Morpho-Physiological Responses of Two Multipurpose Species from the Tropical Dry Forest to Contrasting Light Levels: Implications for Their Nursery and Field Management
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Plant Material
4.3. Light Levels and Experimental Design
4.4. Plant Sampling, Morphological and Physiological Measurements
4.5. Data Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newton, A.C. Restoration of dryland forests in Latin America: The ReForLan project. Ecol. Rest. 2008, 26, 10–13. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- CONAFOR (Comisión Nacional Forestal, Mexico). Estado que Guarda el Sector Forestal en México; Comisión Nacional Forestal: Zapopan, Mexico, 2020; pp. 254–271.
- Torres Rojo, J.M. Factores ambientales y físicos que afectan la supervivencia de siete especies forestales en el Estado de México. Rev. Mex. Cienc. For. 2021, 12, 66–91. [Google Scholar] [CrossRef]
- Chechina, M.; Hamann, A. Choosing species for reforestation in diverse forest communities: Social preference versus ecological suitability. Ecosphere 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Moretti, A.P.; Olguin, F.Y.; Pinazo, M.A.; Graciano, C. Water and light stresses drive acclimation during the establishment of a timber tree under different intensities of rainforest canopy coverage. Cerne 2019, 25, 93–104. [Google Scholar] [CrossRef]
- Dos Santos, U.M.; de Gonçalves, C.J.F.; Feldpausch, T.R. Growth, leaf nutrient concentration and photosynthetic nutrient use efficiency in tropical tree species planted in degraded areas in central Amazonia. For. Ecol. Manag. 2006, 226, 299–309. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Poorter, L.; Bongers, F. Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 2011, 27, 477–489. [Google Scholar] [CrossRef]
- Mayoral, C.; Calama, R.; Sánchez-González, M.; Pardos, M. Modelling the influence of light, water and temperature on photosynthesis in young trees of mixed Mediterranean forests. New For. 2015, 46, 485–506. [Google Scholar] [CrossRef]
- Laborde, J.; Corrales-Ferrayola, I. Direct seeding of Brosimum alicastrum SW. (Moraceae) and Enterolobium cyclocarpum (Jacq.) Griseb. (Mimosaceae) in different habitats in the dry tropics of central Veracruz. Acta Bot. Mex. 2012, 100, 107–134. [Google Scholar] [CrossRef][Green Version]
- Calzavara, A.K.; Rocha, J.S.; Lourenço, G.; Sanada, K.; Medri, C.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Oliveira, H.C. Acclimation responses to high light by Guazuma ulmifolia Lam. (Malvaceae) leaves at different stages of development. Plant Biol. 2017, 19, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Masarovičová, E.; Májeková, M.; Vykouková, I. Functional traits and plasticity of plants. In Handbook of Photosynthesis, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 487–505. [Google Scholar]
- Valladares, F.; Aranda, I.; Sánchez-Gómez, D. La luz como factor ecologico y evolutivo para las plantas y su interaccion con el agua. In Ecología del Bosque Mediterráneo en un Mundo Cambiante, 2nd ed.; Valladares, F., Ed.; Organismo Autónomo de Parques Nacionales: Madrid, Spain, 2004; pp. 335–369. [Google Scholar]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpääs, M.; Matsubara, S.; Pons, T.L. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.D.; Wang, H.; Jiao, D.Y.; Cai, Z.Q. Phenotypic plasticity of seedlings of five tropical tree species in response to different light and nutrient availability. Trop. Ecol. 2016, 57, 727−737. [Google Scholar]
- Kelly, J.; Jose, S.; Nichols, J.D.; Bristow, M. Growth and physiological response of six Australian rainforest tree species to a light gradient. For. Ecol. Manag. 2009, 257, 287–293. [Google Scholar] [CrossRef]
- Moretti, A.P.; Olguin, F.Y.; Pinazo, M.A.; Gortari, F.; Bahima, J.V.; Graciano, C. Supervivencia y crecimiento de un árbol nativo maderable bajo diferentes coberturas de dosel en el Bosque Atlántico, Misiones, Argentina. Ecol. Austral 2019, 29, 99–111. [Google Scholar] [CrossRef]
- Olguin, F.Y.; Moretti, A.P.; Pinazo, M.; Gortari, F.; Bahima, J.V.; Graciano, C. Morphological and physiological plasticity in seedlings of Araucaria angustifolia and Cabralea canjerana is related to plant establishment performance in the rainforest. For. Ecol. Manag. 2020, 460, 117867. [Google Scholar] [CrossRef]
- Endres, L.; Câmara, C.A.; Ferreira, V.M.; Silva, J.V. Morphological and photosynthetic alterations in the Yellow-ipe, Tabebuia chrysotricha (Mart. Ex DC.) Standl., under nursery shading and gas exchange after being transferred to full sunlight. Agrof. Syst. 2010, 78, 287–298. [Google Scholar] [CrossRef]
- Cerqueira, A.F.; Dalmolin, Â.C.; dos Anjos, L.; da Silva Ledo, C.A.; da Costa Silva, D.; Mielke, M.S. Photosynthetic plasticity of young plants of Carpotroche brasiliensis (Raddi) A. Gray, Achariaceae. Trees–Struct. Funct. 2018, 32, 191–202. [Google Scholar] [CrossRef]
- Sanches, M.C.; Marzinek, J.; Bragiola, N.G.; Nascimento, A.R.T. Morpho-physiological responses in Cedrela fissilis Vell. submitted to changes in natural light conditions: Implications for biomass accumulation. Trees–Struct. Funct. 2017, 31, 215–227. [Google Scholar] [CrossRef]
- Bonfil, C.; Trejo, I. Plant propagation and the ecological restoration of Mexican tropical deciduous forests. Ecol. Rest. 2010, 28, 369–376. [Google Scholar] [CrossRef]
- Solares, A.F. Etnobotánica y usos potenciales del cirián (Crescentia alata, H.B.K.) en el estado de Morelos. Polibotánica 2004, 18, 13–31. [Google Scholar]
- Foroughbakhch, R.; Alvarado-Vázquez, M.A.; Hernández-Piñero, J.L.; Rocha-Estrada, A.; Guzmán-Lucio, M.A.; Treviño-Garza, E.J. Establishment, growth and biomass production of 10 tree woody species introduced for reforestation and ecological restoration in northeastern Mexico. For. Ecol. Manag. 2006, 235, 194–201. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Barros, F.V.; Goulart, M.F.; Sá Telles, S.B.; Lovato, M.B.; Valladares, F.; de Lemos-Filho, J.P. Phenotypic plasticity to light of two congeneric trees from contrasting habitats: Brazilian Atlantic Forest versus cerrado (savanna). Plant Biol. 2012, 14, 208–215. [Google Scholar] [CrossRef]
- Robakowski, P. Photosynthetic competition between forest trees. In Handbook of Photosynthesis, 3rd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 475–486. [Google Scholar]
- Alves, P.L.D.C.A.; Magalhães, C.N.A.; Barja, P.R. The phenomenon of photoinhibition of photosynthesis and its importance in reforestation. Bot. Rev. 2002, 68, 193–208. [Google Scholar] [CrossRef]
- Dos Anjos, L.; Oliva, M.A.; Kuki, K.N.; Mielke, M.S.; Ventrella, M.C.; Galvão, M.F.; Pinto, L.M.R. Key leaf traits indicative of photosynthetic plasticity in topical tree species. Trees 2015, 29, 247–258. [Google Scholar] [CrossRef]
- Guzmán-Quesada, J.A.; Cordero-Solórzano, R.A.; Corea-Arias, E. Biomass allocation and gas exchange are affected by light conditions in endangered Cedrela salvadorensis (Meliaceae) seedlings. Rev. Biol. Trop. 2016, 64, 1143–1154. [Google Scholar] [CrossRef]
- Basave-Villalobos, E.; Rosales-Mata, S.; Sigala-Rodríguez, J.Á.; Calixto-Valencia, C.G.; Sarmiento-López, H. Cambios morfo-fisiológicos de plántulas de Prosopis laevigata (Humb. & Bonpl. ex Willd.) M. C. Johnst. ante diferentes ambientes de luz en vivero. Rev. Mex. Cienc. For. 2017, 8, 112–131. [Google Scholar] [CrossRef][Green Version]
- Piña, M.; Arboleda, M.E. Efecto de dos ambientes lumínicos en el crecimiento inicial y calidad de plantas de Crescentia cujete. Bioagro 2010, 22, 61–66. [Google Scholar]
- Khurana, E.; Singh, J.S. Ecology of seed and seedling growth for conservation and restoration of tropical dry forest: A review. Environ. Conserv. 2001, 28, 39–52. [Google Scholar] [CrossRef]
- Naves, V.L.; Rambal, S.; Barbosa, J.P.R.A.D.; de Castro, E.M.; Pasqual, M. Recruitment niches of Enterolobium contortisiliquum (Vell.) Morong: Functional acclimations to light. Forests 2018, 9, 266. [Google Scholar] [CrossRef]
- Hall, A.E. Crop physiological responses to light, photosynthesis, and respiration. In Crop Responses to Environment: Adapting to Global Climate Change, 2nd ed.; Hall, A.E., Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 37–62. [Google Scholar] [CrossRef]
- Morales, A.; Kaiser, E. Photosynthetic acclimation to fluctuating irradiance in plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, R.; Koyama, K. Photosynthetic and morphological acclimation to high and low light environments in Petasites japonicus subsp. giganteus. Forests 2020, 11, 1365. [Google Scholar] [CrossRef]
- Baligar, V.C.; Elson, M.K.; He, Z.; Li, Y.; Paiva, A.Q.; Almeida, A.A.F.; Ahnert, D. Light Intensity effects on the growth, physiological and nutritional parameters of tropical perennial legume cover crops. Agronomy 2020, 10, 1515. [Google Scholar] [CrossRef]
- Chapin, F.S.; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant responses to multiple environmental factors. BioScience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- Hiremath, A.J. Photosynthetic nutrient-use efficiency in three fast-growing tropical trees with differing leaf longevities. Tree Physiol. 2000, 20, 937–944. [Google Scholar] [CrossRef][Green Version]
- Berendse, F.; de Kroon, H.; Braakhekke, W.G. Acquisition, use, and loss of nutrients. In Functional Plant Ecology, 2nd ed.; Pugnaire, F., Valladares, F., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 259–283. [Google Scholar] [CrossRef]
- Müller, C.; Hodecker, B.E.R.; Merchant, A.; de Barros, N.F. Nutritional efficiency of Eucalyptus clones under water stress. Rev. Bras. Cienc. Solo 2017, 41, e0160528. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Poorter, L.; Kwant, R.; Hernández, R.; Medina, E.; Werger, M.J.A. Leaf optical properties in Venezuelan cloud forest trees. Tree Physiol. 2000, 20, 519–526. [Google Scholar] [CrossRef]
- Chapin, F.S.; Schulze, E.D.; Mooney, H.A. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Cetina-Alcalá, V.; Ortega-Delgado, M.; González-Hernández, V.; Vargas-Hernández, J.; Colinas-León, M.; Villegas-Monter, A. Fotosíntesis y contenido de carbohidratos de Pinus greggii Engelm. en respuesta a la poda y al régimen de riego en vivero. Agrociencia 2001, 35, 599–607. [Google Scholar]
- Valverde-Rodríguez, K.; Morales, C.O.; García, E.G. Germinación de semillas de Crescentia alata (Bignoniaceae) en distintas condiciones de temperatura, luminosidad y almacenamiento. Rev. Biol. Trop. 2019, 67, 120–131. [Google Scholar] [CrossRef]
- Viveros-Viveros, H.; Quino-Pascual, K.; Velasco-García, M.V.; Sánchez-Viveros, G.; Bautista, V.E. Variación geográfica de la germinación en Enterolobium cyclocarpum en la costa de Oaxaca, México. Bosque 2017, 38, 317–326. [Google Scholar] [CrossRef][Green Version]
- Torres, A.P.; Lopez, R.G. Medición de luz Diaria Integrada en Invernaderos; HO-238-SW; Purdue Extension, Purdue University: West Lafayette, IN, USA, 2016. [Google Scholar]
- Li, X.; Schmid, B.; Wang, F.; Paine, C.E.T. Net Assimilation rate determines the growth rates of 14 species of subtropical forest trees. PLoS ONE 2016, 11, e0150644. [Google Scholar] [CrossRef] [PubMed]
- Uscola, M.; Salifu, K.F.; Oliet, J.A.; Jacobs, D.F. An exponential fertilization dose-response model to promote restoration of the Mediterranean oak Quercus ilex. New For. 2015, 46, 795–812. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A modern tool for classical plant growth analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2008; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2008. [Google Scholar]

| Variable | p Value | Light Level (PPFD; %) | |||
|---|---|---|---|---|---|
| 25 | 35 | 55 | 70 | ||
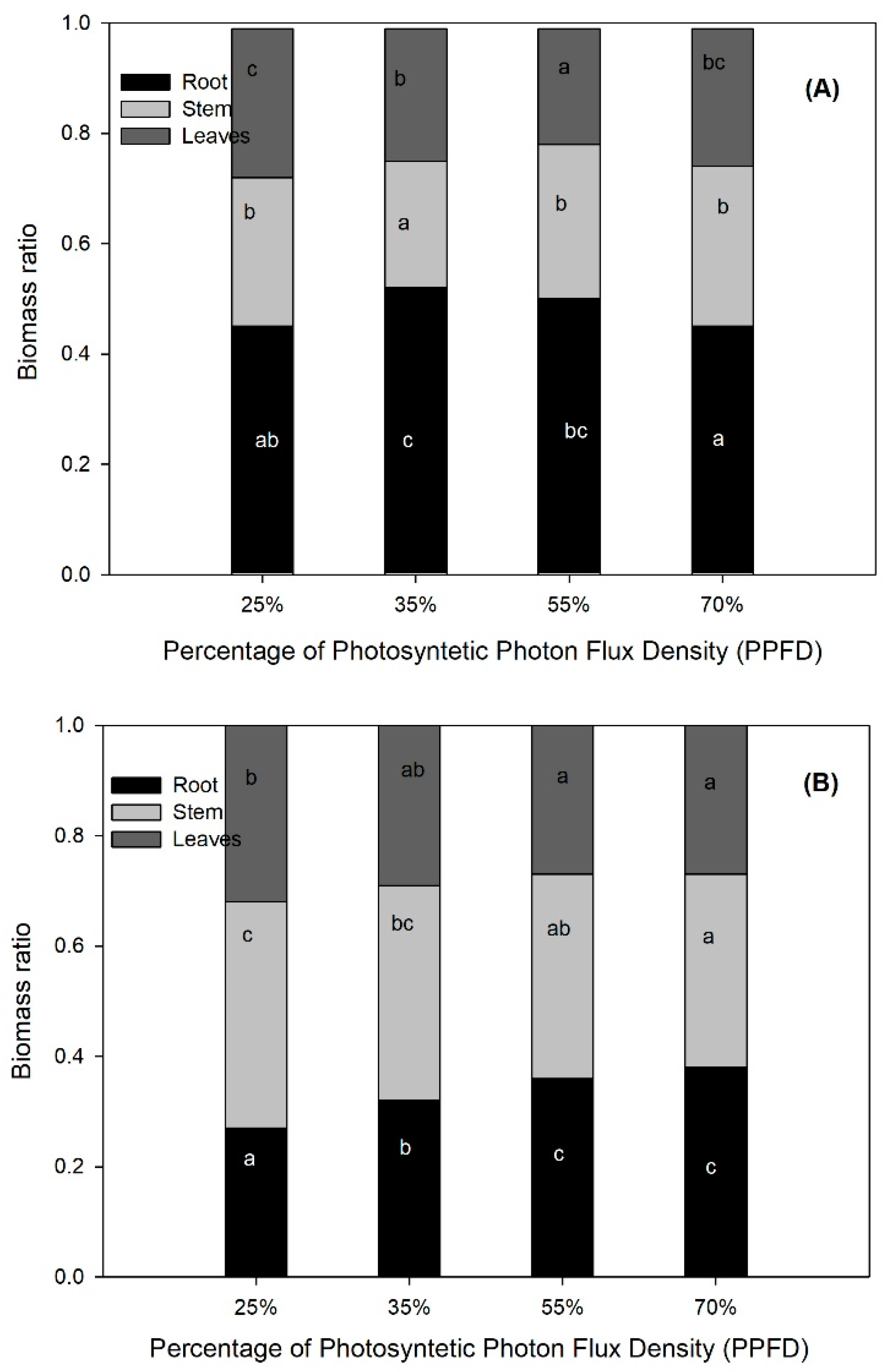
| SH (cm) | 0.0001 | 10.88 b | 8.71 a | 10.75 b | 12.29 c |
| RCD (mm) | 0.0389 | 4.85 a | 5.19 ab | 5.15 ab | 5.75 b |
| LB (g) | 0.0015 | 0.52 a | 0.43 a | 0.47 a | 0.63 b |
| SB (g) | 0.0001 | 0.52 ab | 0.42 a | 0.59 b | 0.71 c |
| RB (g) | 0.0918 | 0.89 | 0.93 | 1.06 | 1.12 |
| TB (g) | 0.0030 | 1.93 a | 1.78 a | 2.12 ab | 2.45 b |
| LA (cm2) | 0.0015 | 146.72 a | 134.01 a | 139.09 a | 163.23 b |
| SLA (cm2 g−1) | 0.0203 | 287.41 ab | 325.16 b | 313.84 b | 264.48 a |
| LAR (cm2 g−1) | 0.0038 | 77.71 b | 77.28 b | 67.27 a | 67.21 a |
| NAR (mg cm−2 day−1) | 0.0044 | 0.14 ab | 0.14 a | 0.16 bc | 0.17 c |
| RGR (mg g−1 day−1) | 0.0055 | 26.88 a | 26.14 a | 27.59 ab | 28.94 b |
| Light Level (PPFD; %) | Nutrient Concentration (%) | Nutrient Content (mg plant−1) | Nutrient Uptake Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | N | P | K | N | P | K | |
| 25 | 2.08 | 0.26 | 0.90 | 40.08 ab | 4.99 a | 13.63 | 11.04 ab | 4.45 a | 6.08 |
| 35 | 1.88 | 0.29 | 0.71 | 33.43a | 5.08 a | 14.69 | 9.05 a | 4.53 a | 6.55 |
| 55 | 1.88 | 0.28 | 0.83 | 39.82 ab | 5.90 ab | 15.00 | 10.96 ab | 5.26 ab | 6.69 |
| 70 | 2.38 | 0.34 | 0.71 | 58.27 b | 8.45 b | 22.08 | 16.47 b | 7.54 b | 9.86 |
| p value | 0.05 | 0.103 | 0.306 | 0.032 | 0.043 | 0.086 | 0.032 | 0.043 | 0.086 |
| Variable | p Value | Light Level (PPFD; %) | |||
|---|---|---|---|---|---|
| 25 | 35 | 55 | 70 | ||
| SH (cm) | <0.0001 | 50.75 c | 45.00 b | 40.08 ab | 36.25 a |
| RCD (mm) | 0.1977 | 5.17 | 5.65 | 5.40 | 5.35 |
| LB (g) | 0.3821 | 1.48 | 1.37 | 1.33 | 1.22 |
| SB (g) | 0.2028 | 1.89 | 1.86 | 1.78 | 1.52 |
| RB (g) | 0.0042 | 1.24 a | 1.48 ab | 1.68 b | 1.60b |
| TB (g) | 0.7046 | 4.62 | 4.70 | 4.79 | 4.33 |
| LA (cm2) | 0.3821 | 220.31 | 203.12 | 198.21 | 181.02 |
| SLA (cm2 g−1) | 0.3220 | 148.58 | 148.71 | 148.76 | 149.36 |
| LAR (cm2 g−1) | 0.0445 | 47.37 b | 43.07 ab | 41.17 a | 40.73 a |
| NAR (mg cm−2 day−1) | 0.1278 | 0.39 | 0.42 | 0.43 | 0.40 |
| RGR (mg g−1 day−1) | 0.4892 | 19.84 | 19.92 | 20.03 | 18.88 |
| Light Level (PPFD; %) | Nutrient Concentration (%) | Nutrient Content (mg) | Nutrient Uptake Efficiency (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | N | P | K | N | P | K | |
| 25 | 2.92 b | 0.27 | 0.95 | 134.75 b | 12.65 | 44.07 | 27.14 b | 7.57 | 14.06 |
| 35 | 2.23 ab | 0.27 | 0.82 | 104.73 ab | 12.57 | 38.58 | 19.82 ab | 7.51 | 12.05 |
| 55 | 1.93 a | 0.27 | 0.80 | 92.21 a | 12.98 | 38.41 | 16.77 a | 7.81 | 11.99 |
| 70 | 1.86 a | 0.30 | 0.94 | 80.32 a | 13.04 | 40.59 | 13.88 a | 7.86 | 12.79 |
| p value | 0.024 | 0.644 | 0.281 | 0.018 | 0.826 | 0.546 | 0.018 | 0.826 | 0.546 |
| Environmental Variable | Light Level (PPFD; %) | ||||
|---|---|---|---|---|---|
| 25 | 35 | 55 | 70 | Open Sky * | |
| Maximum temperature (°C) ¶ | 36.0 ± 3.1 | 36.4 ± 3.0 | 36.6 ± 3.2 | 42.6 ± 4.5 | - |
| Minimum temperature (°C) ¶ | 15.9 ± 1.6 | 14.1 ± 1.5 | 15.5 ± 1.7 | 14.8 ± 1.8 | - |
| Relative humidity (%) ¶ | 27.4 ± 6.4 | 28.3 ± 8.5 | 28.5 ± 8.1 | 26.5 ± 6.2 | - |
| Daily light integral (mol m−2 day−1) | 9.6 | 13.5 | 21.2 | 27.0 | 38.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basave-Villalobos, E.; Cetina-Alcalá, V.M.; Conde-Martínez, V.; López-López, M.Á.; Trejo, C.; Ramírez-Herrera, C. Morpho-Physiological Responses of Two Multipurpose Species from the Tropical Dry Forest to Contrasting Light Levels: Implications for Their Nursery and Field Management. Plants 2022, 11, 1042. https://doi.org/10.3390/plants11081042
Basave-Villalobos E, Cetina-Alcalá VM, Conde-Martínez V, López-López MÁ, Trejo C, Ramírez-Herrera C. Morpho-Physiological Responses of Two Multipurpose Species from the Tropical Dry Forest to Contrasting Light Levels: Implications for Their Nursery and Field Management. Plants. 2022; 11(8):1042. https://doi.org/10.3390/plants11081042
Chicago/Turabian StyleBasave-Villalobos, Erickson, Víctor M. Cetina-Alcalá, Víctor Conde-Martínez, Miguel Á. López-López, Carlos Trejo, and Carlos Ramírez-Herrera. 2022. "Morpho-Physiological Responses of Two Multipurpose Species from the Tropical Dry Forest to Contrasting Light Levels: Implications for Their Nursery and Field Management" Plants 11, no. 8: 1042. https://doi.org/10.3390/plants11081042
APA StyleBasave-Villalobos, E., Cetina-Alcalá, V. M., Conde-Martínez, V., López-López, M. Á., Trejo, C., & Ramírez-Herrera, C. (2022). Morpho-Physiological Responses of Two Multipurpose Species from the Tropical Dry Forest to Contrasting Light Levels: Implications for Their Nursery and Field Management. Plants, 11(8), 1042. https://doi.org/10.3390/plants11081042