Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum, Increase Antioxidant Activity, and Decrease Stress in Tomato Plants
Abstract
:1. Introduction
2. Results
2.1. Incidence and Severity
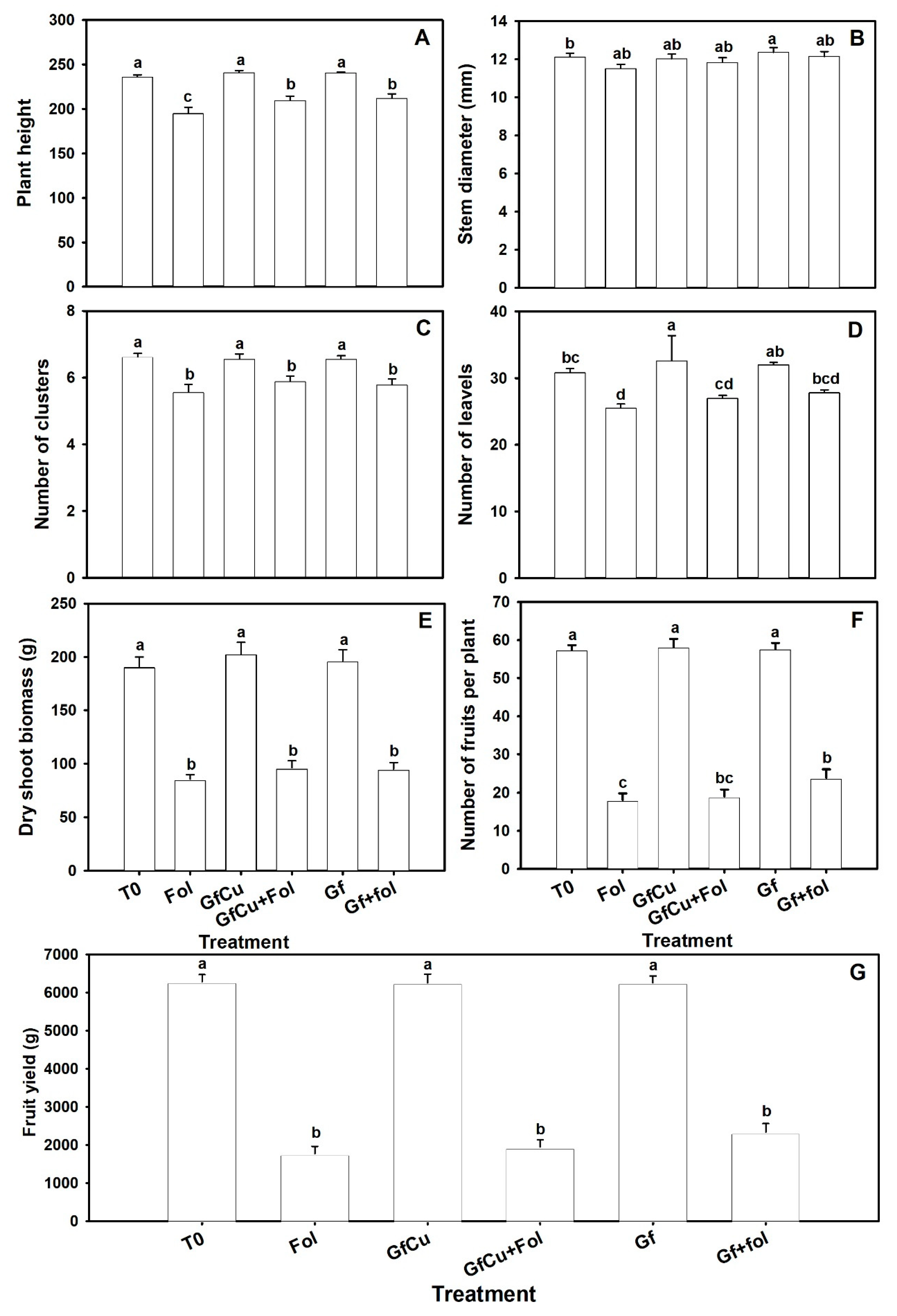
2.2. Agronomical Parameters
2.3. Physiological Variables
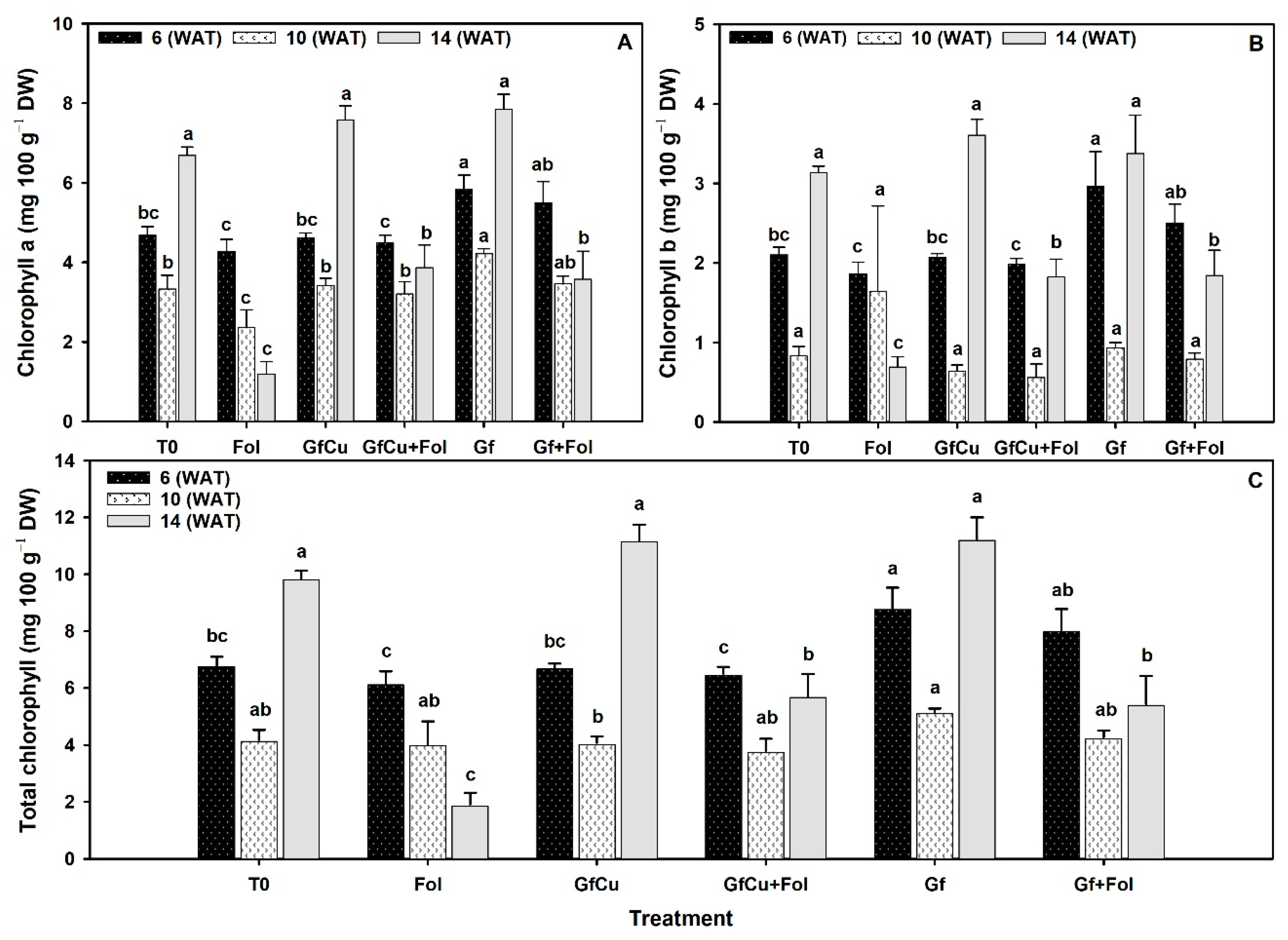
2.4. Photosynthetic Pigments
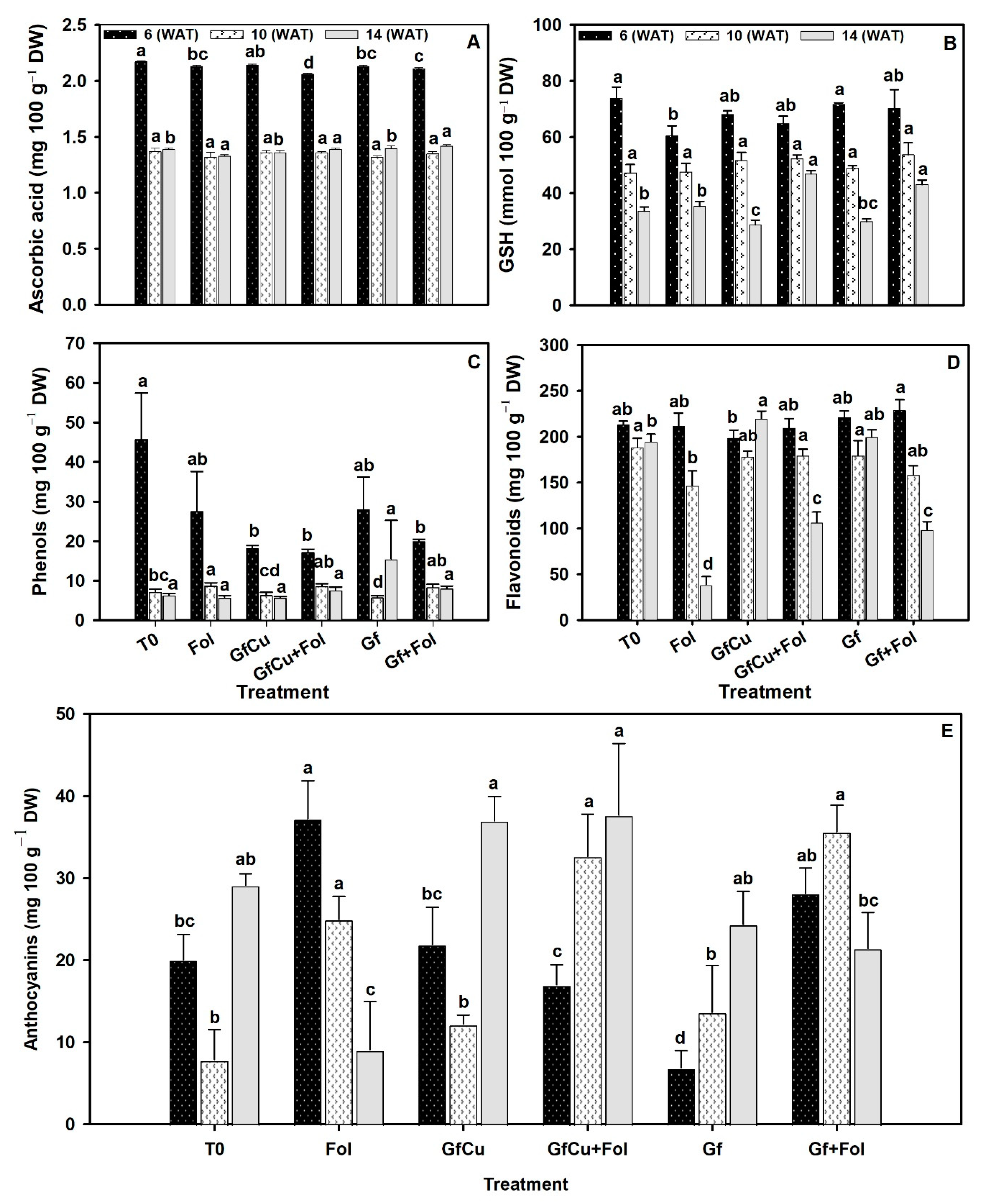
2.5. Antioxidant Compounds
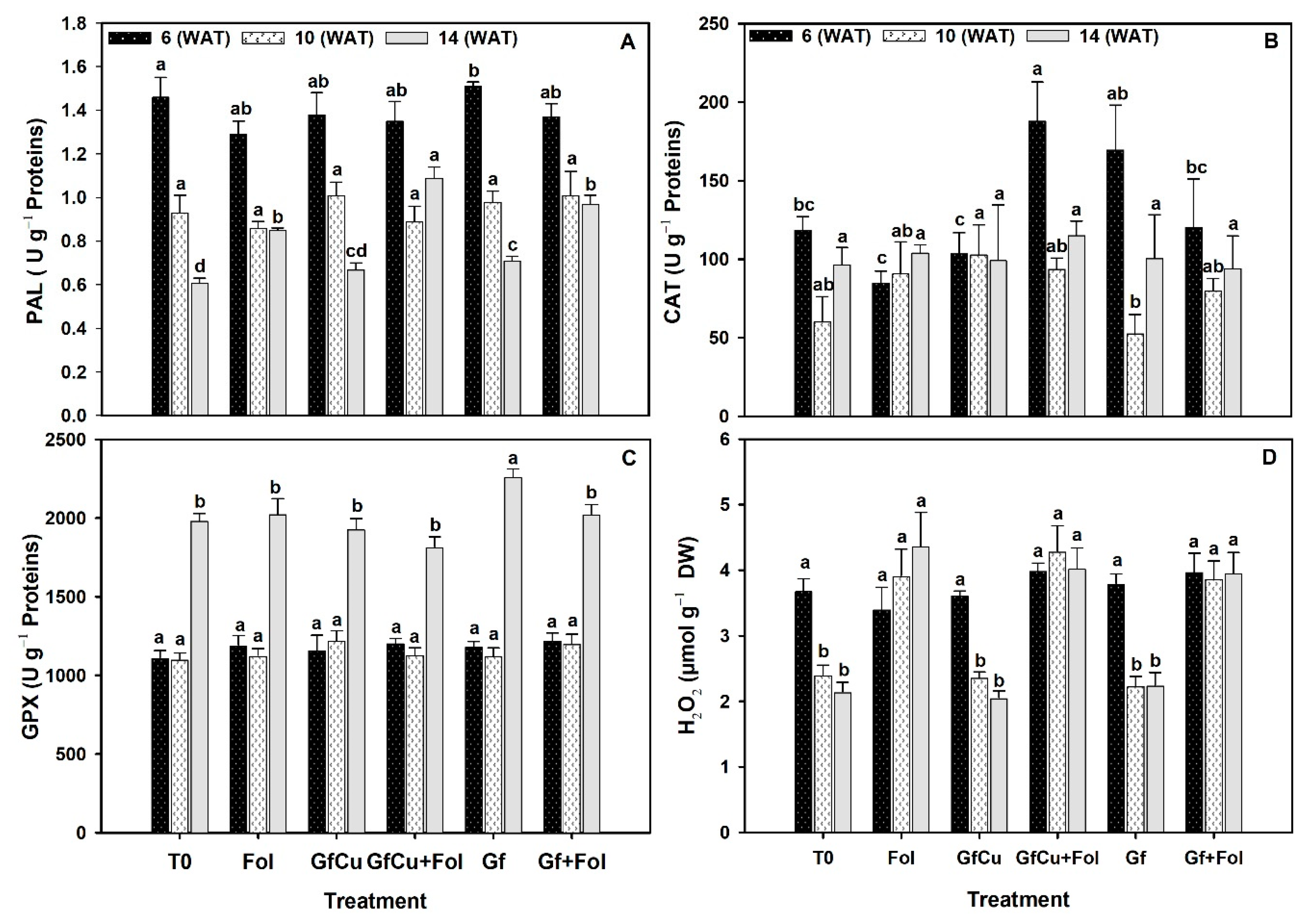
2.6. Enzymatic Activity in Tomato Leaves
3. Discussion
3.1. Impact of F. oxysporum f. sp. lycopersici and Nanocomposites on Plant Growth and Development
3.2. Impact of F. oxysporum f. sp. lycopersici and Nanocomposites on Water Potential and Fluorescence of Chlorophyll a
3.3. Impact of F. oxysporum f. sp. lycopersici and Nanocomposites on Photosynthetic Pigments
3.4. Impact of F. oxysporum f. sp. lycopersici and Nanocomposites on Antioxidant Defense Systems
4. Materials and Methods
4.1. Crop Development
4.2. Treatments
4.3. Inoculation of F. oxysporum f. sp. lycopersici
4.4. Agronomical Parameters
4.5. Physiological Variables
4.6. Biochemical Variables
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kavroulakis, N.; Doupis, G.; Papadakis, I.E.; Ehaliotis, C.; Papadopoulou, K.K. Tolerance of Tomato Plants to Water Stress Is Improved by the Root Endophyte Fusarium Solani FsK. Rhizosphere 2018, 6, 77–85. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does Plant—Microbe Interaction Confer Stress Tolerance in Plants: A Review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, H.B.; Upadhyay, R.S. Role of Fusaric Acid in the Development of ‘Fusarium Wilt’ Symptoms in Tomato: Physiological, Biochemical and Proteomic Perspectives. Plant Physiol. Biochem. 2017, 118, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Taylor, P.W.J.; Chen, D.; Vaghefi, N.; He, J.-Z. Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies. Microorganisms 2023, 11, 263. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.; Jal Pretorius, Z.; Hammond-kosack, K.; Di pietro, A.; Apanu, P.; Rudd, J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, J.-Y.; Han, S.; Hong, H.-J.; Cho, H.; Kim, D.; Kwon, Y.; Kwon, S.-K.; Crüsemann, M.; Bok Lee, Y.; Kim, J.F.; et al. Microbial and Biochemical Basis of a Fusarium Wilt-Suppressive Soil. ISME J. 2016, 10, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chitwood-Brown, J.; Kaur, G.; Labate, J.A.; Vallad, G.E.; Lee, T.G.; Hutton, S.F. Novel Sources of Resistance to Fusarium Oxysporum f. Sp. Lycopersici Race 3 Among Solanum Pennellii Accessions. J. Am. Soc. Hortic. Sci. 2022, 147, 35–44. [Google Scholar] [CrossRef]
- McGovern, R.J. Management of Tomato Diseases Caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Djidonou, D.; Simonne, A.H.; Koch, K.E.; Brecht, J.K.; Zhao, X. Nutritional Quality of Field-Grown Tomato Fruit as Affected by Grafting with Interspecific Hybrid Rootstocks. HortScience 2016, 51, 1618–1624. [Google Scholar] [CrossRef] [Green Version]
- Michielse, C.; Rep, M. Pathogen Profile Update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Srinivas, C.; Nirmala Devi, D.; Narasimha Murthy, K.; Mohan, C.D.; Lakshmeesha, T.R.; Singh, B.; Kalagatur, N.K.; Niranjana, S.R.; Hashem, A.; Alqarawi, A.A.; et al. Fusarium oxysporum f. Sp. Lycopersici Causal Agent of Vascular Wilt Disease of Tomato: Biology to Diversity– A Review. Saudi J. Biol. Sci. 2019, 26, 1315–1324. [Google Scholar] [CrossRef]
- Patel, Z.M.; Mahapatra, R.; Jampala, S.S.M. Role of Fungal Elicitors in Plant Defense Mechanism. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–158. [Google Scholar]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen Derived Elicitors: Searching for Receptors in Plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Dangl, J.L.; Jones, J.D.G. Plant Pathogens and Integrated Defence Responses to Infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef]
- Humbal, A.; Pathak, B. Influence of Exogenous Elicitors on the Production of Secondary Metabolite in Plants: A Review (“VSI: Secondary Metabolites”). Plant Stress 2023, 8, 100166. [Google Scholar] [CrossRef]
- Moreno-Escamilla, J.O.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Núñez-Gastélum, J.A.; González-Aguilar, G.A.; Rodrigo-García, J. Effect of Elicitors in the Nutritional and Sensorial Quality of Fruits and Vegetables. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Elsevier: Amsterdam, The Netherlands, 2018; pp. 71–91. [Google Scholar]
- Juárez-Maldonado, A.; Ortega-Ortiz, H.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-de la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, Y.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Nanomaterials as Novel Elicitors of Plant Secondary Metabolites. In Nanotechnology in Agriculture and Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 113–139. [Google Scholar]
- Selvakesavan, R.K.; Kruszka, D.; Shakya, P.; Mondal, D.; Franklin, G. Impact of Nanomaterials on Plant Secondary Metabolism. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Springer International Publishing: Cham, Switzerland, 2023; pp. 133–170. [Google Scholar]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a New Sustainable Approach for Controlling Crop Diseases and Increasing Agricultural Production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon Nanomaterials against Pathogens; the Antimicrobial Activity of Carbon Nanotubes, Graphene/Graphene Oxide, Fullerenes, and Their Nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, K.R.; Cowley, S.; Martinez, F.O.; Shaw, M.; Minger, S.L.; James, W. Homogeneous Monocytes and Macrophages from Human Embryonic Stem Cells Following Coculture-Free Differentiation in M-CSF and IL-3. Exp. Hematol. 2008, 36, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Sedeño, P.; Villalonga, R.; Pingarrón, J.M. Electroanalytical Methods Based on Hybrid Nanomaterials. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2019; pp. 1–22. [Google Scholar]
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and Toxicity: The Magnitude of the Impact of Nanomaterials in Microorganisms and Plants. J. Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef]
- Jha, S.; Yadav, A. Assessment of Carbon and Fullerene Nanomaterials for Sustainable Crop Plants Growth and Production. In Engineered Nanomaterials for Sustainable Agricultural Production, Soil Improvement and Stress Management; Elsevier: Amsterdam, The Netherlands, 2023; pp. 145–160. [Google Scholar]
- Tortella, G.; Rubilar, O.; Pieretti, J.C.; Fincheira, P.; de Melo Santana, B.; Fernández-Baldo, M.A.; Benavides-Mendoza, A.; Seabra, A.B. Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics 2023, 12, 338. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Hassan, Z.U.; Akram, M.A.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s Uptake and Translocation Mechanisms in Plants via Seed Priming, Foliar Treatment, and Root Exposure: A Review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jing, X.; Lin, S.; Lu, K.; Li, W.; Lu, J.; Li, M.; Gao, S.; Lu, S.; Zhou, D.; et al. Root Hair Apex Is the Key Site for Symplastic Delivery of Graphene into Plants. Environ. Sci. Technol. 2022, 56, 12179–12189. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.R.; Adam, V.; Rizvi, T.F.; Zhang, B.; Ahamad, F.; Jośko, I.; Zhu, Y.; Yang, M.; Mao, C. Nanoparticle–Plant Interactions: Two-Way Traffic. Small 2019, 15, 1901794. [Google Scholar] [CrossRef] [PubMed]
- El-Abeid, S.E.; Ahmed, Y.; Daròs, J.-A.; Mohamed, M.A. Reduced Graphene Oxide Nanosheet-Decorated Copper Oxide Nanoparticles: A Potent Antifungal Nanocomposite against Fusarium Root Rot and Wilt Diseases of Tomato and Pepper Plants. Nanomaterials 2020, 10, 1001. [Google Scholar] [CrossRef]
- Shoala, T. Carbon Nanostructures: Detection, Controlling Plant Diseases and Mycotoxins. In Carbon Nanomaterials for Agri-food and Environmental Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 261–277. ISBN 9780128197868. [Google Scholar]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The Bifunctional Role of Copper Nanoparticles in Tomato: Effective Treatment for Fusarium wilt and Plant Growth Promoter. Sci. Hortic. 2021, 277, 109810. [Google Scholar] [CrossRef]
- Maksimova, Y.G. Microorganisms and Carbon Nanotubes: Interaction and Applications (Review). Appl. Biochem. Microbiol. 2019, 55, 1–12. [Google Scholar] [CrossRef]
- Majumdar, T.D.; Singh, M.; Thapa, M.; Dutta, M.; Mukherjee, A.; Ghosh, C.K. Size-Dependent Antibacterial Activity of Copper Nanoparticles against Xanthomonas Oryzae Pv. Oryzae—A Synthetic and Mechanistic Approach. Colloid Interface Sci. Commun. 2019, 32, 100190. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Mosa, M.A.; Ismail, A.M.; Khalil, A.E. Lignin-Loaded Carbon Nanoparticles as a Promising Control Agent against Fusarium Verticillioides in Maize: Physiological and Biochemical Analyses. Polymers 2023, 15, 1193. [Google Scholar] [CrossRef]
- Bytešníková, Z.; Pečenka, J.; Tekielska, D.; Kiss, T.; Švec, P.; Ridošková, A.; Bezdička, P.; Pekárková, J.; Eichmeier, A.; Pokluda, R.; et al. Reduced Graphene Oxide-Based Nanometal-Composite Containing Copper and Silver Nanoparticles Protect Tomato and Pepper against Xanthomonas euvesicatoria Infection. Chem. Biol. Technol. Agric. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Cabrera, R.I.; Juárez-Maldonado, A. Effect of Carbon-Based Nanomaterials on Fusarium wilt in Tomato. Sci. Hortic. 2022, 291, 110586. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; White, J.C.; Nazir, M.M.; Azizullah; Li, D.; Song, F. Bacillus Altitudinis-Stabilized Multifarious Copper Nanoparticles Prevent Bacterial Fruit Blotch in Watermelon (Citrullus lanatus L.): Direct Pathogen Inhibition, In Planta Particles Accumulation, and Host Stomatal Immunity Modulation. Small 2023, 19, 2207136. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.E.; de Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F.L.W. Endophyte-Mediated Resistance in Tomato to Fusarium Oxysporum Is Independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.-L.; Li, J.; Wang, H.-C.; Zhang, C.-L.; Naeem, M.S. Fullerol Improves Seed Germination, Biomass Accumulation, Photosynthesis and Antioxidant System in Brassica napus L. under Water Stress. Plant Physiol. Biochem. 2018, 129, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.T.; Oates, R.P.; Subbiah, S.; Payton, P.R.; Singh, K.P.; Shah, S.A.; Green, M.J.; Klein, D.M.; Cañas-Carrell, J.E. Carbon Nanotubes Affect Early Growth, Flowering Time and Phytohormones in Tomato. Chemosphere 2020, 256, 127042. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, J.; Wang, R.; Zhang, H.; Xing, B.; Naeem, M.; Yao, T.; Li, R.; Xu, R.; Zhang, Z.; et al. Effects of Graphene Oxide on Tomato Growth in Different Stages. Plant Physiol. Biochem. 2021, 162, 447–455. [Google Scholar] [CrossRef]
- Kralova, K.; Jampilek, J. Applications of Nanomaterials in Plant Disease Management and Protection. In Nanotechnology in Agriculture and Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–296. [Google Scholar]
- Yadeta, K.A.; Thomma, B.P.H.J. The Xylem as Battleground for Plant Hosts and Vascular Wilt Pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Sun, H.; Qu, X. Antibacterial Applications of Graphene-Based Nanomaterials: Recent Achievements and Challenges. Adv. Drug Deliv. Rev. 2016, 105, 176–189. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, H. Cation–π Interactions in Graphene-Containing Systems for Water Treatment and Beyond. Adv. Mater. 2020, 32, 1905756. [Google Scholar] [CrossRef]
- Zhao, D.; Fang, Z.; Tang, Y.; Tao, J. Graphene Oxide as an Effective Soil Water Retention Agent Can Confer Drought Stress Tolerance to Paeonia Ostii without Toxicity. Environ. Sci. Technol. 2020, 54, 8269–8279. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta, M.C.; Chelbi, N.; Lopez-Zaplana, A.; Carvajal, M. Discerning the Mechanism of the Multiwalled Carbon Nanotubes Effect on Root Cell Water and Nutrient Transport. Plant Physiol. Biochem. 2020, 146, 23–30. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, R.; Zhong, Y.; Zhao, X.; Chen, Q.; Zhu, H. Graphene Oxide as a Water Transporter Promoting Germination of Plants in Soil. Nano Res. 2018, 11, 1928–1937. [Google Scholar] [CrossRef]
- Wang, F.; Wu, N.; Zhang, L.; Ahammed, G.J.; Chen, X.; Xiang, X.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; et al. Light Signaling-Dependent Regulation of Photoinhibition and Photoprotection in Tomato. Plant Physiol. 2018, 176, 1311–1326. [Google Scholar] [CrossRef]
- Maqsood, A.; Wu, H.; Kamran, M.; Altaf, H.; Mustafa, A.; Ahmar, S.; Hong, N.T.T.; Tariq, K.; He, Q.; Chen, J.-T. Variations in Growth, Physiology, and Antioxidative Defense Responses of Two Tomato (Solanum lycopersicum L.) Cultivars after Co-Infection of Fusarium oxysporum and Meloidogyne incognita. Agronomy 2020, 10, 159. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Shen, D.; Dong, S.; Chen, C.; Lin, S.; Lu, S.; Xing, B.; Mao, L. Uptake of Graphene Enhanced the Photophosphorylation Performed by Chloroplasts in Rice Plants. Nano Res. 2020, 13, 3198–3205. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Alp, F.N.; Arikan, B.; Elbasan, F.; Cavusoglu, H.; Yildiztugay, E. The Biphasic Responses of Nanomaterial Fullerene on Stomatal Movement, Water Status, Chlorophyll a Fluorescence Transient, Radical Scavenging System and Aquaporin-Related Gene Expression in Zea mays under Cobalt Stress. Sci. Total Environ. 2022, 826, 154213. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Cavusoglu, H.; Arikan, B.; Alp, F.N.; Elbasan, F.; Kucukoduk, M.; Turkan, I. Nanomaterial Sulfonated Graphene Oxide Advances the Tolerance against Nitrate and Ammonium Toxicity by Regulating Chloroplastic Redox Balance, Photochemistry of Photosystems and Antioxidant Capacity in Triticum aestivum. J. Hazard. Mater. 2022, 424, 127310. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, X.; Liu, L.; Chen, C.; Sha, A.; Li, J. Effects of Three Graphene-Based Materials on the Growth and Photosynthesis of Brassica napus L. Ecotoxicol. Environ. Saf. 2022, 234, 113383. [Google Scholar] [CrossRef]
- Marín-Ortiz, J.C.; Gutierrez-Toro, N.; Botero-Fernández, V.; Hoyos-Carvajal, L.M. Linking Physiological Parameters with Visible/near-Infrared Leaf Reflectance in the Incubation Period of Vascular Wilt Disease. Saudi J. Biol. Sci. 2020, 27, 88–99. [Google Scholar] [CrossRef]
- Páramo, L.; Aguirre Becerra, H.; Ramírez Piña, J.E.; Cervantes Chávez, J.A.; Feregrino-Pérez, A.A.; Esquivel, K. Impact of Nanomaterials on Chlorophyll Content in Plants. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Springer International Publishing: Cham, Switzerland, 2023; pp. 69–92. [Google Scholar]
- Meften, M.T.; Ibrahim, S.A.; Mohamed, A.S. The Impact Of Carbon-Nano Tubes On Morphological, Photosynthetic Pigments, Protein Content And Enzyme Activity Of In-Vitro Multiplication Of Cucumber. J. Pharm. Negat. Results 2023, 14, 1361–1366. [Google Scholar]
- Shafiq, F.; Iqbal, M.; Ashraf, M.A.; Ali, M. Foliar Applied Fullerol Differentially Improves Salt Tolerance in Wheat through Ion Compartmentalization, Osmotic Adjustments and Regulation of Enzymatic Antioxidants. Physiol. Mol. Biol. Plants 2020, 26, 475–487. [Google Scholar] [CrossRef]
- Safikhan, S.; Chaichi, M.; Khoshbakht, K.; Amini, A.; Motesharezadeh, B. Application of Nanomaterial Graphene Oxide on Biochemical Traits of Milk Thistle (Silybum Marianum L.) under Salinity Stress. Aust. J. Crop Sci. 2018, 12, 931–936. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Seed Pre-Treatment with Polyhydroxy Fullerene Nanoparticles Confer Salt Tolerance in Wheat Through Upregulation of H2O2 Neutralizing Enzymes and Phosphorus Uptake. J. Soil Sci. Plant Nutr. 2019, 19, 734–742. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2018, 66, 6654–6662. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and Oxidative Burst: Roots in Plant Development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bai, T.; Wei, H.; Gardea-Torresdey, J.L.; Keller, A.; White, J.C. Nanobiotechnology-Based Strategies for Enhanced Crop Stress Resilience. Nat. Food 2022, 3, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Maldonado, A. Influence of Nanomaterials on Non-Enzymatic Antioxidant Defense Activities in Plants. In Nanomaterial Interactions with Plant Cellular Mechanisms and Macromolecules and Agricultural Implications; Springer International Publishing: Cham, Switzerland, 2023; pp. 273–298. [Google Scholar]
- Wu, Q.; Fan, C.; Wang, H.; Han, Y.; Tai, F.; Wu, J.; Li, H.; He, R. Biphasic Impacts of Graphite-Derived Engineering Carbon-Based Nanomaterials on Plant Performance: Effectiveness vs. Nanotoxicity. Adv. Agrochem 2023, 2, 113–126. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Abolhassani, M.; Hadian-Deljou, M.; Feyzi, H.; Akbari, A.; Rasouli, F.; Koçak, M.Z.; Kulak, M.; Gohari, G. Proline-Functionalized Graphene Oxide Nanoparticles (GO-Pro NPs): A New Engineered Nanoparticle to Ameliorate Salinity Stress on Grape (Vitis vinifera L. Cv Sultana). Plant Stress 2023, 7, 100128. [Google Scholar] [CrossRef]
- Borges, C.V.; Orsi, R.O.; Maraschin, M.; Lima, G.P.P. Oxidative Stress in Plants and the Biochemical Response Mechanisms. In Plant Stress Mitigators; Elsevier: Amsterdam, The Netherlands, 2023; pp. 455–468. [Google Scholar]
- Kwon, D.H.; Lee, H.; Park, C.; Hong, S.-H.; Hong, S.H.; Kim, G.-Y.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Hwang, H.-J.; et al. Glutathione Induced Immune-Stimulatory Activity by Promoting M1-Like Macrophages Polarization via Potential ROS Scavenging Capacity. Antioxidants 2019, 8, 413. [Google Scholar] [CrossRef] [Green Version]
- Rai, G.K.; Kumar, P.; Choudhary, S.M.; Singh, H.; Adab, K.; Kosser, R.; Magotra, I.; Kumar, R.R.; Singh, M.; Sharma, R.; et al. Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants 2023, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid Production: Current Trends in Plant Metabolic Engineering and De Novo Microbial Production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, A.G.; Apostolova, E.L.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Ioannis-Dimosthenis, S.; Moustakas, M. Cadmium Toxicity in Salvia Sclarea L.: An Integrative Response of Element Uptake, Oxidative Stress Markers, Leaf Structure and Photosynthesis. Ecotoxicol. Environ. Saf. 2021, 209, 111851. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Alp, F.N.; Arikan, B.; Balci, M.; Parmaksizoglu, Z.; Yildiztugay, E.; Cavusoglu, H. The Effects of Fullerene on Photosynthetic Apparatus, Chloroplast-encoded Gene Expression, and Nitrogen Assimilation in Zea Mays under Cobalt Stress. Physiol. Plant. 2022, 174, e13720. [Google Scholar] [CrossRef] [PubMed]
- González-García, Y.; López-Vargas, E.R.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; González-Morales, S.; Robledo-Olivo, A.; Alpuche-Solís, Á.G.; Juárez-Maldonado, A. Impact of Carbon Nanomaterials on the Antioxidant System of Tomato Seedlings. Int. J. Mol. Sci. 2019, 20, 5858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Karami, A.; Ghorbanpour, M. Single-Wall Carbon Nano Tubes (SWCNTs) Penetrate Thymus Daenensis Celak. Plant Cells and Increase Secondary Metabolite Accumulation in Vitro. Ind. Crops Prod. 2021, 165, 113424. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Carrasco, A.; Boudet, A.M.; Marigo, G. Enhanced Resistance of Tomato Plants to Fusarium by Controlled Stimulation of Their Natural Phenolic Production. Physiol. Plant Pathol. 1978, 12, 225–232. [Google Scholar] [CrossRef]
- Raja, M.; Pandian Prakasa, T.R.; Sharma, M.; Jambhulkar, P.; Sharma, P. Study of Induced Systemic Resistance in Tomato against Fusarium Oxysporum f. Sp Lycopersici Causing Wilt of Tomato. Indian Phytopath 2016, 64, 539–542. [Google Scholar]
- Zhang, X.; Liu, C.-J. Multifaceted Regulations of Gateway Enzyme Phenylalanine Ammonia-Lyase in the Biosynthesis of Phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Sharma, I.; Ahmad, P. Catalase: A Versatile Antioxidant in Plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 131–148. [Google Scholar]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and Comparative Analysis of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Glutathione Peroxidase) in Selected Plants Employing Bioinformatics Approaches. Front. Plant Sci. 2016, 7, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Holghoomi, R.; Sarghein, S.H.; Khara, J.; Hosseini, B. Effect of Functionalized-Carbon Nanotube on Growth Indices in Ocimum Basilicum L. Grown in Vitro. Russ. J. Plant Physiol. 2021, 68, 958–972. [Google Scholar] [CrossRef]
- Li, F.; Sun, C.; Li, X.; Yu, X.; Luo, C.; Shen, Y.; Qu, S. The Effect of Graphene Oxide on Adventitious Root Formation and Growth in Apple. Plant Physiol. Biochem. 2018, 129, 122–129. [Google Scholar] [CrossRef]
- Chauhan, H.; Patel, M.; Patel, P.; Tiwari, S.; Jinal, H.N.; Amaresan, N. Assessment of Copper (Cu) Nanoparticle for Their Biocontrol Activity against Xanthomonas oryzae Pv. Oryzae, Growth Promotion, and Physiology of Rice (Oryza sativa L.) Plants. Lett. Appl. Microbiol. 2023, 76, 1–7. [Google Scholar] [CrossRef]
- Steiner, A.A. A Universal Method for Preparing Nutrient Solutions of a Certain Desired Composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Guel, M.; Reyes-Rodríguez, P.Y.; Cabello-Alvarado, C.J.; Cadenas-Pliego, G.; Ávila-Orta, C.A. Influence of Modified Carbon Black on Nylon 6 Nonwoven fabric and Performance as Adsorbent Material. Nanomaterials 2022, 12, 4247. [Google Scholar] [CrossRef] [PubMed]
- Jardón-Maximino, N.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Lugo-Uribe, L.E.; Cabello-Alvarado, C.; Mata-Padilla, J.M.; Barriga-Castro, E.D. Synthesis of Copper Nanoparticles Stabilized with Organic Ligands and Their Antimicrobial Properties. Polymers 2021, 13, 2846. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.; Cadenas-Pliego, G.; Pérez-Camacho, O.; Comparán-Padilla, V.E.; Cabello-Alvarado, C.J.; Saucedo-Salazar, E. Green Synthesis of Copper Nanoparticles Using Cotton. Polymers 2021, 13, 1906. [Google Scholar] [CrossRef]
- Grattidge, R.; O’Brien, R.G. Occurrence of a Third Race of Fusarium Wilt of Tomatoes in Queensland. Plant Dis. 1982, 66, 165–166. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Hemmingsen, E.A.; Bradstreet, E.D. Hydrostatic Pressure and Osmotic Potencial in Leaves of Mangroves and Some Other Plants. Proc. Natl. Acad. Sci. USA 1964, 52, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.Y.; Yen, G.C. Antioxidant Activity of Phenolic Compounds Isolated from Mesona Procumbens Hemsl. J. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and Growth-Promoting Effect of Selenium on Senescing Lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Yu, Z.; Dahlgren, R.A. Evaluation of Methods for Measuring Polyphenols in Conifer Foliage. J. Chem. Ecol. 2000, 26, 2119–2140. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of a Propolis Extract and Identification of the Main Constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T. Senescencia Foliar: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Łojkowska, E.; Chinou, I. Effect of L-Phenylalanine on PAL Activity and Production of Naphthoquinone Pigments in Suspension Cultures of Arnebia Euchroma (Royle) Johnst. Vitr. Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cota-Ungson, D.; González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum, Increase Antioxidant Activity, and Decrease Stress in Tomato Plants. Plants 2023, 12, 2270. https://doi.org/10.3390/plants12122270
Cota-Ungson D, González-García Y, Cadenas-Pliego G, Alpuche-Solís ÁG, Benavides-Mendoza A, Juárez-Maldonado A. Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum, Increase Antioxidant Activity, and Decrease Stress in Tomato Plants. Plants. 2023; 12(12):2270. https://doi.org/10.3390/plants12122270
Chicago/Turabian StyleCota-Ungson, Diana, Yolanda González-García, Gregorio Cadenas-Pliego, Ángel Gabriel Alpuche-Solís, Adalberto Benavides-Mendoza, and Antonio Juárez-Maldonado. 2023. "Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum, Increase Antioxidant Activity, and Decrease Stress in Tomato Plants" Plants 12, no. 12: 2270. https://doi.org/10.3390/plants12122270
APA StyleCota-Ungson, D., González-García, Y., Cadenas-Pliego, G., Alpuche-Solís, Á. G., Benavides-Mendoza, A., & Juárez-Maldonado, A. (2023). Graphene–Cu Nanocomposites Induce Tolerance against Fusarium oxysporum, Increase Antioxidant Activity, and Decrease Stress in Tomato Plants. Plants, 12(12), 2270. https://doi.org/10.3390/plants12122270








