Hormetic Effect of Glyphosate on the Morphology, Physiology and Metabolism of Coffee Plants
Abstract
:1. Introduction
2. Results
2.1. Morphological and Growth Analyses
2.2. Physiological Analyses
2.3. Biochemical Analyses
3. Discussion
4. Materials and Methods
4.1. Morphological and Growth Analyses
4.2. Physiological Analyses
4.3. Biochemical Analyses
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, I.P.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest Manag. Sci. 2017, 74, 1064–1070. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Abbas, T.; Tanveer, A.; Maqbool, R.; Zohaib, A.; Shehzad, M.A. Glyphosate hormesis in broad-leaved weeds: A challenge for weed management. Arch. Agron. Soil Sci. 2016, 63, 344–351. [Google Scholar] [CrossRef]
- Belz, R.G.; Duke, S.O. Herbicides and plant hormesis. Pest Manag. Sci. 2014, 70, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Streibig, J.C.; Kudsk, P.; Mathiassen, S.K.; Duke, S.O. The Occurrence of Hormesis in Plants and Algae. Dose-Response 2007, 5, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Velini, E.D.; Alves, E.; Godoy, M.C.; Meschede, D.K.; Souza, R.T.; O Duke, S. Glyphosate applied at low doses can stimulate plant growth. Pest Manag. Sci. 2008, 64, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duke, S.; Cedergreen, N.; Belz, R.; Velini, E. Hormesis: Is It an Important Factor in Herbicide Use and Allelopathy? Outlooks Pest Manag. 2006, 17, 29–33. [Google Scholar] [CrossRef]
- dos Santos, J.C.C.; da Silva, D.M.R.; Amorim, D.J.; Sab, M.P.V.; de Almeida Silva, M. Glyphosate hormesis mitigates the effect of water deficit in safflower (Carthamus tinctorius L.). Pest Manag. Sci. 2021, 77, 2029–2044. [Google Scholar] [CrossRef]
- Anunciato, V.M.; Bianchi, L.; Gomes, G.L.G.C.; Velini, E.D.; Duke, S.O.; Carbonari, C.A. Effect of low glyphosate doses on flowering and seed germination of glyphosate-resistant and -susceptible Digitaria insularis. Pest Manag. Sci. 2022, 78, 1227–1239. [Google Scholar] [CrossRef]
- Strandberg, B.; Boutin, C.; Mathiassen, S.K.; Damgaard, C.; Dupont, Y.L.; Carpenter, D.J.; Kudsk, P. Effects of Herbicides on Non-Target Terrestrial Plants. In Pesticide Dose: Effects on the Environment and Target and Non-Target Organisms; Duke, S.O., Kudsk, P., Solomon, K., Eds.; American Chemical Society: Washington, DC, USA, 2017; Volume 1249, pp. 149–166. [Google Scholar]
- Velini, E.D.; Carbonari, C.A.; Trindade, M.L.B.; Gomes, G.L.G.C.; Antuniassi, U.R. Variations in Pesticide Doses under Field Conditions. In Pesticide Dose: Effects on the Environment and Target and Non-Target Organisms; Duke, S.O., Kudsk, P., Solomon, K.R., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1249, pp. 47–60. ISBN 9780841232099. [Google Scholar]
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicide; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.S.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376–1380. [Google Scholar] [CrossRef] [Green Version]
- de María, N.; de Felipe, M.R.; Fernández-Pascual, M. Alterations induced by glyphosate on lupin photosynthetic apparatus and nodule ultrastructure and some oxygen diffusion related proteins. Plant Physiol. Biochem. 2005, 43, 985–996. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.-G.; Lee, K.-W.; Alam, I.; Lee, S.-H.; Bahk, J.D.; Lee, B.-H. Glyphosate-induced oxidative stress in rice leaves revealed by proteomic approach. Plant Physiol. Biochem. 2008, 46, 1062–1070. [Google Scholar] [CrossRef]
- Cedergreen, N.; Olesen, C.F. Can glyphosate stimulate photosynthesis? Pestic. Biochem. Physiol. 2010, 96, 140–148. [Google Scholar] [CrossRef]
- Cedergreen, N. Herbicides can stimulate plant growth. Weed Res. 2008, 48, 429–438. [Google Scholar] [CrossRef]
- Belz, R.G.; Carbonari, C.A.; Duke, S.O. The potential influence of hormesis on evolution of resistance to herbicides. Curr. Opin. Environ. Sci. Health 2022, 27, 100360. [Google Scholar] [CrossRef]
- Camilo dos Santos, J.C.; Ribeiro Silva, D.M.; Jardim Amorim, D.; do Rosário Rosa, V.; Farias dos Santos, A.L.; Domingues Velini, E.; Carbonari, C.A.; de Almeida Silva, M. Glyphosate hormesis attenuates water deficit stress in safflower (Carthamus tinctorius L.) by modulating physiological and biochemical mediators. Sci. Total Environ. 2022, 810, 152204. [Google Scholar] [CrossRef] [PubMed]
- El-Shahawy, T.A.; Sharara, F.A.A. Hormesis Influence of Glyphosate in Between Increasing Growth, Yield and Controlling Weeds in Faba bean. J. Am. Sci. 2011, 7, 139–144. [Google Scholar]
- Nascentes, R.F.; Carbonari, C.A.; Simões, P.S.; Brunelli, M.C.; Velini, E.D.; Duke, S.O. Low doses of glyphosate enhance growth, CO2 assimilation, stomatal conductance and transpiration in sugarcane and eucalyptus. Pest Manag. Sci. 2018, 74, 1197–1205. [Google Scholar] [CrossRef]
- Pincelli-Souza, R.P.; Bortolheiro, F.P.A.P.; Carbonari, C.A.; Velini, E.D.; Silva, M.D.A. Hormetic effect of glyphosate persists during the entire growth period and increases sugarcane yield. Pest Manag. Sci. 2020, 76, 2388–2394. [Google Scholar] [CrossRef]
- de Moraes, C.P.; de Brito, I.P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effect of glyphosate on Urochloa decumbens plants. J. Environ. Sci. Health Part B 2020, 55, 376–381. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Coffee: World Markets and Trade; United States Department of Agriculture Foreign Agricultural Service: Washington, DC, USA, 2022. [Google Scholar]
- Zaidan, U.R.; Campos, R.C.; Faria, R.M.; Zaidan, I.R.; de Souza, W.M.; Santos, R.H.S.; de Freitas, F.C.L. Productivity and grain size of coffee grown in different weed management systems. Acta Sci. Agron. 2022, 44, e55692. [Google Scholar] [CrossRef]
- França, A.C.; Carvalho, F.P.; Fialho, C.M.T.; D’antonino, L.; Silva, A.A.; Santos, J.B.; Ferreira, L.R. Simulated Glyphosate Drift on Acaiá and Catucaí Coffee Cultivars. Planta Daninha 2013, 31, 443–451. [Google Scholar] [CrossRef] [Green Version]
- França, A.C.; Freitas, M.A.M.; Fialho, C.M.T.; Silva, A.A.; Reis, M.R.; Galon, L.; Victoria Filho, R. Growth of Arabica Coffee Cultivars Submitted to Glyphosate Doses. Planta Daninha 2010, 28, 599–607. [Google Scholar] [CrossRef]
- Nelson, S. Glyphosate Herbicide Injury to Coffee; Cooperative Extension Service: Palmer, AK, USA, 2008. [Google Scholar]
- Castanheira, D.T.; Alecrim, A.D.O.; Voltolini, G.B.; Rezende, T.T.; Netto, P.M.; Guimarães, R.J. Growth, Anatomy and Physiology of Coffee Plants Intoxicated by the Herbicide Glyphosate. Coffee Sci. 2019, 14, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Schrübbers, L.C.; Valverde, B.E.; Sørensen, J.C.; Cedergreen, N. Glyphosate spray drift in Coffea arabica—Sensitivity of coffee plants and possible use of shikimic acid as a biomarker for glyphosate exposure. Pestic. Biochem. Physiol. 2014, 115, 15–22. [Google Scholar] [CrossRef]
- De Carvalho, L.B.; Alves, P.L.; Duke, S.O. Hormesis with glyphosate depends on coffee growth stage. An. Da Acad. Bras. De Ciências 2013, 85, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Carvalho, L.B.; Alves, P.L.C.A.; Bianco, S.; De Prado, R. Physiological Dose-Response of Coffee (Coffea arabica L.) Plants to Glyphosate Depends on Growth Stage. Chil. J. Agric. Res. 2012, 72, 182–187. [Google Scholar] [CrossRef]
- Domingues Júnior, A.P. Avaliação Dos Efeitos Do Herbicida Glifosato Sobre o Cafeiro: Respostas Bioquímicas e Fisiológicas. Master’s Dissertation, Universidade Estadual de Campinas, Campinas, Brazil, 2011. [Google Scholar]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A Compelling Platform for Sophisticated Plant Science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef]
- Belz, R.G.; Duke, S.O. Herbicide-Mediated Hormesis. ACS Symp. Ser. 2017, 1249, 135–148. [Google Scholar] [CrossRef]
- Calabrese, E.J. Paradigm lost, paradigm found: The re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ. Pollut. 2005, 138, 378–411. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. The Dose Determines the Stimulation (and Poison): Development of A Chemical Hormesis Database. Int. J. Toxicol. 1997, 16, 545–559. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Fisiologia e Desenvolvimento Vegetal, 6th ed.; Artmed: Porto Alegre, Brazil, 2017. [Google Scholar]
- Yanniccari, M.; Tambussi, E.; Istilart, C.; Castro, A.M. Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 2012, 57, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.L.; Duke, S.O.; Dayan, F.E.; Velini, E.D. Low doses of glyphosate change the responses of soyabean to subsequent glyphosate treatments. Weed Res. 2015, 56, 124–136. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krall, J.P.; Edwards, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Energy dissipation in C3 plants under drought. Funct. Plant Biol. 2002, 29, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Parra, C.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.; Morales, F. Photosynthetic response of Tempranillo grapevine to climate change scenarios. Ann. Appl. Biol. 2012, 161, 277–292. [Google Scholar] [CrossRef]
- Amrhein, N.; Deus, B.; Gehrke, P.; Steinrücken, H.C. The Site of the Inhibition of the Shikimate Pathway by Glyphosate. Plant Physiol. 1980, 66, 830–834. [Google Scholar] [CrossRef] [Green Version]
- Velini, E.D.; Carbonari, C.A.; Trindade, M.L.B.; Gomes, G.L.G.C.; Mesched, D.K.; Duke, S.O. Modo de Ação Do Glyphosate. In Glyphosate: Uso Sustetável; Velini, E.D., Carbonari, C.A., Mechede, D.K., Trindade, M.L.B., Eds.; FEPAF: Borucatu, Brazil, 2012; pp. 39–66. [Google Scholar]
- Schrübbers, L.C.; Valverde, B.E.; Strobel, B.W.; Cedergreen, N. Glyphosate accumulation, translocation, and biological effects in Coffea arabica after single and multiple exposures. Eur. J. Agron. 2016, 74, 133–143. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Gomes, G.L.G.C.; Velini, E.D.; Machado, R.F.; Simões, P.S.; Macedo, G.D.C. Glyphosate Effects on Sugarcane Metabolism and Growth. Am. J. Plant Sci. 2014, 05, 3585–3593. [Google Scholar] [CrossRef] [Green Version]
- Anderson, K.A.; Cobb, W.T.; Loper, B.R. Analytical method for determination of shikimic acid: Shikimic acid proportional to glyphosate application rates. Commun. Soil Sci. Plant Anal. 2001, 32, 2831–2840. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. THE SHIKIMATE PATHWAY. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Tazaki, K.; Minamikawa, T. Occurrence of shikimic and quinic acids in angiosperms. Phytochemistry 1975, 14, 195–197. [Google Scholar] [CrossRef]
- Zabalza, A.; Orcaray, L.; Fernández-Escalada, M.; Zulet-González, A.; Royuela, M. The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic. Biochem. Physiol. 2017, 141, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerril, J.; Duke, S.O.; Lydon, J. Glyphosate effects on shikimate pathway products in leaves and flowers of velvetleaf. Phytochemistry 1989, 28, 695–699. [Google Scholar] [CrossRef]
- Orcaray, L.; Igal, M.; Marino, D.; Zabalza, A.; Royuela, M. The possible role of quinate in the mode of action of glyphosate and acetolactate synthase inhibitors. Pest Manag. Sci. 2010, 66, 262–269. [Google Scholar] [CrossRef]
- Boudet, A.M. Polyphenols: From Plant Adaptation to Useful Chemical Resources. In Recent Advances in Polyphenol Research; Cheynie, V., Sarni-Manchado, P., Quideau, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 3, pp. 41–70. ISBN 9781444337464. [Google Scholar]
- Gomes, G.L.G.C.; Velini, E.D.; Carbonari, C.A. Fosfito de potássio não protege plantas de milho contra os efeitos fitotóxicos do glyphosate1. Pesqui. Agropecuária Trop. 2015, 45, 291–296. [Google Scholar] [CrossRef]
- Kumar, D.; Chapagai, D.; Dean, P.; Davenport, M. Biotic and Abiotic Stress Signaling Mediated by Salicylic Acid. In Elucidation of Abiotic Stress Signaling in Plants; Pandey, G.K., Ed.; Springer: New York, NY, USA, 2015; pp. 329–346. ISBN 9781493922116. [Google Scholar]
- Gazola, T. Ação Do Herbicida Glyphosate Em Biótipos de Digitaria insularis Resistentes e Suscetíveis. Master’s Dissertation, Universidade Estadual Paulista “Júlio de Mesquita Filho”, São Paulo, Brazil, 2017. [Google Scholar]
- Gomes, G.L.G.C. Caracterização Bioquímica e Morfofisiológica de Populações de Buva (Conyza spp.) Resistentes Ao Glyphosate. Doctoral Thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”, São Paulo, Brazil, 2014. [Google Scholar]
- Meschede, D.K.; Velini, E.D.; Tonin, F.G.; Carbonari, C.A. Alterações No Metabolismo Da Cana-de-Açúcar Em Função Da Aplicação de Maturadores. Planta Daninha 2012, 30, 113–119. [Google Scholar] [CrossRef]
- Fernández-Escalada, M.; Zulet-González, A.; Gil-Monreal, M.; Zabalza, A.; Ravet, K.; Gaines, T.; Royuela, M.; Christoffoleti, P.J. Effects of EPSPS Copy Number Variation (CNV) and Glyphosate Application on the Aromatic and Branched Chain Amino Acid Synthesis Pathways in Amaranthus palmeri. Front. Plant Sci. 2017, 8, 1970. [Google Scholar] [CrossRef] [Green Version]
- Gravena, R.; Filho, R.V.; Alves, P.L.C.; Mazzafera, P.; Gravena, A.R. Low glyphosate rates do not affect Citrus limonia (L.) Osbeck seedlings. Pest Manag. Sci. 2009, 65, 420–425. [Google Scholar] [CrossRef]
- Maroli, A.S.; Nandula, V.K.; Dayan, F.E.; Duke, S.O.; Gerard, P.; Tharayil, N. Metabolic Profiling and Enzyme Analyses Indicate a Potential Role of Antioxidant Systems in Complementing Glyphosate Resistance in an Amaranthus palmeri Biotype. J. Agric. Food Chem. 2015, 63, 9199–9209. [Google Scholar] [CrossRef]
- Petersen, I.L.; Hansen, H.C.B.; Ravn, H.W.; Sørensen, J.C.; Sørensen, H. Metabolic effects in rapeseed (Brassica napus L.) seedlings after root exposure to glyphosate. Pestic. Biochem. Physiol. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Zulet, A.; Gil-Monreal, M.; Villamor, J.G.; Zabalza, A.; van der Hoorn, R.; Royuela, M. Proteolytic Pathways Induced by Herbicides That Inhibit Amino Acid Biosynthesis. PLoS ONE 2013, 8, e73847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Escalada, M.; Gil-Monreal, M.; Zabalza, A.; Royuela, M. Characterization of the Amaranthus palmeri Physiological Response to Glyphosate in Susceptible and Resistant Populations. J. Agric. Food Chem. 2016, 64, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velini, E.D.; Meschede, D.K.; Carbonari, C.A.; Trindade, M.L.B. (Eds.) Características e Uso Do Glyphosate. In Glyphosate: Uso Sustetável; FEPAF: Borucatu, Brazil, 2012; pp. 11–16. [Google Scholar]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef] [PubMed]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Hattley, H.O. The Use of Range in Analysis of Variance. Biometrika 1950, 37, 271–280. [Google Scholar] [CrossRef]
- Streibig, J.C. Herbicide bioassay. Weed Res. 1988, 28, 479–484. [Google Scholar] [CrossRef]
- Brain, P.; Cousens, R. An equation to describe dose responses where there is stimulation of growth at low doses. Weed Res. 1988, 29, 93–96. [Google Scholar] [CrossRef]
- Streibig, J.C. Models for Curve-fitting Herbicide Dose Response Data. Acta Agric. Scand. 1980, 30, 59–64. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R Software Package for Dose-Response Studies: The Concept and Data Analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Schabenberger, O.; Tharp, B.E.; Kells, J.J.; Penner, D.; Schabenberger, O.; Tharp, B.E.; Kells, J.J.; Penner, D. STATISTICS Statistical Tests for Hormesis and Effective Dosages in Herbicide Dose Response Mesis (from the Greek for ’Setting into Motion’). Agron. J. 1999, 91, 713–721. [Google Scholar] [CrossRef]
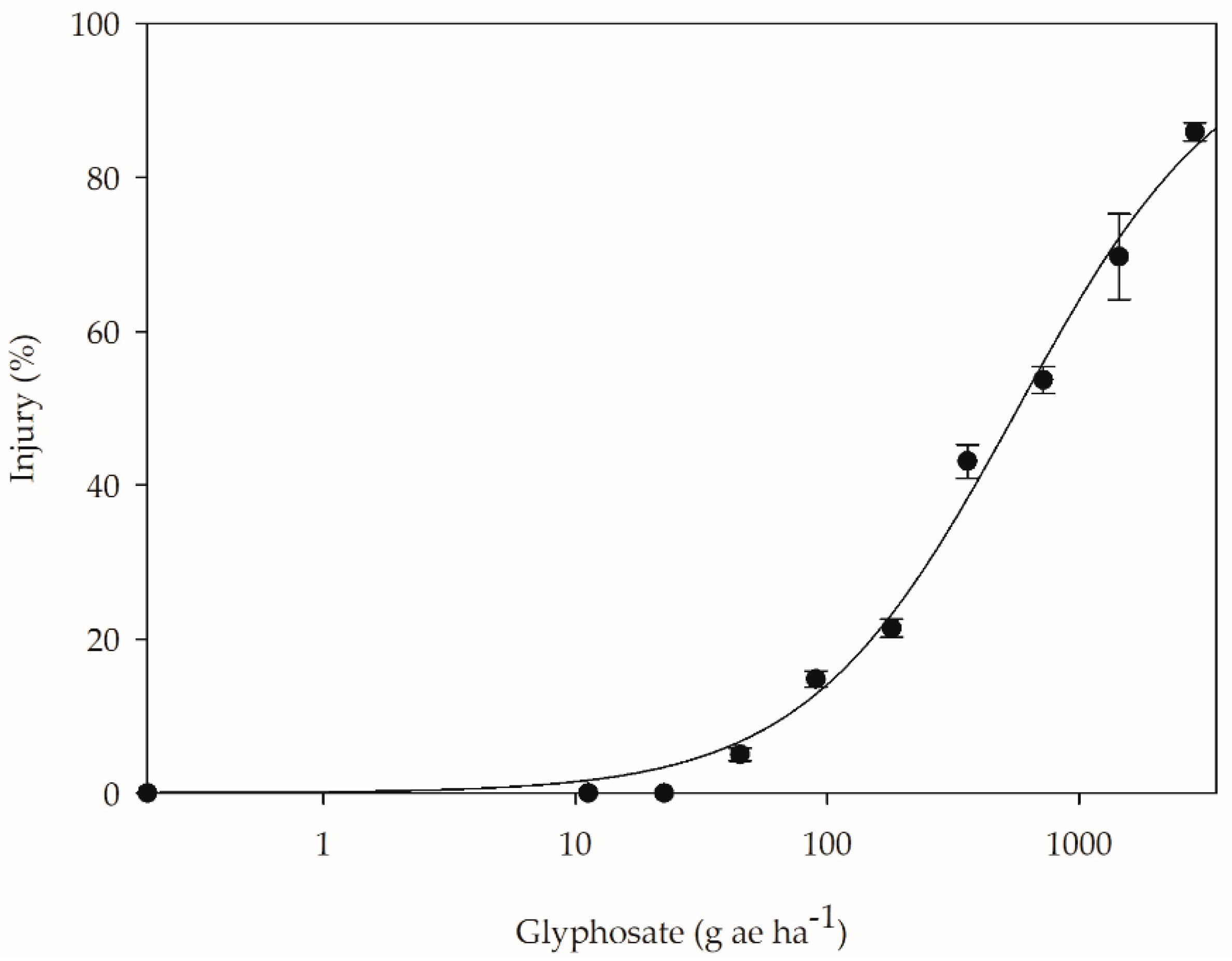

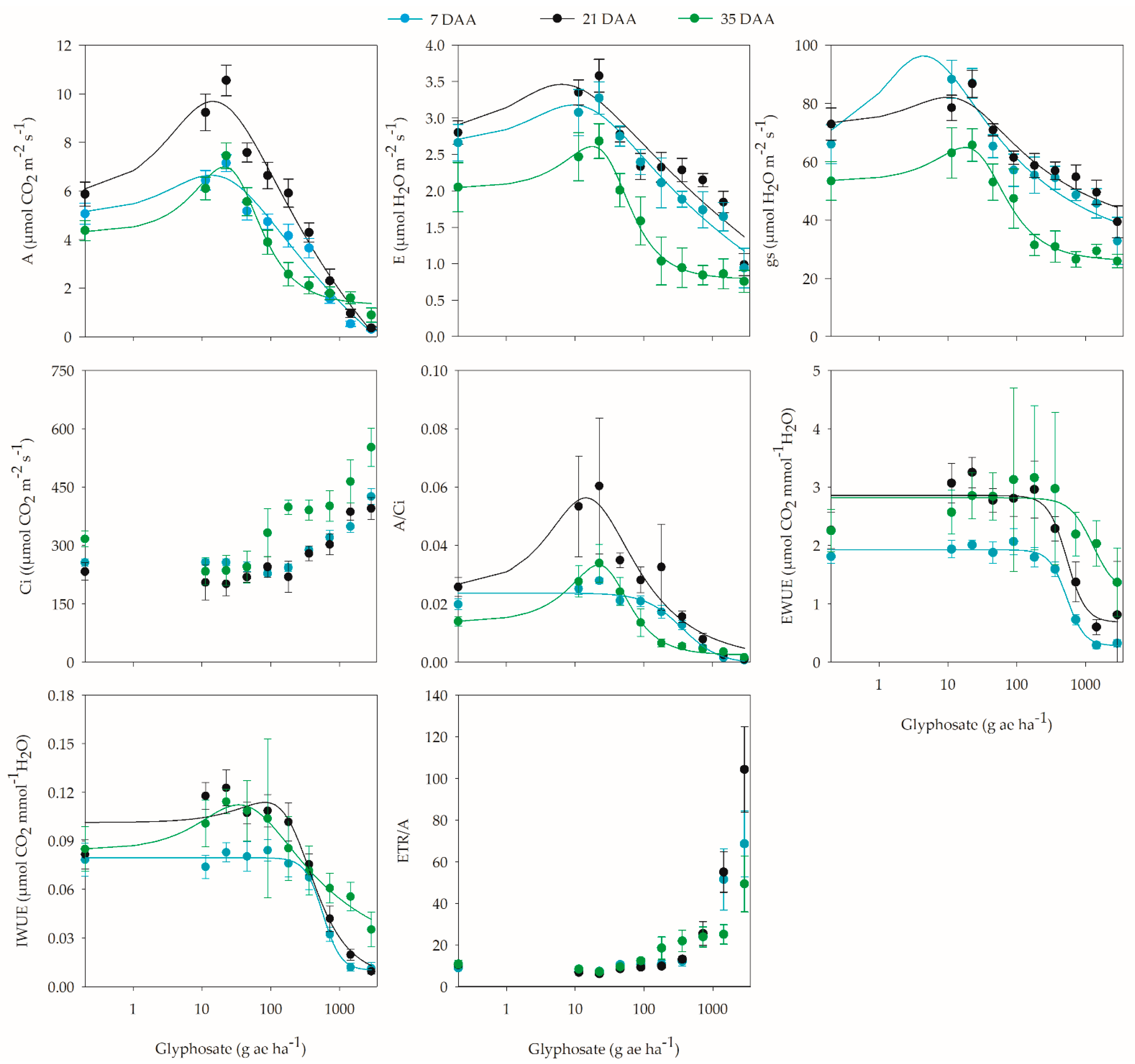

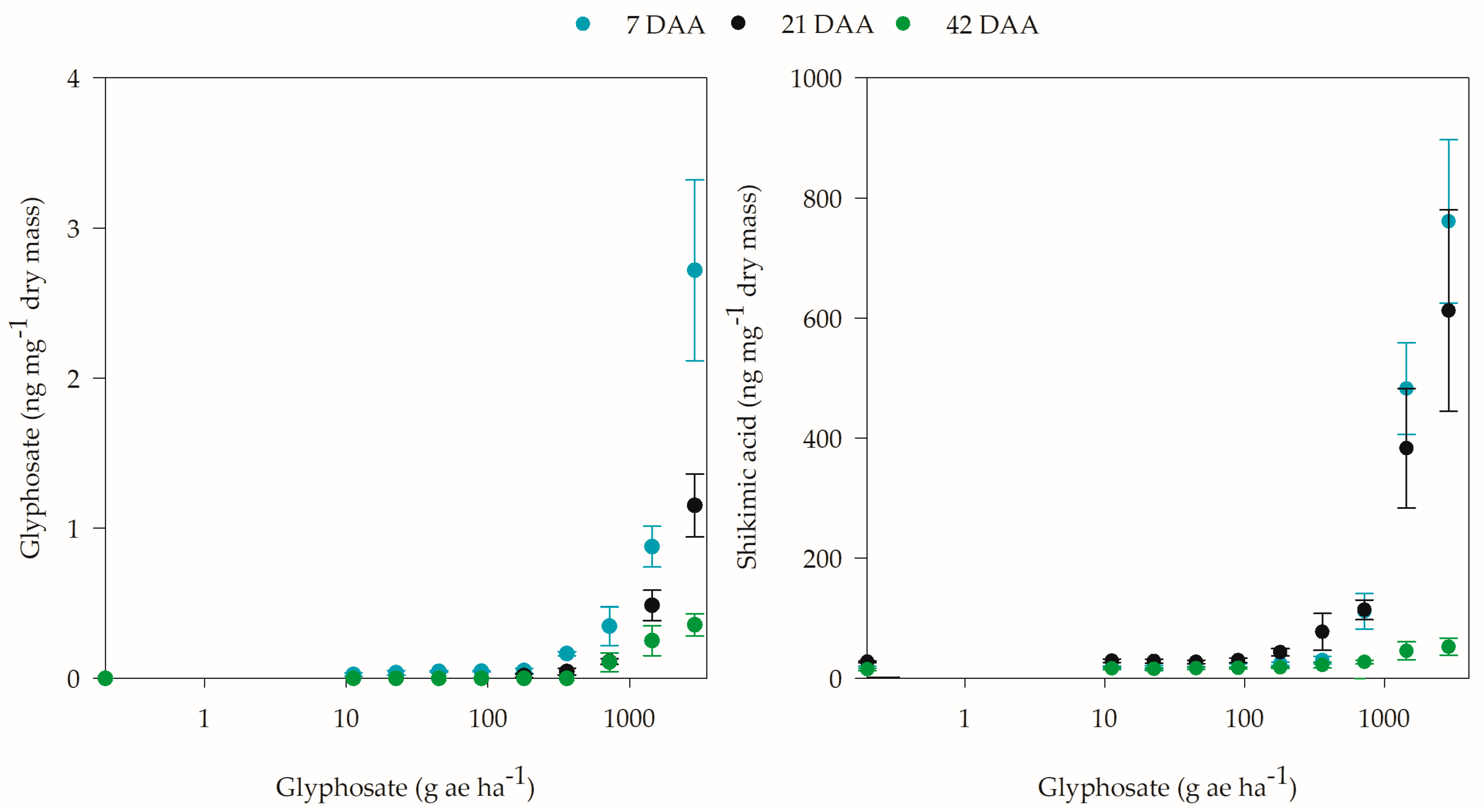
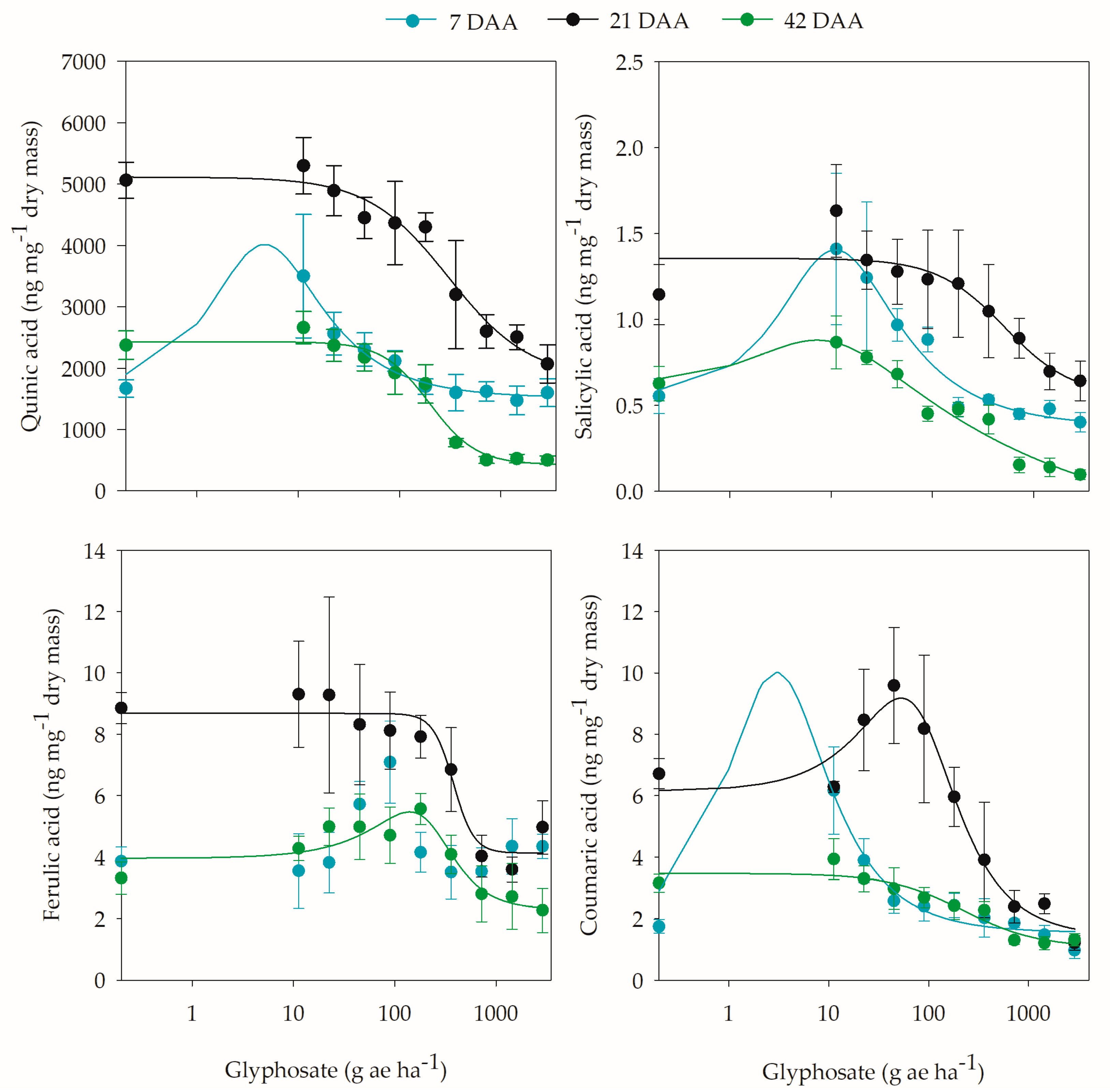

| Variables | Stimulant Dose Range 1 (g ae ha−1) | Maximum Stimulating Dose 2 (g ae ha−1) | Maximum Stimulation (%) | Period of Evaluation 3 |
|---|---|---|---|---|
| Morphological and growth | ||||
| Plant height | 4.6–49.1 | 21.5 | 10.2 | 42 |
| Number of leaves | 6.7–52.8 | 29.9 | 12.2 | 42 |
| Leaf area | 2.3–45.3 | 19.9 | 19.0 | 42 |
| Dry leaf mass | 2.0–47.7 | 17.0 | 14.8 | 42 |
| Stem dry mass | 1.4–54.4 | 14.5 | 13.6 | 42 |
| Total dry mass | 1.9–51.0 | 17.0 | 14.9 | 42 |
| Physiological | ||||
| CO2 assimilation | 0.3–128.8 | 14.4 | 39.6 | 21 |
| Transpiration | 1.7–44.1 | 17.8 | 22.1 | 35 |
| Stomatal conductance | 0.2–49.5 | 4.4 | 31.4 | 7 |
| Carboxylation efficiency | 0.4–87.0 | 22.0 | 58.7 | 35 |
| Intrinsic water use efficiency | 1.7–170.0 | 35.0 | 24.8 | 35 |
| Rate of electron transport | 3.8–197.2 | 50.0 | 14.7 | 7 |
| Photochemical efficiency of PSII | 5.1–194.5 | 55.0 | 14.8 | 7 |
| Biochemical | ||||
| Quinic acid | 0.1–184.0 | 4.6 | 58.3 | 7 |
| Salicylic acid | 0.2–218.0 | 11.0 | 60.5 | 7 |
| Caffeic acid | 11.2–353.0 | 140.0 | 27.7 | 42 |
| Coumaric acid | 0.02–251.0 | 3.0 | 82.6 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, R.N.; Bevilaqua, N.d.C.; Krenchinski, F.H.; Giovanelli, B.F.; Pereira, V.G.C.; Velini, E.D.; Carbonari, C.A. Hormetic Effect of Glyphosate on the Morphology, Physiology and Metabolism of Coffee Plants. Plants 2023, 12, 2249. https://doi.org/10.3390/plants12122249
Costa RN, Bevilaqua NdC, Krenchinski FH, Giovanelli BF, Pereira VGC, Velini ED, Carbonari CA. Hormetic Effect of Glyphosate on the Morphology, Physiology and Metabolism of Coffee Plants. Plants. 2023; 12(12):2249. https://doi.org/10.3390/plants12122249
Chicago/Turabian StyleCosta, Renato Nunes, Natalia da Cunha Bevilaqua, Fábio Henrique Krenchinski, Bruno Flaibam Giovanelli, Vinicius Gabriel Caneppele Pereira, Edivaldo Domingues Velini, and Caio Antonio Carbonari. 2023. "Hormetic Effect of Glyphosate on the Morphology, Physiology and Metabolism of Coffee Plants" Plants 12, no. 12: 2249. https://doi.org/10.3390/plants12122249
APA StyleCosta, R. N., Bevilaqua, N. d. C., Krenchinski, F. H., Giovanelli, B. F., Pereira, V. G. C., Velini, E. D., & Carbonari, C. A. (2023). Hormetic Effect of Glyphosate on the Morphology, Physiology and Metabolism of Coffee Plants. Plants, 12(12), 2249. https://doi.org/10.3390/plants12122249









