The Effect of Dry Hopping Efficiency on β-Myrcene Dissolution into Beer
Abstract
:1. Introduction
2. Results
2.1. Chemical Properties of the Final Product
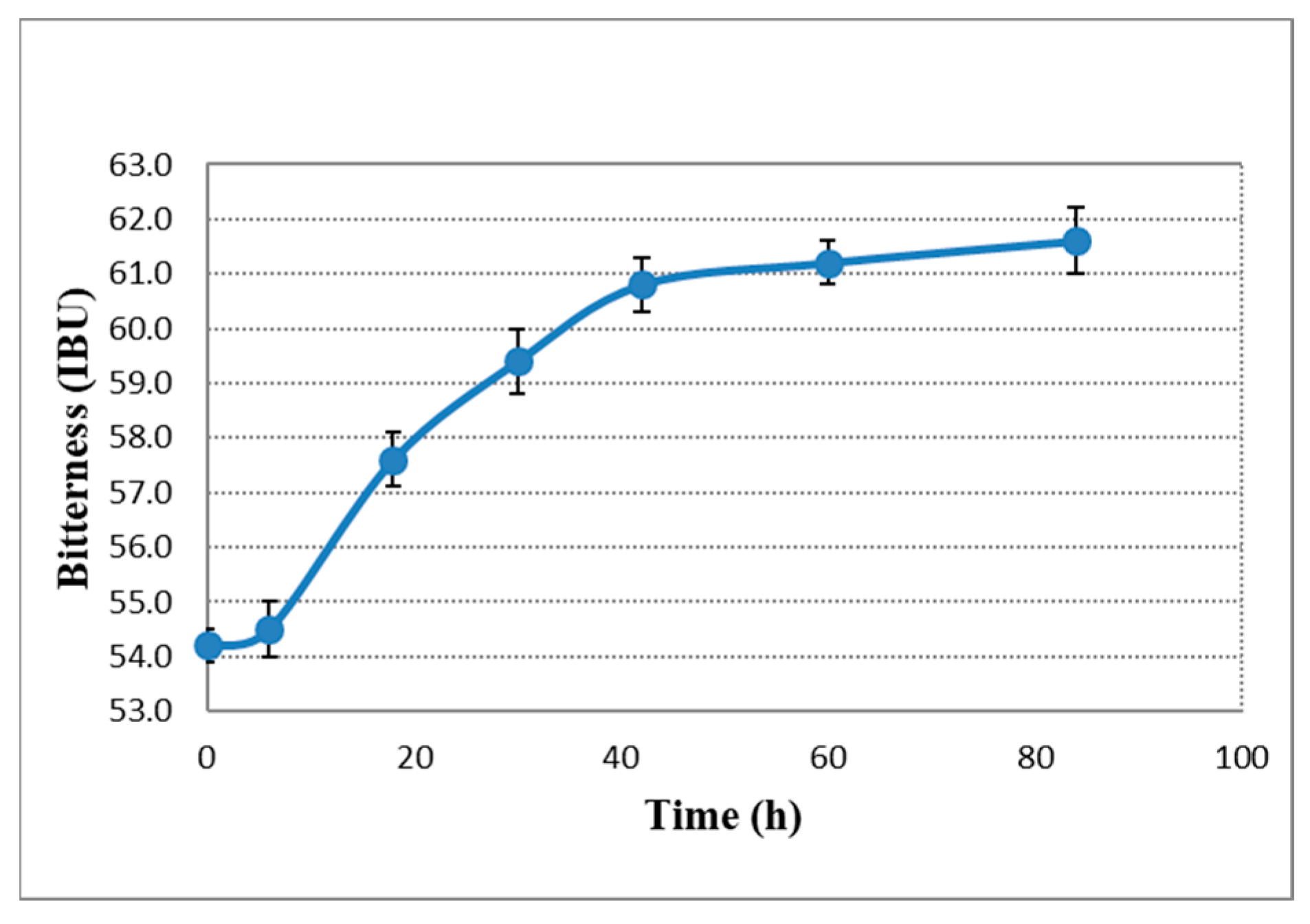
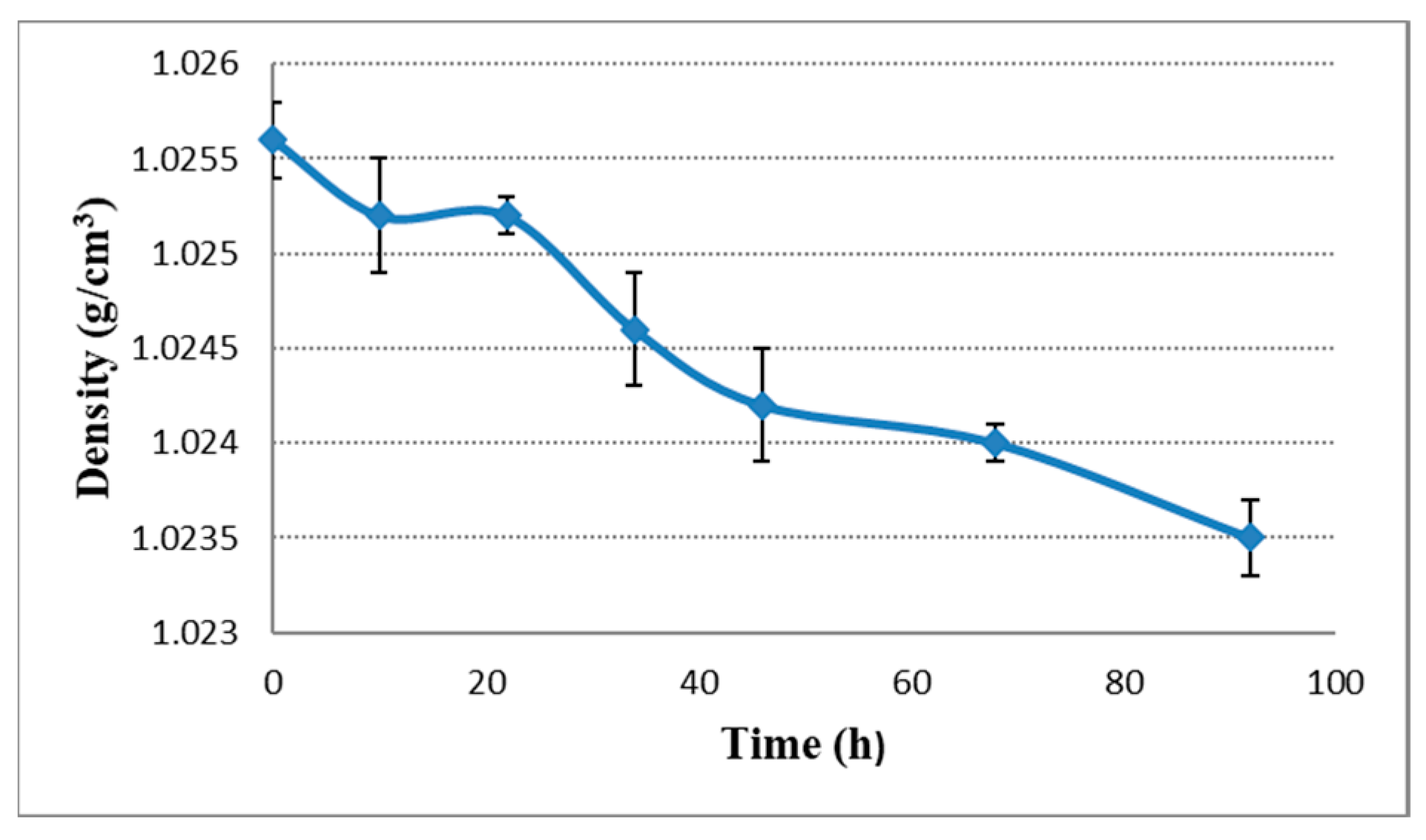
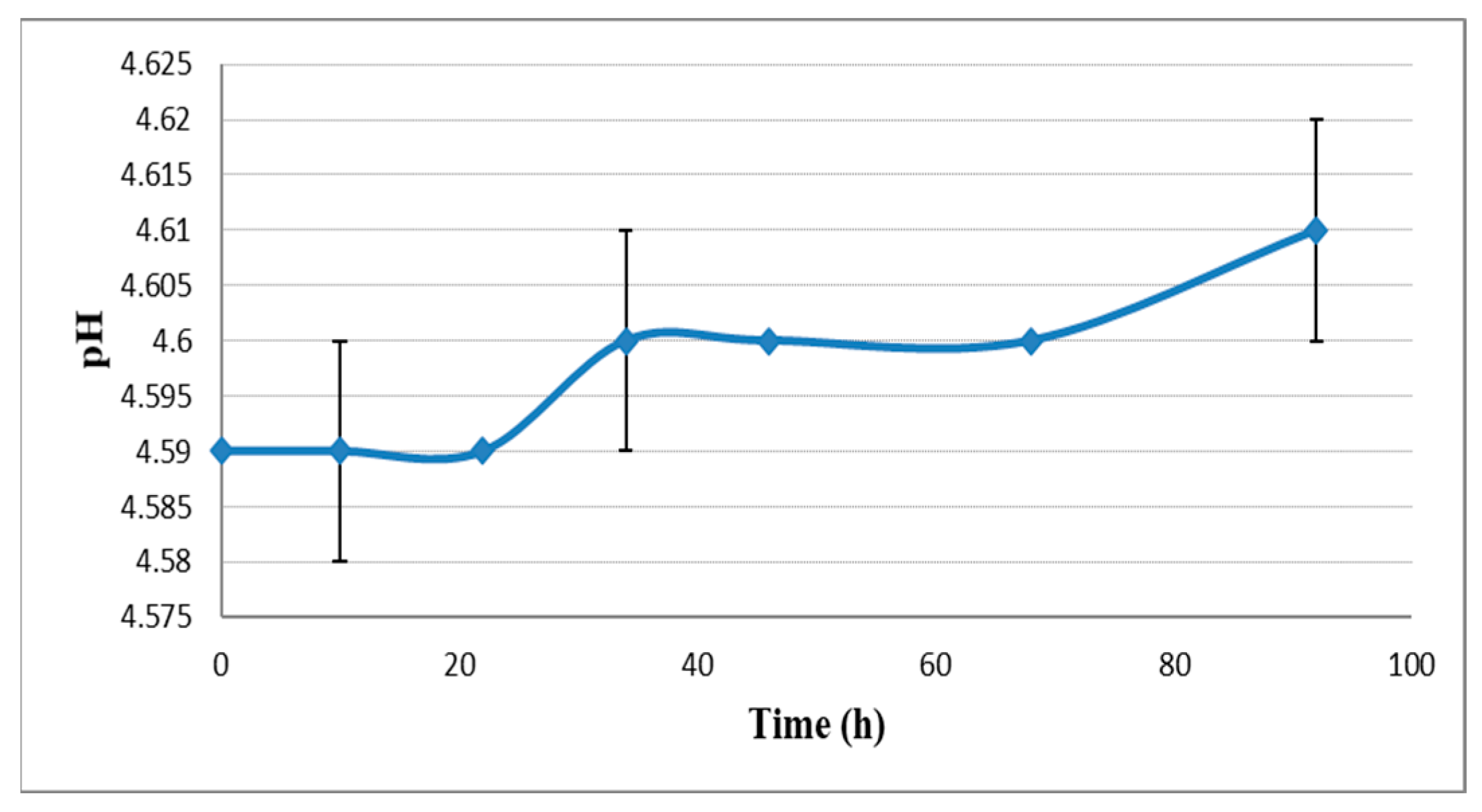
2.2. pH, Bitterness and Density Change during Dry Hopping
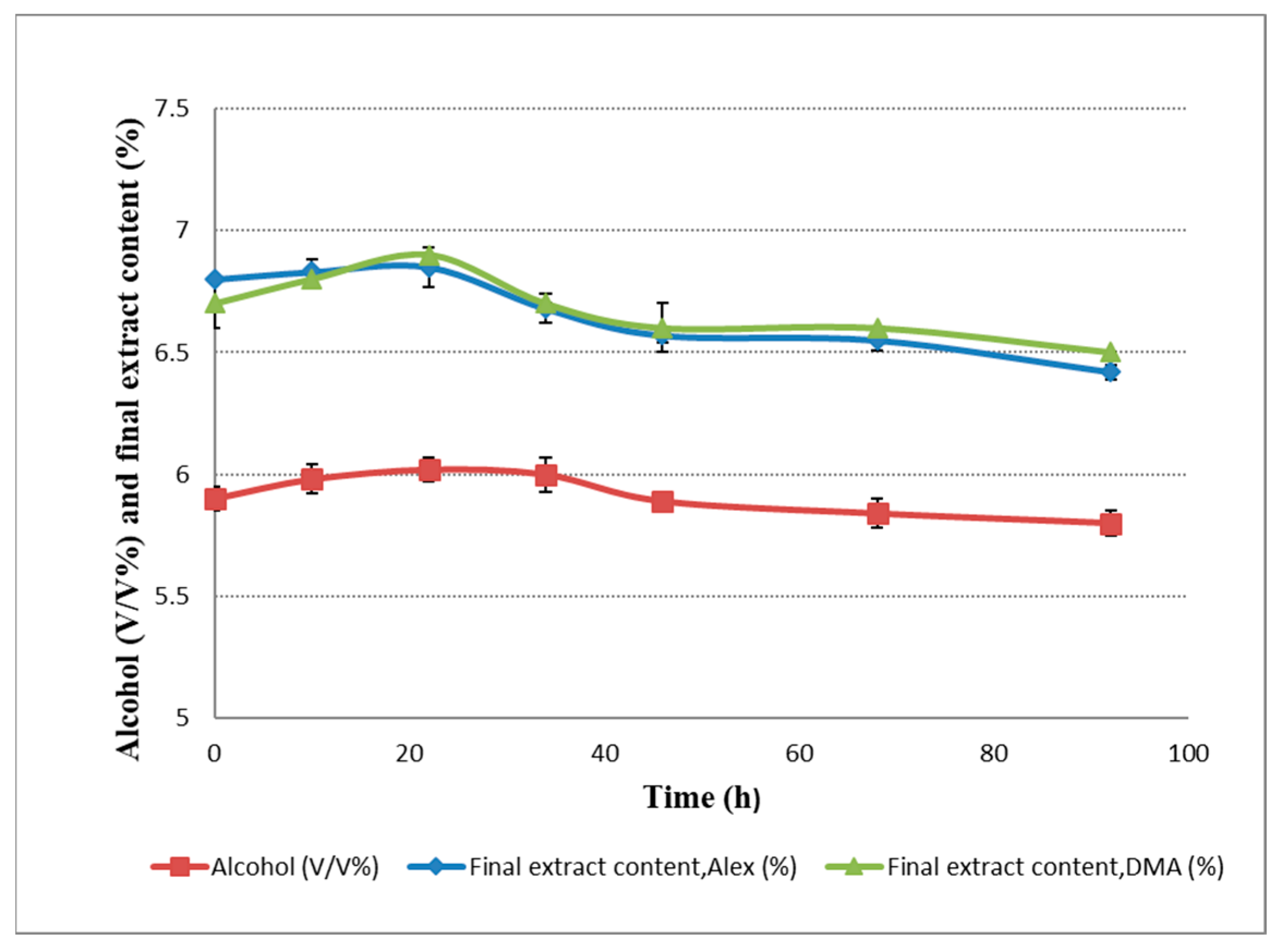
2.3. Alcohol Content and Extract Variations during Dry Hopping
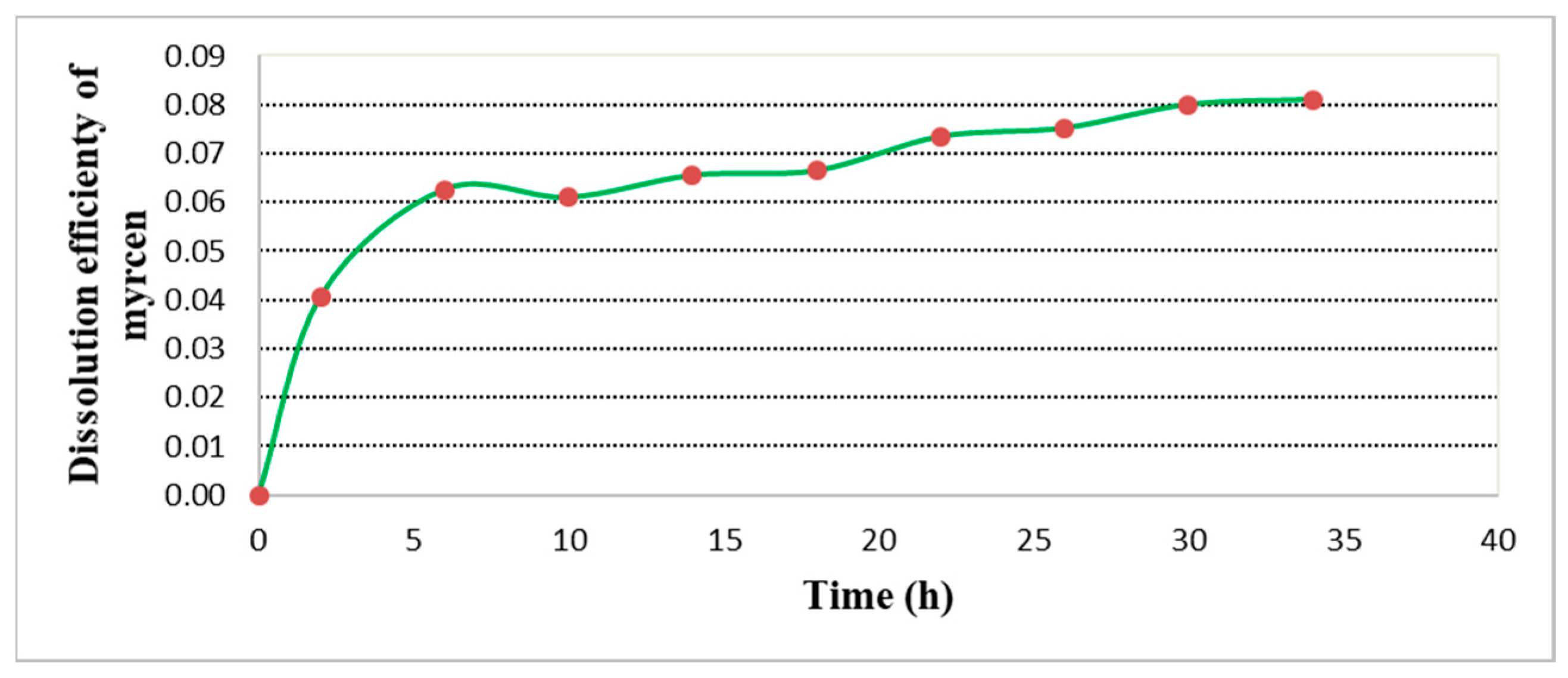
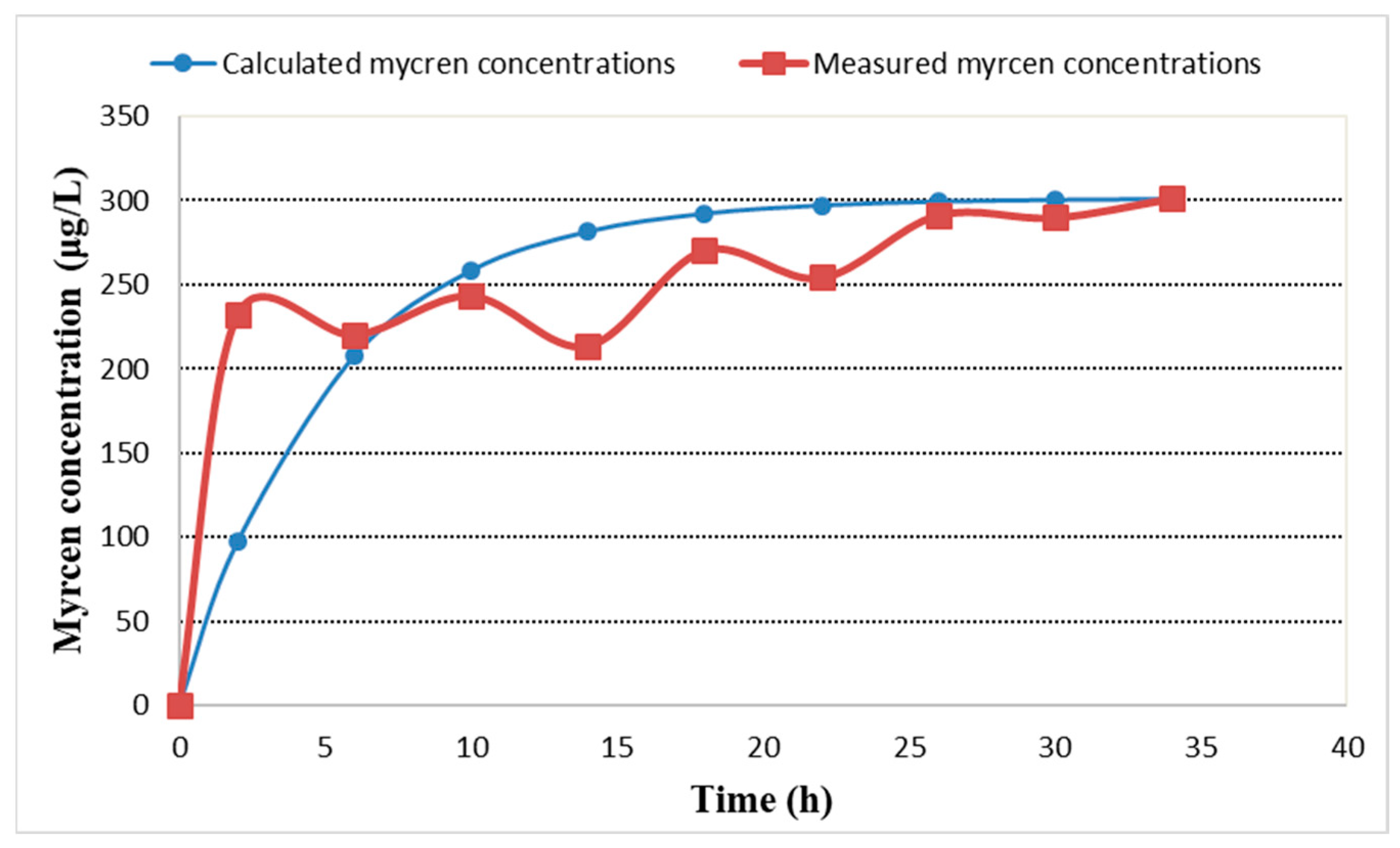
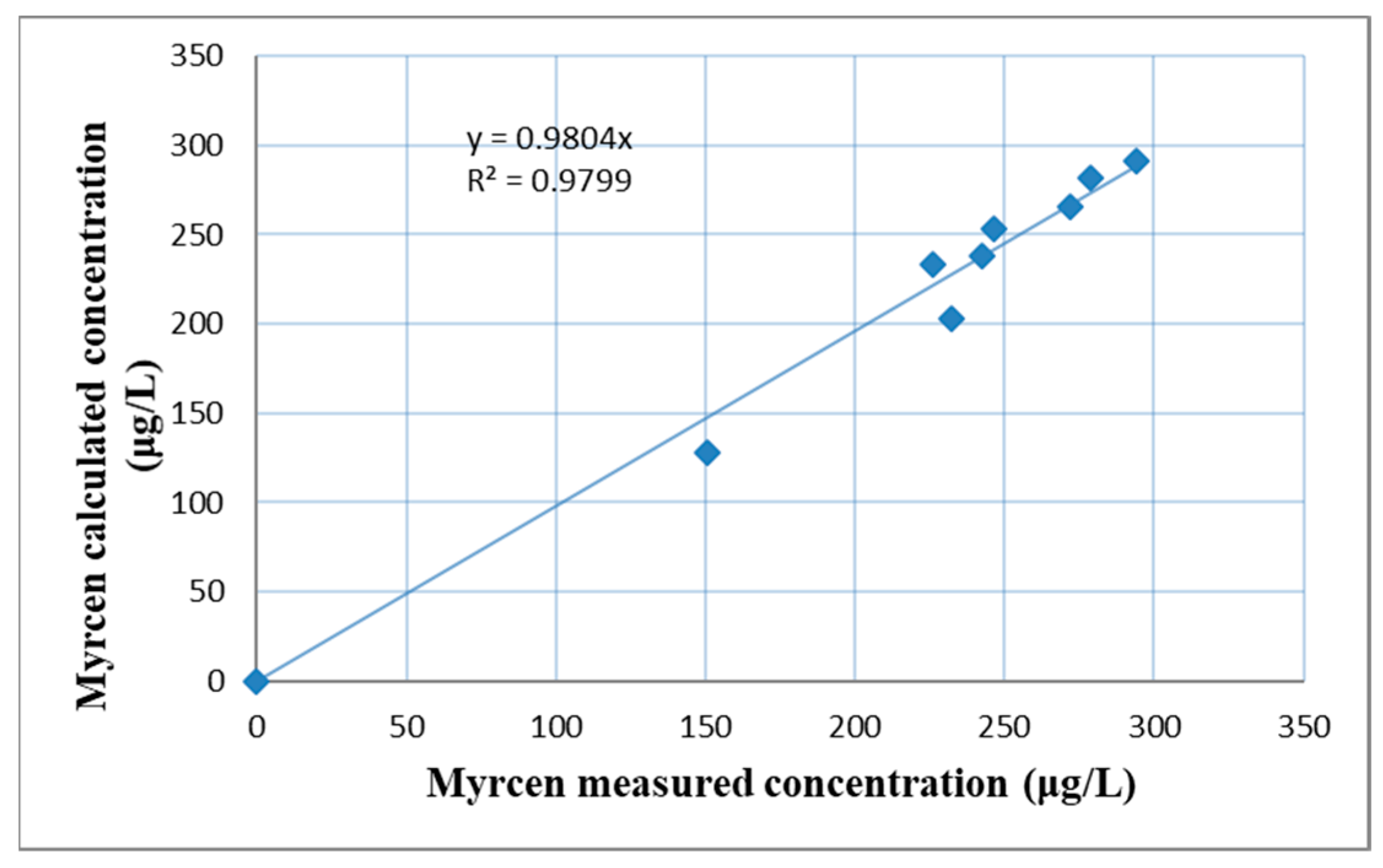
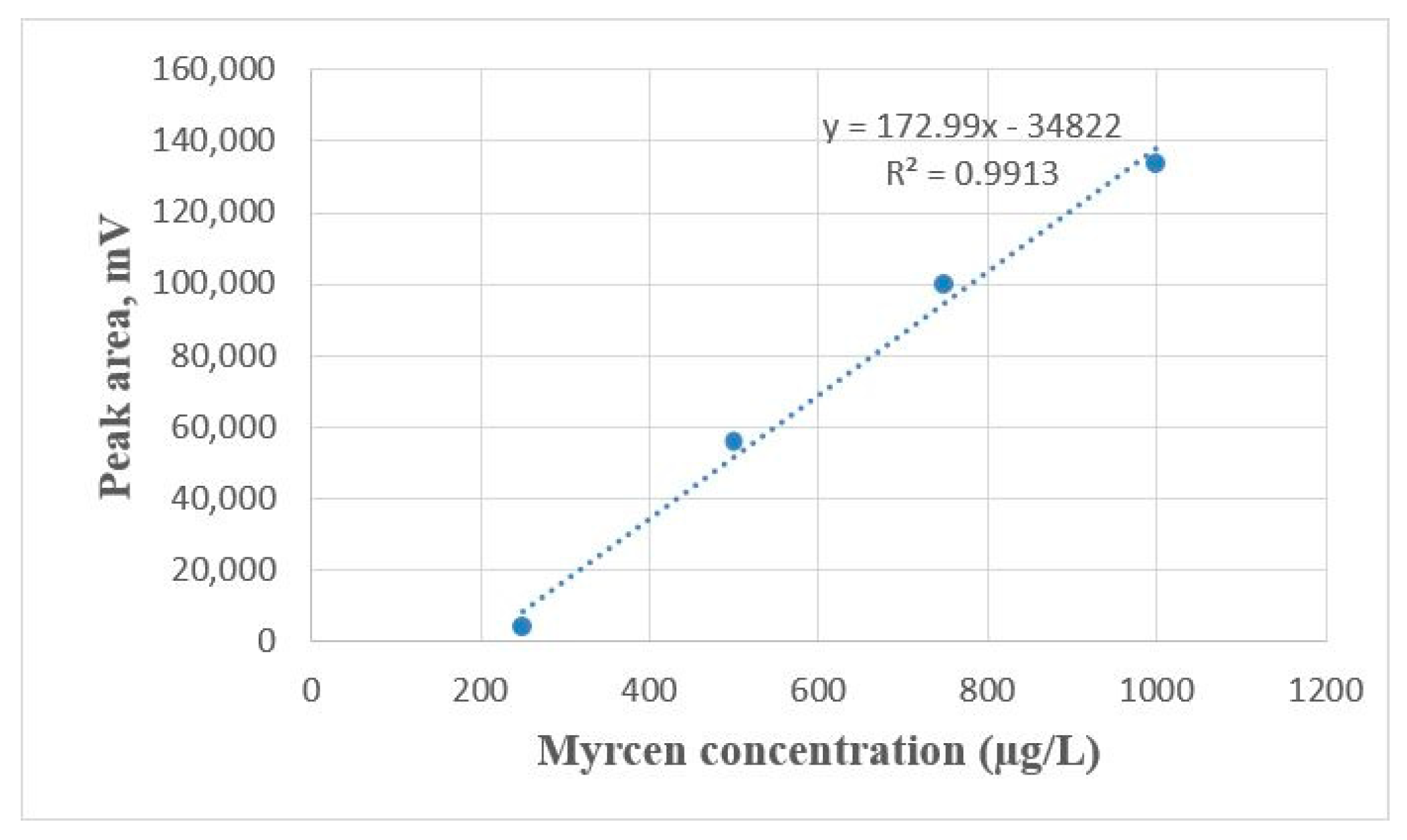
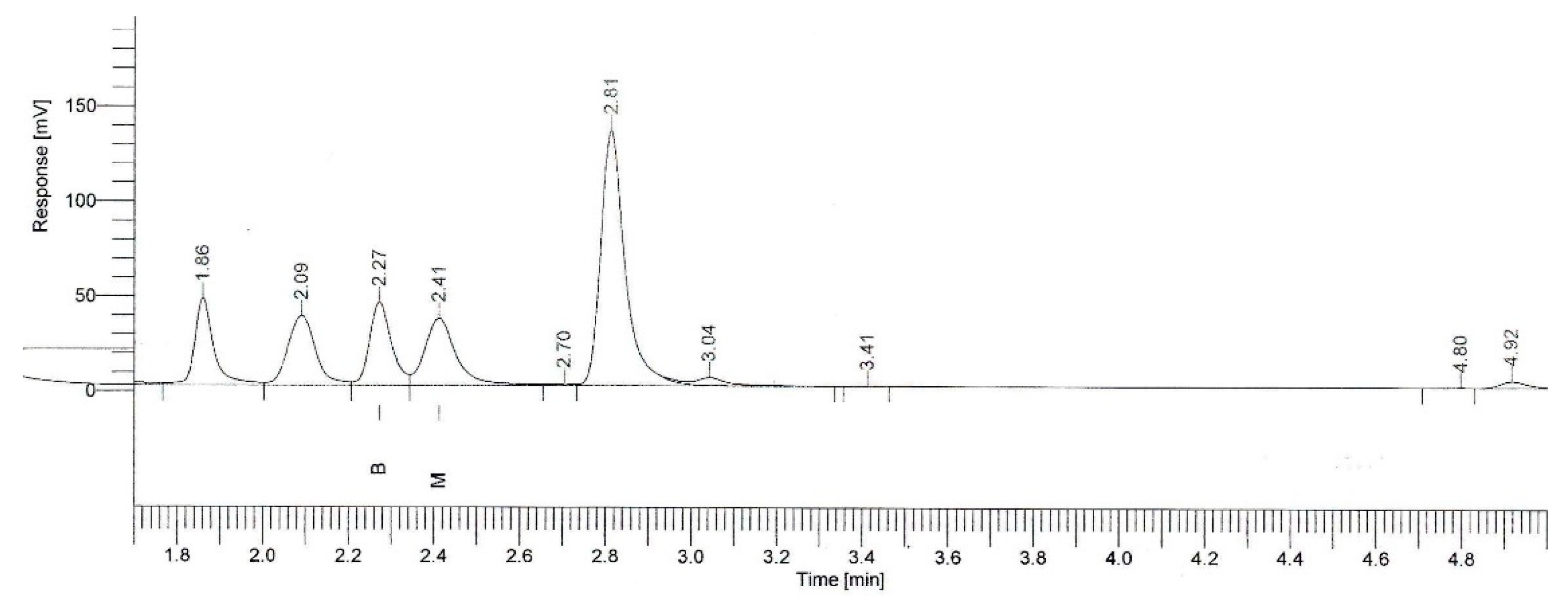
2.4. β-Myrcene Concentration
3. Discussions
4. Materials and Methods
4.1. Raw Materials and Equipment Used
4.2. Wort Preparation
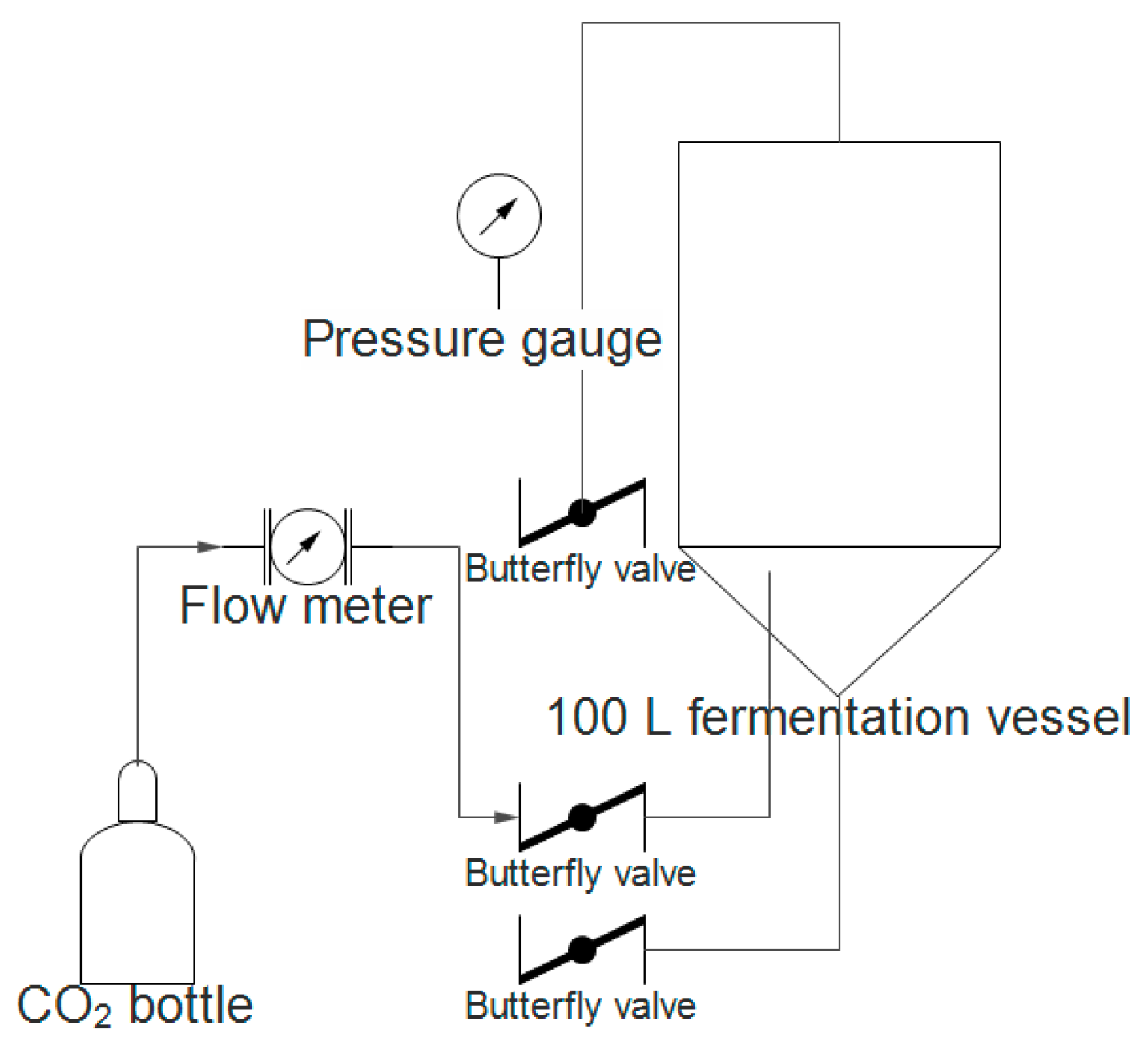
4.3. Dry Hopping
4.4. Analytical Measurements
4.4.1. pH Measurements
4.4.2. Extract, Alcohol Content and Density Measurements
4.4.3. Color Measurement
4.4.4. Bitterness Measurement
4.4.5. β-Myrcene Concentration Measurement
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafontaine, S.; Shellhammer, T.H. Investigating the factors impacting aroma, flavor, and stability in dry-hopped beers. Tech. Q. 2019, 56, 13–26. [Google Scholar]
- Schönberger, C.; Biendl, M.; Schmidt, R.; Mitter, W.; Gahr, A.; Forster, A.; Engelhard, B.; Lutz, A. Hops Their Cultivation, Composition and Usage; Fachverlag Hans Carl: Nürnberg, Germany, 2015; pp. 209–210. [Google Scholar]
- Steele, M. IPA: Brewing Techniques, Recipes and the Evolution of the India Pale Ale; Brewers Publication: Boulder, CO, USA, 2013; pp. 206–209. [Google Scholar]
- Schönberger, C.; Kostelecky, T. 125th Anniversary review: The role of hops in brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Mitter, W.; Cocuzza, S. Revival of a process (dry hopping-basics and techniques). Brew. Beverage Ind. Int. 2013, 3, 28–30. [Google Scholar]
- Harasym, J.; Podeszwa, T. New methods of hopping (dry hopping) and their impact on sensory properties of beer. Acta Innov. 2016, 21, 79–86. [Google Scholar]
- Algazzali, V.A.; Shellhammer, T. Bitterness intensity of oxidized hop acids: Humulinones and hulupones. J. Am. Soc. Brew. Chem. 2016, 74, 36–43. [Google Scholar] [CrossRef]
- Maye, J.P.; Smith, R.; Leker, J. Humulinone formation in hops and hop pellets and its implications for dry hopped beers. Master Brew. Assoc. Am. Tech. Q. 2016, 53, 23–27. [Google Scholar]
- Silva Ferreira, C.; Thibault de Chanvalon, E.; Bodart, E.; Collin, S. Why humulinones are key bitter constituents only after dry hopping: Comparison with other Belgian styles. J. Am. Soc. Brew. Chem. 2018, 76, 236–246. [Google Scholar] [CrossRef]
- Peltz, M.; Shellhammer, T. Ethanol content has little effect on the sensory orthonasal detection threshold of hop compounds in beer. J. Am. Soc. Brew. Chem. 2017, 75, 221–227. [Google Scholar] [CrossRef]
- Rettberg, N.; Biendl, M.; Garbe, L.A. Hop aroma and hoppy beer flavor: Chemical backgrounds and analytical tools—A review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Vollmer, D.M.; Shellhammer, T.H. Influence of hop oil content and composition on hop aroma intensity in dry-hopped beer. J. Am. Soc. Brew. Chem. 2016, 74, 242–249. [Google Scholar] [CrossRef]
- Ramírez, A.; Viveros, J.M. Brewing with Cannabis sativa vs. Humulus lupulus: A review. J. Inst. Brew. 2021, 127, 201–209. [Google Scholar] [CrossRef]
- Steinhaus, M.; Schieberle, P. Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. variety Spalter Select) based on GC−olfactometry and odor dilution techniques. J. Agric. Food Chem. 2000, 48, 1776–1783. [Google Scholar] [CrossRef]
- Rodrigues Arruda, T.; Fontes Pinheiro, P.; Ibrahim Silva, P.; Campos Bernardes, P. Exclusive raw material for beer production? Addressing greener extraction techniques, the relevance, and prospects of hops (Humulus lupulus L.) for the food industry. Food Bioprocess Technol. 2021, 15, 275–305. [Google Scholar] [CrossRef]
- Nance, M.; Setzer, W.N. Volatile components of aroma hops (Humulus lupulus L.) commonly used in beer brewing. J. Brew. Distill. 2011, 2, 16–22. [Google Scholar]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene-What are the potential health benefits of this flavouring and aroma agent. Front. Nutr. 2021, 8, 666–699. [Google Scholar] [CrossRef]
- National Toxicology Program. Technical Report on the Toxicology and Carcinogenesis Studies of β-Myrcene (CAS NO. 123-35-3) in F344/N Rats and B6C3F1 Mice; NTP TR 557. NIH Publication No.; National Toxicology Program Technical Report Series; NIH Publication: Bethesda, MA, USA, 2010.
- Okaru, A.O.; Lachenmeier, D.W. The food and beverage occurrence of furfuryl alcohol and myrcene—two emerging potential human carcinogens? Toxics 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, L.F.; Ross, C.F. Determination of flavour compounds in beer using stir-bar sorptive extraction and solid-phase microextraction. J. Inst. Brew. 2015, 121, 197–203. [Google Scholar] [CrossRef]
- Schmidt, C.; Biendl, M. Headspace Trap GC-MS analysis of hop aroma compounds in beer. Brew. Sci. 2016, 69, 9–15. [Google Scholar]
- Kishimoto, T.; Wanikawa, A.; Kagami, N.; Kawatsura, K. Analysis of Hop-derived terpenoids in beer and evaluation of their behavior using the stir bar–sorptive extraction method with GC-MS. J. Agric. Food Chem. 2005, 53, 4701–4706. [Google Scholar] [CrossRef]
- Adams, T.; Gavin, C.; McGowen, M.; Waddell, W.; Cohen, S.; Feron, V.; Marnett, L.; Munro, I.; Portoghese, P.; Rietjens, I.; et al. The FEMA GRAS assessment of aliphatic and aromatic terpene hydrocarbons used as flavor ingredients. Food Chem. Toxicol. 2011, 49, 2471–2494. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, M.; Grdadolnik, J.; Košir, I.J. Determination of the botanical origin of hops (Humulus lupulus L.) using different analytical techniques in combination with statistical methods. J. Inst. Brew. 2016, 122, 452–461. [Google Scholar] [CrossRef]
- Gordon, S.; Kristen, E. 2021 Style Guidelines; BCJP, Inc.: Boulder, CO, USA, 2022; p. 38. [Google Scholar]
- Cocuzza, S.; Mitter, W. Dry hopping—A study of various parameters consequences of the applied dosing method. Brew. Beverage Ind. Int. 2013, 4, 70–74. [Google Scholar]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Hauser, D.; Van Simaeys, K.R.; Lafontaine, S.R.; Stellhammer, T. Comparison of single-stage and two-stage dry-hopping regimes. J. Am. Soc. Brew. Chem. 2019, 77, 251–260. [Google Scholar] [CrossRef]
- Forster, A.; Gahr, A. On the Fate of Certain Hop Substances during Dry Hopping. Brew. Sci. 2013, 66, 93–103. [Google Scholar]
- Shopska, V.; Denkova-Kostova, R.; Kostov, G. Modeling in brewing—A Review. Processes 2022, 10, 267. [Google Scholar] [CrossRef]
- Shopska, V.; Denkova, R.; Lyubenova, V.; Kostov, G. Kinetic Characteristics of alcohol fermentation in brewing: State of art and control of the fermentation process. In Fermented Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 529–575. [Google Scholar]
- Brendel, S.; Hofmann, T.; Granvog, M. Hop-induced formation of ethyl esters in dry-hopped beer. Food Prod. Processing Nutr. 2020, 2, 18. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a novel multipurpose crop for the Mediterranean region of Europe: Challenges and opportunities of their cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Dietz, C.; Cook, D.; Huismann, M.; Wilson, C.; Ford, R. The multisensory perception of hop essential oil: A review. J. Inst. Brew. 2020, 126, 320–342. [Google Scholar]
- Speers, R.A.; MacIntosh, A.J. Carbon Dioxide Solubility in Beer. J. Am. Soc. Brew. Chem. 2013, 71, 242–247. [Google Scholar] [CrossRef]
- Bamforth, C.W. 125th Anniversary Review: The non-biological instability of beer. J. Inst. Brew. 2011, 117, 488–497. [Google Scholar] [CrossRef]
- Steiner, E.; Becker, T.; Gastl, M. Turbidity and haze formation in beer—Insights and overview. J. Inst. Brew. 2010, 116, 360–368. [Google Scholar] [CrossRef]
- Kahle, E.-M.; Zarnkow, M.; Jacob, F. Beer Turbidity Part 1: A review of factors and solutions. J. Am. Soc. Brew. Chem. 2020, 79, 99–114. [Google Scholar] [CrossRef]
- Karabín, M.; Hanko, V.; Nešpor, J.; Jelínek, L.; Dostálek, P. Hop tannin extract: A promising tool for acceleration of lautering. J. Inst. Brew. 2018, 124, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Gomes, F.O.; Guimarães, B.P.; Ceola, D.; Ghesti, G.F. Advances in dry hopping for industrial brewing: A review. Food Sci. Technol. 2022, 42, 1–8. [Google Scholar] [CrossRef]
- Hahn, C.D.; Lafontaine, S.R.; Pereira, C.B.; Shellhammer, T.H. Evaluation of nonvolatile chemistry affecting sensory bitterness intensity of highly hopped beers. J. Agric. Food Chem. 2018, 66, 3505–3513. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, C.; Hort, J.; Cook, D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem. 2016, 205, 212–220. [Google Scholar] [CrossRef]
- Parkin, E.; Shellhammer, T. Toward understanding the bitterness of dry-hopped Beer. J. Am. Soc. Brew. Chem. 2017, 75, 363–368. [Google Scholar]
- Rutnik, K.; Knez Hrnčič, M.; Jože Košir, I. Hop essential oil: Chemical composition, extraction, analysis, and applications. Food Rev. 2021, 1–23. [Google Scholar] [CrossRef]
- Janish, S. Dry hop best practices: Using science as a guide for process and recipe development. MBAA Tech. Q. 2021, 58, 59–64. [Google Scholar]
- Cocuzza, S. The impact of dry hopping on selected physical and chemical attributes of beer. Brew. Sci. 2019, 72, 118–124. [Google Scholar]
- Połeć, K.; Broniatowski, M.; Wydro, P.; Hąc-Wydro, K. The impact of β-myrcene—The main component of the hop essential oil—On the lipid films. J. Mol. Liq. 2020, 308, 113028. [Google Scholar] [CrossRef]
- Titus, B.M.; Lerno, L.A.; Beaver, J.W.; Byrnes, N.K.; Heymann, H.; Oberholster, A. Impact of dry hopping on beer flavor stability. Foods 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, N.; Yang, A.; Huang, J.; Ren, X.; Xian, M.; Zou, H. Hop bitter acids: Resources, biosynthesis, and applications. Appl. Microbiol. Biotechnol. 2021, 105, 4343–4356. [Google Scholar] [CrossRef] [PubMed]
- Paszkot, J.; Kawa-Rygielska, J.; Anioł, M. Properties of dry hopped dark beers with high xanthohumol content. Antioxidants 2021, 10, 763. [Google Scholar] [CrossRef]
- Habschied, K.; Košir, I.J.; Krstanović, V.; Kumrić, G.; Mastanjević, K. Beer polyphenols—Bitterness, astringency, and off-flavors. Beverages 2021, 7, 38. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.; Lorenzo, J.M. Humulus lupulus L. as a natural source of functional biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- Haslbeck, K.; Bub, S.; Schönberger, C.; Zarnkow, M.; Jacob, F.; Coelhan, M. On the fate of β-myrcene during fermentation the role of stripping and uptake of hop oil components by brewer’s yeast in dry-hopped wort and beer. Brew. Sci. 2017, 70, 159–169. [Google Scholar]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Iniguez, A.B.; Zhu, M.J. Hop bioactive compounds in prevention of nutrition-related noncommunicable diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Wanikawa, A.; Kono, K.; Shibata, K. Comparison of the odor active compounds in unhopped beer and beers hopped with different hop varieties. J. Agric. Food Chem. 2006, 54, 8855–8861. [Google Scholar] [CrossRef] [PubMed]
- EBC Analytica, 9.35—pH of Beer (Formerly Published as IOB Method 9.42) 2004. 23 October 2018. Available online: https://brewup.eu/ebc-analytica/beer/ph-of-beer-formerly-published-as-iob-method-9-42/9.35 (accessed on 10 February 2021).
- EBC Analytica, 9.43.2—Specific Gravity of Beer Using a Density Meter, 2004. 23 October 2018. Available online: https://brewup.eu/ebc-analytica/beer/specific-gravity-of-beer-using-a-density-meter/9.43.2 (accessed on 10 February 2021).
- EBC Analytica, 9.6—Colour of Beer: Spectrophotometric Method (IM), 2004. 23 October 2018. Available online: https://brewup.eu/ebc-analytica/beer/colour-of-beer-spectrophotometric-method-im/9.6 (accessed on 10 February 2021).
- EBC Analytica, 9.8—Bitterness of Beer (IM), 2004. 23 November 2020. Available online: https://brewup.eu/ebc-analytica/beer/bitterness-of-beer-im/9.8 (accessed on 10 February 2021).

| Parameters | Young Beer | After Dry Hopping | BJCP Parameters |
|---|---|---|---|
| Original extract (% w/w) | 15.15 ± 0.03 a* | 15.27 ± 0.02 a | - |
| Final extract (% w/w) | 5.79 ± 0.01 a | 5.58 ± 0.01 a | - |
| Original density (g/cm3) | 1.065 ± 0.001 a | 1.067 ± 0.001 a | 1.050–1.085 |
| Alcohol (% v/v) | 5.9 ± 0.05 a | 5.8 ± 0.05 a | 5.5–9.0 |
| Final density (g/cm3) | 1.025 ± 0.001 a | 1.020 ± 0.001 a | 1.050–1.085 |
| CO2 (g/L) | 3.56 ± 0.02 a | 4.48 ± 0.03 b | - |
| O2 (mg/L) | 0.06 ± 0.01 a | 0.22 ± 0.01 a | - |
| Turbidity (EBC) | 11.19 ± 0.03 a | 16.32 ± 0.04 b | - |
| pH | 4.59 ± 0.01 a | 4.61 ± 0.01 a | - |
| Color | 132.3 ± 0.2 a | 131.7 ± 0.1 a | 50–80 |
| Bitterness (IBU) | 54.5 ± 0.2 a | 64.3 ± 0.4 b | 50–90 |
| β-myrcene (μg/L) | 0 ± 0.00 a | 228 ± 0.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamon, R.V.; Dabija, A.; Ferencz, Á.; Tankó, G.; Ciocan, M.E.; Codină, G.G. The Effect of Dry Hopping Efficiency on β-Myrcene Dissolution into Beer. Plants 2022, 11, 1043. https://doi.org/10.3390/plants11081043
Salamon RV, Dabija A, Ferencz Á, Tankó G, Ciocan ME, Codină GG. The Effect of Dry Hopping Efficiency on β-Myrcene Dissolution into Beer. Plants. 2022; 11(8):1043. https://doi.org/10.3390/plants11081043
Chicago/Turabian StyleSalamon, Rozália Veronika, Adriana Dabija, Ágota Ferencz, György Tankó, Marius Eduard Ciocan, and Georgiana Gabriela Codină. 2022. "The Effect of Dry Hopping Efficiency on β-Myrcene Dissolution into Beer" Plants 11, no. 8: 1043. https://doi.org/10.3390/plants11081043
APA StyleSalamon, R. V., Dabija, A., Ferencz, Á., Tankó, G., Ciocan, M. E., & Codină, G. G. (2022). The Effect of Dry Hopping Efficiency on β-Myrcene Dissolution into Beer. Plants, 11(8), 1043. https://doi.org/10.3390/plants11081043








