [CrossRef]
- Oyenihi, O.R.; Cerf, M.E.; Matsabisa, M.G.; Brooks, N.L.; Oguntibeju, O.O. Effect of kolaviron on islet dynamics in diabetic rats. Saudi J. Biol. Sci. 2022, 29, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Olopade, J.O.; Farombi, E.O. Kolaviron and garcinia kola attenuate doxorubicin-induced cardiotoxicity in wistar rats. J. Complement. Integr. Med. 2017, 15. [Google Scholar] [CrossRef]
- Adedara, I.A.; Awogbindin, I.O.; Anamelechi, J.P.; Farombi, E.O. Garcinia kola seed ameliorates renal, hepatic, and testicular oxidative damage in streptozotocin-induced diabetic rats. Pharm. Biol. 2015, 53, 695–704. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Farombi, E.O.; Pant, A.B. Kolaviron biflavanoids of garcinia kola seeds protect atrazine-induced cytotoxicity in primary cultures of rat leydig cells. Int. J. Toxicol. 2012, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Farombi, E.O.; Kashyap, M.P.; Pant, A.B. Kolaviron protects apoptotic cell death in pc12 cells exposed to atrazine. Free Radic. Res. 2011, 45, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Phytomelatonin: Discovery, content, and role in plants. Adv. Bot. 2014, 2014, 815769. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef]
- Olakowska, E.; Marcol, W.; Kotulska, K.; Lewin-Kowalik, J. The role of melatonin in the neurodegenerative diseases. Bratisl. Lek. Listy. 2005, 106, 171–174. [Google Scholar] [PubMed]
- Ma, H.; Yan, J.; Sun, W.; Jiang, M.; Zhang, Y. Melatonin treatment for sleep disorders in parkinson’s disease: A meta-analysis and systematic review. Front. Aging Neurosci. 2022, 14, 784314. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sidhu, I.P.; Bhatti, G.K. Ameliorative action of melatonin on oxidative damage induced by atrazine toxicity in rat erythrocytes. Mol. Cell. Biochem. 2011, 353, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sarkar, J.; Haldar, C.; Sinha, S. Melatonin reverses fas, e2f-1 and endoplasmic reticulum stress mediated apoptosis and dysregulation of autophagy induced by the herbicide atrazine in murine splenocytes. PLoS ONE 2014, 9, e108602. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. L-carnitine and acetyl-l-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.Y.; Liu, G.H.; Lu, H.B.; Mao, C.Y. L-carnitine and heart disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef]
- Ribas, G.S.; Vargas, C.R.; Wajner, M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 2014, 533, 469–476. [Google Scholar] [CrossRef]
- Lagares, M.A.; da Silva, G.C.; Cortes, S.F.; Moreira, F.H.M.; Neves, F.C.D.; Alves, N.C.; Viegas, R.N.; Diniz, T.F.; Lemos, V.S.; de Rezende, A.S.C.; et al. L-carnitine added to post-thawed semen acts as an antioxidant and a stimulator of equine sperm metabolism. Andrologia 2022, 54, e14338. [Google Scholar] [CrossRef]
- Karam, K.M.; Alebady, A.S.; Al-Nailey, K.G.C.; Al-Delemi, D.H.J. L-carnitine effect on induced hyperlipidemia on premature rats: Fertility profile. J. Med. Life 2022, 15, 124–131. [Google Scholar] [CrossRef]
- Elkomy, A.; Abdelhiee, E.Y.; Fadl, S.E.; Emam, M.A.; Gad, F.A.; Sallam, A.; Alarifi, S.; Abdel-Daim, M.M.; Aboubakr, M. L-Carnitine Mitigates Oxidative Stress and Disorganization of Cytoskeleton Intermediate Filaments in Cisplatin-Induced Hepato-Renal Toxicity in Rats. Front. Pharmacol. 2020, 11, 574441. [Google Scholar] [CrossRef]
- Rashad, W.A.; Saadawy, S.F.; Refaay, N.E. Mitigating effect of l-carnitine against atrazine-induced hepatotoxicity: Histopathological and biochemical analyses in albino rats. Environ. Sci. Pollut. Res. Int. 2023, 30, 22034–22045. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxid. Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium. Adv. Food Nutr. Res. 2021, 96, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. An original discovery: Selenium deficiency and keshan disease (an endemic heart disease). Asia. Pac. J. Clin. Nutr. 2012, 21, 320–326. [Google Scholar] [PubMed]
- Gać, P.; Czerwińska, K.; Macek, P.; Jaremków, A.; Mazur, G.; Pawlas, K.; Poręba, R. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021, 82, 103553. [Google Scholar] [CrossRef]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium deficiency due to diet, pregnancy, severe illness, or COVID-19-A preventable trigger for autoimmune disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in human health and gut microflora: Bioavailability of selenocompounds and relationship with diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef]
- Marins, A.T.; Rodrigues, C.C.R.; de Menezes, C.C.; de Lima Costa Gomes, J.; Costa, M.D.; Nunes, M.E.M.; de Souza Vieira, M.; Donato, F.F.; Zanella, R.; da Silva, L.P.; et al. Integrated biomarkers response confirm the antioxidant role of diphenyl diselenide against atrazine. Ecotoxicol. Environ. Saf. 2018, 151, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Taviano, M.F.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Paterniti Mastrazzo, G.; De Rose, R.F.; Celano, M.F.; Lombardo, G.E.; et al. Phenolic profile, antioxidant and cytotoxic properties of polar extracts from leaves and flowers of isatis tinctoria l.(brassicaceae) growing in Sicily. Plant Biosyst. 2018, 152, 795–803. [Google Scholar] [CrossRef]
- Speranza, J.; Miceli, N.; Taviano, M.F.; Ragusa, S.; Kwiecień, I.; Szopa, A.; Ekiert, H. Isatis tinctoria l.(woad): A review of its botany, ethnobotanical uses, phytochemistry, biological activities, and biotechnological studies. Plants 2020, 9, 298. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jiang, X.; Zhang, L. Optimisation of extraction conditions for polysaccharides from the roots of isatis tinctoria l. By response surface methodology and their in vitro free radicals scavenging activities and effects on IL-4 and IFN-γ MRNA expression in chicken lymphocytes. Carbohydr. Polym. 2011, 86, 1320–1326. [Google Scholar] [CrossRef]
- Kong, X.; Hu, Y.; Rui, R.; Wang, D.; Li, X. Effects of chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int. Immunopharmacol. 2004, 4, 975–982. [Google Scholar] [CrossRef]
- Liao, B.L.; Pan, Y.J.; Zhang, W.; Pan, L.W. Four natural compounds separated from folium isatidis: Crystal structures and antibacterial activity. Chem. Biodivers. 2018, 15, e1800152. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.F.; Hakim, Y.A.E.; Mahmoud, E.A. Alleviating effects of β-glucan in oreochromis niloticus on growth performance, immune reactions, antioxidant, transcriptomics disorders and resistance to aeromonas sobria caused by atrazine. Aquac. Res. 2020, 51, 1801–1812. [Google Scholar] [CrossRef]
- Ali, M.F.; Soliman, A.A.; Gewaily, M.S.; Abdel-Kader, T.Y.; Amer, A.A.; Zaineldin, A.I.; Al-Asgah, N.A.; Younis, E.M.; Abdel-Warith, A.A.; Sewilam, H.; et al. Isatis phytogenic relieved atrazine induced growth retardation, hepato-renal dysfunction, and oxidative stress in nile tilapia. Saudi J. Biol. Sci. 2022, 29, 190–196. [Google Scholar] [CrossRef]
- Bravo, L.J. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- de Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- Milder, I.E.; Arts, I.C.; van de Putte, B.; Venema, D.P.; Hollman, P.C. Lignan contents of dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.I.; Ibern-Gómez, M.; Lamuela-Raventós, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.G.; Zahradka, P.; Taylor, C.G. Lentil-based diets attenuate hypertension and large-artery remodelling in spontaneously hypertensive rats. Br. J. Nutr. 2014, 111, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Bijak, M.; Synowiec, E.; Sitarek, P.; Sliwiński, T.; Saluk-Bijak, J. Evaluation of the cytotoxicity and genotoxicity of flavonolignans in different cellular models. Nutrients 2017, 9, 1356. [Google Scholar] [CrossRef]
- Liu, W.Y.; Liou, S.S.; Hong, T.Y.; Liu, I.M. Protective effects of hesperidin (citrus flavonone) on high glucose induced oxidative stress and apoptosis in a cellular model for diabetic retinopathy. Nutrients 2017, 9, 1312. [Google Scholar] [CrossRef]
- Komsky-Elbaz, A.; Saktsier, M.; Biran, D.; Argov-Argaman, N.; Azaizeh, H.; Landau, Y.S.; Roth, Z. Atrazine-induced toxicity in goat spermatozoa is alleviated to some extent by polyphenol-enriched feed. Chemosphere 2019, 236, 124858. [Google Scholar] [CrossRef]
- Rather, L.J.; Shahid-ul-Islam; Mohammad, F. Acacia nilotica (l.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Zaman, S.U.; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plants Res. 2012, 6, 1492–1496. [Google Scholar]
- Pareek, P.; Choudhry, M.A. Management of type 2 diabetics by Indian gum arabic (acacia nilotica) pods powder. Int. J. Food Nutr. Sci. 2013, 2, 77–83. [Google Scholar]
- Abdulazeez, A.; Balogun, E. Antiulcer effect of acacia nilotica seedpod aqueous extract on experimentally induced ulcer. Afr. J. Biol. Sci. 2022, 4, 86. [Google Scholar] [CrossRef]
- Han, Y.A.; Song, C.W.; Koh, W.S.; Yon, G.H.; Kim, Y.S.; Ryu, S.Y.; Kwon, H.J.; Lee, K.H. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother. Res. 2013, 27, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D. Ginger: Is it ready for prime time? Cancer Prev. Res. 2013, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.H.; Makpol, S.; Abdul Hamid, N.A.; Das, S.; Ngah, W.Z.; Yusuf, Y.A. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef]
- Wang, W.H.; Wang, Z.M. Studies of commonly used traditional medicine-ginger. Zhongguo Zhong Yao Za Zhi. 2005, 30, 1569–1573. [Google Scholar]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Sullivan, D.R.; Fenech, M.; Patch, C.S.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S4–S24. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food. Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef]
- El-Shenawy, N.S.; El-Ahmary, B.; Al-Eisa, R.A. Mitigating effect of ginger against oxidative stress induced by atrazine herbicides in mice liver and kidney. J. Biofertil. Biopestici. 2011, 2. [Google Scholar] [CrossRef]

| Author; Year | Animal Model | Atrazine Dose and Duration of Treatment | Natural Product or Natural Compound Dose and Duration of Treatment | Atrazine Induced Toxicity | Mechanism of Actions |
|---|---|---|---|---|---|
| Dai et al., 2022 [57] | Mice | 50 and 200 mg/kg b.wt./day for 21 days | Lycopene—5 mg/kg b.wt./day for 21 days | Neurotoxicity | Anti-oxidant effect by modulating xenobiotic-sensing nuclear receptors and cytochrome P450 |
| Keshk et al., 2014 [117] | Rats | 400 mg/kg b.wt./day for 3 weeks | Curcumin—400 mg/kg b.wt./day for 3 weeks | Cardiac toxicity | Modulating redox status, mitochondrial function, caspase-3 expression |
| Abo El-Noor et al., 2014 [118] | Rats | 100 mg/kg b.wt./day for 21 days | Curcumin—400 mg/kg/day for 21 days | Nephrotoxicity | By ameliorating the oxidative stress, apoptosis, DNA damage, mitochondrial dysfunction. |
| Ahmed et al., 2022 [122] | Nile tilapia (Oreochromis niloticus) | 1.39 mg/L for 60 days | Panax ginseng essential oil—60 days | Growth inhibition and hepatotoxicity | Anti-oxidant and anti-apoptotic effects |
| Toughan et al., 2017 [131] | Cyprinus carpio L. | 428 μg/L for 40 days | Spirulina (Spirulina platensis)—1% for 40 days | Hepatotoxicity | Anti-oxidant and anti-inflammatory effects |
| Khalil et al., 2017 [132] | Cyprinus carpio L. | 428 μg/L for 40 days | Spirulina (Spirulina platensis)—1% for 40 days | Immunotoxicity | Immune related genes expression modulation and anti-inflammatory effect |
| Hedayatirad et al., 2020 [133] | Adult female Zebra fish | 5 μg/L and 50 μg/L for 28 days | Spirulina (Spirulina platensis)—10 g/kg b.wt./day for 28 days | Immunotoxicity and endocrine disruptor toxicity | Transgenerational antimicrobial effects and immunotoxic suppression |
| Abdel-Warith et al., 2021 [143] | Nile tilapia fish | 1/5 96 h LC50 (1.39 mg/L) for 30 days | Fucoidan—0.8% for 30 days | Growth retardation, hepatic and renal toxicity | Anti-oxidant and anti-inflammatory effects |
| Gomes et al., 2022 [158] | Rhamdia quelen fish | 10 µgL−1 for 96 h | Vitamin C—1 g/kg b.wt for 30 days | Hepatotoxicity | Antioxidant and anti-peroxidase effect |
| Li et al., 2019 [171] | Rats | 50 mg/kg for 45 days | Soybean—isoflavones 10, 50, or 100 mg/kg for 45 days | Neurotoxicity | Autophagy modulation through mTOR-dependent signalling pathway |
| Abdel Aziz et al., 2018 [182] | Rats | 120 mg/kg b.wt. 21 days | Quercetin—10 and 50 mg/kg b.wt/day L-carnitine—200 and 400 mg/kg b.wt for 21 days | Reproductive toxicity and genotoxicity | Anti-oxidant effects |
| Abarikwu et al., 2016 [183] | Rats | 120 mg/kg b.wt./day for 16 days | Quercetin—10 mg/kg b.wt./day for 16 days | Testicular toxicity | Anti-oxidant effects |
| Farombi et al., 2013 [184] | Rats | 120 mg/kg/b.wt./day for 16 day | Quercetin—20 mg/kg/b.wt/day for 16 days | Testicular toxicity | Anti-oxidant effects |
| Abarikwu et al., 2022 [192] | Rats | 50 mg/kg b.wt./day 60 days | Fluted pumpkin seeds extract—25 and 50 mg/kg b.wt/day for 60 days | Testicular toxicity | Antioxidant activity |
| Singh et al., 2008 [199] | Rats | 300 mg/kg b.wt./day for 7,14 and 21 days | Vitamin E—100 mg/kg b.wt/day 7, 14, and 21 days | Genotoxicity | Antioxidant activity |
| Agdam et al., 2017 [201] | Rats | 200 mg/kg b.wt./day for 22 and 48 days | Vitamin E—150 mg/kg/b.wt/day | Testicular toxicity | Promoting antioxidant capacity and endocrine function |
| Griboff et al., 2014 [202] | Shrimp Palaemonetes argentinus | 0.4 mg/L for 21 days | Vitamin E—(16 mg%) for 21 days | Oxidative stress | Antioxidant effects |
| Bhatti et al., 2011 [219] | Rats | 300 mg/kg of bw/day for 21 days | Melatonin—10 mg/kg bw/day for 21 days | Erythrocytes toxicity | Antioxidant effects |
| Sharma et al., 2014 [220] | Mice | 100 mg/kg b.wt./day for 14 days | Melatonin—20 mg/kg b.wt/day for 14 days | Immunotoxicity | Suppression of endoplasmic reticulum stress, Fas-mediated and p53 independent mitochondria-mediated apoptosis and autophagy modulation |
| Rashad et al., 2023 [227] | Rats | 400 mg/kg b.wt./day for 14 days | L-Carnitine—100 mg/kg b.wt/day for 14 days | Hepatotoxicity | Antioxidant, anti-inflammatory, and anti-apoptosis activities |
| Adesiya et al., 2011 [25] | Rat | 120 mg/kg b.wt./day for 16 days | Selenium—0.25 mg/kg b.wt/day for 16 days | Hepatotoxicity | Antioxidant effects |
| Marins et al., 2018 [237] | Fish | 2 or 10 µg/L for 96 h | Selenium compound diphenyl diselenide (PhSe)2 containing diet—3 mg/kg b.wt/day | Hepatotoxicity and reproductive toxicity | Antioxidant effects |
| Ali et al., 2021 [244] | Nile tilapia fish | 1.39 mg/L for 30 days | Isatis diet—1% for 30 days | Hepatotoxicity and renal toxicity | Antioxidant effects |
| Komsky-Elbaz et al., 2019 [252] | Male goat (Capra hircus) | 15 mg/kg b.wt./day for 6 months | Polyphenol A— standard ration for 90 days | Testicular toxicity | Antioxidant effects |
| Ahmed et al., 2022 [56] | Rats | 200 mg/kg b.wt/day for 30 days | Acacia nilotica—400 mg/kg/day for 30 days | Hepatotoxicity, neurotoxicity and genotoxicity | Antioxidant effects |
| El-Shenawy et al., 2011 [264] | Mice | 78.25 mg/kg b.wt./day for 14 days | Ginger extract—120 mg/kg b.wt for 14 days | Hepatotoxicity and renal toxicity | Antioxidant effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Sakr, H.; Al-Huseini, I.; Jetti, R.; Al-Qasmi, S.; Sugavasi, R.; Sirasanagandla, S.R. Atrazine Toxicity: The Possible Role of Natural Products for Effective Treatment. Plants 2023, 12, 2278. https://doi.org/10.3390/plants12122278
Das S, Sakr H, Al-Huseini I, Jetti R, Al-Qasmi S, Sugavasi R, Sirasanagandla SR. Atrazine Toxicity: The Possible Role of Natural Products for Effective Treatment. Plants. 2023; 12(12):2278. https://doi.org/10.3390/plants12122278
Chicago/Turabian StyleDas, Srijit, Hussein Sakr, Isehaq Al-Huseini, Raghu Jetti, Sara Al-Qasmi, Raju Sugavasi, and Srinivasa Rao Sirasanagandla. 2023. "Atrazine Toxicity: The Possible Role of Natural Products for Effective Treatment" Plants 12, no. 12: 2278. https://doi.org/10.3390/plants12122278
APA StyleDas, S., Sakr, H., Al-Huseini, I., Jetti, R., Al-Qasmi, S., Sugavasi, R., & Sirasanagandla, S. R. (2023). Atrazine Toxicity: The Possible Role of Natural Products for Effective Treatment. Plants, 12(12), 2278. https://doi.org/10.3390/plants12122278











