Host Factors Genes BcCLC1 and BcCLC2 Confer Turnip Mosaic Virus Resistance in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis)
Abstract
:1. Introduction
2. Results
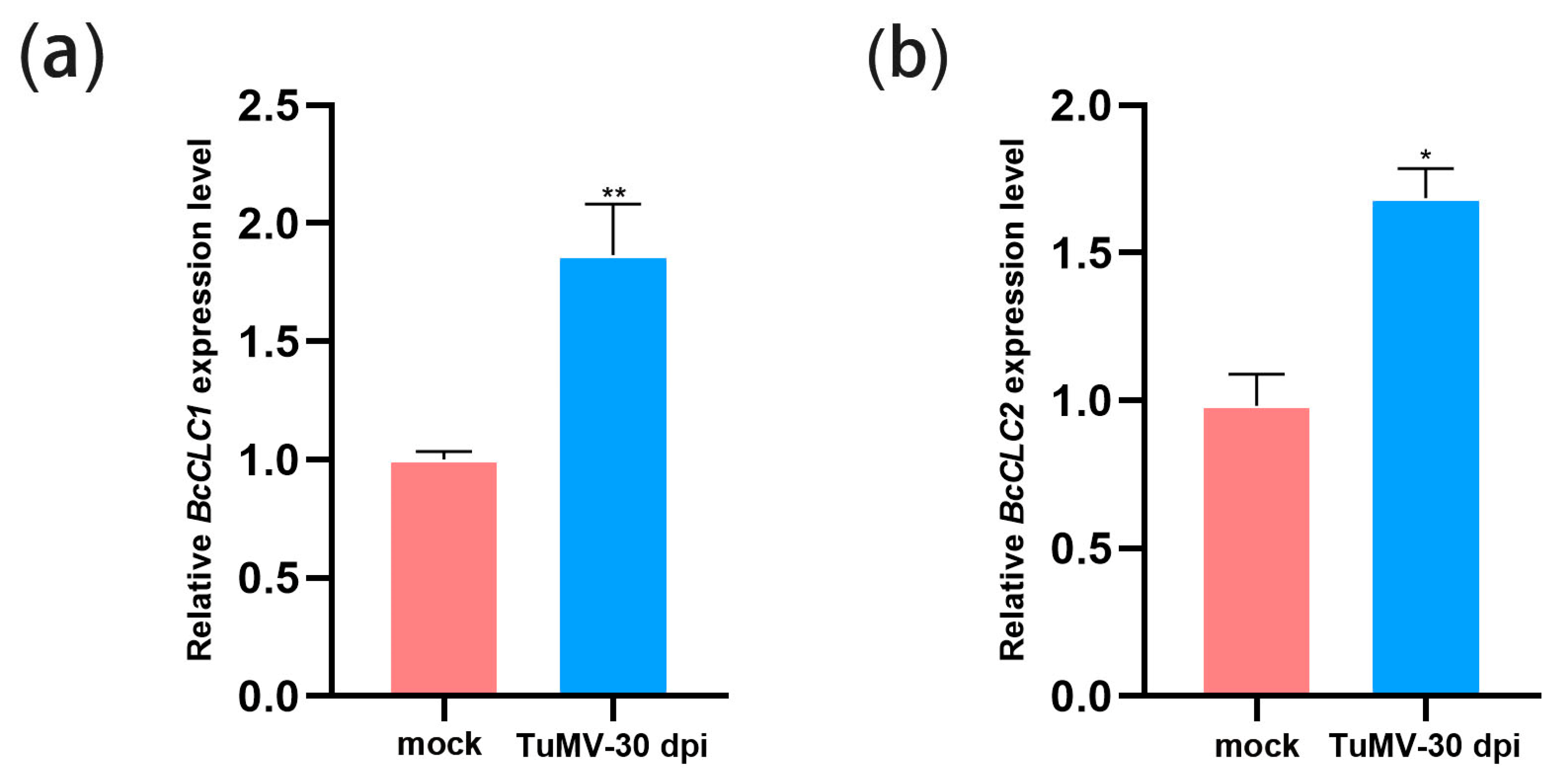
2.1. TuMV Infection Increased the Expression Level of BcCLCs
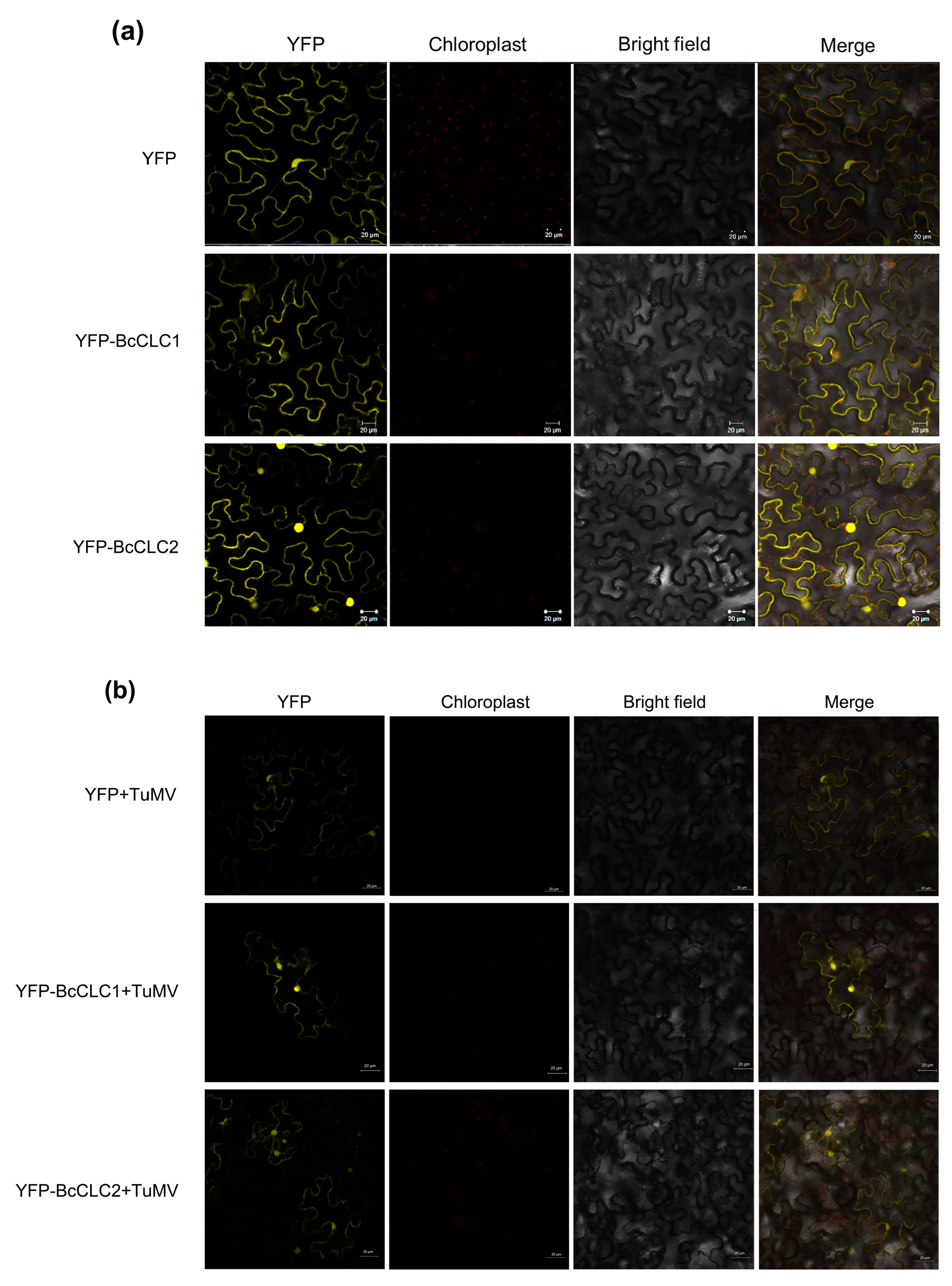
2.2. Subcellular Localization of BcCLCs
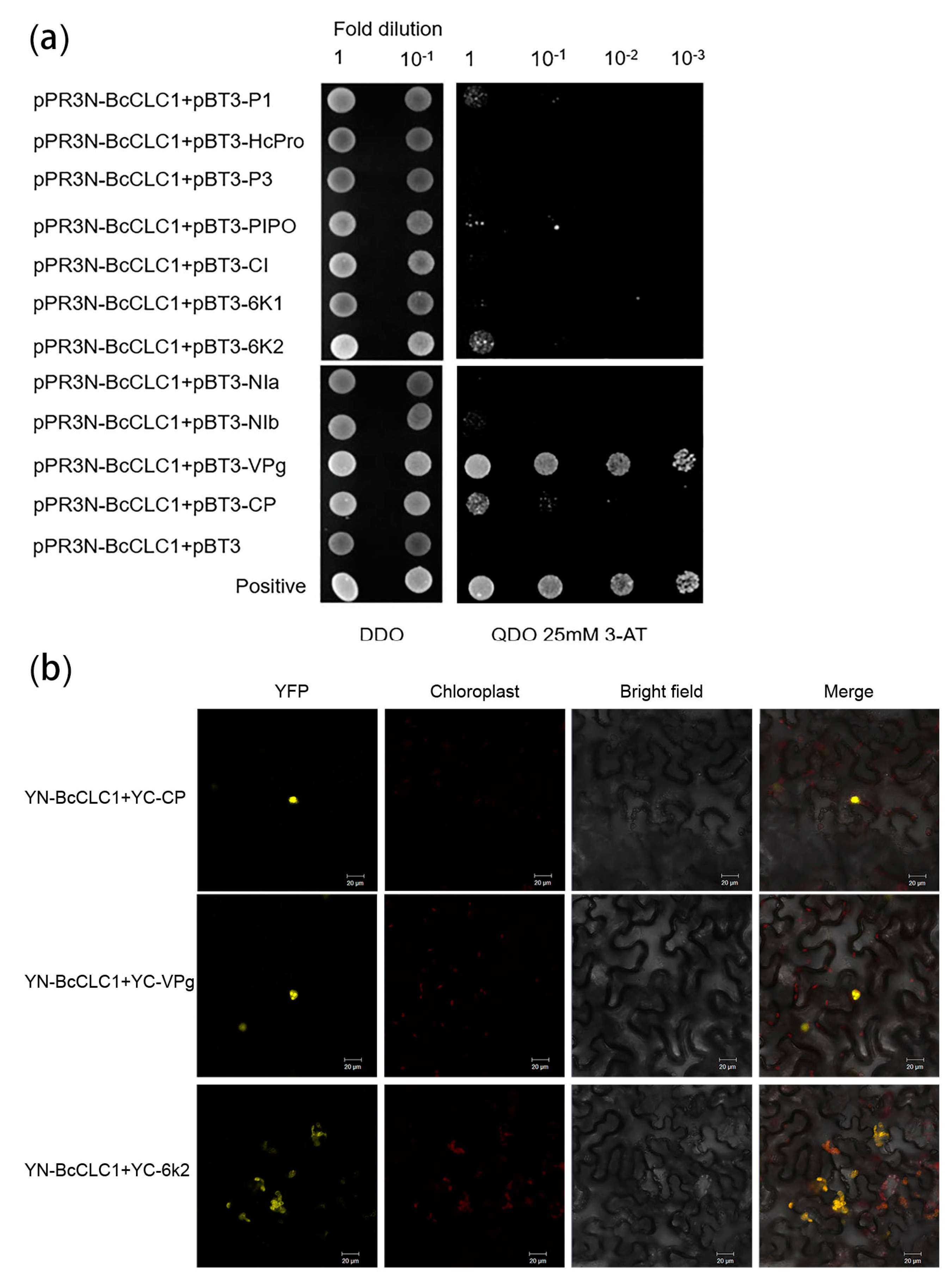
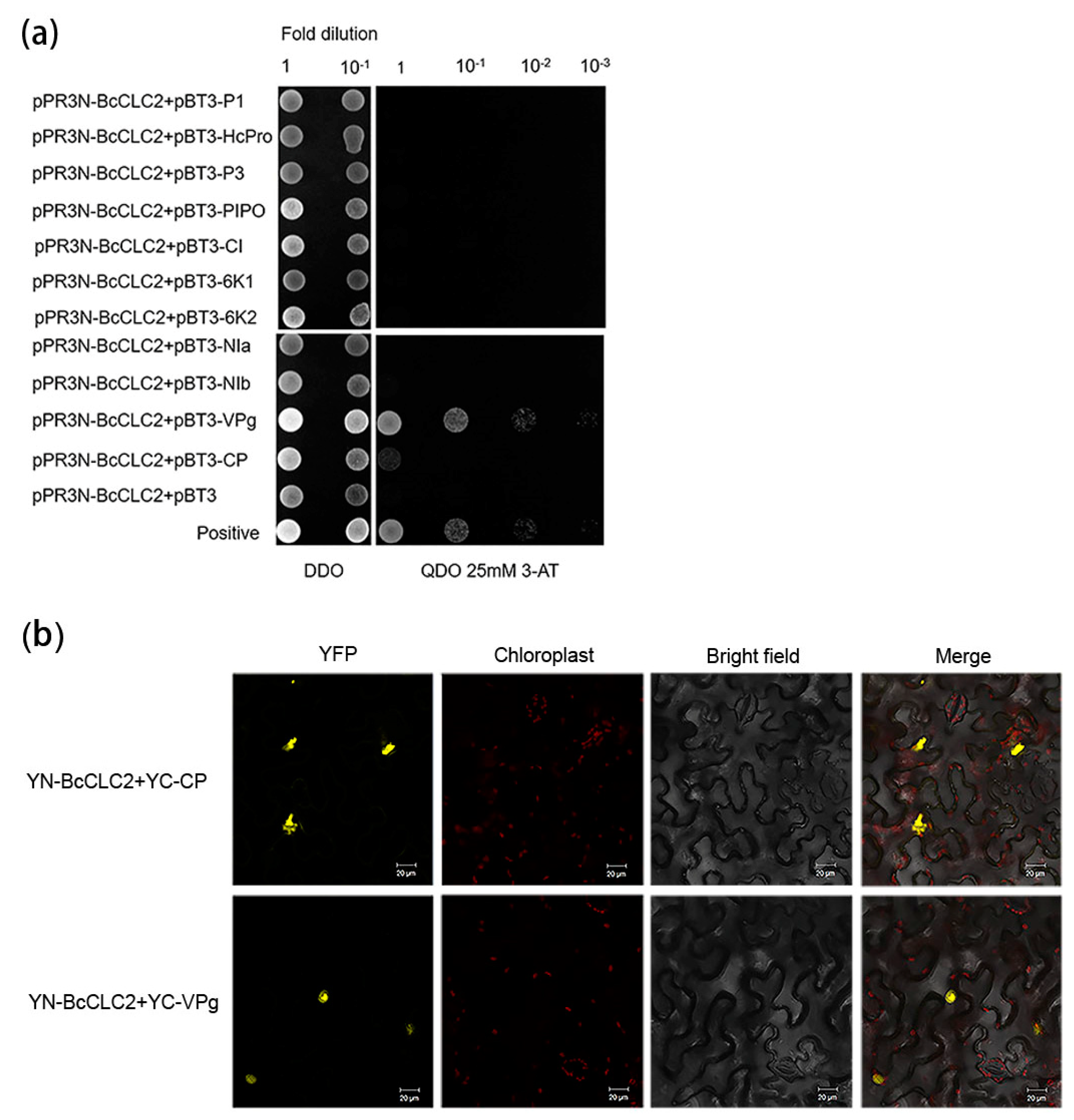
2.3. Analysis of the Interaction between Clathrin Light Chain and the TuMV Protein in NHCC
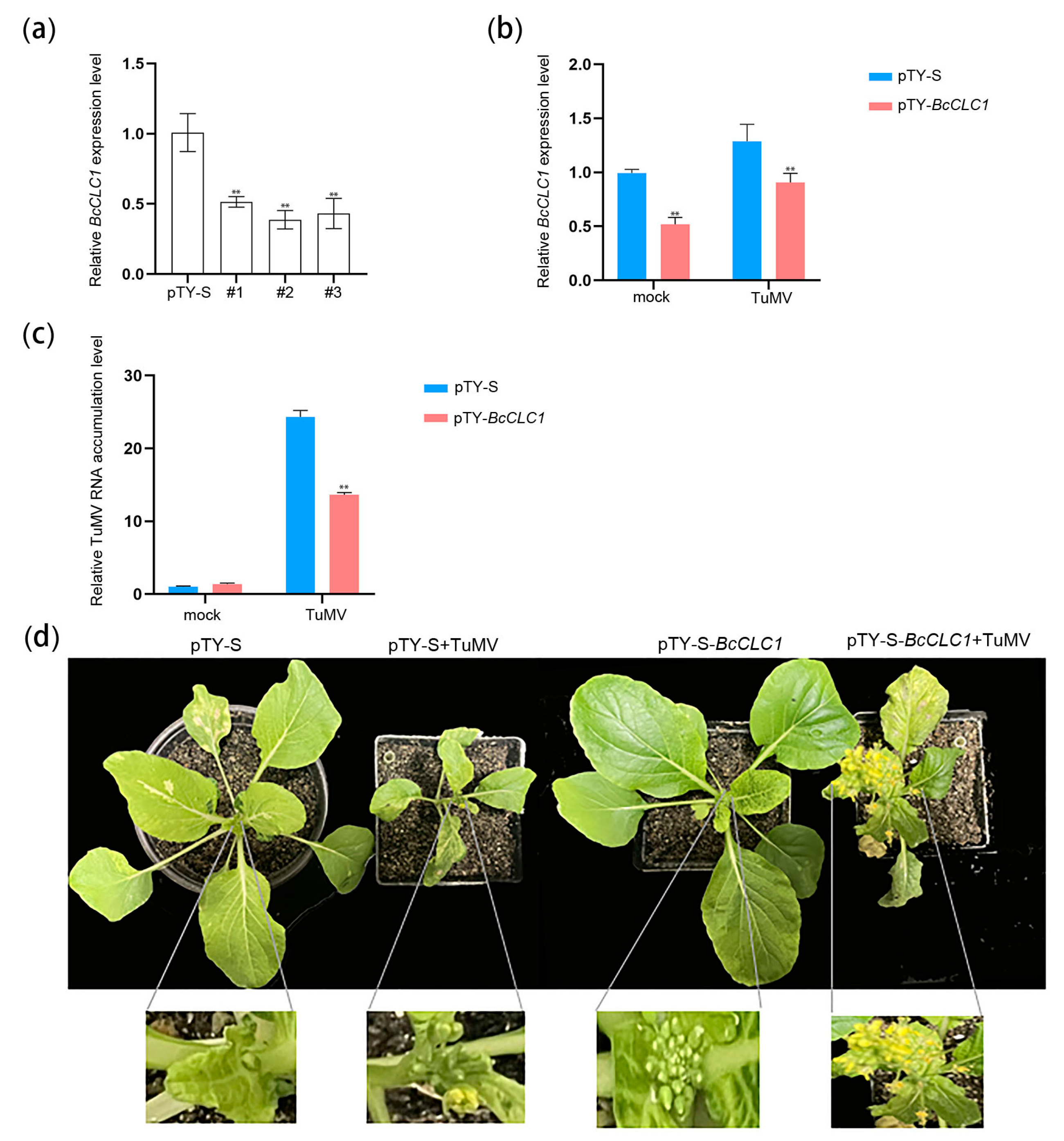
2.4. BcCLC1 Gene Silencing Inhibits the TuMV Infection of NHCC
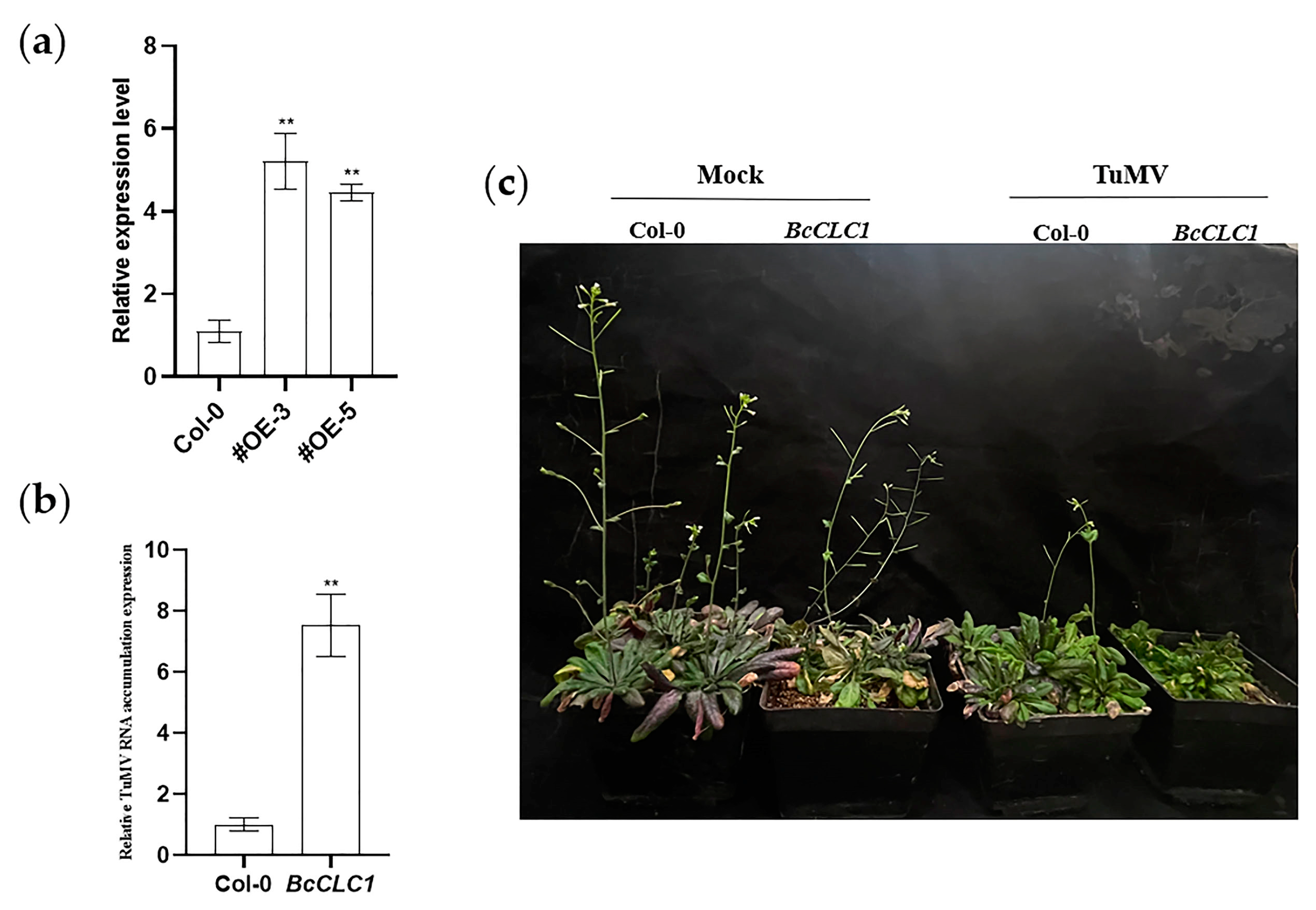
2.5. The Overexpression of BcCLC1 in Arabidopsis Promotes the TuMV Infection
2.6. BcCLC2 Silencing Inhibits TuMV Infection in NHCC
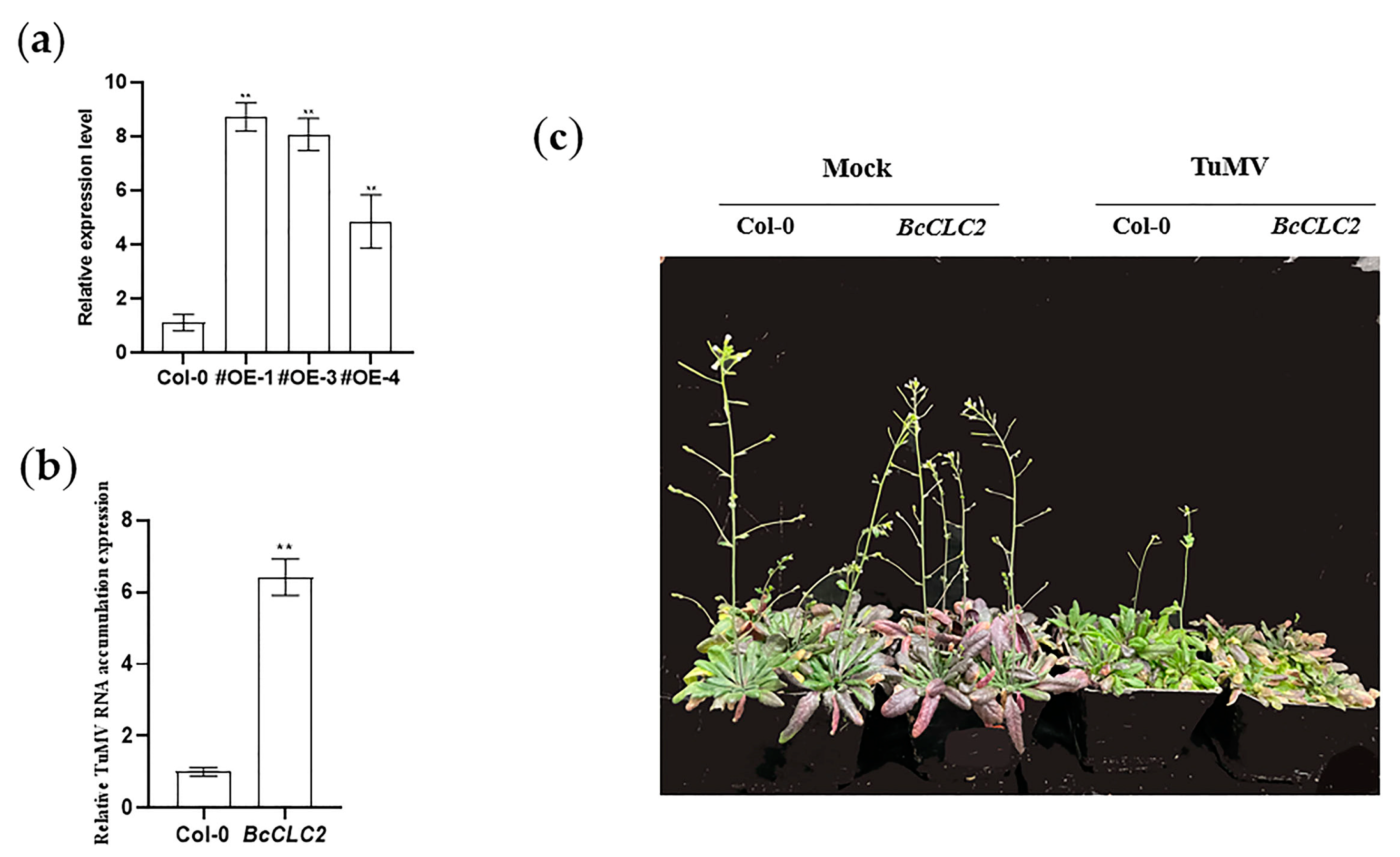
2.7. Overexpression of BcCLC2 Positively Influences the Process of TuMV Infection in NHCC
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Cloning and Plasmid Construction
4.3. Subcellular Localization and BIFC of BcCLCs
4.4. Y2H Analysis
4.5. TuMV Inoculation
4.6. Virus-Induced Gene Silencing Experiment
4.7. Screening for the Overexpression of BcCLCs in Arabidopsis
4.8. RNA Extraction and qRT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dahlberg, J.E. Quantitative electron microscopic analysis of the penetration of VSV into L cells. Virology 1974, 58, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Dales, S. Early events in cell-animal virus interactions. Bacteriol. Rev. 1973, 37, 103–135. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Kartenbeck, J.; Simons, K.; Fries, E. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 1980, 84, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glomb-Reinmund, S.; Kielian, M. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 1998, 248, 372–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abell, B.A.; Brown, D.T. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J. Virol. 1993, 67, 5496–5501. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.J.H.; Ng, M.L. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 2004, 78, 10543–10555. [Google Scholar] [CrossRef] [Green Version]
- Van der Schaar, H.M.; Rust, M.J.; Chen, C.; van der Ende-Metselaar, H.; Wilschut, J.; Zhuang, X.; Smit, J.M. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Path. 2008, 4, e1000244. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, M.; Boll, W.; van Oijen, A.; Hariharan, R.; Chandran, K.; Nibert, M.L.; Kirchhausen, T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 2004, 118, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Johannsdottir, H.K.; Mancini, R.; Kartenbeck, J.; Amato, L.; Helenius, A. Host cell factors and functions involved in vesicular stomatitis virus entry. J. Virol. 2009, 83, 440–453. [Google Scholar] [CrossRef] [Green Version]
- Cureton, D.K.; Massol, R.H.; Saffarian, S.; Kirchhausen, T.L.; Whelan, S.P.J. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Path. 2009, 5, e1000394. [Google Scholar] [CrossRef] [Green Version]
- Peleg-Grossman, S.; Volpin, H.; Levine, A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J. Exp. Bot. 2007, 58, 1637–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.D.; Lazarowitz, S.G. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl. Acad. Sci. USA 2010, 107, 2491–2496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Cui, X.; Chen, H.; Renaud, J.B.; Yu, K.; Chen, X.; Wang, A. Dynamin-Like proteins of endocytosis in plants are coopted by potyviruses to enhance virus infection. J. Virol. 2018, 92, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Cui, X.; Dai, Z.; He, R.; Li, Y.; Yu, K.; Bernards, M.; Chen, X.; Wang, A. A plant RNA virus hijacks endocytic proteins to establish its infection in plants. Plant J. 2020, 101, 384–400. [Google Scholar] [CrossRef] [PubMed]
- Mettlen, M.; Chen, P.H.; Srinivasan, S.; Danuser, G.; Schmid, S.L. Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 2018, 87, 871–896. [Google Scholar] [CrossRef]
- Wang, C.; Yan, X.; Chen, Q.; Jiang, N.; Fu, W.; Ma, B.; Liu, J.; Li, C.; Bednarek, S.Y.; Pan, J. Clathrin light chains regulate clathrin-mediated trafficking, auxin signaling, and development in Arabidopsis. Plant Cell 2013, 25, 499–516. [Google Scholar] [CrossRef] [Green Version]
- Geldner, N.; Richter, S.; Vieten, A.; Marquardt, S.; Torres-Ruiz, R.A.; Mayer, U.; Jurgens, G. Partial loss-of-function alleles reveal a role for GNOM in auxin transport-related, post-embryonic development of Arabidopsis. Development 2004, 131, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Tromas, A.; Braun, N.; Muller, P.; Khodus, T.; Paponov, I.A.; Palme, K.; Ljung, K.; Lee, J.-Y.; Benfey, P.; Murray, J.A.H.; et al. The auxin binding protein 1 is required for differential auxin responses mediating root growth. PLoS ONE 2009, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Kitakura, S.; Vanneste, S.; Robert, S.; Lofke, C.; Teichmann, T.; Tanaka, H.; Friml, J. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 2011, 23, 1920–1931. [Google Scholar] [CrossRef] [Green Version]
- Liming, G. Functional Analysis of Clathrin Light Protein Gene Taclc1 Involved in the Regulation of Resistance for Wheat to Fusarium Head Blight. Master’s Thesis, Shandong Agricultural University, Shandong, China, 2018. [Google Scholar]
- Wang, J.; Virta, V.C.; Riddelle-Spencer, K.; O’Halloran, T.J. Compromise of clathrin function and membrane association by clathrin light chain deletion. Traffic 2003, 4, 891–901. [Google Scholar] [CrossRef]
- Lu, J.; Qu, Y.; Liu, Y.; Jambusaria, R.B.; Han, Z.; Ruthel, G.; Freedman, B.D.; Harty, R.N. Host IQGAP1 and Ebola virus VP40 interactions facilitate virus-like particle egress. J. Virol. 2013, 87, 7777–7780. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Zhao, S.; Liu, H.; Chang, Z.; Li, F.; Zhou, X. Tomato yellow leaf curl virus V3 protein traffics along microfilaments to plasmodesmata to promote virus cell-to-cell movement. Sci. China Life Sci. 2022, 65, 1046–1049. [Google Scholar] [CrossRef]
- Lellis, A.D.; Kasschau, K.D.; Whitham, S.A.; Carrington, J.C. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr. Biol. 2002, 12, 1046–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaad, M.C.; Lellis, A.D.; Carrington, J.C. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 1997, 71, 8624–8631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, R.J.; Tada, H.; Ypma-Wong, M.F.; Dunn, J.J.; Semler, B.L.; Wimmer, E. Construction of a “mutagenesis cartridge” for poliovirus genome-linked viral protein: Isolation and characterization of viable and nonviable mutants. Proc. Natl. Acad. Sci. USA 1988, 85, 519–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellers, J.; Wan, J.; Hong, Y.; Collins, G.B.; Hunt, A.G. In vitro interactions between a potyvirus-encoded, genome-linked protein and RNA-dependent RNA polymerase. J. Gen. Virol. 1998, 79, 2043–2049. [Google Scholar] [CrossRef]
- Goodfellow, I. The genome-linked protein VPg of vertebrate viruses—A multifaceted protein. Curr. Opin. Virol. 2011, 1, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Wang, A. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J. Virol. 2008, 82, 12252–12264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Juárez, S.; Hartweck, L.; Alamillo, J.M.; Simón-Mateo, C.; Pérez, J.J.; Fernández-Fernández, M.R.; Olszewski, N.E.; García, J.A. Identification of secret agent as the O-GlcNAc transferase that participates in Plum pox virus infection. J. Virol. 2005, 79, 9381–9387. [Google Scholar] [CrossRef] [Green Version]
- Lõhmus, A.; Hafrén, A.; Mäkinen, K. Coat protein regulation by CK2, CPIP, HSP70 and CHIP is required for Potato virus A replication and coat protein accumulation. J. Virol. 2017, 91, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; He, R.; Bernards, M.A.; Wang, A. The cis-expression of the coat protein of turnip mosaic virus is essential for viral intercellular movement in plants. Mol. Plant Pathol. 2020, 21, 1194–1211. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, X.D.; Wang, Q.; Gao, L.W.; Yang, Y.; Xiao, D.; Liu, T.K.; Li, Y.; Hou, X.L.; Zhang, C.W. Efficient virus-induced gene silencing in Brassica rapa using a turnip yellow mosaic virus vector. Biol. Plant. 2018, 62, 826–834. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Lyu, S.; Wang, Y.; E, L.; Liu, T.; Hou, X.; Li, Y.; Zhang, C. Host Factors Genes BcCLC1 and BcCLC2 Confer Turnip Mosaic Virus Resistance in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis). Plants 2023, 12, 2269. https://doi.org/10.3390/plants12122269
Yuan M, Lyu S, Wang Y, E L, Liu T, Hou X, Li Y, Zhang C. Host Factors Genes BcCLC1 and BcCLC2 Confer Turnip Mosaic Virus Resistance in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis). Plants. 2023; 12(12):2269. https://doi.org/10.3390/plants12122269
Chicago/Turabian StyleYuan, Mengguo, Shanwu Lyu, Yaolong Wang, Liu E, Tongkun Liu, Xilin Hou, Ying Li, and Changwei Zhang. 2023. "Host Factors Genes BcCLC1 and BcCLC2 Confer Turnip Mosaic Virus Resistance in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis)" Plants 12, no. 12: 2269. https://doi.org/10.3390/plants12122269
APA StyleYuan, M., Lyu, S., Wang, Y., E, L., Liu, T., Hou, X., Li, Y., & Zhang, C. (2023). Host Factors Genes BcCLC1 and BcCLC2 Confer Turnip Mosaic Virus Resistance in Non-Heading Chinese Cabbage (Brassica campestris ssp. chinensis). Plants, 12(12), 2269. https://doi.org/10.3390/plants12122269







