Algal Ocelloids and Plant Ocelli
Abstract
1. Introduction
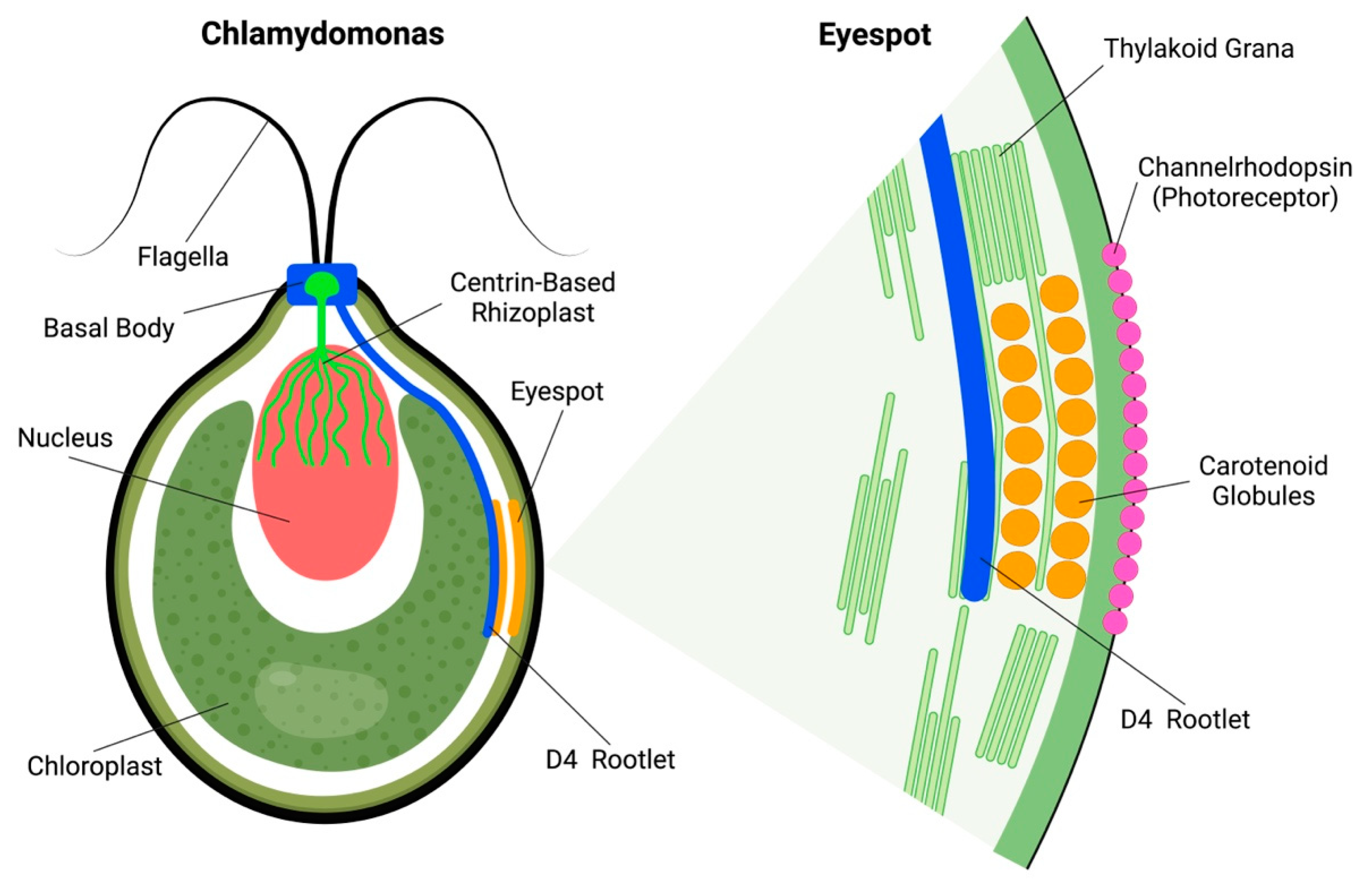
2. Chlamydomonas Algal Eyespot: Rhizoplast and Rootlet Connections
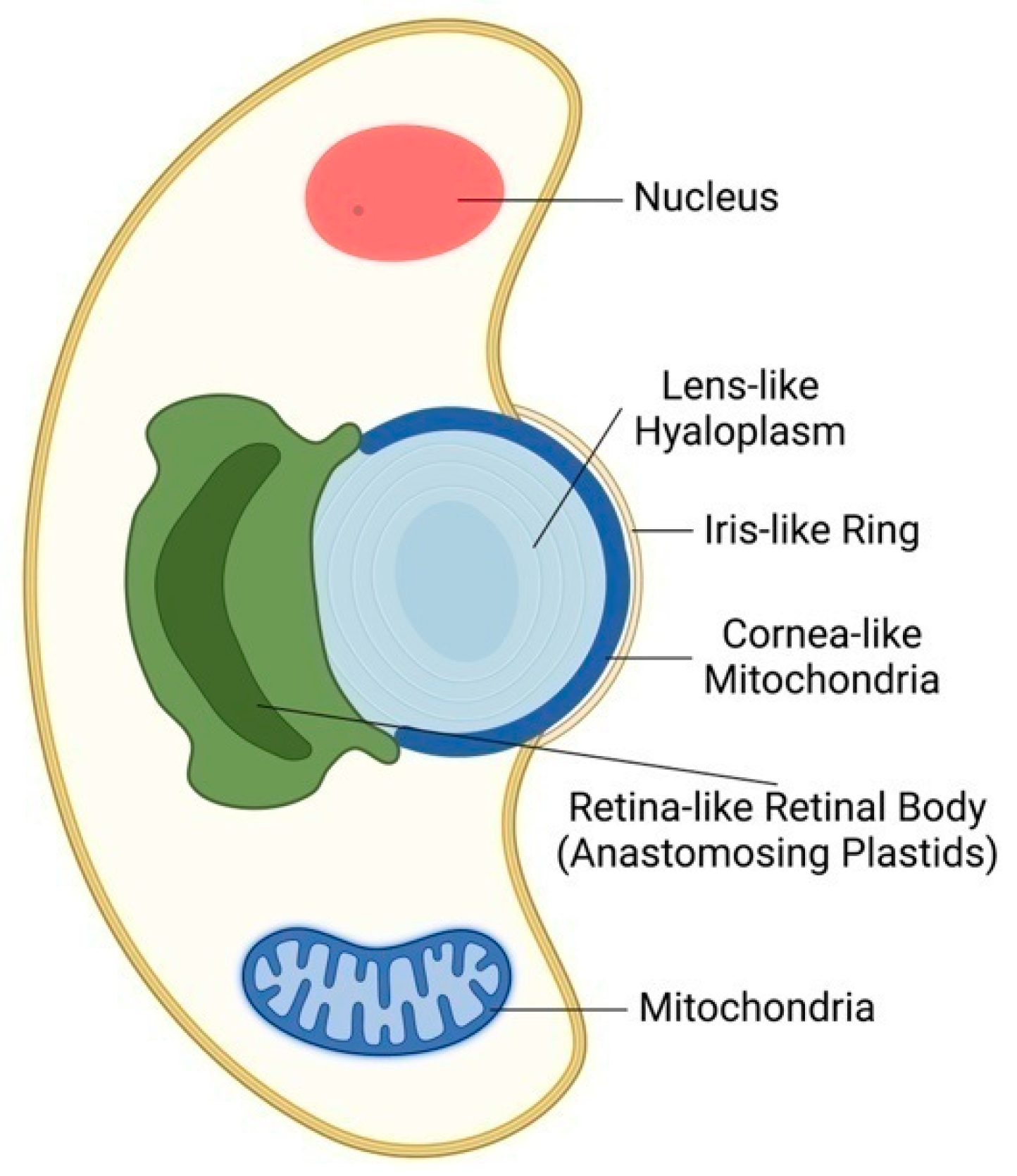
3. From Algal Ocelloids to Plant Ocelli
4. Bacterial Vision: Cyanobacterium Synechocystis
5. Plant Vision: Boquila trifoliata
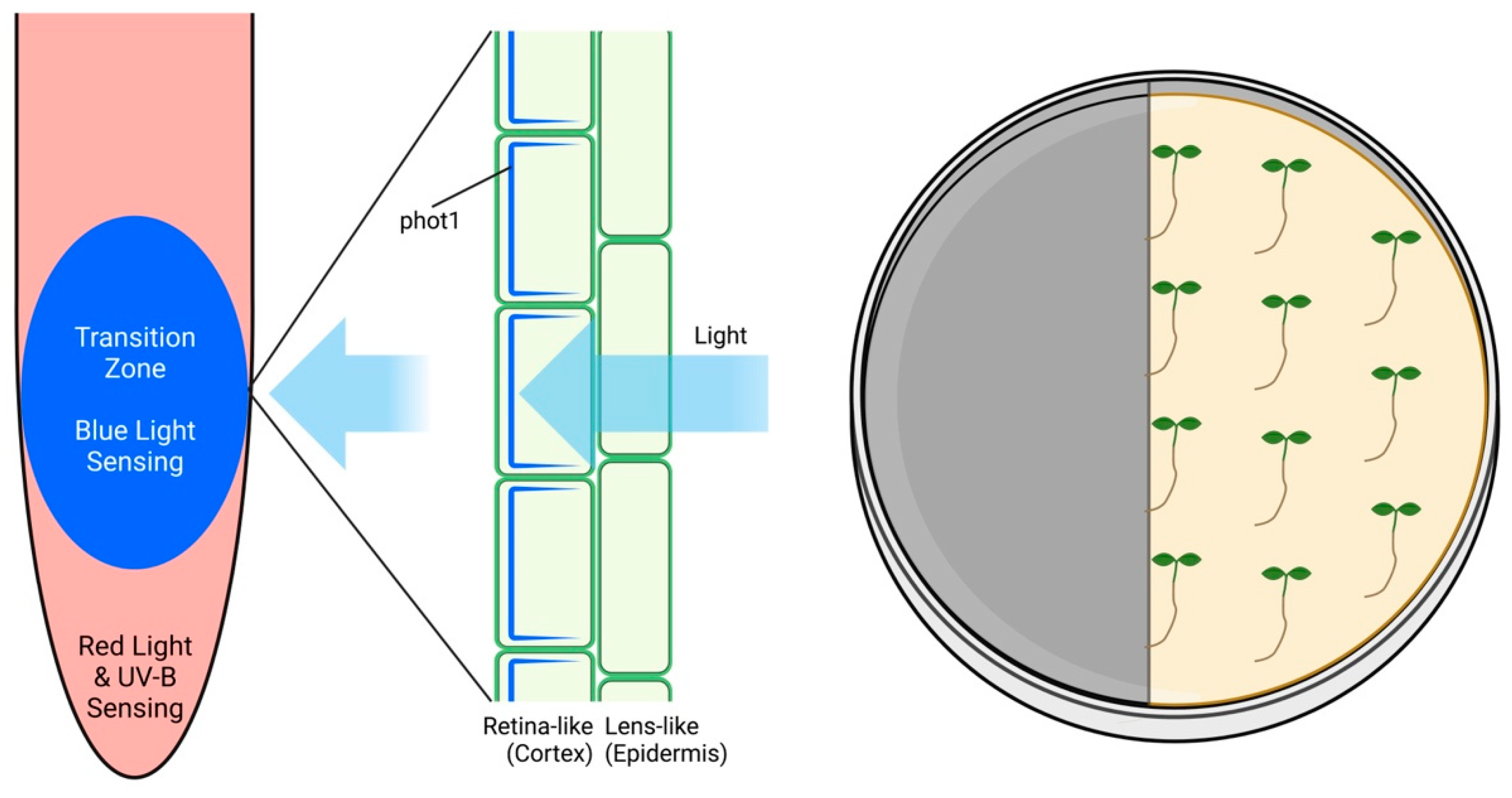
6. Root Apex Vision: Root Skototropism
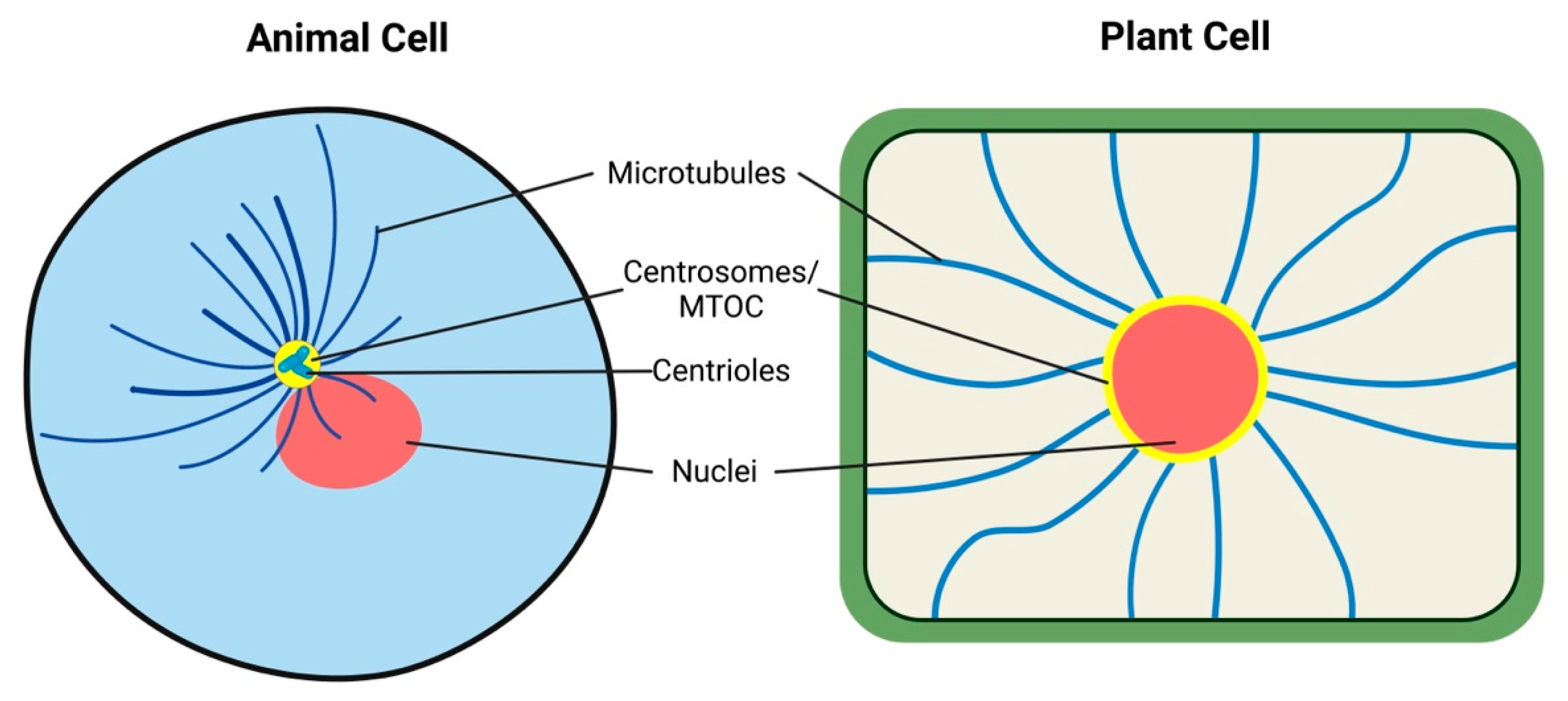
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilsson, D.-E.; Bok, M.J. Low-resolution vision—At the hub of eye evolution. Integr. Comp. Biol. 2017, 57, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, D.-E. The diversity of eyes and vision. Annu. Rev. Vis. Sci. 2021, 7, 19–41. [Google Scholar] [CrossRef]
- Nilsson, D.E. The evolution of visual roles—Ancient vision versus object vision. Front. Neuroanat. 2022, 16, 789375. [Google Scholar] [CrossRef]
- Richards, T.A.; Gomes, S.L. How to build a microbial eye. Nature 2015, 523, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Takaku, Y.; Hwang, J.S.; Horiguchi, T.; Suga, H.; Gehring, W.; Ikeo, K.; Gojobori, T. Function and evolutionary origin of unicellular camera-type eye structure. PLoS ONE 2015, 10, e0118415. [Google Scholar] [CrossRef]
- Galindo, L.J.; Milner, D.S.; Gomes, S.L.; Richards, T.A. A Light-sensing system in the common ancestor of the fungi. Curr. Biol. 2022, 32, 3146–3153.e3. [Google Scholar] [CrossRef] [PubMed]
- Haberlandt, G. Die Lichtsinnesorgane der Laubblätter; Engelmann: Leipzig, Germany, 1905. [Google Scholar]
- Baluška, F.; Mancuso, S. Vision in plants via plant-specific ocelli? Trends Plant Sci. 2016, 21, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Baluška, F. Plant ocelli for visually guided plant behavior. Trends Plant Sci. 2017, 22, 5–6. [Google Scholar] [CrossRef]
- Strong, D.R.; Ray, T.S. Host tree location behavior of a tropical vine (Monstera gigantea) by skototropism. Science 1975, 190, 804–806. [Google Scholar] [CrossRef]
- Salisbury, J.L.; Baron, A.; Surek, B.; Melkonian, M. Striated Flagellar roots: Isolation and partial characterization of a calcium-modulated contractile organelle. J. Cell Biol. 1984, 99, 962–970. [Google Scholar] [CrossRef]
- Salisbury, J.L.; Baron, A.T.; Sanders, M.A. The Centrin-based cytoskeleton of Chlamydomonas reinhardtii: Distribution in interphase and mitotic cells. J. Cell Biol. 1988, 107, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.L.; Adler, S.A.; Spanier, J.G.; Jarvik, J.W. Nucleus-basal body connector in Chlamydomonas: Evidence for a role in basal body segregation and against essential roles in mitosis or in determining cell polarity. Cell Motil. Cytoskelet. 1989, 14, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Lechtreck, K.-F.; Melkonian, M. An update on fibrous flagellar roots in green algae. In The Cytoskeleton of Flagellate and Ciliate Protists; Springer: Vienna, Austria, 1991; pp. 38–44. [Google Scholar]
- Salisbury, J.L. Roots. J. Eukaryot. Microbiol. 1998, 45, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, S.K. Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic 2003, 4, 443–451. [Google Scholar] [CrossRef]
- Geimer, S.; Melkonian, M. Centrin scaffold in Chlamydomonas reinhardtii revealed by immunoelectron microscopy. Eukaryot. Cell 2005, 4, 1253–1263. [Google Scholar] [CrossRef]
- Mahen, R. The structure and function of centriolar rootlets. J. Cell Sci. 2021, 134. [Google Scholar] [CrossRef]
- Owa, M.; Furuta, A.; Usukura, J.; Arisaka, F.; King, S.M.; Witman, G.B.; Kamiya, R.; Wakabayashi, K. Cooperative binding of the outer arm-docking complex underlies the regular arrangement of outer arm dynein in the axoneme. Proc. Natl. Acad. Sci. USA 2014, 111, 9461–9466. [Google Scholar] [CrossRef]
- Mittelmeier, T.M.; Thompson, M.D.; Öztürk, E.; Dieckmann, C.L. Independent localization of plasma membrane and chloroplast components during eyespot assembly. Eukaryot. Cell 2013, 12, 1258–1270. [Google Scholar] [CrossRef]
- Mittelmeier, T.M.; Boyd, J.S.; Lamb, M.R.; Dieckmann, C.L. Asymmetric properties of the Chlamydomonas reinhardtii cytoskeleton direct rhodopsin photoreceptor localization. J. Cell Biol. 2011, 193, 741–753. [Google Scholar] [CrossRef][Green Version]
- Boyd, J.S.; Gray, M.M.; Thompson, M.D.; Horst, C.J.; Dieckmann, C.L. the daughter four-membered microtubule rootlet determines anterior-posterior positioning of the eyespot in Chlamydomonas reinhardtii. Cytoskeleton 2011, 68, 459–469. [Google Scholar] [CrossRef]
- Baluška, F.; Lyons, S. Energide–cell body as smallest unit of eukaryotic life. Ann. Bot. 2018, 122, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Lyons, S. Archaeal origins of eukaryotic cell and nucleus. Biosystems 2021, 203, 104375. [Google Scholar] [CrossRef] [PubMed]
- Morishita, J.; Tokutsu, R.; Minagawa, J.; Hisabori, T.; Wakabayashi, K. Characterization of Chlamydomonas reinhardtii mutants that exhibit strong positive phototaxis. Plants 2021, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Misawa, Y.; Mochiji, S.; Kamiya, R. Reduction-oxidation poise regulates the sign of phototaxis in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2011, 108, 11280–11284. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Isu, A.; Ueki, N. Channelrhodopsin-dependent photo-behavioral responses in the unicellular green alga Chlamydomonas reinhardtii. Optogenetics 2021, 1293, 21–33. [Google Scholar]
- Schmidt, M.; Geßner, G.; Luff, M.; Heiland, I.; Wagner, V.; Kaminski, M.; Geimer, S.; Eitzinger, N.; Reißenweber, T.; Voytsekh, O.; et al. Proteomic analysis of the eyespot of Chlamydomonas reinhardtii provides novel insights into its components and tactic movements. Plant Cell 2006, 18, 1908–1930. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef]
- Ueki, N.; Ide, T.; Mochiji, S.; Kobayashi, Y.; Tokutsu, R.; Ohnishi, N.; Yamaguchi, K.; Shigenobu, S.; Tanaka, K.; Minagawa, J.; et al. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 5299–5304. [Google Scholar] [CrossRef]
- Kreimer, G. The green algal eyespot apparatus: A primordial visual system and more? Curr. Genet. 2009, 55, 19–43. [Google Scholar] [CrossRef]
- Hegemann, P. Algal sensory photoreceptors. Annu. Rev. Plant Biol. 2008, 59, 167–189. [Google Scholar] [CrossRef]
- Hegemann, P. Vision in microalgae. Planta 1997, 203, 265–274. [Google Scholar] [CrossRef]
- Holland, E.M.; Harz, H.; Uhl, R.; Hegemann, P. Control of phobic behavioral responses by rhodopsin-induced photocurrents in Chlamydomonas. Biophys. J. 1997, 73, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Sineshchekov, O.A.; Spudich, J.L. Sensory rhodopsin signaling in green flagellate algae. In Handbook of Photosensory Receptors; Wiley-VCH: Weinheim, Germany, 2005; pp. 25–42. [Google Scholar] [CrossRef]
- Berthold, P.; Tsunoda, S.P.; Ernst, O.P.; Mages, W.; Gradmann, D.; Hegemann, P. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell 2008, 20, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Tanno, Y.; Kato, S.; Ozasa, K.; Wakazaki, M.; Sato, M.; Toyooka, K.; Maoka, T.; Ishikawa, T.; Maeda, M.; et al. Carotenoid accumulation in the eyespot apparatus required for phototaxis is independent of chloroplast development in Euglena gracilis. Plant Sci. 2020, 298, 110564. [Google Scholar] [CrossRef]
- Kato, S.; Ozasa, K.; Maeda, M.; Tanno, Y.; Tamaki, S.; Higuchi-Takeuchi, M.; Numata, K.; Kodama, Y.; Sato, M.; Toyooka, K.; et al. Carotenoids in the eyespot apparatus are required for triggering phototaxis in Euglena gracilis. Plant J. 2020, 101, 1091–1102. [Google Scholar] [CrossRef]
- Hyams, J.S. The Euglena paraflagellar rod: Structure, relationship to other flagellar components and preliminary biochemical characterization. J. Cell Sci. 1982, 55, 199–210. [Google Scholar] [CrossRef]
- Ozasa, K.; Kang, H.; Song, S.; Tamaki, S.; Shinomura, T.; Maeda, M. Regeneration of the eyespot and flagellum in Euglena gracilis during cell division. Plants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Burki, F. The eukaryotic tree of life from a global phylogenomic perspective. Cold Spring Harb. Perspect. Biol. 2014, 6, a016147. [Google Scholar] [CrossRef]
- Francis, D. On the eyespot of the dinoflagellate, Nematodinium. J. Exp. Biol. 1967, 47, 495–501. [Google Scholar] [CrossRef]
- Gavelis, G.S.; Hayakawa, S.; White III, R.A.; Gojobori, T.; Suttle, C.A.; Keeling, P.J.; Leander, B.S. Eye-like ocelloids are built from different endosymbiotically acquired components. Nature 2015, 523, 204–207. [Google Scholar] [CrossRef]
- Nilsson, D.-E.; Marshall, J. Lens eyes in protists. Curr. Biol. 2020, 30, R458–R459. [Google Scholar] [CrossRef] [PubMed]
- Colley, N.J.; Nilsson, D.-E. Photoreception in phytoplankton. Integr. Comp. Biol. 2016, 56, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Lenn, T.; Kampmann, R.; Meissner, M.V.; Esteves, T.; Temerinac-Ott, M.; Korvink, J.G.; Lowe, A.R.; Mullineaux, C.W.; Wilde, A. Cyanobacteria use micro-optics to sense light direction. eLife 2016, 5, e12620. [Google Scholar] [CrossRef] [PubMed]
- Schuergers, N.; Mullineaux, C.W.; Wilde, A. Cyanobacteria in motion. Curr. Opin. Plant Biol. 2017, 37, 109–115. [Google Scholar] [CrossRef]
- Dieckmann, C.; Mittelmeier, T. Life in focus. eLife 2016, 5, e14169. [Google Scholar] [CrossRef]
- Nilsson, D.-E.; Colley, N.J. Comparative Vision: Can bacteria really see? Curr. Biol. 2016, 26, R369–R371. [Google Scholar] [CrossRef]
- Kessler, J.O.; Nedelcu, A.M.; Solari, C.A.; Shelton, D.E. Cells acting as lenses: A possible role for light in the evolution of morphological asymmetry in multicellular volvocine algae. In Evolutionary Transitions to Multicellular Life; Springer: Berlin/Heidelberg, Germany, 2015; pp. 225–243. [Google Scholar]
- Harwood, T.V.; Zuniga, E.G.; Kweon, H.; Risser, D.D. The cyanobacterial taxis protein HmpF regulates type IV pilus activity in response to light. Proc. Natl. Acad. Sci. USA 2021, 118, e2023988118. [Google Scholar] [CrossRef]
- Timsit, Y.; Lescot, M.; Valiadi, M.; Not, F. Bioluminescence and photoreception in unicellular organisms: Light-signalling in a bio-communication perspective. Int. J. Mol. Sci. 2021, 22, 11311. [Google Scholar] [CrossRef]
- Allaf, M.M.; Peerhossaini, H. Cyanobacteria: Model microorganisms and beyond. Microorganisms 2022, 10, 696. [Google Scholar] [CrossRef]
- White, J.; Yamashita, F. Boquila trifoliolata mimics leaves of an artificial plastic host plant. Plant Signal. Behav. 2022, 17, 1977530. [Google Scholar] [CrossRef]
- Mo, M.; Yokawa, K.; Wan, Y.; Baluška, F. How and why do root apices sense light under the soil surface? Front. Plant Sci. 2015, 6, 775. [Google Scholar] [CrossRef] [PubMed]
- Yokawa, K.; Fasano, R.; Kagenishi, T.; Baluška, F. Light as stress factor to plant roots—Case of root halotropism. Front. Plant Sci. 2014, 5, 718. [Google Scholar] [CrossRef]
- Burbach, C.; Markus, K.; Zhang, Y.; Schlicht, M.; Baluška, F. Photophobic behavior of maize roots. Plant Signal. Behav. 2012, 7, 874–878. [Google Scholar] [CrossRef]
- Yokawa, K.; Kagenishi, T.; Kawano, T.; Mancuso, S.; Baluška, F. Illumination of Arabidopsis roots induces immediate burst of ROS production. Plant Signal. Behav. 2011, 6, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ding, G.; Yokawa, K.; Baluška, F.; Li, Q.-F.; Liu, Y.; Shi, W.; Liang, J.; Zhang, J. An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. Sci. Rep. 2013, 3, 1273. [Google Scholar] [CrossRef] [PubMed]
- Yokawa, K.; Kagenishi, T.; Baluška, F. Root photomorphogenesis in laboratory-maintained Arabidopsis seedlings. Trends Plant Sci. 2013, 18, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Černý, M.; Pavlů, J.; Zemánková, J.; Skalák, J.; Plačková, L.; Brzobohatý, B. Roles of proteome dynamics and cytokinin signaling in root to hypocotyl ratio changes induced by shading roots of Arabidopsis seedlings. Plant Cell Physiol. 2015, 56, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Silva-Navas, J.; Moreno-Risueno, M.A.; Manzano, C.; Pallero-Baena, M.; Navarro-Neila, S.; Téllez-Robledo, B.; Garcia-Mina, J.M.; Baigorri, R.; Gallego, F.J.; del Pozo, J.C. D-Root: A system for cultivating plants with the roots in darkness or under different light conditions. Plant J. 2015, 84, 244–255. [Google Scholar] [CrossRef]
- Lacek, J.; García-González, J.; Weckwerth, W.; Retzer, K. Lessons learned from the studies of roots shaded from direct root illumination. Int. J. Mol. Sci. 2021, 22, 12784. [Google Scholar] [CrossRef]
- Miotto, Y.E.; da Costa, C.T.; Offringa, R.; Kleine-Vehn, J.; Maraschin, F.D.S. Effects of light intensity on root development in a D-root growth system. Front. Plant Sci. 2021, 12, 778382. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, S.; Bao, W.; Xue, X.; Ma, Z.; Yokawa, K.; Baluška, F.; Wan, Y. Expression of root genes in Arabidopsis seedlings grown by standard and improved growing methods. Int. J. Mol. Sci. 2017, 18, 951. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yamashita, F.; Njimona, I.; Baluška, F. Root and hypocotyl growth of Arabidopsis seedlings grown under different light conditions and influence of TOR kinase inhibitor AZD. Int. J. Biotechnol. Mol. Biol. Res. 2022, 12, 22–30. [Google Scholar] [CrossRef]
- Wan, Y.-L.; Eisinger, W.; Ehrhardt, D.; Kubitscheck, U.; Baluska, F.; Briggs, W. The subcellular localization and blue-light-induced movement of Phototropin 1-GFP in etiolated seedlings of Arabidopsis thaliana. Mol. Plant 2008, 1, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Jasik, J.; Wang, L.; Hao, H.; Volkmann, D.; Menzel, D.; Mancuso, S.; Baluška, F.; Lin, J. the signal transducer NPH3 integrates the Phototropin1 photosensor with PIN2-based polar auxin transport in Arabidopsis root phototropism. Plant Cell 2012, 24, 551–565. [Google Scholar] [CrossRef]
- Baluška, F.; Mancuso, S.; Volkmann, D.; Barlow, P.W. Root Apex transition zone: A signalling–response nexus in the root. Trends Plant Sci. 2010, 15, 402–408. [Google Scholar] [CrossRef]
- Baluška, F.; Mancuso, S. Root apex transition zone as oscillatory zone. Front. Plant Sci. 2013, 4, 354. [Google Scholar] [CrossRef]
- Baluška, F.; Yamashita, F.; Mancuso, S. Root apex cognition: From neuronal molecules to root-fungal networks. In Rhizobiology: Molecular Physiology of Plant Roots; Springer: Cham, Switzerland, 2021; pp. 1–24. [Google Scholar]
- Mäthger, L.M.; Roberts, S.B.; Hanlon, R.T. Evidence for distributed light sensing in the skin of cuttlefish, Sepia officinalis. Biol. Lett. 2010, 6, 600–603. [Google Scholar] [CrossRef]
- Kingston, A.C.N.; Kuzirian, A.M.; Hanlon, R.T.; Cronin, T.W. Visual phototransduction components in cephalopod chromatophores suggest dermal photoreception. J. Exp. Biol. 2015, 218, 1596–1602. [Google Scholar] [CrossRef]
- Yerramilli, D.; Johnsen, S. Spatial vision in the purple sea urchin Strongylocentrotus purpuratus (Echinoidea). J. Exp. Biol. 2010, 213, 249–255. [Google Scholar] [CrossRef]
- Sumner-Rooney, L.; Rahman, I.A.; Sigwart, J.D.; Ullrich-Lüter, E. Whole-body photoreceptor networks are independent of ‘lenses’ in brittle stars. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172590. [Google Scholar] [CrossRef]
- Garm, A.; Nilsson, D.-E. Visual navigation in starfish: First evidence for the use of vision and eyes in starfish. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133011. [Google Scholar] [CrossRef] [PubMed]
- Korsvig-Nielsen, C.; Hall, M.; Motti, C.; Garm, A. Eyes and negative phototaxis in juvenile crown-of-thorns starfish, Acanthaster Species Complex. Biol. Open 2019, 8, bio041814. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, D.E.; Gislén, L.; Coates, M.M.; Skogh, C.; Garm, A. Advanced optics in a jellyfish eye. Nature 2005, 435, 201–205. [Google Scholar] [CrossRef]
- Gehring, W.J. New perspectives on eye development and the evolution of eyes and photoreceptors. J. Hered. 2005, 96, 171–184. [Google Scholar] [CrossRef]
- Duanmu, D.; Bachy, C.; Sudek, S.; Wong, C.-H.; Jiménez, V.; Rockwell, N.C.; Martin, S.S.; Ngan, C.Y.; Reistetter, E.N.; van Baren, M.J.; et al. Marine algae and land plants share conserved phytochrome signaling systems. Proc. Natl. Acad. Sci. USA 2014, 111, 15827–15832. [Google Scholar] [CrossRef] [PubMed]
- Albrecht-Buehler, G. In defense of “nonmolecular” cell biology. Int. Rev. Cytol. 1990, 120, 191–241. [Google Scholar] [CrossRef] [PubMed]
- Albrecht-Buehler, G. Rudimentary form of cellular “vision”. Proc. Natl. Acad. Sci. USA 1992, 89, 8288–8292. [Google Scholar] [CrossRef]
- Albrecht-Buehler, G. Cellular infrared detector appears to be contained in the centrosome. Cell Motil. Cytoskelet. 1994, 27, 262–271. [Google Scholar] [CrossRef]
- Albrecht-Buehler, G. Changes of cell behavior by near-infrared signals. Cell Motil. Cytoskelet. 1995, 32, 299–304. [Google Scholar] [CrossRef]
- Albrecht-Buehler, G. Altered drug resistance of microtubules in cells exposed to infrared light pulses: Are microtubules the “nerves” of cells? Cell Motil. Cytoskelet. 1998, 40, 183–192. [Google Scholar] [CrossRef]
- Baluška, F.; Volkmann, D.; Barlow, P.W. Nuclear components with microtubule-organizing properties in multicellular eukaryotes: Functional and evolutionary considerations. Int. Rev. Cytol. 1997, 175, 91–135. [Google Scholar] [CrossRef]
- Mazia, D. The chromosome cycle and the centrosome cycle in the mitotic cycle. Int. Rev. Cytol. 1987, 100, 49–92. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, F.; Baluška, F. Algal Ocelloids and Plant Ocelli. Plants 2023, 12, 61. https://doi.org/10.3390/plants12010061
Yamashita F, Baluška F. Algal Ocelloids and Plant Ocelli. Plants. 2023; 12(1):61. https://doi.org/10.3390/plants12010061
Chicago/Turabian StyleYamashita, Felipe, and František Baluška. 2023. "Algal Ocelloids and Plant Ocelli" Plants 12, no. 1: 61. https://doi.org/10.3390/plants12010061
APA StyleYamashita, F., & Baluška, F. (2023). Algal Ocelloids and Plant Ocelli. Plants, 12(1), 61. https://doi.org/10.3390/plants12010061





