Biocontrol Efficacy of Endophyte Pseudomonas poae to Alleviate Fusarium Seedling Blight by Refining the Morpho-Physiological Attributes of Wheat
Abstract
1. Introduction
2. Results
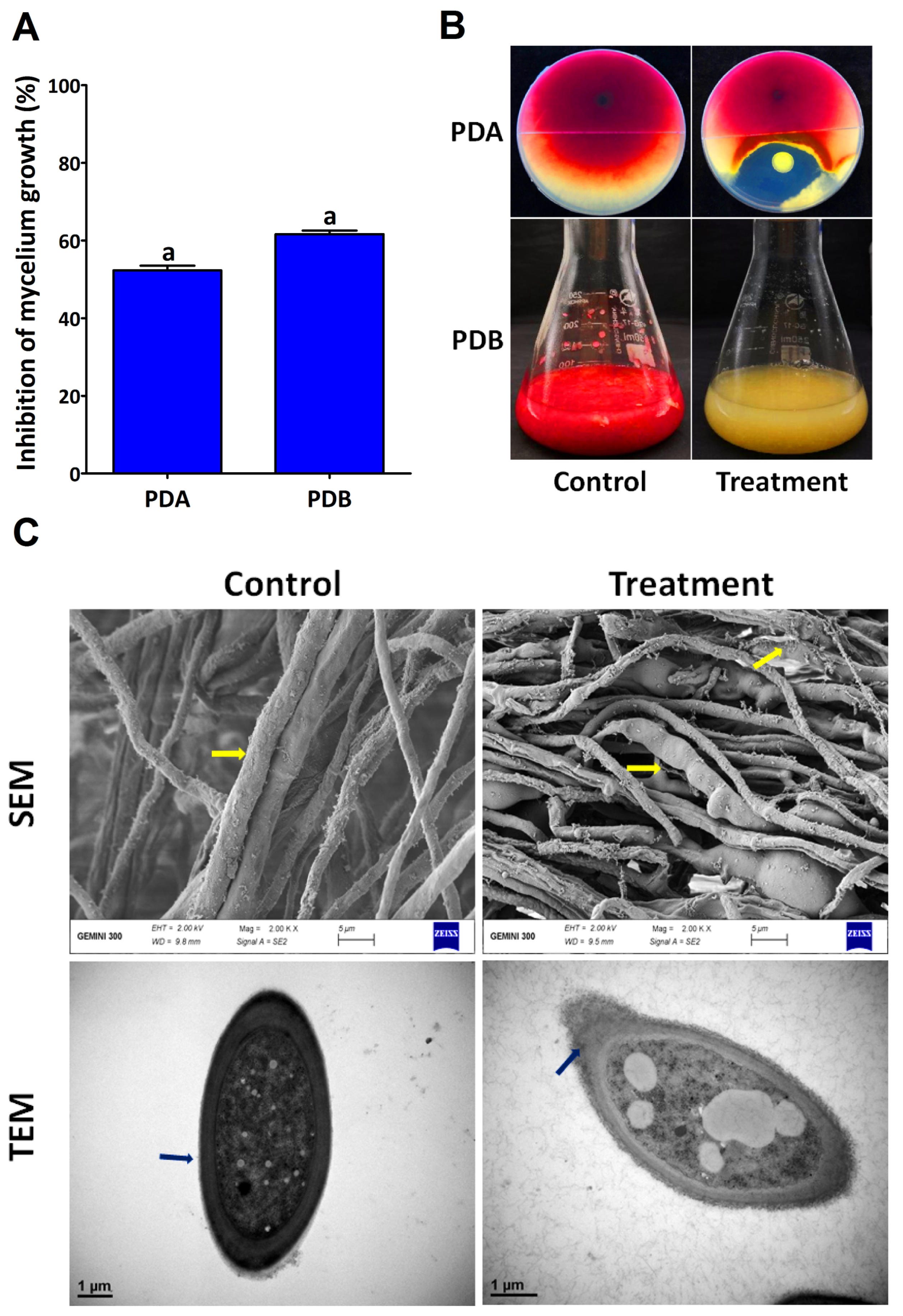
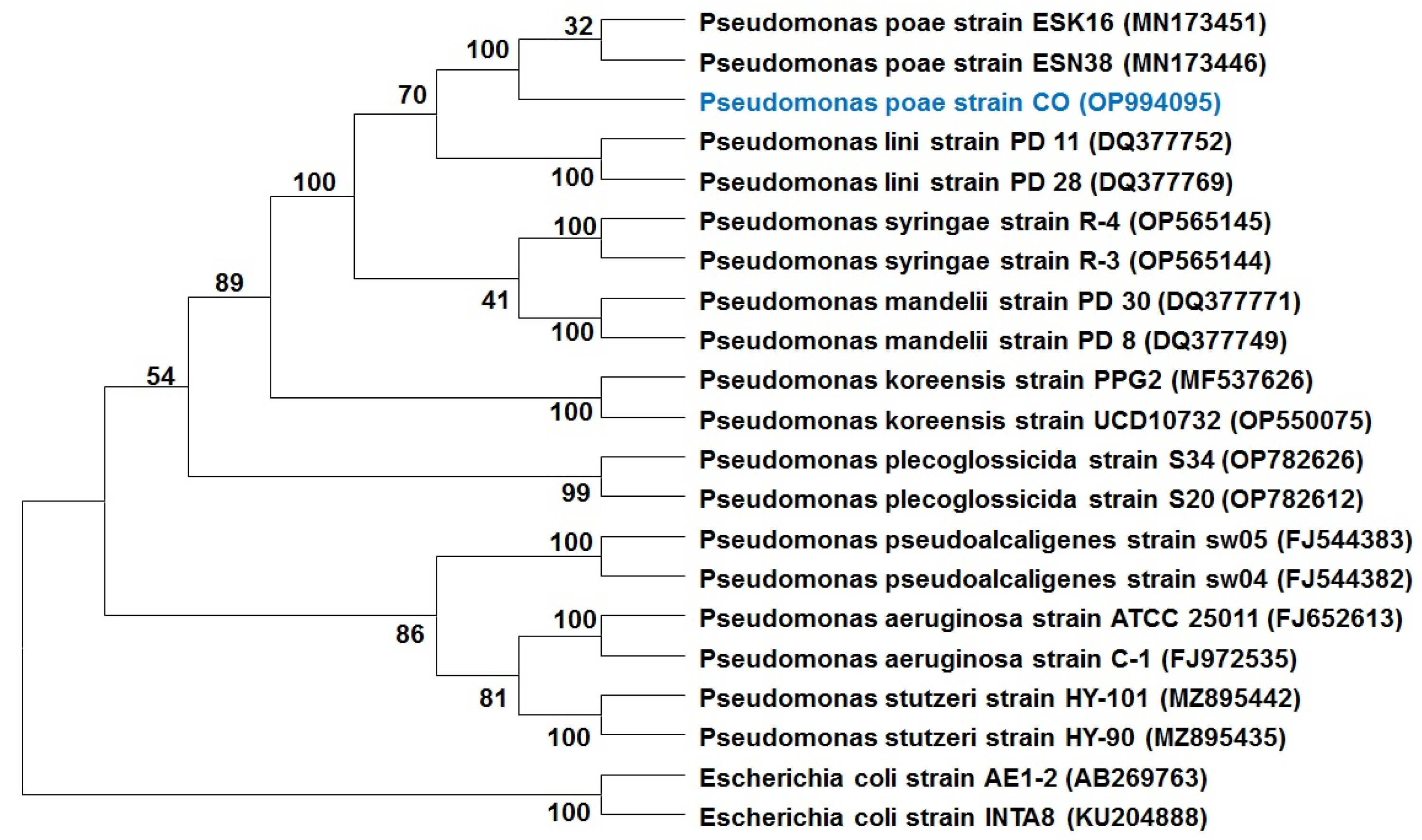
2.1. In Vitro Antifungal Activity and Identification of the Endophytic Bacterium
2.2. Suppression of FSB under Greenhouse Conditions
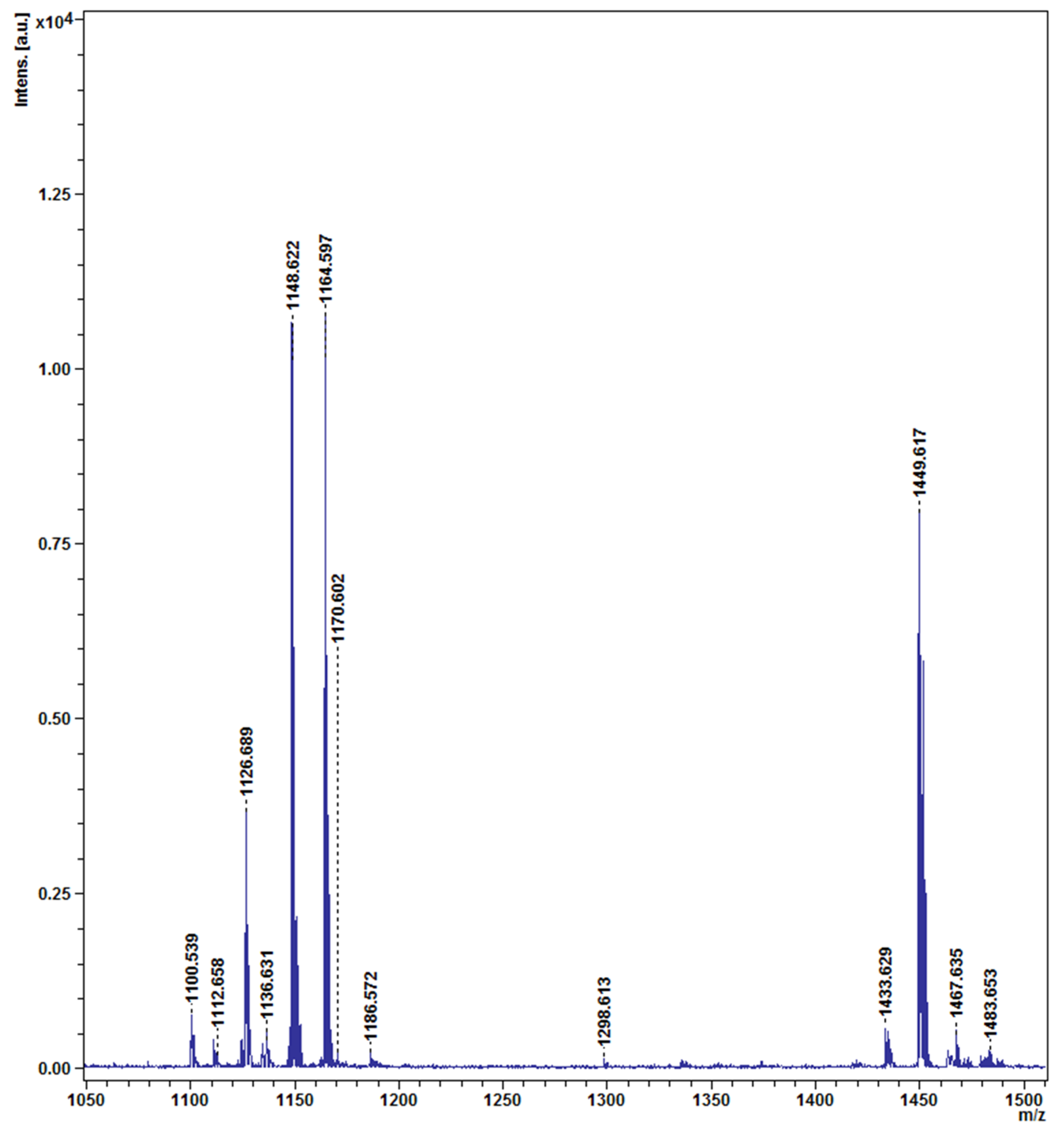
2.3. Antifungal Mechanism
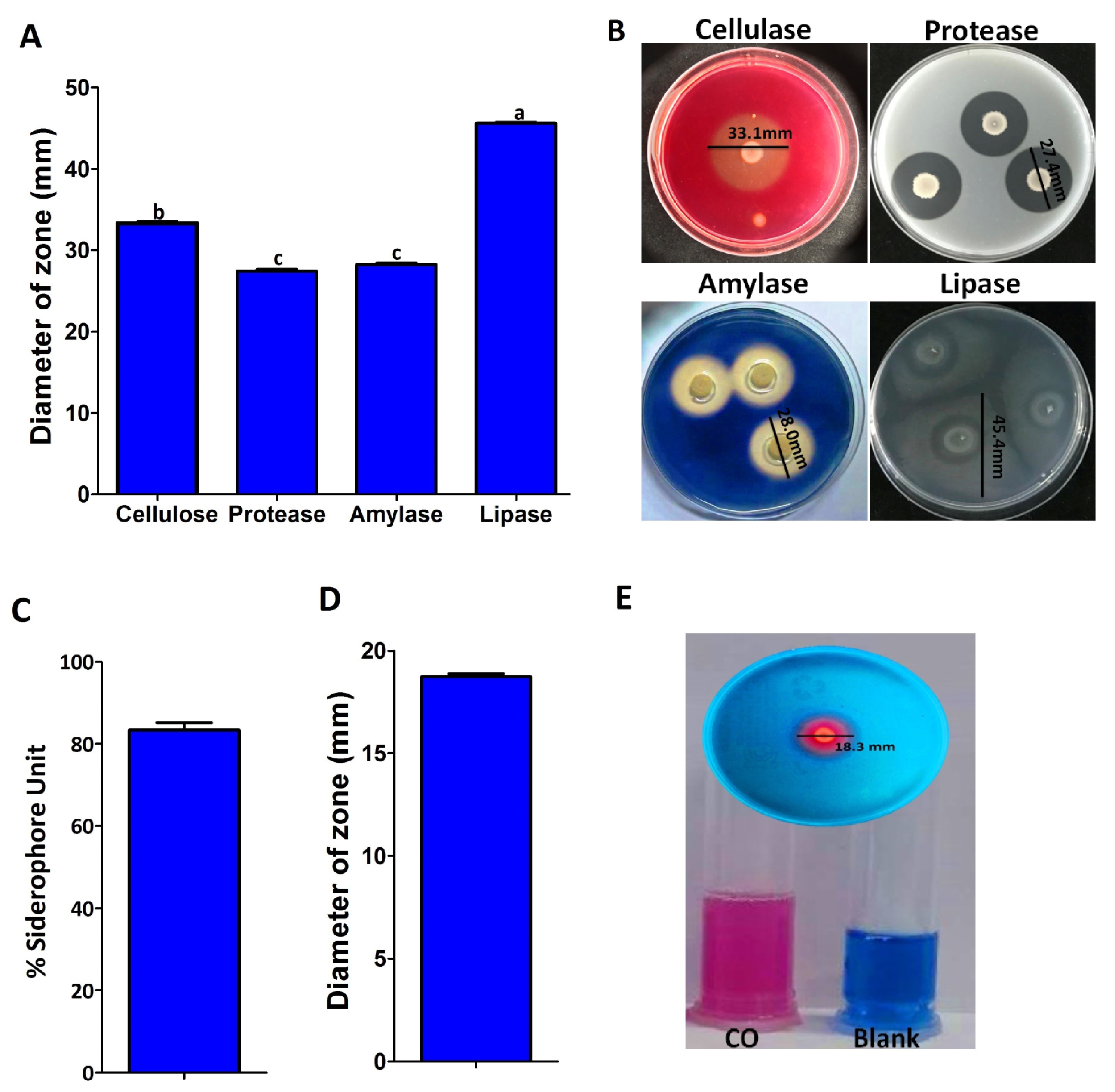
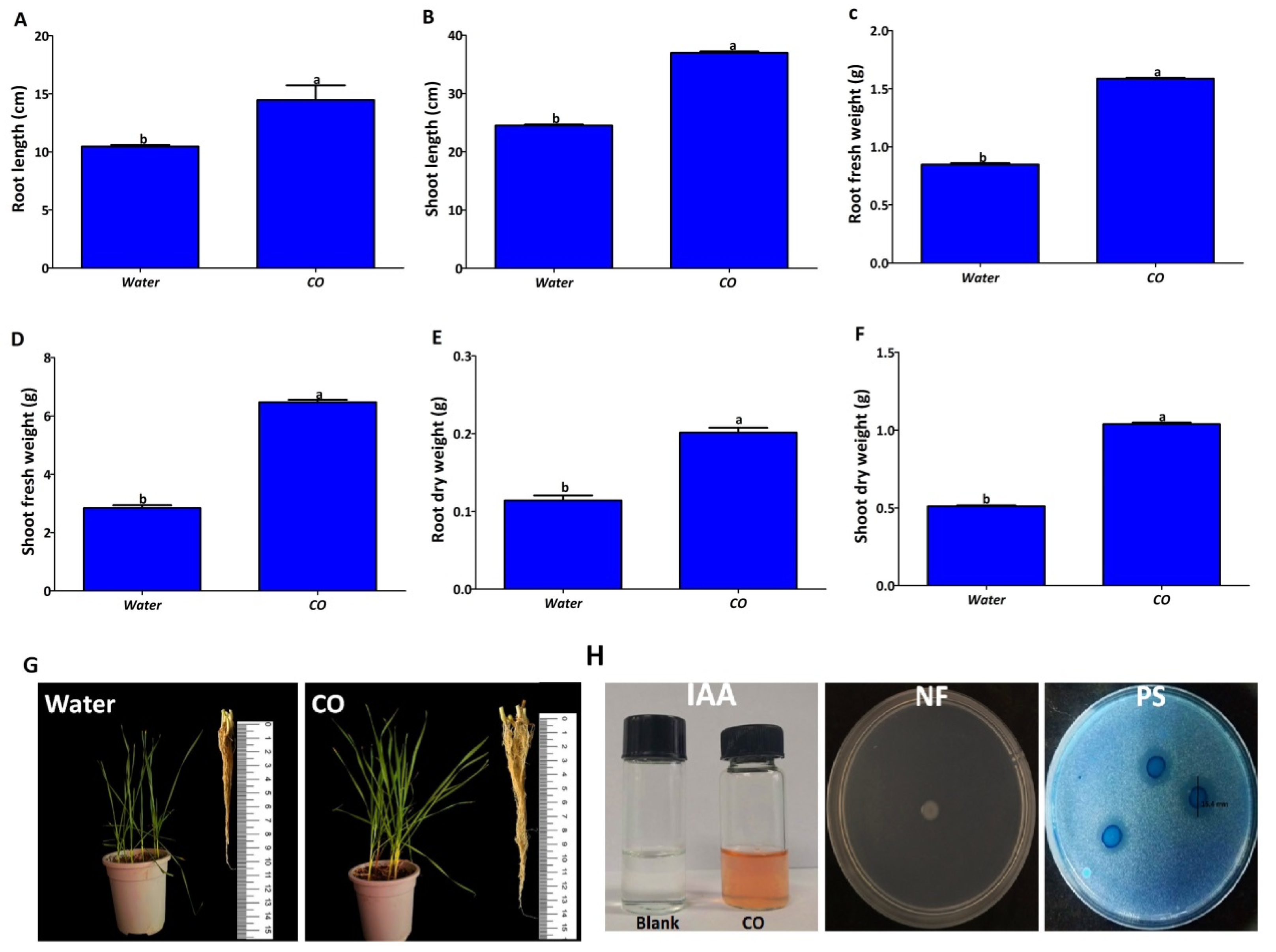
2.4. Improvement of Wheat Seedling Growth and Growth Promotion Properties
3. Discussion
4. Materials and Methods
4.1. Isolation of Endophytic Bacteria
4.2. Identification of Endophytic Bacterium
4.3. In Vitro Antifungal Activity Assay
4.3.1. Inhibition of Mycelium Growth in Solid Medium
4.3.2. Inhibition of Mycelium Growth in Liquid Culture
4.3.3. Cell Wall Morphology
4.4. Antifungal Activity of Endophytic Bacterial Metabolites against Strain PH-1
4.4.1. Preparation of Cell-Free Supernatants
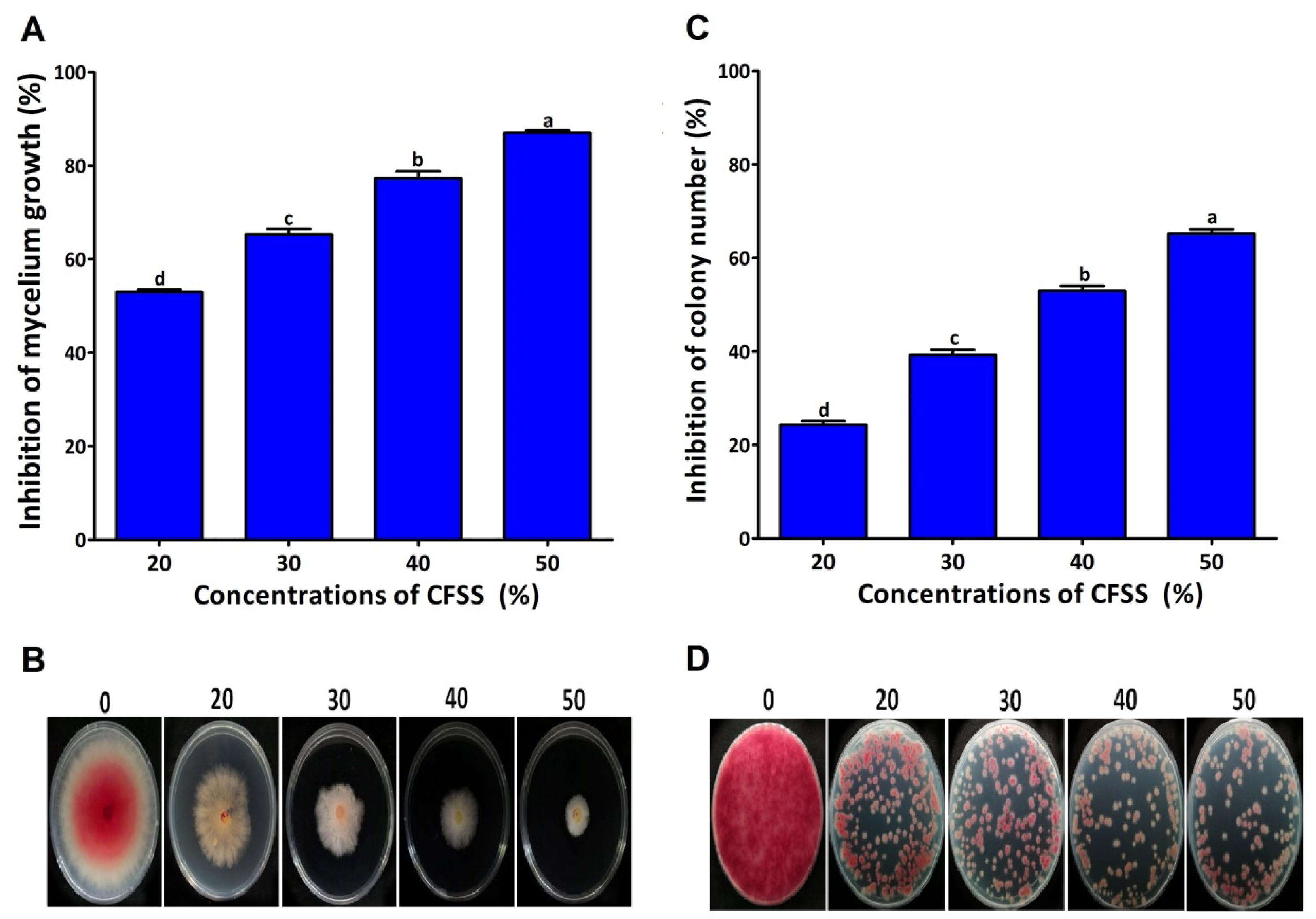
4.4.2. Effect of CFSs of P. poae on Mycelium Growth
4.4.3. Inhibition of Fungal Colony Number
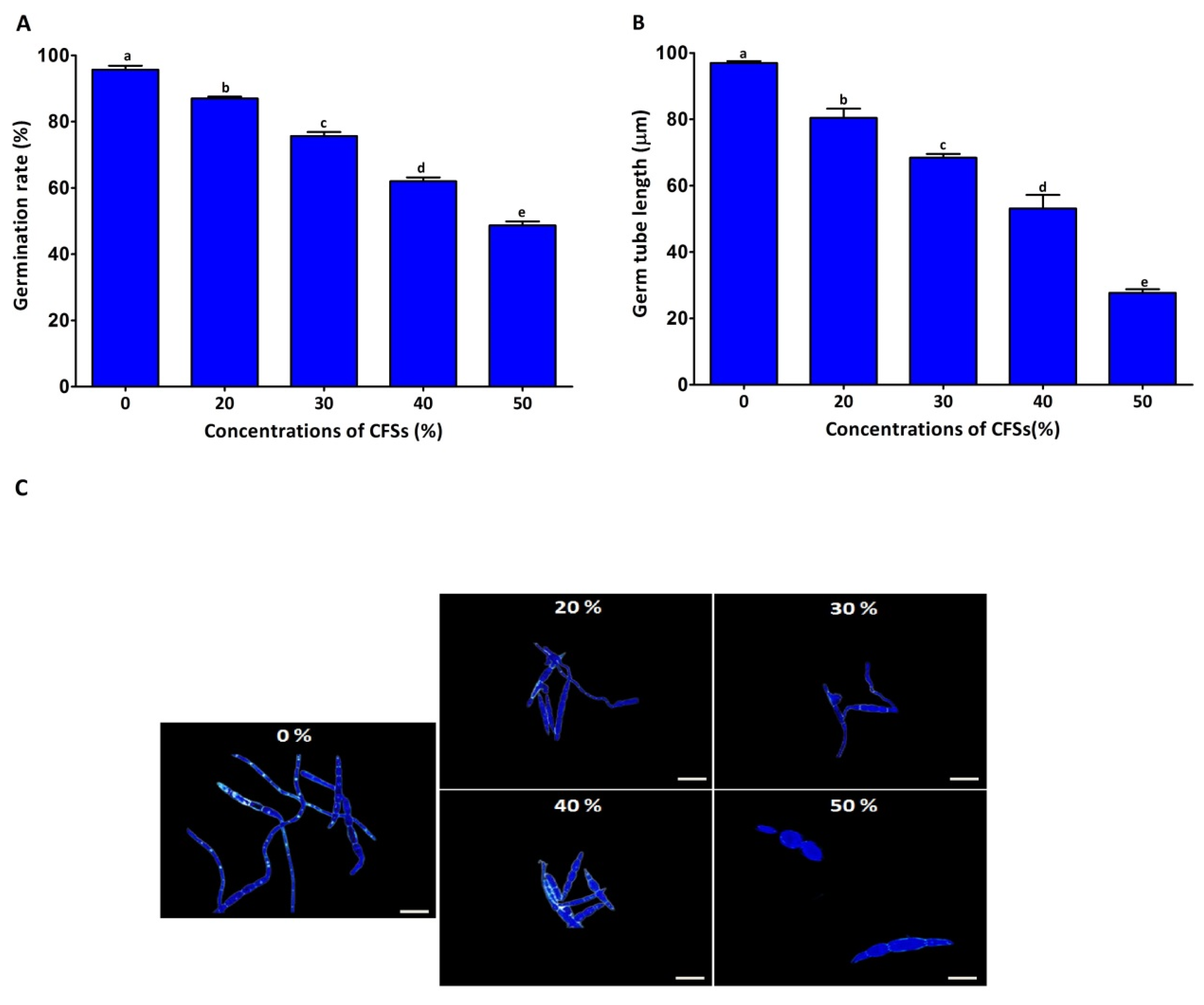
4.4.4. Effect of CFS on Spore Germination and Length of Germ Tubes
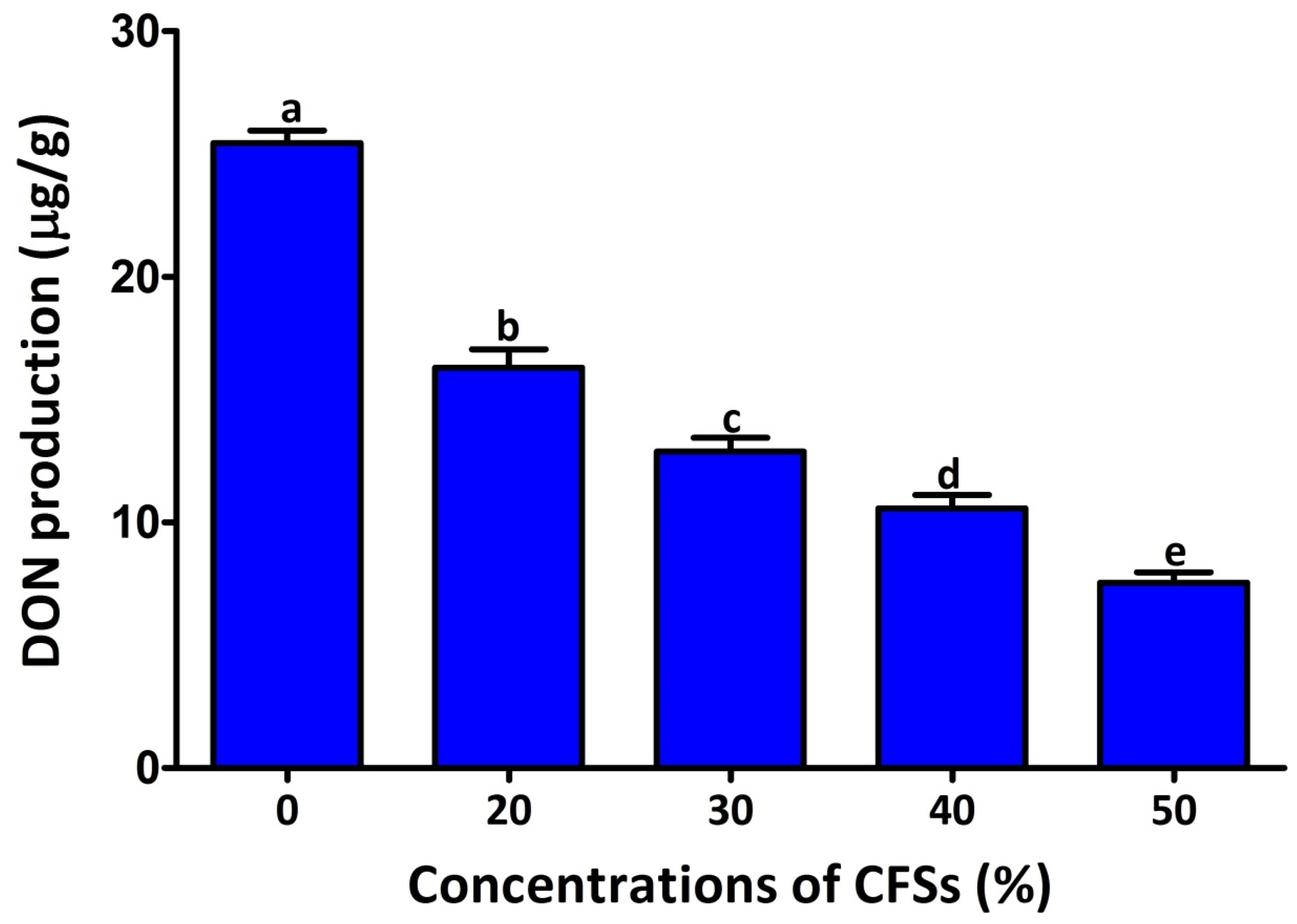
4.4.5. Effect of CFSs on Deoxynivalenol Production
4.5. Seedling Protection from FSB
4.6. Antifungal Properties of Endophytic Bacterium
4.6.1. Protease Activity
4.6.2. Amylase Activity
4.6.3. Cellulolytic Activity
4.6.4. Lipolytic Activity
4.6.5. Siderophores Assay
4.6.6. Detection of Lipopeptides Produced from CO Cells
4.7. Improve Seedling Growth and Plant Growth-Promoting Properties
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miransari, M.; Smith, D. Sustainable wheat (Triticum aestivum L.) production in saline fields: A review. Crit. Rev. Biotechnol. 2019, 39, 999–1014. [Google Scholar] [CrossRef]
- Teli, B.; Jyotika, P.; Md, M.; Rashid, A.; Abdul, K.J.; Anirudha, C. Omics insight on Fusarium head blight of wheat for translational research perspective. Curr. Genomics. 2020, 21, 411–428. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, S.; Li, X.; Xu, J.-R.; Schultzhaus, Z.; Jin, Q.A. Gin4-like protein kinase GIL1 involvement in hyphal growth, asexual development, and pathogenesis in Fusarium graminearum. Int. J. Mol. Sci. 2017, 18, 424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, G.; Zhu, Z.; Chen, L.; Niu, H.; He, W.; Tong, H.; Song, J.; Zhang, Y.; Ma, D. Investigation and Genome-Wide Association Analysis of Fusarium Seedling Blight Resistance in Chinese Elite Wheat Lines. Front Plant Sci. 2021, 12, 777. [Google Scholar] [CrossRef]
- Abbas, A.; Yli-Mattila, T. Biocontrol of Fusarium graminearum, a causal agent of Fusarium head blight of wheat, and deoxynivalenol accumulation: From in vitro to in planta. Toxins 2022, 22, 299. [Google Scholar] [CrossRef]
- Tang, X.; Yangjing, G.; Zhuoma, G.; Guo, X.; Cao, P.; Yi, B.; Wang, W.; Ji, D.; Pasquali, M.; Baccelli, I.; et al. Biological characterization and in vitro fungicide screenings of a new causal agent of wheat Fusarium head blight in Tibet, China. Front. Microbiol. 2022, 13, 941734. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wan, J.; Lu, J.; Wang, X.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Chitosan coatings on lecithin stabilized emulsions inhibit mycotoxin production by Fusarium pathogens. Food Control. 2018, 92, 276–285. [Google Scholar] [CrossRef]
- Lakshmeesha, T.R.; Kalagatur, N.K.; Mudili, V.; Mohan, C.D.; Rangappa, S.; Prasad, B.D.; Ashwini, B.S.; Hashem, A.; Alqarawi, A.A.; Malik, J.A. Biofabrication of zinc oxide nanoparticles with Syzygium aromaticum flower buds extract and finding its novel application in controlling the growth and mycotoxins of Fusarium graminearum. Front. Microbiol. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Dunlap, C.A.; Bowman, M.J.; Chulze, S.N. Bacillus velezensis RC 218 as a biocontrol agent to reduce Fusarium head blight and deoxynivalenol accumulation: Genome sequencing and secondary metabolite cluster profiles. Microbiol Res. 2016, 192, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Heng, J.; Qin, S.; Bian, K. A comprehensive understanding of the biocontrol potential of Bacillus velezensis LM2303 against Fusarium head blight. PLoS ONE 2018, 13, e0198560. [Google Scholar] [CrossRef]
- Udayashankar, A.; Nayaka, S.C.; Reddy, M.; Srinivas, C. Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control. 2011, 59, 114–122. [Google Scholar]
- Di, Y.N.; Kui, L.; Singh, P.; Liu, L.F.; Xie, L.Y.; He, L.L.; Li, F.S. Identification and characterization of Bacillus subtilis B9: A diazotrophic plant growth-promoting endophytic bacterium isolated from sugarcane root. J. Plant Growth Regul. 2022, 7, 1720–1737. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; AbdAllah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Chinnaswamy, A.; Coba De La Peña, T.; Stoll, A.; De La Peña Rojo, D.; Bravo, J.; Rincón, A.; Lucas, M.; Pueyo, J. A nodule endophytic Bacillus megaterium strain isolated from Medicago polymorpha enhances growth, promotes nodulation by Ensifer medicae and alleviates salt stress in alfalfa plants. Ann. Appl. Biol. 2018, 172, 295–308. [Google Scholar] [CrossRef]
- Pathak, P.; Rai, V.K.; Can, H.; Singh, S.K.; Kumar, D.; Bhardwaj, N.; Roychowdhury, R.; de Azevedo, L.C.; Kaushalendra, V.H. Plant-endophyte interaction during biotic stress management. Plants 2022, 11, 2203. [Google Scholar] [CrossRef] [PubMed]
- Ansary, W.R.; Prince, F.R.K.; Haque, E.; Sultana, F.; West, H.M.; Rahman, M.; Mondol, A.M.; Akanda, A.M.; Rahman, M.; Clarke, M.L. Endophytic Bacillus spp. from medicinal plants inhibit mycelial growth of Sclerotinia sclerotiorum and promote plant growth. Z Naturforsch. 2018, 73, 247–256. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.; Bergen, M.; English, C.; Elmore, M.; Kharwar, R.N.; White, J.F. Bacterial endophytes from rice cut grass (Leersia oryzoides L.) increase growth, promote root gravitropic response, stimulate root hair formation, and protect rice seedlings from disease. Plant Soil 2018, 422, 223–238. [Google Scholar] [CrossRef]
- Chen, B.; Luo, S.; Wu, Y.; Ye, J.; Wang, Q.; Xu, X.; Pan, F.; Khan, K.Y.; Feng, Y.; Yang, X. The effects of the endophytic bacterium Pseudomonas fluorescens Sasm05 and IAA on the plant growth and cadmium uptake of Sedum alfredii Hance. Front. Microbiol. 2017, 8, 2538. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, M.; Kumar, A.; Singh, A.; Pandey, K. Endophytic bacteria in plant disease management. In Microbial endophytes; Woodhead Publishing: Soston, UK, 2020; pp. 61–89. [Google Scholar]
- Ali, M.A.; Luo, J.; Ahmed, T.; Zhang, J.; Xie, T.; Dai, D.; Jiang, J.; Zhu, J.; Hassan, S.; Alorabi, J.A. Pseudomonas bijieensis strain XL17 within the P. corrugata subgroup producing 2, 4-diacetylphloroglucinol and lipopeptides controls bacterial canker and gray mold pathogens of kiwifruit. Microorganisms. 2022, 10, 425. [Google Scholar] [CrossRef]
- Xia, W.; Du, Z.; Cui, Q.; Dong, H.; Wang, F.; He, P.; Tang, Y. Biosurfactant produced by novel Pseudomonas sp. WJ6 with biodegradation of n-alkanes and polycyclic aromatic hydrocarbons. J. Hazard Mater. 2014, 276, 489–498. [Google Scholar]
- Yin, J.; Zhang, X.; Ma, D.; Lu, C.; He, Y.; Zhao, M.; Zhu, Y. A study on the sensitivity and synergistic effect of prochloraz and cypermethrin on Gibberella zeae in wheat. Acta Agric. Univ. Jiangxiensis 2018, 40, 920–924. [Google Scholar]
- Xia, Y.; Zhu, Y.; Ma, D.; Liu, L.; Yin, J. Isolation and identification of antagonistic strain of Fusarium graminearum. Acta Agric. Univ. Jiangxiensis 2019, 41, 33–42. [Google Scholar]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens. Microorganisms 2022, 9, 596. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Hu, X.; Ji, F.; Shi, J. Isolation, identification of antagonistic bacteria AF0907 against Fusarium graminearum and its characteristics. Jiangsu J. Agric. Sci. 2013, 29, 517–522. [Google Scholar]
- Zhang, P.; Zhu, Y.; Ma, D.; Xu, W.; Zhou, J.; Yan, H.; Yang, L.; Yin, J. Screening, identification, and optimization of fermentation conditions of an antagonistic endophyte to wheat head blight. Agronomy 2019, 9, 476. [Google Scholar] [CrossRef]
- Zachow, C.; Jahanshah, G.; de Bruijn, I.; Song, C.; Ianni, F.; Pataj, Z.; Gerhardt, H.; Pianet, I.; Lämmerhofer, M.; Berg, G. The novel lipopeptide poaeamide of the endophyte Pseudomonas poae RE* 1-1-14 is involved in pathogen suppression and root colonization. Mol. Plant. Microbe Interact. 2015, 28, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms-A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Hernández-León, R.; González-Rodríguez, A.; Tapia-Torres, Y. Phosphorus Recycling, Biocontrol, and Growth Promotion Capabilities of Soil Bacterial Isolates from Mexican Oak Forests: An Alternative to Reduce the Use of Agrochemicals in Maize Cultivation. Appl. Microbiol. 2022, 2, 965–980. [Google Scholar] [CrossRef]
- Ren, Y.; Yao, M.; Chang, P.; Sun, Y.; Li, R.; Meng, D.; Xia, X.; Wang, Y. Isolation and characterization of a Pseudomonas poae JSU-Y1 with patulin degradation ability and biocontrol potential against Penicillium expansum. Toxicon 2021, 195, 1–6. [Google Scholar] [CrossRef]
- Dagher, F.; Nickzad, A.; Zheng, J.; Hoffmann, M.; Déziel, E. Characterization of the biocontrol activity of three bacterial isolates against the phytopathogen Erwinia amylovora. Microbiologyopen 2021, 10, e1202. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Potential for control of seedling blight of wheat caused by Fusarium graminearum and related species using the bacterial endophyte Bacillus mojavensis. Biocontrol Sci. Technol. 2007, 17, 81–94. [Google Scholar] [CrossRef]
- Hao, Q.; Albaghdady, D.M.D.; Xiao, Y.; Xiao, X.; Mo, C.; Tian, T.; Wang, G. Endophytic Metarhizium anisopliae is a potential biocontrol agent against wheat Fusarium head blight caused by Fusarium graminearum. JPP 2021, 10, 875–885. [Google Scholar] [CrossRef]
- da Silva Ribeiro, A.; Polonio, J.C.; Costa, A.T.; Dos Santos, C.M.; Rhoden, S.A.; Azevedo, J.L.; Pamphile, J.A. Bioprospection of culturable endophytic fungi associated with the ornamental plant Pachystachys lutea. Curr. Microbiol. 2018, 75, 588–596. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Alsohim, A.S.; Taylor, T.B.; Barrett, G.A.; Gallie, J.; Zhang, X.X.; Altamirano-Junqueira, A.E.; Johnson, L.J.; Rainey, P.B.; Jackson, R.W. The biosurfactant viscosin produced by Pseudomonas fluorescens SBW 25 aids spreading motility and plant growth promotion. Environ. Microbiol. 2014, 16, 2267–2281. [Google Scholar] [CrossRef]
- Nielsen, T.; Sørensen, D.; Tobiasen, C.; Andersen, J.B.; Christophersen, C.; Givskov, M.; Sørensen, J. Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl. Environ. Microbiol. 2002, 68, 3416–3423. [Google Scholar] [CrossRef]
- Firdous, J.; Subhash, J.B. Screening of cultivable endophytic bacterial isolates for their plant growth promoting activity in rice. Indian J. Agric. Sci. 2017, 51, 413–418. [Google Scholar] [CrossRef]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.M.; Devaraj, S.; Benson, A.; Sa, T. Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of plant growth promotion and antioxidant activity under salt stress. J. Appl. Res. Med. Aromat. Plants 2016, 3, 71–77. [Google Scholar]
- William, D.T.; Ivan, A.P.; Klaus, P. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef]
- Gupta, G.; Panwar, J.; Akhtar, M.S.; Jha, P.N. Endophytic nitrogen-fixing bacteria as biofertilizer. Sustain. Agric. Rev. 2012, 11, 183–221. [Google Scholar]
- James, E.K.; Olivares, F.L. Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. CRC Crit. Rev. Plant Sci. 1998, 17, 77–119. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Mei, Y.; Wang, L.; Wang, X.; Xu, Q.; Peng, S.; Zhou, Y.; Wei, C. Isolation, diversity, and growth-promoting activities of endophytic bacteria from tea cultivars of Zijuan and Yunkang-10. Front. Microbiol. 2018, 9, 1848. [Google Scholar] [CrossRef]
- Masum, M.; Liu, L.; Yang, M.; Hossain, M.; Siddiqa, M.; Supty, M.; Ogunyemi, S.; Hossain, A.; An, Q.; Li, B. Halotolerant bacteria belonging to operational Bacillus amyloliquefaciens in biocontrol of the rice brown stripe pathogen Acidovorax oryzae. J. Appl. Microbiol. 2018, 125, 1852–1867. [Google Scholar] [CrossRef] [PubMed]
- Vinayarani, G.; Prakash, H. Growth promoting rhizospheric and endophytic bacteria from Curcuma longa L. as biocontrol agents against rhizome rot and leaf blight diseases. Plant Pathol. J. 2018, 34, 218. [Google Scholar] [CrossRef]
- Ibrahim, E.; Luo, J.; Ahmed, T.; Wu, W.; Yan, C.; Li, B. Biosynthesis of silver nanoparticles using onion endophytic bacterium and its antifungal activity against rice pathogen Magnaporthe oryzae. J. Fungi 2020, 6, 294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Liu, J.; Xu, L.; Ji, P.; Sun, L.; Pan, H.; Jiang, B.; Li, L. Identification of a biocontrol agent Bacillus vallismortis BV23 and assessment of effects of its metabolites on Fusarium graminearum causing corn stalk rot. Biocontrol Sci. Technol. 2019, 29, 263–275. [Google Scholar] [CrossRef]
- Kheiri, A.; Jorf, S.M.; Malihipour, A.; Saremi, H.; Nikkhah, M. Application of chitosan and chitosan nanoparticles for the control of Fusarium head blight of wheat (Fusarium graminearum) in vitro and greenhouse. Int. J. Biol. Macromo. 2016, 93, 1261–1272. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef]
- Wu, D.; Lu, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Influence of nonionic and ionic surfactants on the antifungal and mycotoxin inhibitory efficacy of cinnamon oil nanoemulsions. Food Funct. 2019, 10, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Yasmin, H.; Mumtaz, S.; Jabeen, Z.; Naz, R.; Nosheen, A.; Hassan, M.N. Multitrait Pseudomonas spp. isolated from monocropped wheat (Triticum aestivum) suppress Fusarium root and crown rot. Phytopathology 2020, 110, 582–592. [Google Scholar] [CrossRef]
- Shin, S.; Kim, K.-H.; Kang, C.-S.; Cho, K.-M.; Park, C.S.; Okagaki, R.; Park, J.C. A simple method for the assessment of Fusarium head blight resistance in Korean wheat seedlings inoculated with Fusarium graminearum. Plant Pathol. J. 2014, 30, 25. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.-J.; Tang, H.-Y.; Wang, W.-Q.; Yuan, T.-L.; Wei, W.-Q.; Pang, B.; Gong, X.-M.; Wang, S.-F.; Li, Y.-J.; Zhang, D. A linear nonribosomal octapeptide from Fusarium graminearum facilitates cell-to-cell invasion of wheat. Nat. Commun. 2019, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Ntabo, R.M.; Nyamache, A.K.; Lwande, W.; Kabii, J.; Nonoh, J. Enzymatic activity of endophytic bacterial isolates from selected mangrove plants in Kenya. Open Microbiol. J. 2018, 12, 354–363. [Google Scholar] [CrossRef]
- Bjelić, D.; Marinković, J.; Tintor, B.; Mrkovački, N. Antifungal and plant growth promoting activities of indigenous rhizobacteria isolated from maize (Zea mays L.) rhizosphere. Soil Sci. Plant Anal. 2018, 49, 88–98. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.L.; Bergen, M.S.; Kowalski, K.P.; White, J.F. Fungal disease prevention in seedlings of rice (Oryza sativa) and other grasses by growth-promoting seed-associated endophytic bacteria from invasive Phragmites australis. Microorganisms 2018, 6, 21. [Google Scholar] [CrossRef]
- Rajkumar, M.; Nagendran, R.; Lee, K.J.; Lee, W.H.; Kim, S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006, 62, 741–748. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Diksha, V.; Thakur, K.; Gupta, C.A. study on nitrogen fixing ability of soil isolates and quantitative estimation of K solubilization by soil and plant isolates. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2641–2647. [Google Scholar]

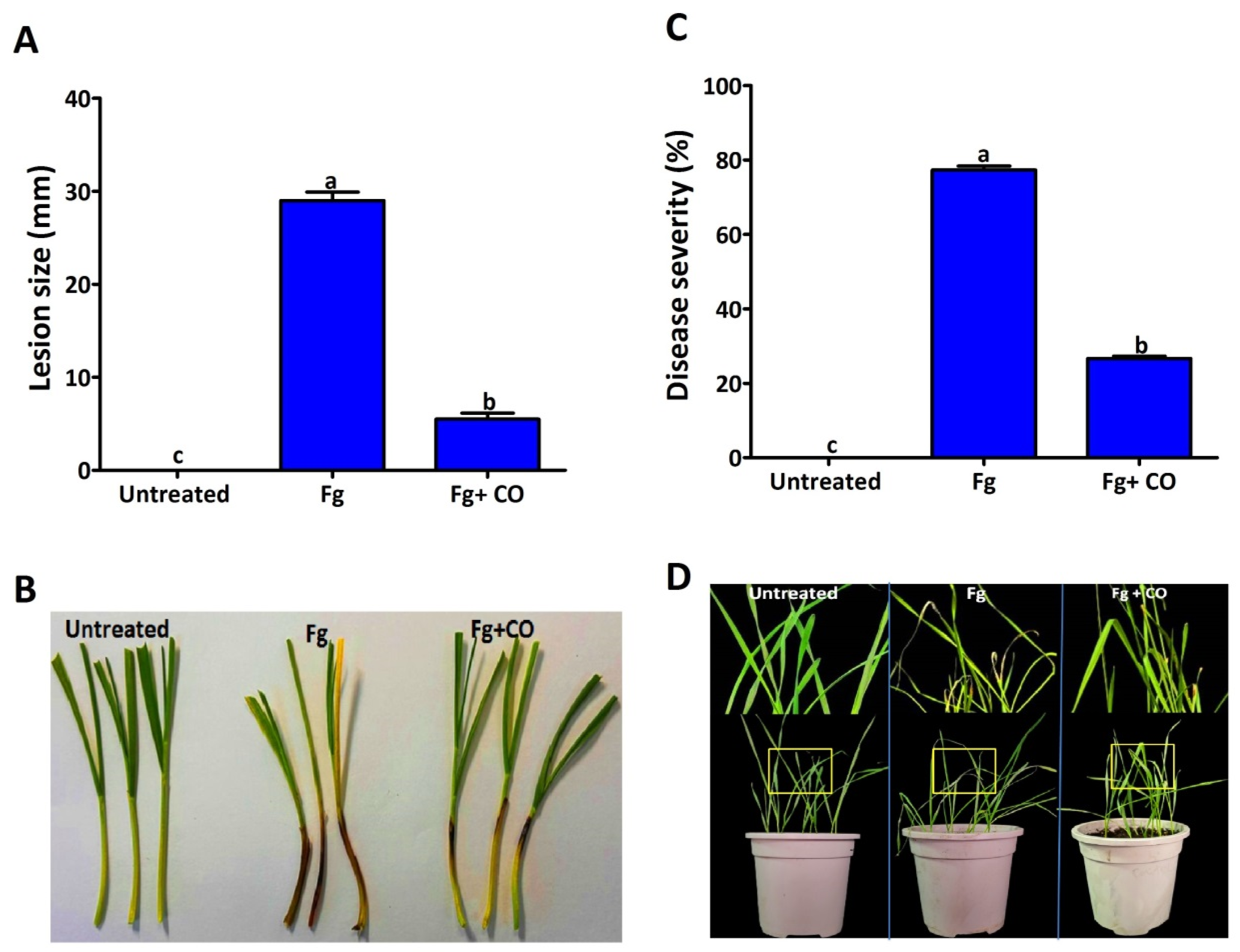
| Treatment | Disease Assessment and Growth Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sg (%) | Pre (%) | Post (%) | Rl (cm) | Sl (cm) | Fw (g) | Dw(g) | Slvi | Swvi | |
| Control | 93.33 ± 1.66 a | 6.66 ± 2.88 a | 0.00 ± 0.00 a | 5.27 ± 0.11 a | 8.90 ± 0.26 a | 0.82 ± 0.04 a | 0.19 ± 0.01 a | 1322.00 ± 7.22 a | 17.68 ± 0.35 a |
| Fg + CO | 70.00 ± 2.88 b | 30.00 ± 5.00 b | 7.10 ± 5.00 b | 3.50 ± 0.10 b | 4.90 ± 0.56 b | 0.55 ± 0.01 b | 0.08 ± 0.00 b | 602.67 ± 16.11 b | 5.24 ± 0.45 b |
| Fg | 15.00 ± 2.88 c | 85.00 ± 5.00 c | 80.33 ± 17.61 c | 1.47 ± 0.49 c | 2.00 ± 0.22 c | 0.21 ± 0.01 c | 0.03 ± 0.00 c | 52.97 ± 1.05 c | 0.35 ± 7.74 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, E.; Nasser, R.; Hafeez, R.; Ogunyemi, S.O.; Abdallah, Y.; Khattak, A.A.; Shou, L.; Zhang, Y.; Ahmed, T.; Atef Hatamleh, A.; et al. Biocontrol Efficacy of Endophyte Pseudomonas poae to Alleviate Fusarium Seedling Blight by Refining the Morpho-Physiological Attributes of Wheat. Plants 2023, 12, 2277. https://doi.org/10.3390/plants12122277
Ibrahim E, Nasser R, Hafeez R, Ogunyemi SO, Abdallah Y, Khattak AA, Shou L, Zhang Y, Ahmed T, Atef Hatamleh A, et al. Biocontrol Efficacy of Endophyte Pseudomonas poae to Alleviate Fusarium Seedling Blight by Refining the Morpho-Physiological Attributes of Wheat. Plants. 2023; 12(12):2277. https://doi.org/10.3390/plants12122277
Chicago/Turabian StyleIbrahim, Ezzeldin, Raghda Nasser, Rahila Hafeez, Solabomi Olaitan Ogunyemi, Yasmine Abdallah, Arif Ali Khattak, Linfei Shou, Yang Zhang, Temoor Ahmed, Ashraf Atef Hatamleh, and et al. 2023. "Biocontrol Efficacy of Endophyte Pseudomonas poae to Alleviate Fusarium Seedling Blight by Refining the Morpho-Physiological Attributes of Wheat" Plants 12, no. 12: 2277. https://doi.org/10.3390/plants12122277
APA StyleIbrahim, E., Nasser, R., Hafeez, R., Ogunyemi, S. O., Abdallah, Y., Khattak, A. A., Shou, L., Zhang, Y., Ahmed, T., Atef Hatamleh, A., Abdullah Al-Dosary, M., M. Ali, H., Luo, J., & Li, B. (2023). Biocontrol Efficacy of Endophyte Pseudomonas poae to Alleviate Fusarium Seedling Blight by Refining the Morpho-Physiological Attributes of Wheat. Plants, 12(12), 2277. https://doi.org/10.3390/plants12122277









